Abstract
Cocoa is a rich source bioactive compounds, i.e., flavan-3-ols, and its consumption has been associated with several beneficial effects, such as the positive modulation of the hemostasis targeted by the platelet function. However, these phenolic compounds have a very low bioavailability and extensively undergo phase I and II metabolism, with the appearing into the bloodstream of (epi)catechin conjugates and phenyl-γ-valerolactones and their conjugates, at different times.
The aims of this study were to explore the effect of dark chocolate on platelet function and to investigate the relationship between this interplay and flavan-3-ol derived metabolites.
Eighteen healthy male volunteers ingested 50 g of 90% cocoa chocolate within 5 minutes. Blood samples were collected immediately before chocolate ingestion (T0) and 4 hours afterwards (T1). Platelet function analyzer (PFA)-100 closure time was assessed using collagen/adenosine-5′-diphosphate (COL/ADP) and collagen/epinephrine (COL/EPI) cartridges. Plasma flavan-3-ol metabolites were identified and quantified by means of liquid chromatography coupled to a triple quadrupole mass spectrometer (UHPLC-ESI-MS/MS).
Results evidenced a significant increase of COL/ADP-induced PFA-100 closure time, but not COL/EPI, 4 hours after ingestion of dark chocolate. Total plasma structurally-related (epi)catechin metabolite (SREM) concentration significantly increased at T1, together with 4 out of the 6 detected metabolites. Total phenyl-γ-valerolactone concentrations remained unchanged. Spearman correlations evidenced a strong correlation between COL/ADP closure time and SREMs, mainly led by (epi)catechin-sulfate isomers.
These data confirm that the potential beneficial effect of dark chocolate on primary hemostasis may be mediated by flavan-3-ol circulating metabolites.
Keywords: cocoa, flavan-3-ols, hemostasis, platelet function analyzer (PFA)-100, phenolic metabolite, phenyl-γ-valerolactones
1. Introduction
Reliable evidence exists to suggest that consumption of flavan-3-ol-rich food sources may generate a kaleidoscope of health benefits, including improvement of vascular function and blood pressure.[1,2] Cocoa is rich in flavan-3-ols, a class of flavonoids which includes monomers like (−)-epicatechin and (+)-catechin, and polymers like procyanidins. Monomers are able to cross the gut epithelium, to be absorbed and to peak into the bloodstream within 1 or 2 hours after ingestion, mainly as glucuronide, sulfate, or methyl conjugates[3] (hereinafter referred as structurally-related (epi)catechin metabolites, SREMs). Dimers and oligomers are poorly absorbed and undergo, as well as monomers, an intense gut microbiota metabolism, with the generation of lower molecular weight compounds, such as phenyl-γ-valerolactones.[3] These smaller metabolites appear into the bloodstream, together with their conjugated forms, at later times, namely from 3 to 24 hours after food consumption, easily reaching micromolar concentrations.[4] For these reasons, there is still a debate regarding the endorsement of flavan-3-ols and the derived metabolites as main responsible of the effects of cocoa intake on primary and secondary hemostasis in healthy subjects.[5–9] Recent evidence shows that dietary flavan-3-ols may positively modulate hemostasis, acting through mechanisms that both directly and indirectly influence platelet function.[10] Other than primary hemostasis, platelets play also an important physiological role in many atherothrombotic, inflammatory, and neoplastic diseases.[11] Platelets actively interact with leucocytes and endothelial cells, release procoagulant factors, and also produce cytokines and chemokines which ultimately interplay with the immune response.[12] A preliminary intervention study from our group showed a significant influence of dark chocolate consumption by healthy volunteers on secondary hemostasis, but it has not been yet fully ascertained which of the compounds may be correlated to the potential anti-thrombotic effects through attenuation of the clotting cascade.[13]
Therefore, this study was aimed at investigating the acute impact of dietary intake of cocoa on primary hemostasis and determining whether these changes may be associated with flavan-3-ol circulating metabolites.
2. Materials and methods
The study was carried out on 18 healthy men volunteers enrolled from the laboratory staff (age: 36 ± 10 years; body mass index (BMI): 22 ± 3 kg/m2), who ingested 50 g of commercial dark (90% cocoa) chocolate (Noir Prodigieux, Lindt, Kilchberg, Switzerland), in a maximum time of 5 minutes. The nutritional characteristics of the 50 g of dark chocolate were as follows: 1242 kJ (i.e., 296 kcal), 27.5 g of lipids (15 g saturated fat acids), 7 g of carbohydrates (3.5 g of sugars), 5 g of proteins, and 7.5 gallic acid equivalents of polyphenols. Blood was drawn early in the morning, immediately before chocolate ingestion (T0) and 4 hours afterwards (T1). Volunteers were asked to fast for 8 hours before blood samples collection and to abstain from eating and drinking any food or beverage except water between the 2 sampling points. Exclusion criteria were BMI >30 kg/m2, blood pressure (BP) >140/90 mmHg, allergies to peanut or cocoa, current intake of dietary supplements, vegan/vegetarian diet. Blood sampling was carried out by the same expert phlebotomist, into evacuated blood tubes containing 0.109 mol/L K2EDTA or 0.109 mol/L buffered sodium citrate (Vacutest Kima, Padova, Italy). All participants provided an informed consent for participation to this study, which was carried out in accordance with the Declaration of Helsinki and was approved by the local ethical committee (University Hospital of Verona; 971CESC, July 25, 2016).
2.1. Laboratory methods
Platelet function (i.e., adhesion and generation of stable aggregates) was investigated using whole blood collected immediately before dark chocolate ingestion and 4 hours afterwards, using a Platelet Function Analyzer 100 (PFA-100; Siemens Healthcare Diagnostics Products; Marburg, Germany GmbH) instrument. This device alternatively uses equine type I collagen and epinephrine bitartrate (collagen/epinephrine) and equine type I collagen and adenosine-5′-diphosphate (collagen/ADP) as agonists.[14] The platelet function event is detected as an end-point of adhesion, activation and aggregation, and expressed as a closure time of both collagen/epinephrine and collagen/ADP. The local reference ranges are 82 to 164 seconds and 62 to 116 seconds for collagen/ADP and for collagen/epinephrine, respectively, while the total imprecision values of both assay components are 5.5% and 4.3% for collagen/epinephrine and for collagen/ADP, respectively.[15]
Concerning circulating polyphenols assessment, plasma samples of all volunteers were extracted using a solid phase extraction (SPE) method, as reported elsewhere.[4] Flavan-3-ol metabolites were measured in extracted plasma samples by a UHPLC DIONEX Ultimate 3000 equipped with a triple quadrupole TSQ Vantage mass spectrometer (Thermo Fisher Scientific Inc., San José, CA) fitted with a heated-ESI (H-ESI) (Thermo Fisher Scientific Inc., San José, CA) probe. Chromatographic separation and ionization parameters were set as previously reported.[16] Quantification was performed with calibration curves of standards, when available. When not available, the conjugated metabolites were quantified with the most structurally similar compound. Phenyl-γ-valerolactone conjugates were synthesized in house.[16]
2.2. Statistics
The Kolmogorov–Smirnov test was used to test normality of distribution for all variables. Significance of differences between T0 and T1 was tested with paired Wilcoxon signed-rank test and the correlation between values was assessed with Spearman test. All values are reported as median ± interquartile range (IQR) and the reported P values are 2-tailed, with a P value <.05 indicating statistical significance. Statistical analyses were performed with IBM SPSS 25.0 Statistics (IBM SPSS, Inc., Chicago, IL).
3. Results
A total of 13 phenolic compounds were identified and quantified in plasma samples of healthy men volunteers (Table 1). The retention times and spectrometric characteristics of the detected compounds are reported in Table 2. A significant increase in the sum of SREMs was observed at T1 compared with T0 (1.99 ± 0.90 μmol/L vs 0.99 ± 1.57, P = .021, respectively) (Fig. 1). Particularly, a significant increase, after 4 hours chocolate consumption, was evidenced in plasma for compounds 1 (P = .004), 3 (P = .043), 5 (P = .035), and 6 (P = .039) (Fig. 1). No significant differences were observed between the sum of all phenyl-γ-valerolactone concentrations measured at T0 and T1 (0.85 ± 1.45 vs 1.11 ± 1.13 μmol/L, P = .54, respectively) (Fig. 2). Among phenyl-γ-valerolactones, compound 7 was detected in very few plasma samples and it was excluded from the results due to its high inter-individual variability. A slight, but significant (P = .007) decrease of compound 9 has been observed at T1 compared with baseline (Fig. 2).
Table 1.
Baseline anthropometric and biochemical characteristics of healthy volunteers.
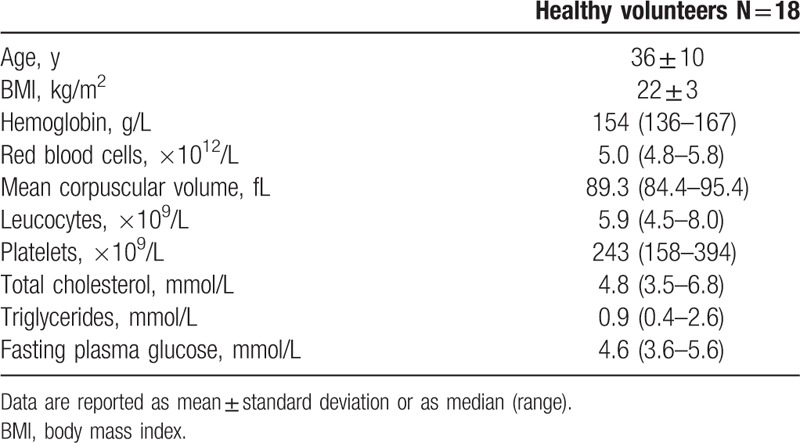
Table 2.
(Epi)catechin and phenyl-γ-valerolactone metabolites detected in plasma samples.
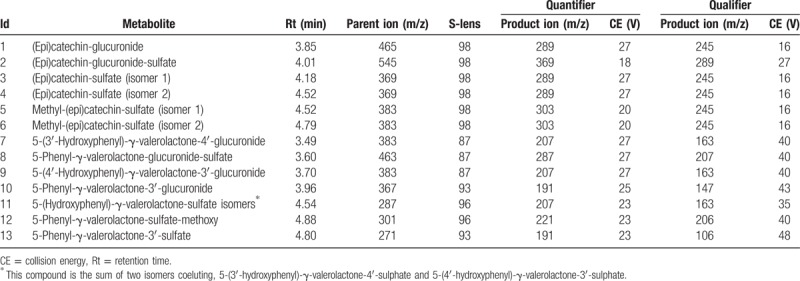
Figure 1.
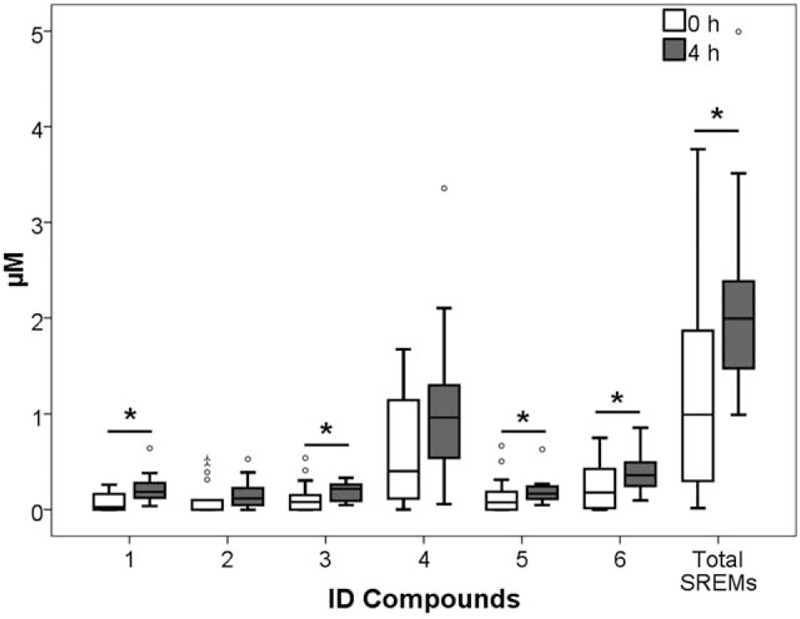
Structurally-related (epi)catechin metabolite (SREM) concentrations before cocoa intake (0 hour) and 4 hours afterwards (4 hours). ∗P < .05; 1, (epi)catechin-glucuronide; 2, (epi)catechin-glucuronide-sulfate; 3, (epi)catechin-sulfate (isomer 1); 4, (epi)catechin-sulfate (isomer 2); 5, methyl-(epi)catechin-sulfate (isomer 1); 6, methyl-(epi)catechin-sulfate (isomer 2).
Figure 2.
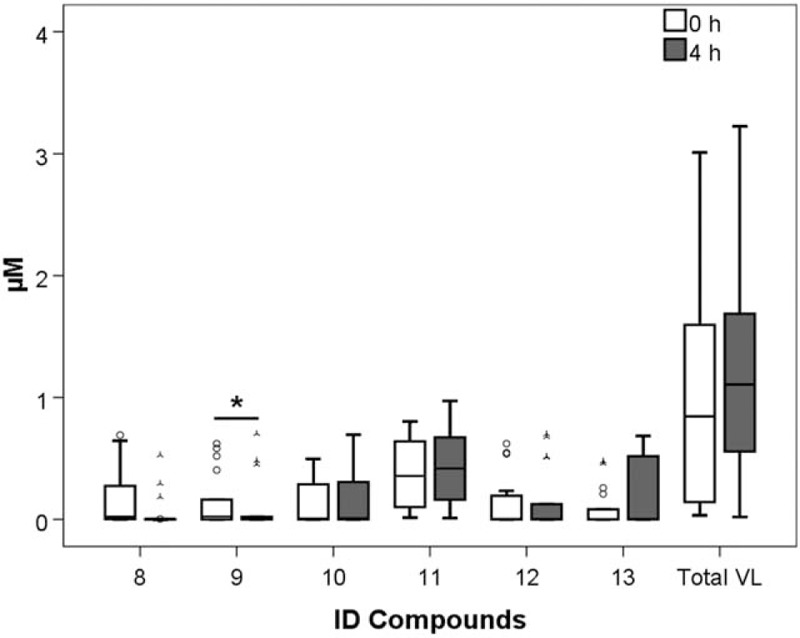
Phenyl-γ-valerolactones (VL) concentrations before cocoa intake (0 hour) and 4 hours afterwards (4 hours). Legend: 7, 5-(3′-hydroxyphenyl)-γ-valerolactone-4′-glucuronide; 8, 5-phenyl-γ-valerolactone-glucuronide-sulfate; 9, 5-(4′-hydroxyphenyl)-γ-valerolactone-3′-glucuronide; 10, 5-phenyl-γ-valerolactone-3′-glucuronide; 11, 5-(hydroxyphenyl)-γ-valerolactone-sulfate isomers; 12, 5-phenyl-γ-valerolactone-sulfate-methoxy; and 13, 5-phenyl-γ-valerolactone-3′-sulfate. ∗P < .05.
Collagen/ADP-induced closure time was found to be significantly increased 4 hours after dark chocolate ingestion (98.5 ± 13.0 vs 114.5 ± 22.0 seconds, P < .001), while the closure time of collagen/epinephrine remained unchanged (128.5 ± 27.0 vs 122.5 ± 34.0 seconds, P = .33) (Fig. 3).
Figure 3.
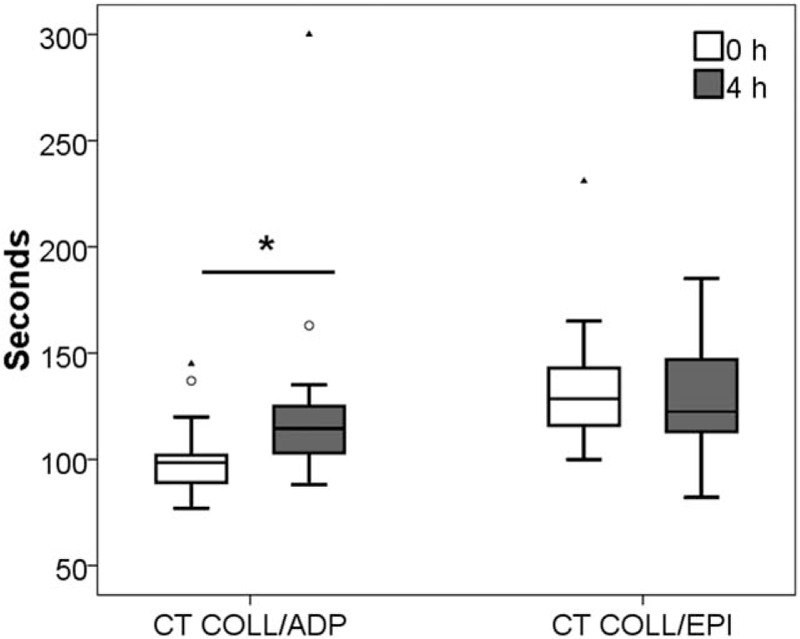
Closure time (CT) induced by collagen and adenosine-5′-diphosphate (COLL/ADP) and collagen/epinephrine (COLL/EPI) before cocoa intake (0 hour) and 4 hours afterwards (4 hours). ∗P < .05.
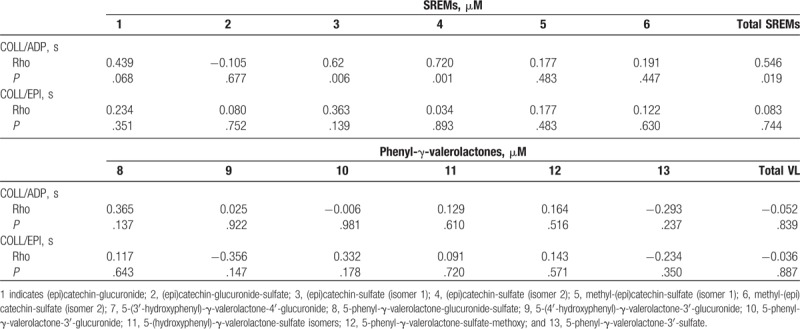
Among SREMs, the 4 hours concentration increase of compound 3 significantly correlated with the 4 hours increase of compound 4 (rho = 0.64, P = .004). Intriguingly, the increased concentration of both compounds 3 and 4 significantly correlated with the increase of total SREMs (rho = 0.653, P = .003 and rho = 0.94, P < .001, respectively) (data not shown). No significant correlations have been found among the phenyl-γ-valerolactone delta values.
Interestingly, the increase of collagen/ADP closure time was found to significantly correlate with the increase of total SREMs (rho = 0.55, P = .019), as well as with compounds 3 (rho = 0.62, P = .006) and 4 (rho = 0.72, P = .001) (Table 3). No significant correlations have been found among the phenyl-γ-valerolactones and the considered coagulation outcomes.
Table 3.
Spearman correlations among structurally-related (epi)catechin metabolite (SREM) or phenyl-γ-valerolactone (VL) metabolite delta concentration increase detected in plasma samples and closure time of both collagen/epinephrine and collagen/ADP.
Age and BMI have not been found to significantly correlate with delta closure time of collagen/epinephrine or delta collagen/ADP closure time values (P > .05).
4. Discussion
The results of this study confirm that cocoa polyphenols may exert a favorable activity on the platelet function in healthy men subjects, especially with respect to adhesion and stable aggregate formation. More specifically, an acute inhibitory effect of cocoa (i.e., 14% prolongation of closure time) was observed on adhesion and aggregation triggered by ADP/collagen, 4 hours after cocoa intake. The highly significant correlation observed between closure time prolongation and plasma SREMs also suggests that this inhibitory effect may be mediated by these compounds. Phenyl-γ-valerolactone plasma concentrations were low after 4 hours cocoa intake and seemed not to have relevant influence on platelet inhibition activity.
To the best of our knowledge, only 1 intervention study has previously investigated the acute effect of dark chocolate consumption on platelet function using PFA-100.[17] A significant increase in closure time of both ADP/collagen and EPI/collagen was observed 2 hours after chocolate consumption, while only ADP/collagen closure time was significantly prolonged still 6 hours after chocolate intake.[17] These findings are in agreement with our data and suggest that the potential inhibitory effect of flavan-3-ol metabolites on platelet function may be time-dependent. Nevertheless, flavan-3-ols, or the deriving metabolites, had not been assayed in the mentioned study and no assumptions could be made concerning the influence of individual metabolites and the clinical outcome. The work of Bordeaux et al,[18] where a 24-hour diet recall was administered to evaluate the consumption of chocolate-based products, reported a significant increase of PFA closure time between individuals consuming a relatively modest amount of commercial chocolate when compared with those who abstained from chocolate consumption. Concerning the effects of dark chocolate long-term consumption on cardiovascular risk events, most of the evidence of the beneficial effects are inferred from epidemiological studies,[19] as there is a lack of long-term (>18 weeks) dietary intervention studies.[20,21] In a recent study, an algorithm was set-up to estimate the long-term effects of dark chocolate consumption in a population with metabolic syndrome at high risk of cardiovascular disease.[22] Results showed that a daily consumption of dark chocolate—at least 60% to 70% in cocoa—might prevent 70 non-fatal cardiovascular events and 15 cardiovascular-related deaths per 10,000 population treated over 10 years.[22]
Some mechanisms were proposed to explain the putative inhibitory effects of flavonoids on platelet function. Cocoa flavanols were shown to exert antioxidant properties by directly increasing nitric oxide (NO) bioavailability,[23] mainly through inhibition of protein kinase C-dependent NADPH oxidase[24] and the inhibition of the vasoconstrictor endothelin-1.[25] NO is a potent platelet inhibitor, since it inhibits the generation of platelet isoprostanes through NOX2 gene down-regulation,[26] thus reducing platelet adhesion.[27] Moreover, high NO availability was shown to inhibit platelet reactivity, by especially inhibiting platelet aggregation,[28] secretion,[29] adhesion,[23,30] and fibrinogen binding to its receptor, GpIIb-IIIa.[31] Rein et al[5] also showed that the expression of fibrinogen receptor GPIIb-IIIa was significantly decreased in healthy individuals consuming a cocoa beverage.
These findings provide a reasonable explanation to our data. Reliable evidence has been provided that a NO-dependent platelet activation is more stably inhibited when triggered by ADP than ephinephrine in normal subjects.[32,33] Interestingly, it has also been observed that the epinephrine-mediated activation of fibrinogen receptor GPIIb-IIIa is much lower than that achievable with ADP in healthy subjects.[34] Therefore, the strong flavonoid-mediated NO generation and GPIIb-IIIa inhibition probably explains why we failed to observe a stable prolongation of collagen/epinephrine closure time on PFA-100 after dark chocolate intake, since both these biological pathways are more strongly targeted by ADP than by epinephrine. Notably, these findings may be of substantial clinical significance, since a very recent and large longitudinal community-based cohort study showed that platelet hyperreactivity to ADP in health subjects free of cardiovascular disease is strongly associated with future risk of arterial thrombosis, while a similar association could not be observed for platelet hyperreactivity to epinephrine.[35]
The statistically significant positive correlation observed between SREM plasma concentrations and ADP/collagen closure times seems to endorse these early-absorbed flavan-3-ol metabolites as main mediators of platelet inhibition. Furthermore, among the SREMs, (epi)catechin-sulfate isomers have shown higher circulating levels compared with the other (epi)catechin metabolites and significantly correlated with ADP/collagen closure time delta values. Not being influenced by age or BMI, the ADP/collagen closure time seems to be really mainly influenced by the increase of (epi)catechin-sulfate isomers circulating levels.
Clearly, our pilot study has several limitations, first of which regards the study population. As already reported, absorption, distribution, metabolism, and elimination (ADME) of flavan-3-ols are clearly influenced by gut microbiota metabolism[3,4,36] and a wider sample size should have been considered. Moreover, the decision to include only healthy man volunteers in the intervention limited the target population and the completeness of results. On the other hand, this choice allowed to study and discuss the potential anti-thrombotic effects of chocolate intake in a more homogeneous group, as previous studies demonstrated that sex specific hormones deeply influence flavonoids absorption and metabolism,[37] as well as platelet response.[38] The 4 hour-time collection point might be considered as a limitation of the study for the quantification of the phenyl-γ-valerolactones in plasma samples, as they are generated after interaction of flavan-3-ols with the colonic microbiota in the large intestine also more than 4-hour after chocolate intake.[3] Additionally, as the volunteers of this short study had not been asked to abstain from flavan-3-ol rich food sources during the days before the test, the presence of their metabolites, at low concentrations, in their system even at T0 was expected.[39]
Finally, the use of a single marker of platelet function, namely the PFA-100 might be influenced by other changes such as hematocrit, platelet count, and von Willebrand factor level and activity. Although we did not measure these other components, our group previously demonstrated that the consumption of chocolate did not affect those parameters.[13,40] We also did not undertake serial time-course collections to interrogate time-course effects. However, this can form the basis of a subsequent study.
In conclusion, due to the essential role played by platelets in the pathophysiology of both arterial and venous thrombosis,[41] we may conclude that dark chocolate consumption may be beneficial for subjects at increased risk of thrombosis and that its action may be mediated by the appearance of SREMs into systemic circulation.[42–44]
Author contributions
Conceptualization: Martina Montagnana, Emmanuel J. Favaloro, Daniele Del Rio, Giuseppe Lippi.
Data curation: Martina Montagnana, Elisa Danese, Donato Angelino, Pedro Mena, Alice Rosi, Marco Benati, Gian Luca Salvagno, Giuseppe Lippi.
Formal analysis: Martina Montagnana, Elisa Danese, Donato Angelino, Alice Rosi, Marco Benati, Matteo Gelati.
Funding acquisition: Giuseppe Lippi.
Investigation: Martina Montagnana.
Methodology: Martina Montagnana, Elisa Danese, Donato Angelino, Pedro Mena, Alice Rosi, Marco Benati, Matteo Gelati, Gian Luca Salvagno.
Resources: Gian Luca Salvagno.
Supervision: Martina Montagnana, Emmanuel J. Favaloro, Daniele Del Rio, Giuseppe Lippi.
Validation: Martina Montagnana, Elisa Danese, Donato Angelino, Giuseppe Lippi.
Visualization: Giuseppe Lippi.
Writing – original draft: Martina Montagnana, Elisa Danese, Daniele Del Rio, Giuseppe Lippi.
Writing – review & editing: Martina Montagnana, Giuseppe Lippi.
Footnotes
Abbreviations: ADME = absorption, distribution, metabolism, and elimination, ADP = adenosine-5′-diphosphate, COL/ADP = collagen/adenosine-5′-diphosphate, COL/EPI = collagen/epinephrine, IQR = interquartile range, NO = nitric oxide, PFA = platelet function analyzer, SPE = solid phase extraction, SREM = structurally-related (epi)catechin metabolite.
MM, ED, and DA have contributed equally to this work.
DDR and GL share senior authorship in this work.
The authors have no conflicts of interest to declare.
References
- [1].Davison K, Berry NM, Misan G, et al. Dose-related effects of flavanol-rich cocoa on blood pressure. J Hum Hypertens 2010;24:568–76. [DOI] [PubMed] [Google Scholar]
- [2].Aprotosoaie A, Miron A, Trifan A, et al. The cardiovascular effects of cocoa polyphenols—an overview. Diseases 2016;4:pii: E39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Borges G, Ottaviani JI, van der Hooft JJJ, et al. Absorption, metabolism, distribution and excretion of (-)-epicatechin: a review of recent findings. Mol Aspects Med 2018;61:18–30. [DOI] [PubMed] [Google Scholar]
- [4].Castello F, Costabile G, Bresciani L, et al. Bioavailability and pharmacokinetic profile of grape pomace phenolic compounds in humans. Arch Biochem Biophys 2018;646:1–9. [DOI] [PubMed] [Google Scholar]
- [5].Rein D, Paglieroni TG, Wun T, et al. Cocoa inhibits platelet activation and function. Am J Clin Nutr 2000;72:30–5. [DOI] [PubMed] [Google Scholar]
- [6].Ottaviani JI, Balz M, Kimball J, et al. Safety and efficacy of cocoa flavanol intake in healthy adults: a randomized, controlled, double-masked trial. Am J Clin Nutr 2015;102:1425–35. [DOI] [PubMed] [Google Scholar]
- [7].Ostertag LM, Kroon PA, Wood S, et al. Flavan-3-ol-enriched dark chocolate and white chocolate improve acute measures of platelet function in a gender-specific way-a randomized-controlled human intervention trial. Mol Nutr Food Res 2013;57:191–202. [DOI] [PubMed] [Google Scholar]
- [8].Hamed MS, Gambert S, Bliden KP, et al. Dark chocolate effect on platelet activity, C-reactive protein and lipid profile: a pilot study. South Med J 2008;101:1203–8. [DOI] [PubMed] [Google Scholar]
- [9].Innes AJ, Kennedy G, McLaren M, et al. Dark chocolate inhibits platelet aggregation in healthy volunteers. Platelets 2003;14:325–7. [DOI] [PubMed] [Google Scholar]
- [10].Holt RR, Actis-Goretta L, Momma TY, et al. Dietary flavanols and platelet reactivity. J Cardiovasc Pharmacol 2006;47:S187–96. [DOI] [PubMed] [Google Scholar]
- [11].Dovizio M, Alberti S, Guillem-Llobat P, et al. Role of platelets in inflammation and cancer: Novel therapeutic strategies. Basic Clin Pharmacol Toxicol 2014;114:118–27. [DOI] [PubMed] [Google Scholar]
- [12].Mancuso ME, Santagostino E. Platelets: much more than bricks in a breached wall. Br J Haematol 2017;178:209–19. [DOI] [PubMed] [Google Scholar]
- [13].Montagnana M, Danese E, Salvagno GL, et al. Short-term effect of dark chocolate consumption on routine haemostasis testing. Int J Food Sci Nutr 2017;68:613–6. [DOI] [PubMed] [Google Scholar]
- [14].Favaloro EJ. Clinical utility of closure times using the platelet function analyzer-100/200. Am J Hematol 2017;92:398–404. [DOI] [PubMed] [Google Scholar]
- [15].Cho YU, Jang S, Park CJ, et al. Variables that affect Platelet Function Analyzer-100 (PFA-100) closure times and establishment of reference intervals in Korean adults. Ann Clin Lab Sci 2008;38:247–53. [PubMed] [Google Scholar]
- [16].Brindani N, Mena P, Calani L, et al. Synthetic and analytical strategies for the quantification of phenyl-γ-valerolactone conjugated metabolites in human urine. Mol Nutr Food Res 2017;61:6–10. [DOI] [PubMed] [Google Scholar]
- [17].Holt RR, Schramm DD, Keen CL, et al. Chocolate consumption and platelet function. JAMA 2002;287:2212–3. [DOI] [PubMed] [Google Scholar]
- [18].Bordeaux B, Yanek LR, Moy TF, et al. Casual chocolate consumption and inhibition of platelet function. Prev Cardiol 2007;10:175–80. [DOI] [PubMed] [Google Scholar]
- [19].Kwok CS, Boekholdt SM, Lentjes MA, et al. Habitual chocolate consumption and risk of cardiovascular disease among healthy men and women. Heart 2015;101:1279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Taubert D, Roesen R, Schömig E. Effect of cocoa and tea intake on blood pressure: a meta-analysis. Arch Intern Med 2007;167:626–34. [DOI] [PubMed] [Google Scholar]
- [21].Larsson SC. Coffee, tea, and cocoa and risk of stroke. Stroke 2014;45:309–14. [DOI] [PubMed] [Google Scholar]
- [22].Zomer E, Owen A, Magliano DJ, et al. The effectiveness and cost effectiveness of dark chocolate consumption as prevention therapy in people at high risk of cardiovascular disease: best case scenario analysis using a Markov model. BMJ 2012;344:e3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Flammer AJ, Hermann F, Sudano I, et al. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation 2007;116:2376–82. [DOI] [PubMed] [Google Scholar]
- [24].Pignatelli P, di Santo S, Buchetti B, et al. Polyphenols enhance platelet nitric oxide by inhibiting protein kinase C-dependent NADPH oxidase activation: effect on platelet recruitment. FASEB J 2006;20:1082–9. [DOI] [PubMed] [Google Scholar]
- [25].Gasper A, Hollands W, Casgrain A, et al. Consumption of both low and high (-)-epicatechin apple puree attenuates platelet reactivity and increases plasma concentrations of nitric oxide metabolites: a randomized controlled trial. Arch Biochem Biophys 2014;559:29–37. [DOI] [PubMed] [Google Scholar]
- [26].Carnevale R, Loffredo L, Pignatelli P, et al. Dark chocolate inhibits platelet isoprostanes via NOX2 down-regulation in smokers. J Thromb Haemost 2012;10:125–32. [DOI] [PubMed] [Google Scholar]
- [27].De Graaf JC, Banga JD, Moncada S, et al. Nitric oxide functions as an inhibitor of platelet adhesion under flow conditions. Circulation 1992;85:2284–90. [DOI] [PubMed] [Google Scholar]
- [28].Schafer A, Alexander R, Handin R, et al. Inhibition of platelet function by organic nitrate vasodilators. Blood 1980;55:649–54. [PubMed] [Google Scholar]
- [29].Lieberman EH, O’Neill S, Mendelsohn ME. S-nitrosocysteine inhibition of human platelet secretion is correlated with increases in platelet cGMP levels. Circ Res 1991;68:1722–8. [DOI] [PubMed] [Google Scholar]
- [30].Sneddon JM, Vane JR. Endothelium-derived relaxing factor reduces platelet adhesion to bovine endothelial cells. Proc Natl Acad Sci USA 1988;85:2800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mendelsohn ME, O’Neill S, George D, et al. Inhibition of fibrinogen binding to human platelets by S-nitroso-N-acetylcysteine. J Biol Chem 1990;265:19028–34. [PubMed] [Google Scholar]
- [32].Harris SN, Rinder CS, Rinder HM, et al. Nitroprusside inhibition of platelet function is transient and reversible by catecholamine priming. Anesthesiology 1995;83:1145–52. [DOI] [PubMed] [Google Scholar]
- [33].Kirkby NS, Lundberg MH, Chan MV, et al. Blockade of the purinergic P2Y12 receptor greatly increases the platelet inhibitory actions of nitric oxide. Proc Natl Acad Sci USA 2013;110:15782–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shattil SJ, Budzynski A, Scrutton MC. Epinephrine induces platelet fibrinogen receptor expression, fibrinogen binding, and aggregation in whole blood in the absence of other excitatory agonists. Blood 1989;73:150–8. [PubMed] [Google Scholar]
- [35].Puurunen MK, Hwang SJ, Larson MG, et al. ADP platelet hyperreactivity predicts cardiovascular disease in the FHS (Framingham Heart Study). J Am Heart Assoc 2018;7:e008522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mena P, Ludwig IA, Tomatis VB, et al. Inter-individual variability in the production of flavan-3-ol colonic metabolites: preliminary elucidation of urinary metabotypes. Eur J Nutr 2018;In press, DOI:10.1007/s00394-018-1683-4. [DOI] [PubMed] [Google Scholar]
- [37].Cassidy A, Minihane AM. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am J Clin Nutr 2017;105:10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Leng XH, Hong SY, Larrucea S, et al. Platelets of female mice are intrinsically more sensitive to agonists than are platelets of males. Arterioscler Thromb Vasc Biol 2004;24:376–81. [DOI] [PubMed] [Google Scholar]
- [39].Calani L, Del Rio D, Luisa Callegari M, et al. Updated bioavailability and 48 h excretion profile of flavan-3-ols from green tea in humans. Int J Food Sci Nutr 2012;63:513–21. [DOI] [PubMed] [Google Scholar]
- [40].Montagnana M, Danese E, Montolli V, et al. Acute effect of dark chocolate on red blood cell distribution width. Eur J Intern Med 2017;37:e29–30. [DOI] [PubMed] [Google Scholar]
- [41].Lippi G, Favaloro EJ. Venous and arterial thromboses: two sides of the same coin? Semin Thromb Hemost 2018;44:239–48. [DOI] [PubMed] [Google Scholar]
- [42].Gresele P, Bury L, Falcinelli E. Inherited platelet function disorders: algorithms for phenotypic and genetic investigation. Semin Thromb Hemost 2016;42:292–305. [DOI] [PubMed] [Google Scholar]
- [43].Gremmel T, Frelinger AL, Michelson AD. Platelet physiology. Semin Thromb Hemost 2016;42:191–204. [DOI] [PubMed] [Google Scholar]
- [44].Lassila R. Platelet function tests in bleeding disorders. Semin Thromb Hemost 2016;42:185–90. [DOI] [PubMed] [Google Scholar]


