Abstract
Background:
The prognostic role of neutrophil-to-lymphocyte ratio (NLR) in esophageal cancer (EC) remains controversial.
Methods:
The aim of this study was to evaluate the association between NLR and oncologic outcome of EC patients through a meta-analysis. A systematic search was performed in PubMed, Web of Science and Embase for relevant studies. Meta-analysis was performed using hazard ratio (HR) and95% confidence interval (CI) as effect measures.
Results:
Finally, 33 articles with 11,039patients were included in our study. The synthesized results indicated that the elevated NLR was negatively related to overall survival (OS) (HR = 1.39, 95% CI: 1.23–1.54). When the patients were stratified according to country, pathological type, treatment strategies, sample size, and different HR estimate method, high NLR was also significantly correlated with poor OS. Similarly, elevated NLR was also associated with shorter disease-free survival (DFS), progress-free survival (PFS), relapse-free survival (RFS), and cancer-specific survival (CSS).
Conclusion:
The elevated pretreatment NLR is associated with poor oncological outcomes in patients with EC. NLR may be a significant predictive biomarker in EC. Further large-cohort studies are needed to confirm these findings.
Keywords: esophageal cancer, meta-analysis, neutrophil to lymphocyte ratio, prognosis
1. Introduction
Esophageal cancer (EC)is one of the most common malignances with high mortality, which caused an estimated 477,900 new cases and 375,000deaths in China, 2015.[1] Esophageal squamous cell carcinoma (SCC) and adenocarcinoma (AC) are the 2 dominant pathological types. SCC is endemic particularly in eastern countries, while AC is more popular in western countries.[2] Although there have been advances in the diagnostic and treatment technologies, the prognosis of EC is still poor with 5-year survival rate about 20%.[3] The tumor-nodes-metastasis (TNM) stage is the most important prognostic factor. However, many studies have found that the prognosis is different even for patients within the same TNM stage. Therefore, it is important to identify other prognostic factors and to determine the optimal treatment strategies for the improvement of 5-year survival rate.
More and more evidences have found that the systematic inflammation response plays a key role in tumorigenesis and progression. Cancer-related inflammation can cause DNA damage, promote angiogenesis and cell proliferation, influence tumor cell invasion and metastasis.[4,5] The biochemical or hematological markers have been proposed as measurement of the systemic inflammatory response among patients with cancer. The most common used parameters included Glasgow Prognostic Score (GPS), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), and neutrophil-to-lymphocyte ratio (NLR). The prognostic roles of these parameters have been demonstrated in various cancers, which implies the elevated systemic inflammation is correlated with poorer outcome.[6–9]
NLR, calculated as neutrophil counts divided by lymphocyte counts, is suggested as a useful prognostic marker for solid cancers. However, the prognostic significance of NLR in EC is still controversial. Several studies demonstrated that NLR had prognostic value in localized and advanced EC.[10,11] However, some studies reported that high serum NLR had no prognostic value in patients with EC.[12,13] The possible reason is due to the variance in the study design, sample size, follow-up time and treatment strategies. Therefore, it is necessary to perform a meta-analysis to comprehensively and systematically evaluate the prognostic value of NLR in the oncologic outcomes of EC patients.
2. Materials and methods
2.1. Search strategy
We performed the meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 guidelines.[14] PubMed, Embase, and Web of Science databases were searched for relevant articles until June, 2018. The main search terms included: “NLR” (e.g., “neutrophil lymphocyte ratio”, “neutrophil to lymphocyte ratio”, “neutrophil-to-lymphocyte ratio”) and “esophageal neoplasm” (e.g., “esophageal cancer”, “esophageal carcinoma”, “EC”) AND “prognosis” (or “survival”). Both full text and MeSH search for keywords were used. The publication language was limited to English. The reference lists and related articles in each identified publication were also reviewed for potential studies. Ethical approval is not required because this is a study based on aggregate data and did not involve humans.
2.2. Inclusion and exclusion criteria
Articles were included if they met the following criteria:
-
(1)
patients with ECs were histopathologically confirmed, including SCC and AC;
-
(2)
the NLR was reported by blood test before treatment;
-
(3)
Hazard ratio (HRs) and 95% CIs for the associations between pretreatment NLR and survival outcomes were reported.
Articles were excluded if they met the following criteria:
-
(1)
abstracts, letters, editorials, reviews, or case reports;
-
(2)
studies were not available in English;
-
(3)
studies had overlapping or repeat data;
-
(4)
studies concerned non-human or non-clinical research;
-
(5)
studies did not present the cut-off value for NLR.
2.3. Date extraction and quality assessment
Two researchers (Xiangwei Zhang and Yuanzhu Jiang) reviewed the eligible articles independently. Articles that could not be categorized based on title and abstract alone were retrieved for full-text review. The following items were recorded for each study: first author, year of publication, country, total number of cases and gender, follow-up time, cut-off value, treatment strategy, cancer type, and survival data. HRs and 95% CIs were obtained directly from individual articles. HRs were extracted preferentially from multivariable analyses if possible. Otherwise, HRs from univariate analyses were used for analysis.
The quality of the included studies was assessed through the Newcastle-Ottawa Quality Assessment Scale (NOS), which consists of 3 parts: selection (0–4 points), comparability (0–2 points), and outcome assessment (0–3 points).[15] The maximum score is 9 points and NOS score ≥7wasassigned as high-quality studies. Any disagreements in data extraction or quality assessment were resolved by joint discussion.
2.4. Statistical analysis
The pooled HRs and 95% CIs were used to evaluate the relationship between NLR and prognosis. A pooled HR>1 indicated a worse prognosis in EC patients with high level of NLR. Cochran's Q test and Higgins I2statistic were undertaken to assess the heterogeneity of the included studies. A P <.10 for Q test or I2> 50% for I2 test suggested significant heterogeneity among the included studies and a random-effect model (Der Simonian–Laird method) was used to pool the outcome.[16] Otherwise, a fixed-effect model (Mantel–Haenszel method) was adopted.[17] Subgroup analyses using variables as country, histology, treatment, cut-off value, sample size and HR analysis method, were conducted to find sources of heterogeneity among studies. Sensitivity analysis was conducted to test the robustness of pooled outcomes of these studies by removing an individual study in sequence. Begg funnel plot and the Egger linear regression tests were used to assess the publication bias.[18,19] All the statistical analyses were performed using STATA statistical software version 12.0 (STATA, College Station, TX). All P values were 2-sided. A P <.05 was considered statistically significant.
3. Results
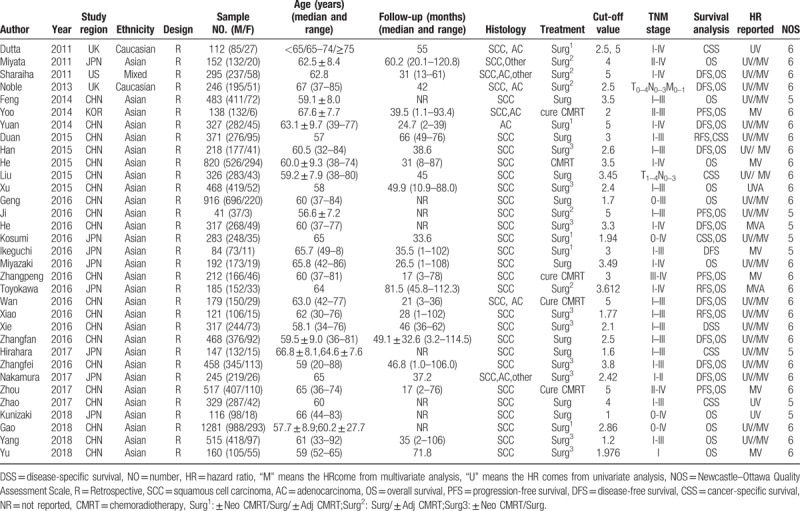
3.1. The characteristics of included studies
The flow diagram of literature selection was presented in Figure 1. A total of 452 articles were searched from Pubmed, Embase, and Web of science based on the search strategy. Finally, 33studies with a total of 11,039 patients, published between 2011 and 2018, were included in our meta-analysis according to the inclusion and exclusion criteria.[10–13,20–48]Table 1 summarized the general characteristics of the primary studies involving the prognosis of NLR to EC. All the included studies were retrospectively designed. Among these studies, participants in 30 studies were Asians, in 2 studies were Caucasians, and in 1 study was mixed ethnicity. Twenty-one studies (63.6%) were from China, 8 studies (24.2%)from Japan, less than 15% from UK, US, and Korean. Participants in 25 studies (75.8%)were patients with SCC. None of these studies included patients treated with non curative intent. Twenty-nine studies (87.9%) included patients who underwent surgical resection with or without chemoradiotherapy. Only 4 studies (12.1%) included patients who underwent curative chemoradiotherapy alone. The cut-off values applied in the studies were not consistent ranging from 1.6 to 5. Twenty studies (60.6%) used a NLR cutoff value greater than 2.5, while thirteen studies (39.4%) used an NLR cutoff value less than 2.5. Twenty-six studies (78.8%) reported the relationship of EC and OS, 10 studies (30.3%) on EC and disease-free survival (DFS) 4 studies (12.1%) on EC and progress-free survival (PFS), 3 studies (9.1%) on EC and relapse-free survival (RFS), 7 studies (21.2%) on EC and cancer-specific survival (CSS), and 1 study (3.0%) on EC and disease-specific survival (DSS). Three studies reported the data of odds ratio (OR) and 1 study only reported the data of relative risk (RR), so we use the OR/RR to replace HR when pooled the data. HRs/OR/RR and 95%CIs were reported directly in all the studies.
Figure 1.
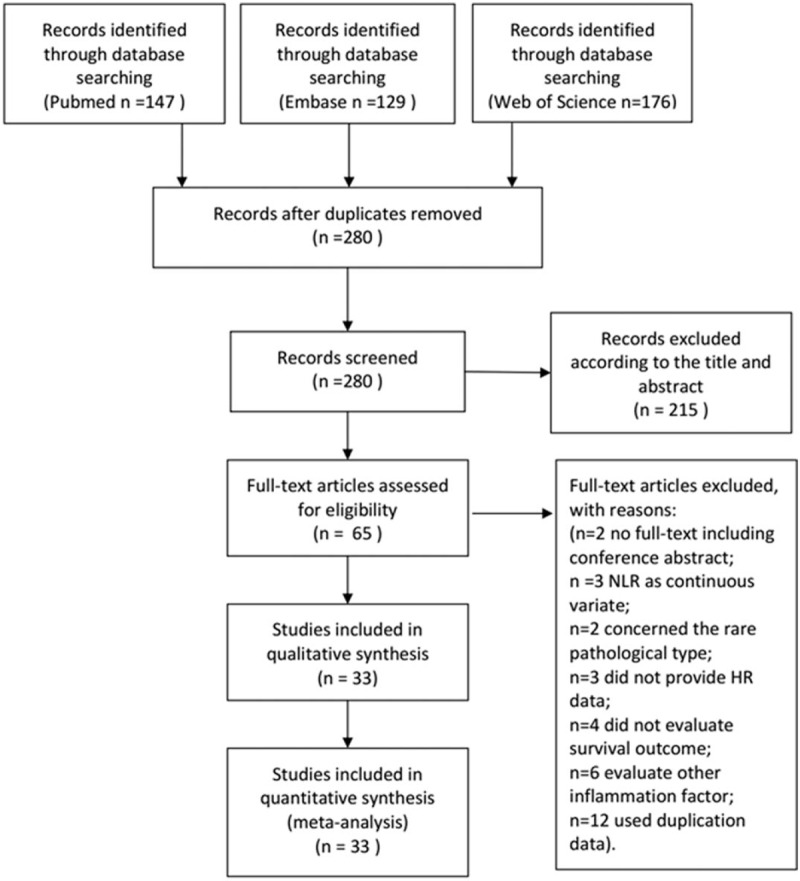
Flow chat of literature search and selection.
Table 1.
Main characteristics of all the studies included in the meta-analysis.
3.2. NLR and OS in EC
There was significant heterogeneity among studies for HRs (I2 = 66.70%; Ph < 0.001) in the 26 studies evaluating OS, so a random-effect model was performed to calculate the pooled HR and its 95% CI. The pooled HR of 1.390 (95% CI: 1.235–1.545) indicated that patients with elevated NLR had poor OS (Fig. 2).
Figure 2.
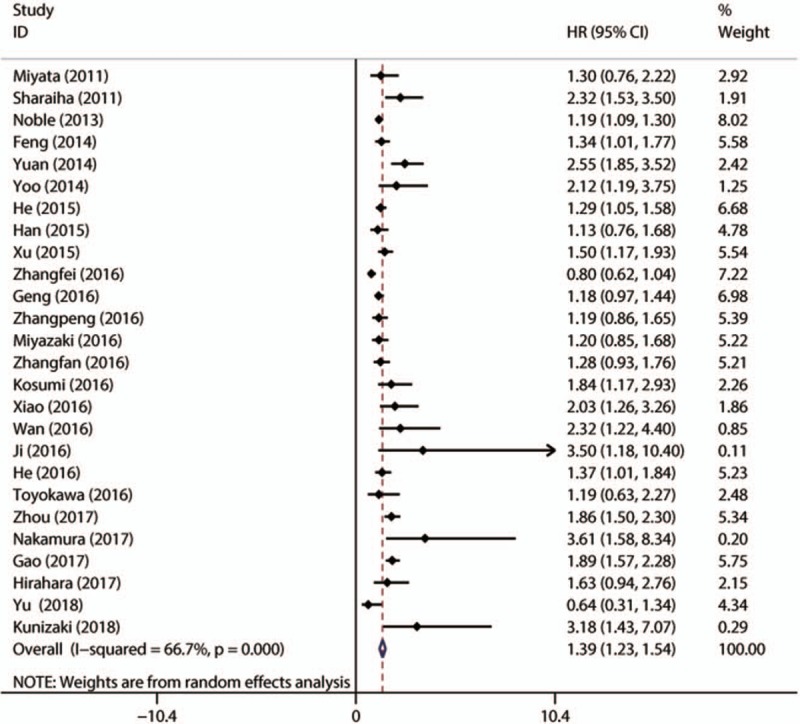
Meta-analysis of the association between elevated NLR and OS in patients with EC. EC = esophageal cancer, NLR = neutrophil-to-lymphocyte ratio, OS = overall survival
3.3. NLR and DFS in EC
There were 10 studies with 2837patients presenting the HR and 95% CI of NLR to DFS. The combined data showed that elevated NLR was associated with short DFS (HR = 1.409; 95% CI: 1.123–1.695, P <.001) with obvious heterogeneity (I2 = 74.2%, Ph < 0.001) (Fig. 3).
Figure 3.
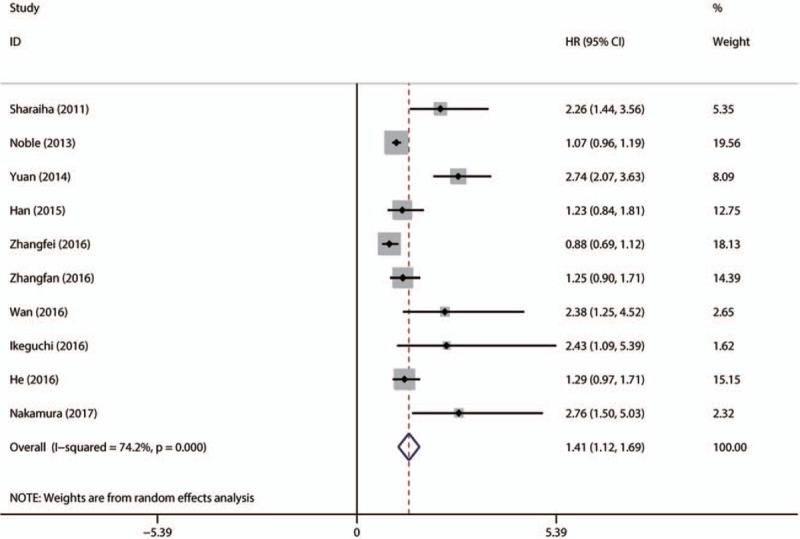
Forest plot of studies evaluating the association between NLR and DFS in EC patients. DFS = disease-free survival, EC = esophageal cancer, NLR = neutrophil-to-lymphocyte ratio.
3.4. NLR and PFS/RFS in EC
No obvious heterogeneity was found among studies evaluating PFS/RFS, so a fixed-effect model was performed to calculate the pooled HR and its 95% CI. The pooled HR of related studies showed that the elevated NLR was associated with shorter PFS (HR = 1.398; 95% CI: 1.147–1.649, P <.001) and RFS (HR = 1.509; 95% CI: 1.113–1.905, P <.001) (Fig. 4).
Figure 4.
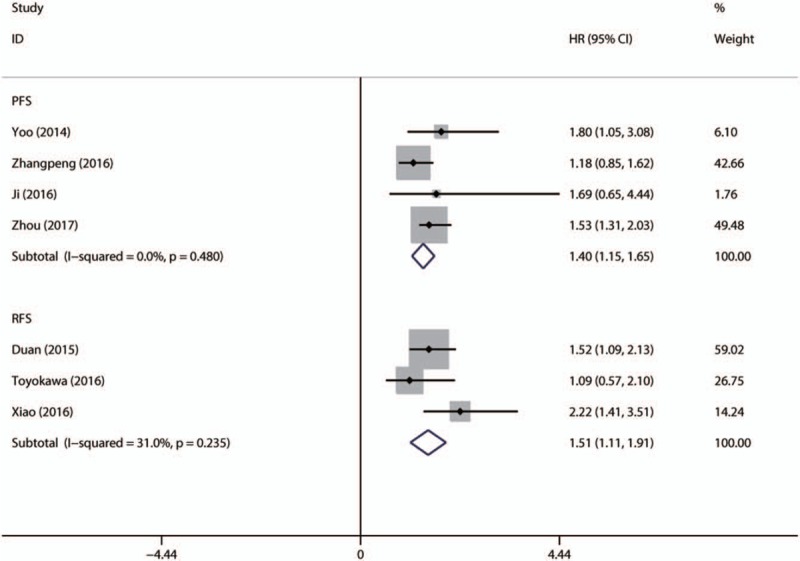
Forest plot of studies evaluating the association between NLR and PFS/RFS in EC patients. EC = esophageal cancer, NLR = neutrophil-to-lymphocyte ratio, PFS = progress-free survival, RFS = relapse-free survival.
3.5. NLR and CSS/disease-specific survival in EC
7 studies with 1885patients reported the data of pretreatment NLR and CSS/DSS in EC. Elevated NLR was associated with poor CSS/DSS (HR = 1.380; 95% CI: 1.065–1.694, P <.001) without obvious heterogeneity (I2 = 50.6%, Ph = 0.306) (Fig. 5).
Figure 5.
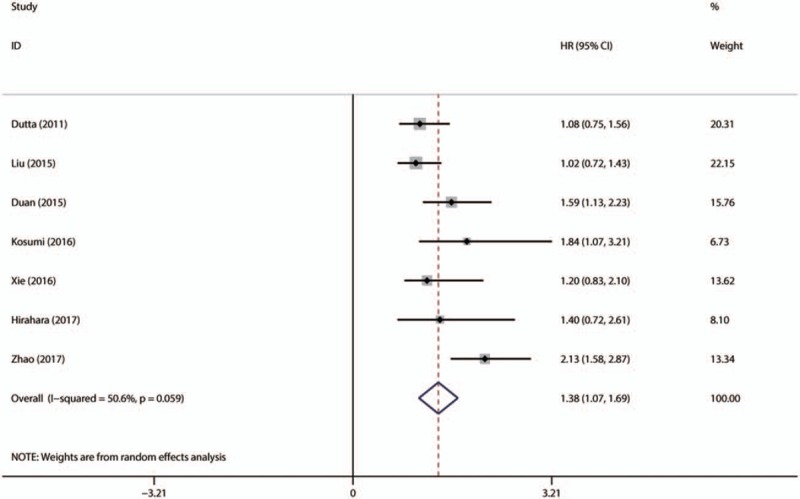
Forest plot of studies evaluating the association between NLR and CSS/DSS in EC patients. CSS = cancer-specific survival, DSS = disease-specific survival, EC = esophageal cancer, NLR = neutrophil-to-lymphocyte ratio.
3.6. Subgroup analyzes
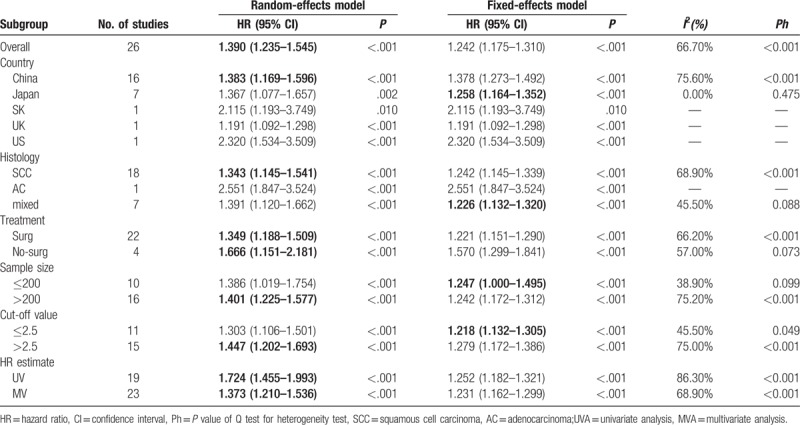
In consideration of the high heterogeneity, we performed subgroup analyses to identify the source of heterogeneity. Subgroup analyses by country revealed that NLR was a negative predictor of OS for patients from different countries. NLR was negatively associated with OS in separated SCC and AC patients. To treatment strategies, we found the pooled HR for patients receiving surgery was 1.349 (95% CI: 1.251–1.694, I2 = 66.2%, Ph < 0.001), and the pooled HR was 1.666 (95% CI: 1.151–2.181, I2 = 57.0%, Ph = 0.073) for patients treated by chemotherapy or radiotherapy. Because the NLR cut-off values were different among the included studies, we performed subgroup analysis based on different cut-off values. The data demonstrated that the pooled HR was 1.218 (95%CI: 1.132–1.305, I2 = 45.5%, Ph = 0.049) for cut-off value ≤2.5 and 1.447 (95%CI: 1.202–1.693 I2 = 75.0%, Ph < 0.001) for cut-off value >2.5. In addition, subgroup analyses results stratified by sample size (<200 and ≥200) and HR estimated method (Univariate analysis and Multivariate analysis were in Table 2.
Table 2.
Subgroup analyses of pooled Hazard ratios (HRs) reflecting the association between PLR and OSin EC patients.
Considering the limited number of related studies providing data for NLR and DFS, PFS, RFS, and CSS, there is no need to conduct other subgroup analyses.
3.7. Sensitivity analyses
Sensitivity analysis was conducted by omitting each single study to identify the influence of the individual study on the pooled HRs for OS. The results were not substantially changed when any study was excluded, indicating the robustness of our findings (Fig. 6).
Figure 6.
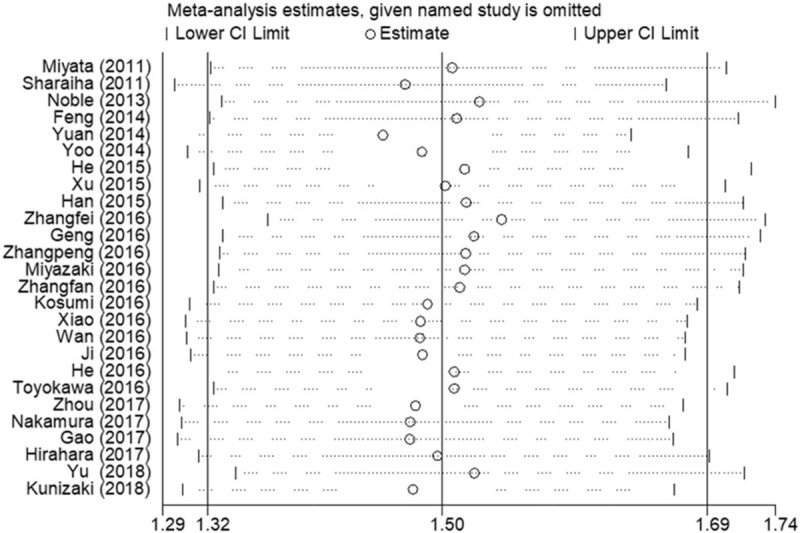
Sensitivity analysis on the relationship between NLR and OS in EC. EC = esophageal cancer, NLR = neutrophil-to-lymphocyte ratio, OS = overall survival.
3.8. Publication bias
Begg funnel plot and Egger test linear regression test were conducted to evaluate the publication bias. Publication bias was detected for OS (Pr>|z| = 0.201 for Begg test and P = .003 for Egger test). Therefore, we performed the “trim and fill” analysis for studies focusing on OS. It was estimated that 4 studies evaluating the prognostic value of NLR to OS remained unpublished (Fig. 7). The adjusted result (HR = 1.402, 95% CI: 1.237–1.590) was similar to our pooled results. Additionally, publication bias was also found in terms of DFS (Begg test, Pr>|z| = 0.297 or Egger test, P = .043). Because of the limited number of included studies, publication bias was not evaluated on NLR to PFS, RFS or CSS.
Figure 7.
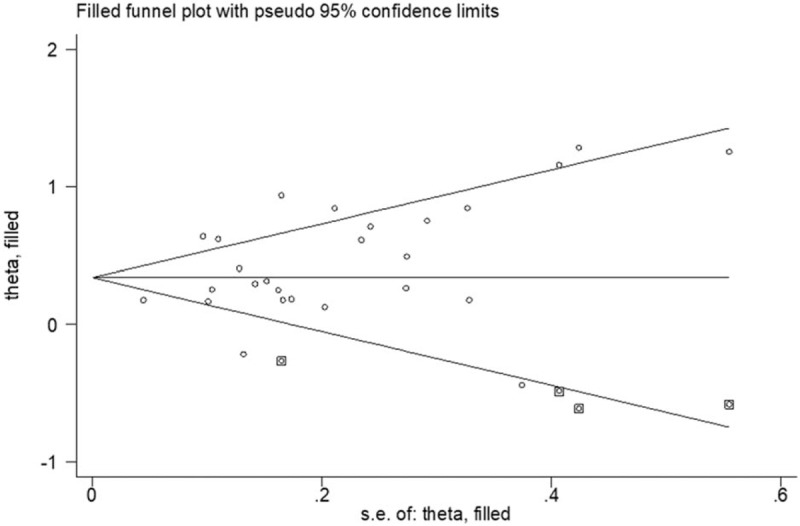
Funnel plot adjusted using a trim and fill method for OS. OS = overall survival.
4. Discussion
This meta-analysis aimed to examine the associations between elevated pretreatment NLR and oncologic outcomes of EC patients. Our results, including 33 individual studies of 11,039 patients, indicated that elevated NLR significantly predicted poor OS (1.390, 95% CI: 1.235–1.545) of EC patients. Although heterogeneity existed, the prognostic significance was not weakened by subgroup analyses stratified by country, histology, treatment method, sample size, cut-off value of NLR and HR estimation method. Subgroup analysis indicated the result was significant for patients from different countries. Poor OS with elevated NLR could be found both in SCC and AC. There was also a significant association between NLR and sample size ≤200 or >200. Cut-off values of elevated NLR were various. We performed subgroup analyses based on NLR cut-off values and found that patients with a low NLR had a better OS, regardless of the NLR cut-off values. Subgroup analysis stratified by HR estimation method also revealed that NLR had a negative effect on OS. Similarly, we found that elevated NLR was also related with shorter DFS, PFS, RFS, or CSS. According to our inclusion and exclusion criteria, all the included articles were retrospectively observational studies, so we did not statistically analyze the impact of study design on the outcomes. Taking all these into consideration, NLR may be a significant biomarker in the prognosis of EC.
Inflammation is a hallmark of cancer. The mechanism between inflammation and tumor progression has not been figured out exactly. Tumor-associated systemic inflammatory response is believed to correlate with prognosis and survival outcomes in cancer patients by promoting angiogenesis and distal spread, suppressing antitumor immunity and impacting response to anti-cancer therapies.[4] As an important part of systemic inflammatory response, tumor-infiltrating neutrophils have been recognized as an important element in tumor progression with various tumor-promoting features. More and more evidences demonstrate that tumor-infiltrating neutrophils might be associated with a poor prognosis through the promotion of angiogenesis, cell mobility, and migration.[49,50] Lymphocytes as crucial elements in innate and adaptive immune system play a vital role in the process of T cell-mediated anti-tumor response. The tumor infiltrating T cells could induce cytokine secretion such as IL-4 and IL-5 to regulate the angiogenesis, proliferation, apoptosis, and metastasis of cancer. The decrease in lymphocyte number could weak the immune response against tumors. Lymphocytopenia has been demonstrated to predict a poor prognosis in terms of survival.[51,52] Taken together, increase of NLR, the relative value of a combined elevated neutrophil or decreased lymphocyte could predict the potential suppression of host immune response and surveillance to tumor cells. Hence, NLR, which is available from blood routine test in daily clinical work, may be a promising prognostic marker for the clinical decision-making process to improve the survivals.
The prognostic role of NLR has also been researched in other cancers. Mu et al conducted a meta-analysis and found that multiple myeloma patients with higher NLR were more likely to have poorer prognosis than those with lower NLR.[53] Takenaka et al found higher NLR was associated with poorer OS, DSS, PFS, and distant metastasis-free survival in nasopharyngeal carcinoma.[54] Szor et al revealed an association of high NLR with older age, male gender, lower 5-year overall survival (OS), increased depth of tumor invasion, positive nodal involvement in resected gastric cancer patients.[55] In addition to NLR, the prognostic role of other parameters, such as platelet to lymphocyte ratio (PLR), trophoblast cell surface antigen, prognostic nutritional index (PNI) has also been researched in cancer patients. In our previous meta-analysis, we found high PLR was is associated with poor OS in patients with EC. PLR may be a significant predictive biomarker in patients with EC.[56] Xu et al found trophoblast cell surface antigen 2 overexpression was a predictive factor to the prognosis of solid cancers.[57] Zhao et al found PNI was allowed to function as an efficient indicator for the prognosis of patients with digestive system carcinomas.[58]
Besides the intrinsic defects of meta-analysis, there were some limitations in our research. First, all of the included studies were retrospective, which was more prone to some biases. Second, this research was limited to articles published in English language and some studies that only provided a Kaplan–Meier curve were also excluded, which could lead to the publication bias. Third, some included studies evaluated the prognostic role of NLR in multivariate analysis, whereas some used univariate analysis, which may impair the accuracy when the data were pooled. More important, significant heterogeneity was observed in the results due to confounding factors, such as the source of patients, pathological types, treatment strategies, duration of follow-up, sample size, cut-off value of NLR, statistic method. Though we performed subgroup analyses and sensitive analyses, but it could not completely explain the heterogeneity. The significant heterogeneity may affect the interpretation of the results. Further meta-analyses including additional studies and large sample sizes are needed to correct for publication bias and heterogeneity.
In conclusion, evaluation of NLR is a cost-effective method that is widely available in daily clinical work. Furthermore, it is an effective prognostic factor, as high values of this biomarker are related to aggressive tumor characteristics and poor survival outcomes. This ratio can be used to stratify the risk of patients within the same disease stage and is a promising prognostic marker for the clinical decision-making process on EC therapy. However, because of the limitations listed above, more studies with well-designed and large-scale are needed to confirm the conclusion in future.
Author contributions
Conceptualization: Xiangwei Zhang, Shaowei Sang.
Data curation: Xiangwei Zhang, Yuanzhu Jiang, Yang Wang, Zhaoyang Wang.
Formal analysis: Xiangwei Zhang, Yuanzhu Jiang, Yang Wang, Shaowei Sang, Lin Zhang.
Funding acquisition: Yuanzhu Jiang, Linping Zhao, Xianbiao Xue.
Investigation: Linping Zhao, Xianbiao Xue.
Methodology: Xiangwei Zhang, Yuanzhu Jiang, Yang Wang, Xianbiao Xue, Shaowei Sang, Lin Zhang.
Resources: Yang Wang, Linping Zhao.
Software: Zhaoyang Wang, Xianbiao Xue, Shaowei Sang.
Validation: Yuanzhu Jiang, Linping Zhao.
Writing – original draft: Xiangwei Zhang, Yuanzhu Jiang.
Writing – review & editing: Shaowei Sang, Lin Zhang.
Footnotes
Abbreviations: CSS = cancer-specific survival, DFS = disease-free survival, EC = esophageal cancer, HR = hazard ratio, NLR = neutrophil-to-lymphocyte ratio, OS = overall survival, PFS = progress-free survival, PLR = platelet to lymphocyte ratio, PNI = prognostic nutritional index, RFS = relapse-free survival.
XZ and YJ contributed equally to this work.
The work was supported by the Provincial Science and Technology Development Plan of Shandong (2016GSF201107) and Health Technology Development Program of Shandong Province (2016WS0433).
The authors have no conflicts of interest to disclose.
References
- [1].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [2].Domper Arnal MJ, Ferrandez Arenas A, Lanas Arbeloa A. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol 2015;21:7933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271–89. [DOI] [PubMed] [Google Scholar]
- [4].Mierke CT. The fundamental role of mechanical properties in the progression of cancer disease and inflammation. Rep Prog Phys Phys Soc (Great Br) 2014;77:076602. [DOI] [PubMed] [Google Scholar]
- [5].Kidane D, Chae WJ, Czochor J, et al. Interplay between DNA repair and inflammation, and the link to cancer. Crit Rev Biochem Mol Biol 2014;49:116–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Proctor MJ, Morrison DS, Talwar D, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer 2011;104:726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhou X, Du Y, Huang Z, et al. Prognostic value of PLR in various cancers: a meta-analysis. PloS One 2014;9:e101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Eo WK, Chang HJ, Kwon SH, et al. The lymphocyte-monocyte ratio predicts patient survival and aggressiveness of ovarian cancer. J Cancer 2016;7:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tohme S, Sukato D, Chalhoub D, et al. Neutrophil-lymphocyte ratio is a simple and novel biomarker for prediction of survival after radioembolization for metastatic colorectal cancer. Ann Surg Oncol 2015;22:1701–7. [DOI] [PubMed] [Google Scholar]
- [10].Sharaiha RZ, Halazun KJ, Mirza F, et al. Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol 2011;18:3362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Feng J-F, Huang Y, Chen Q-X. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol 2014;12:58–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Miyata H, Yamasaki M, Kurokawa Y, et al. Prognostic value of an inflammation-based score in patients undergoing pre-operative chemotherapy followed by surgery for esophageal cancer. Exp Ther Med 2011;2:879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Han L-H, Jia Y-B, Song Q-X, et al. Prognostic significance of preoperative lymphocyte-monocyte ratio in patients with resectable esophageal squamous cell carcinoma. Asian Pacific J Cancer Prevent APJCP 2015;16:2245–50. [DOI] [PubMed] [Google Scholar]
- [14].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg (Lond, Engl) 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [15].Goebell PJ, Kamat AM, Sylvester RJ, et al. Assessing the quality of studies on the diagnostic accuracy of tumor markers. Urol Oncol 2014;32:1051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [17].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Nat Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [18].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [19].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin Res ed) 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dutta S, Crumley ABC, Fullarton GM, et al. Comparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative resection of oesophageal cancer. World J Surg [Article] 2011;35:1861–6. [DOI] [PubMed] [Google Scholar]
- [21].Noble F, Hopkins J, Curtis N, et al. The role of systemic inflammatory and nutritional blood-borne markers in predicting response to neoadjuvant chemotherapy and survival in oesophagogastric cancer. Med Oncol (Northwood Lond Engl) 2013;30:596. [DOI] [PubMed] [Google Scholar]
- [22].Yoo EJ, Park JC, Kim EH, et al. Prognostic value of neutrophil-to-lymphocyte ratio in patients treated with concurrent chemoradiotherapy for locally advanced oesophageal cancer. Dig Liver Dis 2014;46:846–53. [DOI] [PubMed] [Google Scholar]
- [23].Yuan D, Zhu K, Li K, et al. The preoperative neutrophil-lymphocyte ratio predicts recurrence and survival among patients undergoing R0 resections of adenocarcinomas of the esophagogastric junction. J Surg Oncol 2014;110:333–40. [DOI] [PubMed] [Google Scholar]
- [24].Duan H, Zhang X, Wang F-X, et al. Prognostic role of neutrophil-lymphocyte ratio in operable esophageal squamous cell carcinoma. World J Gastroenterol 2015;21:5591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].He Y, Xu X, Li S, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with esophageal cancer in a high incidence area in China. Arch Med Res 2015;46:557–63. [DOI] [PubMed] [Google Scholar]
- [26].Jin-Shi L, Ying H, Xun Y, et al. A nomogram to predict prognostic values of various inflammatory biomarkers in patients with esophageal squamous cell carcinoma. Am J Cancer Res [Article] 2015;5:2180–9. [PMC free article] [PubMed] [Google Scholar]
- [27].Xu XL, Yu HQ, Hu W, et al. A novel inflammation-based prognostic score, the C-reactive protein/albumin ratio predicts the prognosis of patients with operable esophageal squamous cell carcinoma. PloS One 2015;10:e0138657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Geng Y, Shao Y, Zhu D, et al. Systemic immune-inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: a propensity score-matched analysis. Sci Rep 2016;6:39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ji WH, Jiang YH, Ji YL, et al. Prechemotherapy neutrophil: lymphocyte ratio is superior to the platelet: lymphocyte ratio as a prognostic indicator for locally advanced esophageal squamous cell cancer treated with neoadjuvant chemotherapy. Dis Esophagus 2016;29:403–11. [DOI] [PubMed] [Google Scholar]
- [30].Kosumi K, Baba Y, Ishimoto T, et al. Neutrophil/lymphocyte ratio predicts the prognosis in esophageal squamous cell carcinoma patients. Surg Today [Article] 2016;46:405–13. [DOI] [PubMed] [Google Scholar]
- [31].Masahide I, Yusuke K, Kyoichi K, et al. Evaluation of prognostic markers for patients with curatively resected thoracic esophageal squamous cell carcinomas. Mol Clin Oncol [Article] 2016;5:767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Miyazaki T, Sakai M, Sohda M, et al. Prognostic significance of inflammatory and nutritional parameters in patients with esophageal cancer. Anticancer Res 2016;36:6557–62. [DOI] [PubMed] [Google Scholar]
- [33].Peng Z, Mian X, Lei Z, et al. Comparison of two inflammation-based prognostic scores in patients with thoracic esophageal cancer undergoing chemoradiotherapy. Int J Clin Exp Med [Article] 2016;9:1764–71. [Google Scholar]
- [34].Takahiro T, Naoshi K, Tatsuro T, et al. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer [Article] 2016;16:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wan G-X, Chen P, Cai X-J, et al. Elevated red cell distribution width contributes to a poor prognosis in patients with esophageal carcinoma. Clin Chim Acta [Article] 2016;452:199–203. [DOI] [PubMed] [Google Scholar]
- [36].Xiao Q, Zhang B, Deng X, et al. The preoperative neutrophil-to-lymphocyte ratio is a novel immune parameter for the prognosis of esophageal basaloid squamous cell carcinoma. PloS One 2016;11:e0168299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Xie X, Luo KJ, Hu Y, et al. Prognostic value of preoperative platelet-lymphocyte and neutrophil-lymphocyte ratio in patients undergoing surgery for esophageal squamous cell cancer. Dis Esophagus 2016;29:79–85. [DOI] [PubMed] [Google Scholar]
- [38].Zhang F, Chen Z, Wang P, et al. Combination of platelet count and mean platelet volume (COP-MPV) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patients. Tumour Biol 2016;37:9323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang F, Sun P, Wang Z-q, et al. Low preoperative albumin-globulin score predicts favorable survival in esophageal squamous cell carcinoma. Oncotarget 2016;7:30550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hirahara N, Matsubara T, Kawahara D, et al. Prognostic significance of preoperative inflammatory response biomarkers in patients undergoing curative thoracoscopic esophagectomy for esophageal squamous cell carcinoma. Eur J Surg Oncol 2017;43:493–501. [DOI] [PubMed] [Google Scholar]
- [41].Nakamura K, Yoshida N, Baba Y, et al. Elevated preoperative neutrophil-to-lymphocytes ratio predicts poor prognosis after esophagectomy in T1 esophageal cancer. Int J Clin Oncol 2017;22:469–75. [DOI] [PubMed] [Google Scholar]
- [42].Zhou XL, Li YQ, Zhu WG, et al. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for patients with locally advanced esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Sci Rep 2017;7:42581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhao Q, Chen S, Feng J-F. A novel inflammation-based prognostic index for patients with esophageal squamous cell carcinoma: neutrophil lymphocyte ratio/albumin ratio. Oncotarget 2017;8:103535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kunizaki M, Tominaga T, Wakata K, et al. Clinical significance of the C-reactive protein-to-albumin ratio for the prognosis of patients with esophageal squamous cell carcinoma. Mol Clin Oncol 2018;8:370–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gao GD, Sun B, Wang XB, et al. Neutrophil to lymphocyte ratio as prognostic indicator for patients with esophageal squamous cell cancer. Int J Biol Markers 2017;32:e409–14. [DOI] [PubMed] [Google Scholar]
- [46].Yang Y, Xu H, Zhou L, et al. Platelet to lymphocyte ratio is a predictive marker of prognosis and therapeutic effect of postoperative chemotherapy in non-metastatic esophageal squamous cell carcinoma. Clin Chim Acta 2018;479:160–5. [DOI] [PubMed] [Google Scholar]
- [47].He YF, Luo HQ, Wang W, et al. Preoperative NLR and PLR in the middle or lower ESCC patients with radical operation. Eur J Cancer Care 2017;26:2. [DOI] [PubMed] [Google Scholar]
- [48].Yu X, Wen Y, Lin Y, et al. The value of preoperative Glasgow Prognostic Score and the C-Reactive Protein to Albumin Ratio as prognostic factors for long-term survival in pathological T1N0 esophageal squamous cell carcinoma. J Cancer 2018;9:807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol 2013;23:200–7. [DOI] [PubMed] [Google Scholar]
- [50].Reid MD, Basturk O, Thirabanjasak D, et al. Tumor-infiltrating neutrophils in pancreatic neoplasia. Mod Pathol 2011;24:1612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Germano G, Allavena P, Mantovani A. Cytokines as a key component of cancer-related inflammation. Cytokine 2008;43:374–9. [DOI] [PubMed] [Google Scholar]
- [52].Eerola AK, Soini Y, Paakko P. Tumour infiltrating lymphocytes in relation to tumour angiogenesis, apoptosis and prognosis in patients with large cell lung carcinoma. Lung Cancer (Amsterdam, Netherlands) 1999;26:73–83. [DOI] [PubMed] [Google Scholar]
- [53].Mu S, Ai L, Fan F, et al. Prognostic role of neutrophil-lymphocyte ratio in multiple myeloma: a dose-response meta-analysis. OncoTargets Ther 2018;11:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Takenaka Y, Kitamura T, Oya R, et al. Prognostic role of neutrophil-lymphocyte ratio in nasopharyngeal carcinoma: a meta-analysis. PloS One 2017;12:e0181478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Szor DJ, Dias AR, Pereira MA, et al. Prognostic role of neutrophil/lymphocyte ratio in resected gastric cancer: a systematic review and meta-analysis. Clinics (Sao Paulo, Brazil) 2018;73:e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhang X, Wang Y, Zhao L, et al. Prognostic value of platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review and meta-analysis. Int J Biol Markers 2018;1724600818766889. [DOI] [PubMed] [Google Scholar]
- [57].Xu P, Zhao Y, Liu K, et al. Prognostic role and clinical significance of trophoblast cell surface antigen 2 in various carcinomas. Cancer Manag Res 2017;9:821–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhao Y, Xu P, Kang H, et al. Prognostic nutritional index as a prognostic biomarker for survival in digestive system carcinomas. Oncotarget 2016;7:86573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


