Abstract
Background:
Vitamin K antagonists (VKAs) may have potential antitumor effects in prostate cancer. However, the findings of observational studies are inconsistent. The purpose of the present study was to estimate the quantitative association between VKAs use and prostate cancer risk by combining the results of all eligible observational studies.
Methods:
PubMed and Web of Science database were searched from inception until May, 2018. A DerSimonian random-effects model was used to combine the studies. Study heterogeneity was measured using the chi-squared and I2 statistics.
Results:
Six eligible studies were eventually included in our meta-analysis. There was an inverse but not statistically significant association between ever use of VKAs and the risk of prostate cancer (relative risk [RR] 0.84, 95% confidence interval [CI] 0.70–1.01, P = .063) with large heterogeneity across studies (P < .001 for heterogeneity, I2 = 94.6%). When analysis restricted to long term of VKAs user (>3 years), the pooled risk estimate was 0.83 (0.77–0.90) without obvious heterogeneity (P = .597, I2 = 0.0%).
Conclusion:
This meta-analysis indicates that VKAs use may be associated with a decreased risk of prostate cancer, especially in long-term users.
Keywords: meta-analysis, prostate cancer, risk, vitamin K antagonist
1. Introduction
Prostate cancer is one of the most common cancers that affect the male population worldwide.[1] According to Torre report published in 2015, there were 1,111,700 new cases and 307,500 deaths estimated to have occurred in 2012.[2] Well-established risk factors for prostate cancer include increased age, race/ethnicity (African–American or Jamaican), and a positive family history.[3] Additional but less established risk factors include physical activity, body mass index, and some dietary factors.[4–7]
Emerging studies have indicated that the coagulation cascade and thrombocytes implicated in the development and progression of cancer, and that anticoagulant drugs could in theory improve prognosis.[8,9] For instance, activated platelets release secretory factors, such as proangiogenic regulatory proteins, chemokines, and microparticles within the microenvironment, to promote tumor growth and metastasis.[10] In addition, thrombocytes coating circulating tumor cells offer a physical shield around tumor cells and, and thereby protecting from immune elimination.[10,11] Vitamin K antagonists (VKAs) are widely used anticoagulants worldwide for a range of indications, including atrial fibrillation, prosthetic heart valves, and venous thromboembolism.[12,13] There is evidence that VKAs, especially warfarin, have strong antitumor and anti-metastatic effects in different experimental cancer model systems.[14,15] Also, in observational studies, such as case-control and cohort studies, the potential relationship between VKAs use and prostate cancer risk has been investigated with inconsistent findings.[16,17]
Up to now, no meta-analysis has been published regarding the relationship between VKAs use and prostate cancer risk. The purpose of the present study was to estimate the quantitative association between VKAs use and prostate cancer risk by performing a meta-analysis of case-control and cohort studies.
2. Materials and methods
2.1. Search strategy
Two investigators (JDL and HZM) independently performed an electronic literature search using PubMed and Web of Science databases from their inception until May 15, 2018 for studies comparing the incidence of prostate cancer between VKAs users and non-users. There was no restriction on publication language or date. The following keywords were used in search strategy: (“anticoagulants” OR “vitamin K antagonist” OR “warfarin” OR “coumadin”) and (“prostate cancer” OR “prostate neoplasm”). Reference lists of included publications, as well as those relevant reviews, were examined for identification of additional potentially relevant studies. This study was approved by the Institutional Ethics Committee of the First Affiliated Hospital of Zhejiang University.
2.2. Study selection
We included observational studies comparing data on prostate cancer risk between VKAs users and non-users. Studies were included if that reported incidence of prostate cancer, included patients treated with VKAs, and compared VKAs users with non-users. Studies were excluded if they did not provide the information of VKAs used, were animal or cell line studies, did not compare VKAs users with non-users, or did not report data about prostate cancer. This systematic review was performed in accordance with the indications of Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.[18]
2.3. Study quality assessment
A 9-star Newcastle-Ottawa Scale (NOS) (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) was used to evaluate the quality of each selected study by 2 independent reviewers (JDL and HZM). NOS judges a study based on 3 subscales: selection (4 items), comparability (1 item), and exposure/outcome (3 items). Each item is awarded 1 point, except for comparability (2 points). A study with ≥7 awarded points is considered as high quality.
2.4. Data extraction
Two authors (JDL and HZM) independently extracted data from the selected studies into a standardized pre-defined form. Any disagreement was resolved by consensus. The following information were extracted: study population characteristics (e.g., sample size, average age of population), duration of follow-up (for cohort studies), region in which the study was performed, parameters related to VKA therapy (e.g., anticoagulation duration, type of VKAs), matched or adjusted variables in study design and analysis, and methods of exposure and outcome assessment.
2.5. Statistical analysis
The primary outcome was the prevalence or incidence of prostate cancer, comparing VKAs ever users with never users. Considering prostate cancer is a rare disease, the odds ratio (OR) is a close estimator of the relative risk (RR). RR with its 95% confidence interval (CI) was used for summary analysis.
The meta-analysis was performed using STATA 10.0 (StataCorp, College Station, TX). When combining studies, the DerSimonian random-effects model[19] was used to account for study heterogeneity. Study heterogeneity was measured using the chi-squared and I2 statistics, with chi-squared P ≤.1 and I2 ≥50% indicating the presence of significant heterogeneity.[20] Because anticoagulation exposure duration could mediate the effect of VKAs on prostate cancer, we also performed preplanned sensitivity (divided according to the duration of VKAs use) analysis. A funnel plot was inspected visually to assess the possibility of publication bias.
3. Results
3.1. Literature search and study characteristics
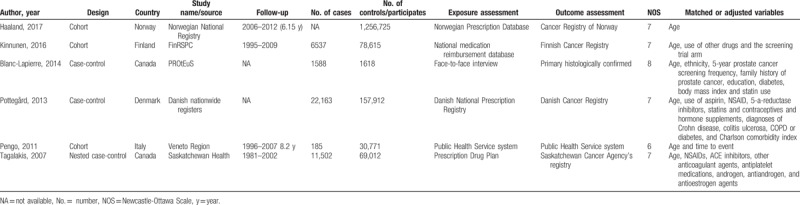
The detailed steps of literature search are presented in Fig. 1. After excluding 201 duplicates and 706 studies that were obviously not relevant (e.g., reviews, non-human studies, and studies concerning other types of cancer), 12 studies were assessed by full text reading. Four studies that reported survival as outcome, 1 study that reported heart valve replacement as exposure, and 1 study that was lack of enough data were further removed. Six eligible studies[16,17,21–24] were eventually included in this meta-analysis of the association between VKAs use and prostate cancer risk. These studies (3 cohort, 1 nested case-control, and 2 case-control studies) were performed in Canada (n = 2) and Europe (n = 4). All of the included studies were published between 2007 and 2017. Assessment of exposure and outcome was mainly based on medical records or databases. The study quality scores, assessed by the NOS, ranged from 6 to 8. Table 1 shows the characteristics of each study included in this meta-analysis.
Figure 1.
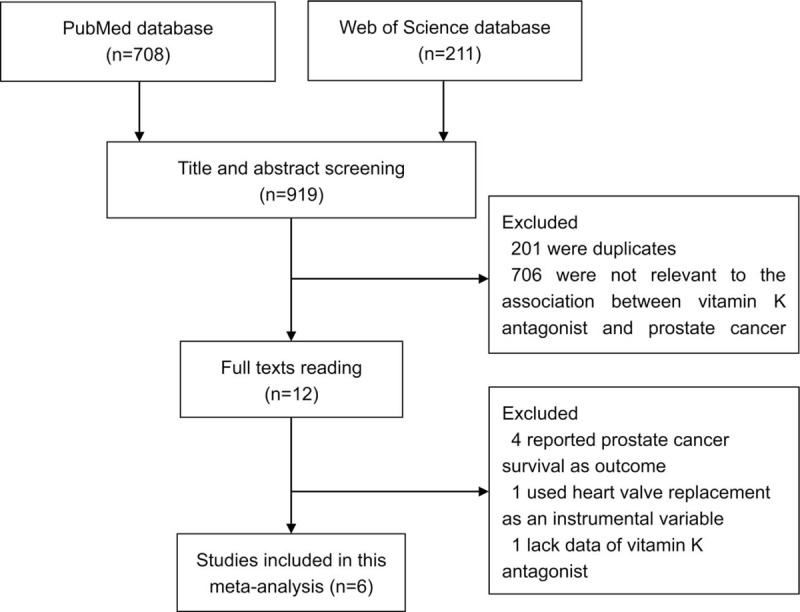
Flow diagram of study selection process.
Table 1.
Main characteristics of studies included in this meta-analysis.
3.2. Overall and subgroup analysis
The multivariable-adjusted RRs for each study and for the combination of all included studies are shown in Fig. 2. Six studies were included in the summary analysis. Pooled risk estimate was calculated with a DerSimonian random-effects model. There was an inverse but not statistically significant association of ever use of VKAs with the risk of prostate cancer (RR 0.84, 95% CI 0.70–1.01, P = .063). Statistically significant heterogeneity was observed across studies (P < .001 for heterogeneity, I2 = 94.6%).
Figure 2.
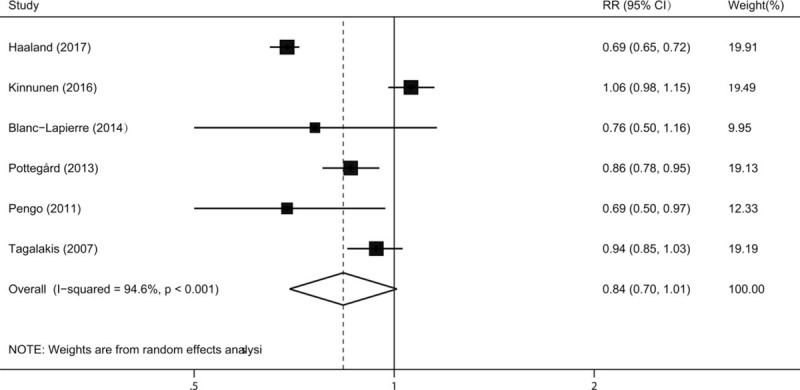
Relative risk for incident prostate cancer in vitamin K antagonists users compared with non-users.
Next, we performed subgroup analyses by study design and geographical region. When stratified by study region, the RRs (95% CIs) were 0.93 (0.85–1.02) and 0.82 (0.64–1.05) for studies performed in North America and Europe, respectively. In the subgroup analyses separated by study design, a more pronounced association was detected in case-control studies (RR 0.85, 95% CI 0.78–0.94) than that in cohort studies (RR 0.84, 95% CI 0.85–1.09).
3.3. Sensitivity analysis
We firstly evaluated the impact of each study on the combined RR by repeating the meta-analysis after omitting each study in turn. The summary RRs (95% CIs) ranged from 0.80 (0.67–0.94) to 0.91 (0.80–1.03) by omitting the studies by Kinnunen et al.[16] and Haaland et al.[21], respectively (Fig. 3). In addition, we evaluated the effect of long-term use of VKAs on the risk of prostate cancer. Four studies[17,22–24] provided data for VKAs use >3 years and the pooled risk estimate of these studies using a DerSimonian random-effects model was 0.83 (0.77–0.90) without obvious heterogeneity (P = .597, I2 = 0.0%) (Fig. 4). Considering reverse causation bias, we included studies with at least 6-month latency period. Four studies[17,21–23] were eligible and the pooled risk estimate using a DerSimonian random-effects model was 0.75 (0.64–0.89) with significant heterogeneity across studies (P = .002, I2 = 80.3%).
Figure 3.
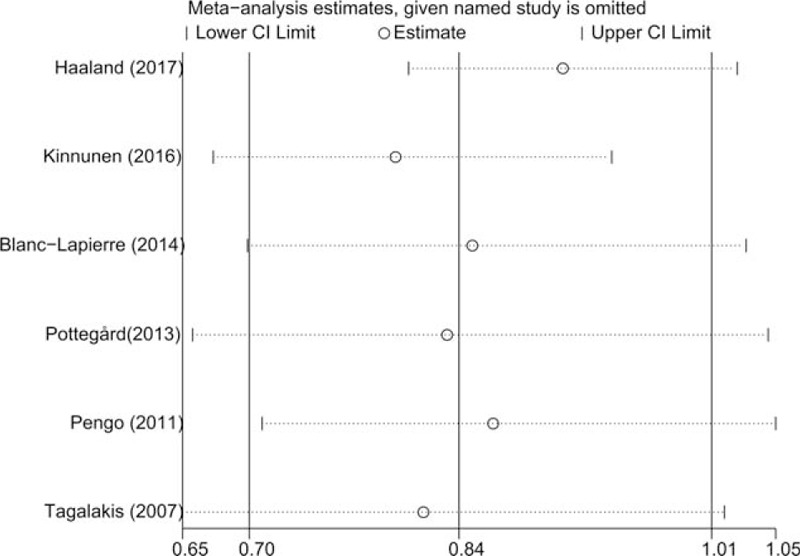
Sensitivity analysis was performed by repeating the meta-analysis after omitting each study in turn.
Figure 4.
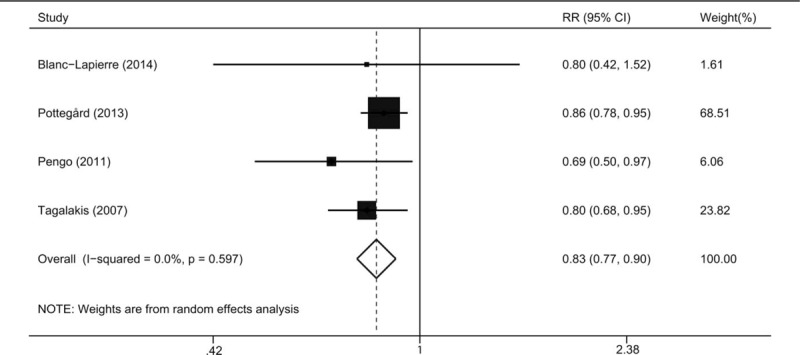
Relative risk for incident prostate cancer in long term vitamin K antagonists users.
3.4. Publication bias
A funnel plot (Fig. 5) is a scatter plot of the studies included in this meta-analysis (represented by black dots) in a space defined by effect size (on the x-axis; scale displayed on top of the plot) and standard error (on the y-axis). A certain degree of asymmetry was observed on funnel plot, which indicated that some publication bias might exist.
Figure 5.
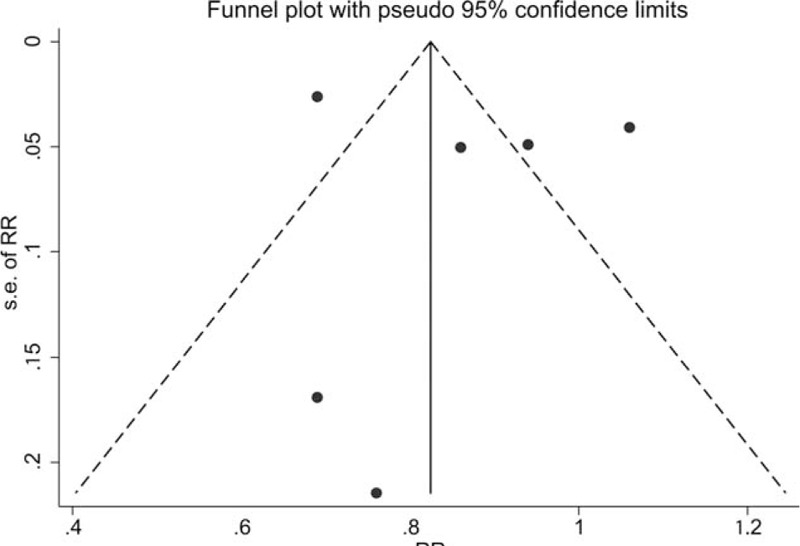
A funnel plots of studies assessing incident prostate cancer in vitamin K antagonists users compared with non-users.
4. Discussion
This systematic review and meta-analysis summarized the results of observational studies on the relationship between use of VKAs and prostate cancer risk, including 3 cohort studies, 1 nested case-control studies, and 2 case-control studies. The summary results indicated that VKAs use might be associated with a reduced risk of prostate cancer, especially for those long-term users.
Obvious heterogeneity was observed among the selected studies, which would weaken the findings of this study. The heterogeneity might attribute to the differences in study design, population source, the method of exposure, and outcome assessment, and so on. However, in the analysis of long-term VKAs users, there was no significant heterogeneity and the pooled effect size estimate was statistically significant. We speculated that when analysis restricted to certain population, the heterogeneity was reduced.
An inverse association between VKAs use and prostate cancer risk is biologically plausible. Choo et al[25] reported that fucoidan had anticancer effects on prostate cancer cells through inhibition of PI3K/Akt and MAPK pathway. The study by Rui et al[26] indicated that fucoidan had anti-tumor and anti-angiogenic effects in both cell-based assays and mouse xenograft model by regulating JAK-STAT3 pathway. Tew et al[27] found that vitamin K epoxide reductase (VKOR) expression was not expressed or expressed at very low levels in cancerous cells compared with that in benign human prostate epithelial cells, implying a possible role for VKOR in mediating the effect of VKAs on prostate cancer risk. Neubauer et al[28] found that warfarin had dose-dependent inhibitory effect of on the metastasis of the PAIII prostatic adenocarcinoma in the rat. All these findings imply a new concept that VKAs are potential chemopreventive and chemotherapeutic agents for prostate cancer.
In our meta-analysis, an inverse but not statistically significant association was observed in overall analysis. By contrast, in sensitivity analysis, we observed that long-term VKAs users had a significant reduced risk of prostate cancer, which indicated that chronic use of VKAs, instead of short-term VKAs use, had a protective effect on prostate cancer. In addition, venous thrombosis embolism (VTE) has been reported to be associated with cancer incidence.[29,30] In our study, when analysis restricted to studies with a 6-month latency period, we also found a significant inverse association between VKAs exposure and prostate cancer risk, which implied a less possibility of reverse causation. However, these secondary analyses were based on sub-population and hence the findings should be treated with caution.
Several important limitations should to be considered in interpreting the findings of current meta-analysis. First, the quality of a meta-analysis mainly depended on the included studies. A meta-analysis is unable to solve the problem of inadequate control of all known confounders in original studies. Second, the current evidence on this association remains limited. Only 6 studies were eligible for this meta-analysis. Third, we could not quantify a dose–response association since dose data were not available in most included studies. Fourth, patients treated with VAKs are under increased medical surveillance, which may cause detection bias. Lastly, although we have performed a comprehensive literature search, some inevitable publication bias may exist, because gray literature (i.e., conference abstracts) is difficult to find and small negative studies are less likely to be published.
5. Conclusion
This meta-analysis indicates that VKAs use may be associated with a decreased risk of prostate cancer, especially in long-term users. Further large well designed randomized controlled trials with long follow-up are warranted to further evaluate the impact of VKAs use on the risk of prostate cancer.
Author contributions
Conceptualization: Jin-Dan Luo, Jie Luo, Jun Chen, Hong-Zhou Meng.
Data curation: Jin-Dan Luo, Jie Luo, Chong Lai, Hong-Zhou Meng.
Formal analysis: Jin-Dan Luo, Jie Luo, Chong Lai, Jun Chen, Hong-Zhou Meng.
Investigation: Jin-Dan Luo, Jie Luo, Hong-Zhou Meng.
Methodology: Jin-Dan Luo, Jie Luo, Hong-Zhou Meng.
Resources: Jin-Dan Luo, Chong Lai, Hong-Zhou Meng.
Software: Jin-Dan Luo, Chong Lai, Hong-Zhou Meng.
Supervision: Jin-Dan Luo, Jun Chen, Hong-Zhou Meng.
Validation: Jin-Dan Luo, Hong-Zhou Meng.
Visualization: Jin-Dan Luo, Hong-Zhou Meng.
Writing – original draft: Jin-Dan Luo, Jie Luo.
Writing – review & editing: Jin-Dan Luo, Hong-Zhou Meng.
Project administration: Jun Chen, Hong-Zhou Meng.
Funding acquisition: Hong-Zhou Meng.
Footnotes
Abbreviations: CI = confidence interval, NOS = Newcastle-Ottawa Scale, OR = odds ratio, RR = relative risk, VKAs = vitamin K antagonists.
This study was supported by the Zhejiang Provincial Natural Science Foundation of China (Grant Number LY17H160019), the Scientific Research Fund of Zhejiang Provincial Education Department (Grant Number Y201737704), the Public Welfare Technology Application Research Project of Zhejiang Province (Grant Number 2016C33065), and the Medical and Health Science and Technology Project of Zhejiang Province (Grant Number 2016142965).
The authors report no conflicts of interest.
References
- [1].Attard G, Parker C, Eeles RA, et al. Prostate cancer. Lancet 2016;387:70–82. [DOI] [PubMed] [Google Scholar]
- [2].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [3].Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 2006;98:529–34. [DOI] [PubMed] [Google Scholar]
- [4].Liu Y, Hu F, Li D, et al. Does physical activity reduce the risk of prostate cancer? A systematic review and meta-analysis. Eur Urol 2011;60:1029–44. [DOI] [PubMed] [Google Scholar]
- [5].Liu B, Mao Q, Cao M, et al. Cruciferous vegetables intake and risk of prostate cancer: a meta-analysis. Int J Urol 2012;19:134–41. [DOI] [PubMed] [Google Scholar]
- [6].Xu X, Cheng Y, Li S, et al. Dietary carrot consumption and the risk of prostate cancer. Eur J Nutr 2014;53:1615–23. [DOI] [PubMed] [Google Scholar]
- [7].Rodriguez C, Freedland SJ, Deka A, et al. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev 2007;16:63–9. [DOI] [PubMed] [Google Scholar]
- [8].McCulloch P, George WD. Warfarin inhibition of metastasis: the role of anticoagulation. Br J Surg 1987;74:879–83. [DOI] [PubMed] [Google Scholar]
- [9].Bobek V, Boubelik M, Kovarik J, et al. Inhibition of adhesion breast cancer cells by anticoagulant drugs and cimetidine. Neoplasma 2003;50:148–51. [PubMed] [Google Scholar]
- [10].Wang S, Li Z, Xu R, et al. Human cancer and platelet interaction, a potential therapeutic target. Int J Mol Sci 2018;19:pii: E1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tesfamariam B. Involvement of platelets in tumor cell metastasis. Pharmacol Ther 2016;157:112–9. [DOI] [PubMed] [Google Scholar]
- [12].Dossett LA, Riesel JN, Griffin MR, et al. Prevalence and implications of preinjury warfarin use: an analysis of the National Trauma Databank. Arch Surg 2011;146:565–70. [DOI] [PubMed] [Google Scholar]
- [13].Keeling D, Baglin T, Tait C, et al. Guidelines on oral anticoagulation with warfarin - fourth edition. Br J Haematol 2011;154:311–24. [DOI] [PubMed] [Google Scholar]
- [14].Kirane A, Ludwig KF, Sorrelle N, et al. Warfarin blocks Gas6-mediated Axl activation required for pancreatic cancer epithelial plasticity and metastasis. Cancer Res 2015;75:3699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Williamson RC, Lyndon PJ, Tudway AJ. Effects of anticoagulation and ileal resection on the development and spread of experimental intestinal carcinomas. Br J Cancer 1980;42:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kinnunen PT, Murtola TJ, Talala K, et al. Warfarin use and prostate cancer risk in the Finnish Randomized Study of Screening for Prostate Cancer. Scand J Urol 2016;50:413–9. [DOI] [PubMed] [Google Scholar]
- [17].Blanc-Lapierre A, Weiss D, Parent ME. Use of oral anticoagulants and risk of prostate cancer: a population-based case-control study in Montreal, Canada. Cancer Causes Control 2014;25:1159–66. [DOI] [PubMed] [Google Scholar]
- [18].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [20].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [21].Haaland GS, Falk RS, Straume O, et al. Association of warfarin use with lower overall cancer incidence among patients older than 50 years. JAMA Intern Med 2017;177:1774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pottegard A, Friis S, Hallas J. Cancer risk in long-term users of vitamin K antagonists: a population-based case-control study. Int J Cancer 2013;132:2606–12. [DOI] [PubMed] [Google Scholar]
- [23].Pengo V, Noventa F, Denas G, et al. Long-term use of vitamin K antagonists and incidence of cancer: a population-based study. Blood 2011;117:1707–9. [DOI] [PubMed] [Google Scholar]
- [24].Tagalakis V, Tamim H, Blostein M, et al. Use of warfarin and risk of urogenital cancer: a population-based, nested case-control study. Lancet Oncol 2007;8:395–402. [DOI] [PubMed] [Google Scholar]
- [25].Choo GS, Lee HN, Shin SA, et al. Anticancer effect of fucoidan on DU-145 prostate cancer cells through inhibition of PI3K/Akt and MAPK pathway expression. Mar Drugs 2016;14:pii: E126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rui X, Pan HF, Shao SL, et al. Anti-tumor and anti-angiogenic effects of Fucoidan on prostate cancer: possible JAK-STAT3 pathway. BMC Complement Altern Med 2017;17:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tew BY, Pal SK, He M, et al. Vitamin K epoxide reductase expression and prostate cancer risk. Urol Oncol 2017;35:112.e13–8. [DOI] [PubMed] [Google Scholar]
- [28].Neubauer BL, Bemis KG, Best KL, et al. Inhibitory effect of warfarin on the metastasis of the PAIII prostatic adenocarcinoma in the rat. J Urol 1986;135:163–6. [DOI] [PubMed] [Google Scholar]
- [29].Sorensen HT, Mellemkjaer L, Steffensen FH, et al. The risk of a diagnosis of cancer after primary deep venous thrombosis or pulmonary embolism. N Engl J Med 1998;338:1169–73. [DOI] [PubMed] [Google Scholar]
- [30].Baron JA, Gridley G, Weiderpass E, et al. Venous thromboembolism and cancer. Lancet 1998;351:1077–80. [DOI] [PubMed] [Google Scholar]


