Supplemental Digital Content is available in the text
Keywords: meta-analysis, poststroke depression, sertraline
Abstract
Background:
Morbidity of poststroke depression (PSD) remains high worldwide. Additionally, PSD causes multiple sequelae. Although sertraline has been reported to be effective in treating PSD, many studies remain inconsistent.
Methods:
PubMed, Embase, Scopus, Cochrane Central Register of Controlled Trials, Clinical trials. gov, Wan fang Data (Chinese), VIP (Chinese), and CNKI (Chinese) were retrieved from inception to April 2017. Randomized controlled trials (RCTs) and self-controlled trials (SCTs) were recruited, which met the inclusion criteria in our study. The depression rating scores, the incidence of PSD, activities of daily living (ADL), neurological impairment scores, and adverse effects were assessed.
Results:
Around 11 studies were recruited in our work, including 1258 participants. For trials enrolled, the results were depicted: the reduction of depression rating scores was significant in sertraline groups (WMD −6.38; 95% CI −8.63 to −4.14; P < .00001); the incidence of PSD was significantly lower in sertraline groups (RR 0.48; 95%CI 0.35–0.67; P < .0001); there was obvious improvement of ADL (WMD 11.48; 95% CI 4.18–18.78; P = .002 <0.05) and neurological impairment (WMD −3.44; 95% CI −6.66 to −0.21; P = .04 <0.05); no significant difference between sertraline and control groups in the morbidity of adverse events (RR 0.94; 95% CI 0.83–1.06; P = .33 >0.05). However, in sensitivity analyses, the conclusions of the reduction of depression rating scores and the improvement of ADL were altered.
Conclusions:
The study suggests that sertraline has a potentially protective role compared with control groups and demonstrates sertraline is safe. However, the reduction of depression rating scores and the improvement of ADL should be considered carefully.
1. Introduction
The incidence of poststroke depression (PSD) is very high around the world.[1–3] A meta-analysis of epidemiology revealed 31% of patients had depression during 5 years following the stroke, in which 61 cohort studies and 25,488 patients were enrolled.[4] PSD could impair the cognitive level and activities of daily living (ADL), cause negative sequela effects on the recovery of patients and increase the burden of caregivers.[2] The etiological mechanisms of PSD remain obscure, including psychological, social and biological factors.[5]
Sertraline, one of the selective serotonin reuptake inhibitors (SSRIs), has been proved effective for the treatment and prophylaxis of PSD, but it is still controversial. One study showed the treatment of sertraline had no statistical significance in both major and minor depression episodes.[6] However, positive effects were shown in other studies.[7,8] Sertraline had significant preventive effects in comparison with placebo and the participants experienced fewer side events in one double blind, randomized, and placebo-controlled study.[9] But Burn's study described neither of the Scandinavian Stroke Scale (SSS) and Barthel Index (BI) changed during the intervention.[10] Up to now, there was no evidence to confirm the efficacy of sertraline.
There are several systematic reviews of SSRIs in the treatment and prevention of PSD.[11–17] Due to the significant heterogeneity of the methods used in these studies, the results could not be pooled.[16] Another study indicated that SSRIs could improve the symptoms of PSD, but the prophylactic efficacy was never confirmed.[12] To our knowledge, we have never found a paper which described the efficacy of sertraline or assessed the improvement of neurological impairment and the ADL after using sertraline. Therefore, further systematic reviews and meta-analyses are necessary to solve such concerns.
Our meta-analysis was conducted to evaluate the efficacy of sertraline in the treatment and prevention of PSD. All randomized controlled trials (RCTs) and self-controlled trials (SCTs) of sertraline in the treatment and prophylaxis of PSD were included in our work. Additionally, studies published in either English or Chinese were included.
2. Methods
2.1. Data sources and search strategy
We retrieved Medline via PubMed, Embase, Scopus, Cochrane Central Register of Controlled Trials, Clinical Trials. gov, CNKI (Chinese), Wan fang (Chinese), and VIP database (Chinese) from inception to April 2017. The search terms were as follows: “‘randomized controlled trial’, ‘controlled clinical trial’, ‘randomized’, ‘placebo’, ‘drug therapy’, ‘randomly’ or ‘trial’ ” and “ humans ” and “ ‘stroke’, ‘cerebral hemorrhage’, ‘cerebral infarction’, ‘apoplexy’, ‘brain vascular accident’, ‘cerebrovascular accident’, ‘cerebral stroke’, ‘brain infarction’, ‘intracranial hemorrhages’ or ‘hemiplegia’ ” and “sertraline” and “depression.” Both English and Chinese papers were included. We also searched references and contacted with authors to get the detailed data if necessary.
2.2. Selection criteria
RCTs and SCTs were enrolled, in which patients were with a clinical diagnosis of stroke at baseline, control arms included usual care or placebo and the experimental arms were treated with sertraline at any dose, by any mode of delivery. Drugs and therapies with mixed effects, duplicates, secondary analyses, or letters and outcome data unavailable or incomplete were excluded.
Two team members scrutinized the abstract and title of each literature independently. A third investigator was discussed with if necessary.
2.3. Outcome measures
The primary outcomes: the depression rating scores measured by multiple depression rating scales, in which the Hamilton Depression Scale (HAMD) was preferred; the incidence of PSD.
The secondary outcomes: the ADL was evaluated by the Barthel Index (BI),[18] Functional Independence Measure (FIM),[19] the WHO Well-Being Scale,[20] or 36-Item Short Form Health Survey (SF-36);[21] neurological impairment was assessed by the Scandinavian Stroke Scale (SSS)[22] or National Institutes of Health Stroke Scale (NIHSS);[23] adverse effects were assessed by Treatment Emergent Symptom Scale (TESS), the Udvalg for Kliniske Undersøgelser (UKU) Side Effect Rating Scale,[24] or the incidence of side effects.
2.4. Data extraction and quality assessment
Using the designed table, 2 researchers extracted data and scrutinized all articles independently. The process was divided into 2 parts.
In the first phase, the information was abstracted as follows: author identification, year of publication, geographic location of the study, study funding source, type of study design (prospective or retrospective, randomized or observational, presence and type of control, blinded or open-label), study population, sample size, follow-up duration, antidepressants used (dose, time, and when to take it) and the results.
In the second phase, 2 reviewers independently assessed the quality of the studies, which was based on the widely used Joanna Briggs Institute (JBI) Reviewers Manual: 2008 Edition,[25] including randomization, blinding, allocation concealment, description of withdraws/dropouts, baseline consistency, the reliability of measurement and analysis of data. Opinion was sought from a third reviewer if the first 2 reviewers could not reach an agreement.
2.5. Statistical analysis
Statistical analyses were carried out by RevMan 5.3 software (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014). The continuous outcomes were described by weighted mean differences (WMD) with 95% confidence intervals (CIs), and risk ratio (RR) was used to describe categorical data with 95% CIs. P < .05 was used as a cutoff for statistical significance. Statistical heterogeneity of trials was evaluated by I2.[26] If we observed I2 > 50% or P < .10, we used a random-effect model to calculate the pooled estimates. On the contrary, we used a fixed-effect model.
Subgroup analyses were conducted based on the participants who were with depression or not at recruitment, the mean age (>70 vs < 70), the gender (male/female >1 vs male/female <1) and the type of control (RCT vs SCT).
We excluded the studies with low quality (nonrandomized and non-double-blind comparison) in sensitivity analyses. Publication bias was assessed by funnel plot regression method and Egger statistical test that was carried out by Stata 12.0 and P < .10 was considered as statistically asymmetry.[27]
We analyzed the data about previous studies which were published early in our research, so ethical approval and patient consent were not necessary and therefore not provided.
3. Results
3.1. Study selection and characteristics
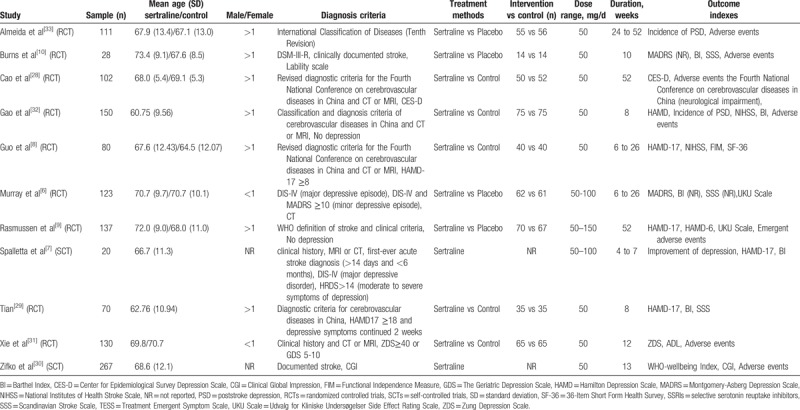
We searched 8 databases and found 743 relevant studies, 34 from PubMed, 49 from Embase, 259 from Scopus, 26 from Cochrane Central Register of Controlled Trials, 1 from Clinical trials. gov, 100 from CNKI, 123 from Wan fang (Chinese) and 132 from VIP Database (Chinese). Around 108 duplicates were removed. After scrutinizing titles and abstracts, 570 articles were excluded, and 65 full texts were searched. Two full texts were not accessible to us. We obtained 63 full texts, 11 articles were included in the final study (Fig. 1),[6–10,28–33] in which 7 articles were about the treatment of PSD,[6–8,28–31] 4 articles were about the prevention of PSD,[9,10,32,33] 9 articles were RCTs,[6,8–10,28,29,31–33] and 2 articles were SCTs.[7,30] Around 1258 participants were enrolled totally in our study, in which 971 participants were randomly enrolled in RCTs and 287 participants were included in SCTs. According to the results of studies, 4 studies consisted of 36 trials.[6,9,10,33] Firstly, according to participants’ degree of depressive episodes (major depressive episode and minor depressive episode), one study included 2 trials.[6] Secondly, judging from the follow-up duration (24 and 52 weeks), 2 trials were included in one study.[33] Thirdly, the categories of side effects were divided into 33 kinds, including 28 common adverse events[6,9] as well as 5 emergent adverse events.[9,10] The detailed characteristics of each trial were shown in Table 1.
Figure 1.
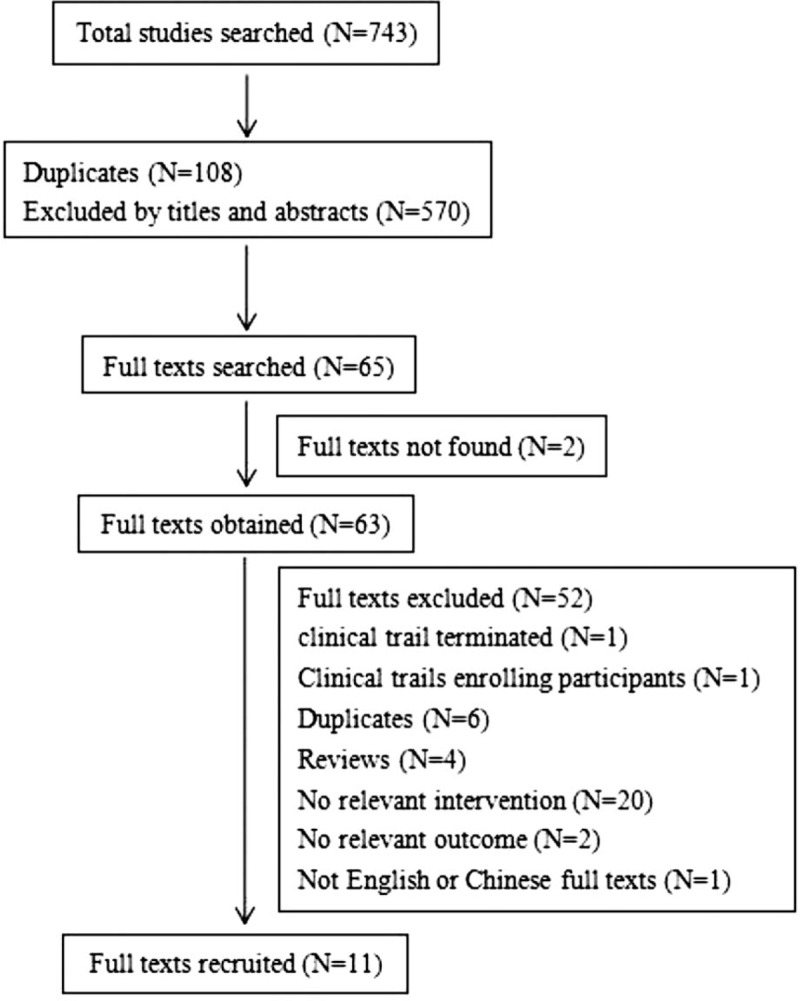
Results of the paper search.
Table 1.
Detailed demographic and clinical characteristics of recruited studies.
3.2. Primary outcomes
3.2.1. Depression rating scores at the end of treatment
Around 6 studies were included,[6–8,28,29,31] one of which was SCT.[7] Moreover, one study had 2 trials.[6] The pooled effect described a significant advantage of sertraline groups over control groups (WMD −6.38; 95% CI, −8.63 to −4.14; P < .00001; I2 84.0%; Fig. 2).
Figure 2.
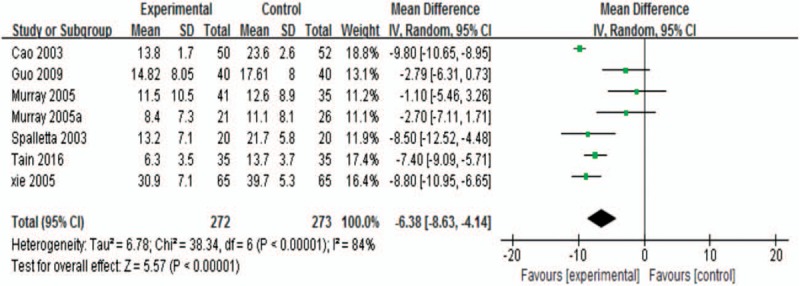
Depression rating scores at the end of treatment.
Subgroup: mean age (>70 vs <70)
For patients whose mean age was >70, there was no statistically significant effect of sertraline (WMD −4.48; 95% CI, −9.78 to 0.83; P = .10 >0.05; I2 85.0%). However, for patients whose mean age was <70, sertraline groups were more improved than control groups (WMD −7.42; 95% CI, −10.01 to −4.84; P < .00001; I2 84.0%). There was no heterogeneity between subgroups (I2 0.0%; Supplemental Fig. 1A).
Subgroup: gender (male/female >1 vs male/female <1)
It was in favor of sertraline groups for male/female >1 at baseline (WMD −7.42; 95% CI, −10.01 to −4.84; P < .00001; I284.0%). However, there was no statistical significance for trials in which male/female was <1 (WMD −4.48; 95% CI, −9.78 to −0.83; P = .10 >0.05; I2 85.0%). No heterogeneity was between subgroups (I2 0.0%; Supplemental Fig. 1B).
Subgroup: type of control (RCTs vs SCTs).
Significant statistical differences were between the experimental and control groups in the subgroup of RCTs (WMD −6.05; 95% CI, −8.54 to −3.55; P < .00001; I2 87.0%). And there was only one study in the subgroup of SCTs.[7] There was low heterogeneity between subgroups (I2 3.3%; Supplemental Fig. 1C).
3.2.2. The Incidence of PSD at the end of treatment
There were 3 studies which described the incidence of PSD.[9,32,33] Pooled analysis revealed a reduced risk in sertraline groups with low statistical heterogeneity (RR 0.48; 95% CI, 0.35–0.67; P < .0001; I2 34.0%; Fig. 3).
Figure 3.
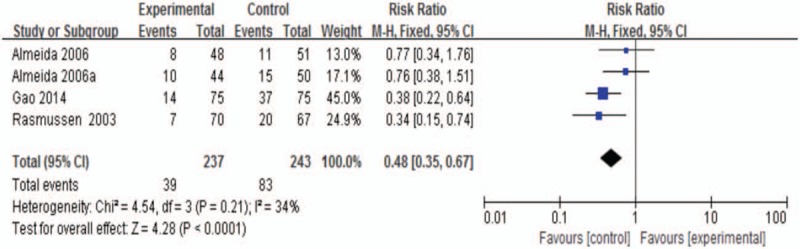
The incidence of PSD at the end of treatment. PSD = poststroke depression.
3.3. Secondary outcomes
3.3.1. Activities of daily living at the end of treatment
Seven studies were analyzed together.[7,8,10,29–32] The pooled analysis showed that there was greatly better improvement in the intervention groups with high heterogeneity (WMD 11.48; 95% CI, 4.18–18.78; P = .002 <0.05; I2 97.0%; Fig. 4)
Figure 4.
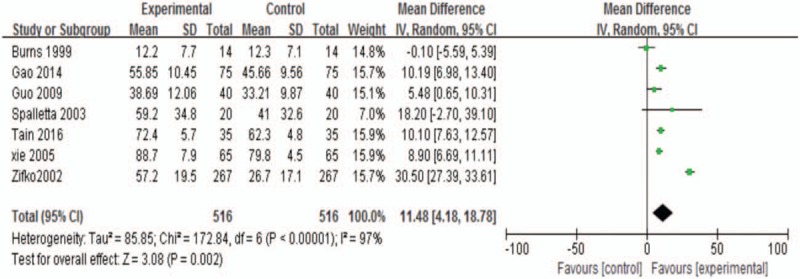
ADL at the end of treatment. ADL = activities of daily living.
Subgroup: depression at recruitment (depression vs no depression)
The WMD was 14.27 for patients with depression at recruitment (95% CI, 4.67 to 23.88; P = .004 <0.05; I2 97.0%). For patients with no depression at recruitment, no statistical significance was found in the pooled analysis (WMD 5.27; 95% CI, −4.80 to 15.34; P = .31 >0.05; I2 97.0%). There was a better effect for patients with depression at recruitment. There was low heterogeneity between subgroups (I2 37.8%; Supplemental Fig. 2A).
Subgroup: mean age (>70 vs <70)
There was no statistical significance for the mean age >70 (WMD 4.74; 95% CI, −4.06–13.53; P = .29 >0.05; I2 89.0%). In addition, for the mean age <70, it was in favor of sertraline groups (WMD 14.59; 95% CI, 4.50–24.68; P = .005 <0.05; I2 97.0%). There was a better effect in the mean age <70. There was no heterogeneity between subgroups (I2 52.0%; Supplemental Fig. 2B).
Subgroup: gender (male/female >1 vs male/female <1)
There was no statistical significance in the male/female <1 (WMD 19.67; 95% CI, −1.50–40.84; P = .07 >0.05; I2 99.0%). The effect of sertraline was greater than control in the male/female >1 (WMD 7.31; 95% CI 3.35–11.27; P = .0003 <0.05; I2 74.0%). Low heterogeneity existed between subgroups (I2 21.0%; Supplemental Fig. 2C).
Subgroup: type of control (RCTs vs SCTs)
Significant statistical differences in the subgroup of RCTs (WMD 7.64; 95% CI, 4.85–10.43; P < .00001; I2 72.0%). And there were only 2 studies in the subgroup of SCTs (WMD 28.87; 95% CI, 20.69–37.04; P < .00001; I2 23.0%).[7,30] There was high heterogeneity between subgroups (I2 95.7%; Supplemental Fig. 2D).
3.3.2. Neurological impairment scores at the end of treatment
There were 4 trials in the analysis.[8,10,29,32] The WMD was −3.44 (95% CI, −6.66 to −0.21; P = .04 <0.05; Fig. 5). Sertraline groups were a little more improved than control groups with a significant heterogeneity (I2 82.0%).
Figure 5.
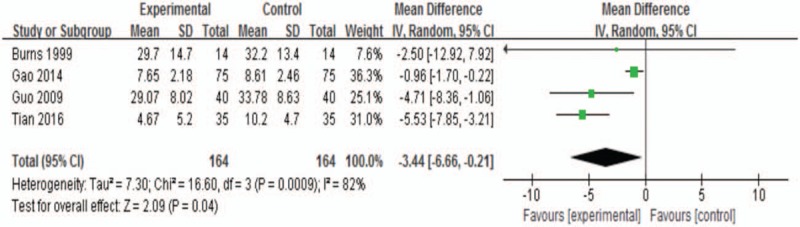
Neurological impairment scores at the end of treatment.
3.3.3. Adverse events at the end of treatment
Side effects were reported in the majority of enrolled papers.[6,9,10,28,31–33] 38 trials were enrolled and revealed that there was no statistical significance between sertraline and the control groups (RR 0.94; 95% CI, 0.83 to 1.06; P = .33 >0.05; Fig. 6). There was moderate heterogeneity among trials (I2 45.0%).
Figure 6.
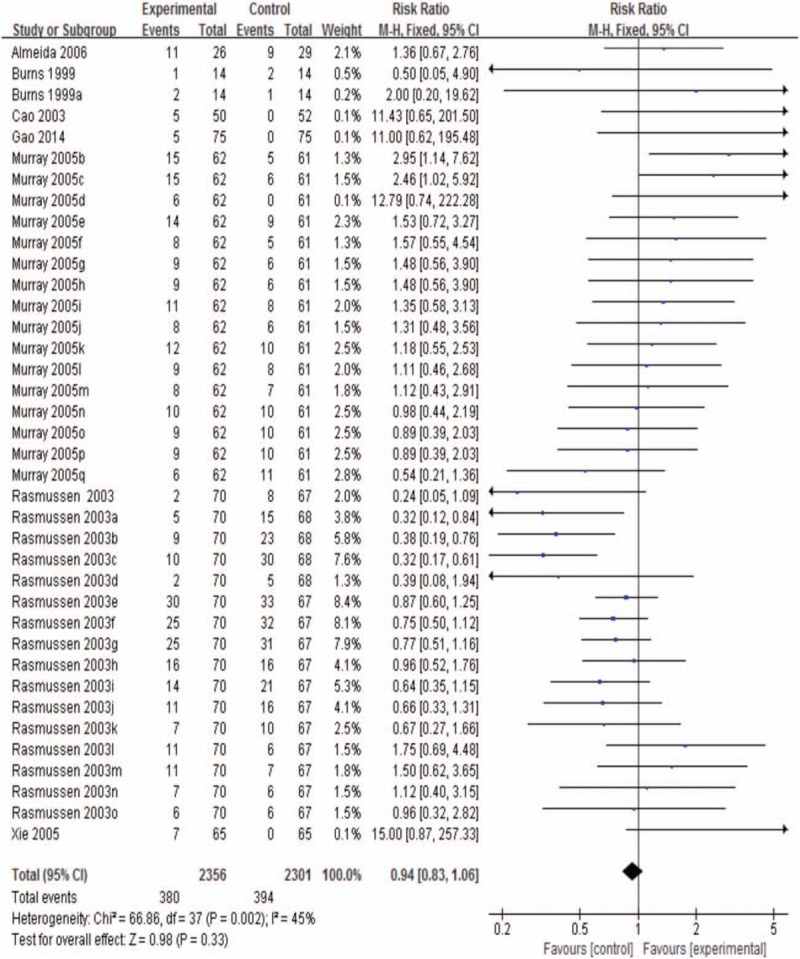
Adverse events at the end of treatment.
3.3.3.1. Common adverse events at the end of treatment
The pooled data consisted of 7 studies and 33 trials,[10,6,9,28,31–33] and the RR was 1.07 (95% CI, 0.94–1.22; P = .31 >0.05; I2 27.0%; Fig. 7). The results showed no statistical significance between sertraline and the control groups. Common adverse events included gastrointestinal symptoms, headache, dizziness, and increased hepatic alanine aminotransferase and aspartate aminotransferase levels.
Figure 7.
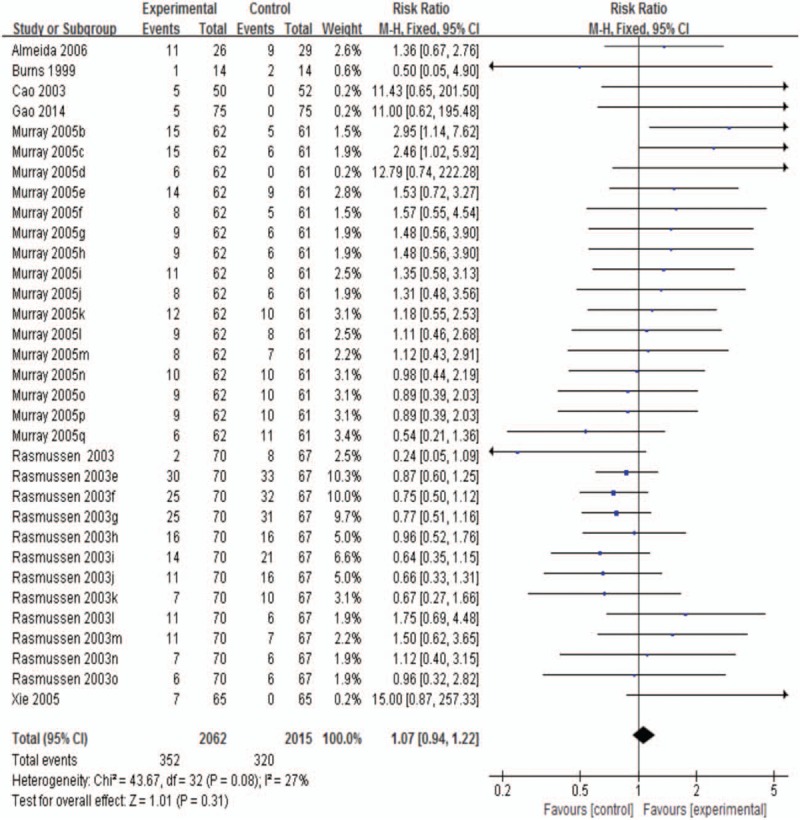
Common adverse events at the end of treatment.
3.3.3.2. Emergent adverse events at the end of treatment
The data analysis consisted of 2 studies,[9,10] including 5 trials. There was a lower risk in sertraline arms with no statistical heterogeneity (RR 0.37; 95% CI, 0.25–0.55; P < .00001; I2 0%; Fig. 8). Emergent adverse events included severe cardiovascular events, recurrent stroke, hospitalization, and death.
Figure 8.
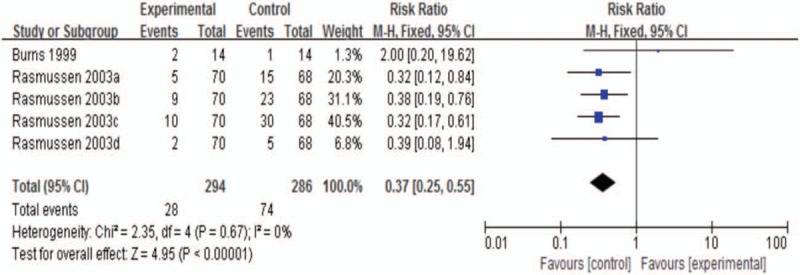
Emergent adverse events at the end of treatment.
3.4. Sensitivity analysis
In the sensitivity analyses, the conclusions of pooled analyses were robust except for the results of depression rating scores and ADL. The nonrandomized and non-double-blind designed studies were excluded,[7,28–32] and the pooled results were changed as follows: the depression rating scores from baseline to endpoint were altered significantly (WMD −2.28; 95% CI, −4.16–0.04; P = .05; I2 0%; Supplemental Fig. 3); the incidence of PSD was changed (RR 0.57; 95% CI, 0.37–0.88; P = .01 <0.05; I2 31%; Supplemental Fig. 4); the pooled analysis of ADL was altered (WMD 2.80; 95% CI, −2.66–8.26; P = .32 >0.05; I2 57.0%; Supplemental Fig. 5); after removing low quality of studies, the WMD of neurological impairment scores was −4.47 (95% CI, −7.91 to −1.02; P = .01 <0.05; I2 0%; Supplemental Fig. 6); the RR of adverse effects in the treatment of PSD was 0.90 (95% CI, 0.79–1.02; P = .09 >0.05; I2 40.0%; Supplemental Fig. 7); there was still no statistical difference in common adverse effects from baseline to endpoint (RR 1.02; 95% CI, 0.89 to 1.16; P = .79 >0.05; I2 14.0%; Supplemental Fig. 8).
3.5. Quality assessment and publication bias
Quality assessment of the studies enrolled was shown in Table 2, which was based on JBI Reviewers Manual (2008).[25] There was a moderate quality of trials enrolled and 5 studies contained randomization, allocation concealment or blinding.[6,8–10,33] Visual inspection of invert funnel plots of adverse effects was symmetrical (Fig. 9). The Egger test also showed that the outcomes were not affected by publication bias (t = 2.59; P = .014 >0.10).
Table 2.
Results of quality assessment.
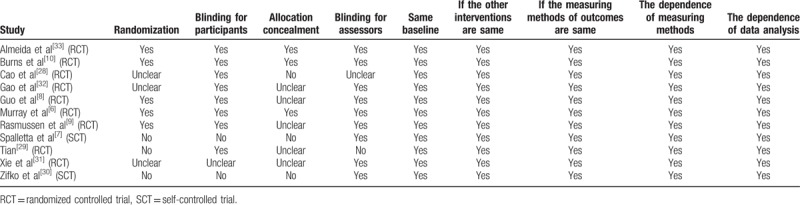
Figure 9.
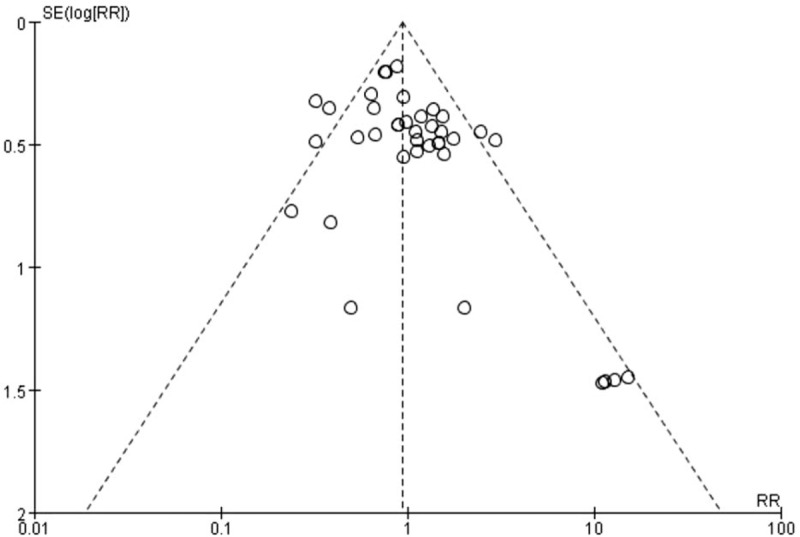
Invert funnel plots of adverse events at the end of treatment.
4. Discussion
Around 11 studies and 1258 participants were enrolled in our study, which included 41 trials. We found that treatment with sertraline benefited participants and all outcomes were positive, including depression rating scores, the incidence of PSD, neurological impairment scores, and ADL at the end of treatment. This confirmed that treatment with sertraline was safe and even protective for stroke patients in our research. However, there existed significant heterogeneity in depression rating scores and neurological impairment scores as well as ADL at the end of treatment. Furthermore, the pooled results of the depression rating scores and ADL were altered in sensitivity analyses, which should be interpreted cautiously.
Our meta-analysis revealed that sertraline was beneficial to reducing depression rating scores, especially for the male patients (mean age <70) with PSD, which is contrary to Xu et al[13] that describes female patients are more beneficial. However, one-third of studies enrolled in a systematic review indicates female sex as a risk factor for PSD,[34] and the findings were replicated in a review of 23 studies,[35] including 18,374 stroke patients. In the sensitivity analysis, after removing the low quality of studies,[7,28,29,31] the improvement of depression did not exist in sertraline compared with the control, which is consistent with Chen and Guo.[36] Low quality of trials included and the limited population enrolled in our research may affect the validity of results and lead to biases. Caution should be taken to interpret the conclusion. Therefore, the efficacy of sertraline in the treatment of PSD was uncertain and more high quality and multicenter RCTs were needed to solve the problem.
Our research indicated that sertraline was protective and safe for participants. The early use of sertraline contributed to the prevention of PSD, which consists with a systematic review that the SSRIs reduced RR of the morbidity of PSD.[17] Furthermore, sertraline was well tolerated for common adverse events and even reduced the occurrence rate of emergent side events, which is different with a previous meta-analysis,[13] due to different types of antidepressants and tricyclic antidepressants used in that research. Therefore, types of antidepressants have a significant influence on the incidence of adverse effects. Hence treatment with sertraline was safe and there existed significant protective effects on PSD.
Sertraline effectively improved ADL and neurological impairment. After removing the low quality of researches, the conclusion of ADL was altered, which is consistent with the meta-analyses of Xu et al[13] and Chen et al.[37] Small samples of trials included may lead to high risk of bias and overestimation.[38]
There are some limitations in our study. Firstly, 2 full texts were not able to obtain, so certain data may be lost. Secondly, the participants who were serious cognitive impairment, aphasia and the severe stroke were excluded in the studies recruited, therefore we could not find who were more beneficial to the treatment with sertraline. Thirdly, the doses of sertraline were different. Hence, we could not determine the proper dose of sertraline. Fourthly, multiple factors may lead to high heterogeneity, for example, different scales, types of control and demographic characteristics as well as small samples of some studies enrolled. Finally, the mean confidence intervals of some pooled analyses were wide, so the reality of the effects was not ensured. Moreover, the duration of intervention was not adequate, which should be continued after recovery of PSD for at least 6 months.[39]
One of our strengths was that a fairly wide range of online databases was searched, including references, unpublished trials and studies documented in both English and Chinese. Secondly, sufficient sensitivity analyses and enough subgroup analyses were conducted to ensure the reliability and robustness of the results. In addition, we only aimed at the efficacy of sertraline, so the methodological heterogeneity was reduced. Furthermore, the statistical heterogeneity was relatively low and more outcomes were analyzed, including the efficacy of sertraline, functional abilities as well as adverse events, which contributes to clinicians and patients to make a better choice. In the future, it is necessary for us to develop more detailed and rigorous intervention projects as well as preclinical medicine to promote the clinical practice.
5. Conclusions
Taken together, our meta-analysis clarified the inconsistency of sertraline's role on PSD in previous studies. Furthermore, our findings prompted that sertraline is safe and effective in the treatment and prevention of PSD. However, the improvement of PSD and ADL should be explained cautiously. Moreover, our study provides limitations and inspirations for further researches. Therefore, more multicenter, larger sample, and more result indexes designed RCTs are needed to evaluate the protective role of sertraline on PSD.
Author contributions
Rongfang Feng collected and analyzed the data, and wrote the paper. Peng Wang analyzed the data, and participated in writing of the manuscript. Chenhao Gao assisted with the data analyses. Rongfang Feng and Peng Wang conceived and designed this study. Chenhao Gao, Jianbo Yang, Ling Li, Zhenxiang Zhang and Shiguang Wang modified the paper. Zixiao Chen, Yaoyao Yang, Jiawei Jiao, Mengmeng Li and Bo Fu extracted data. All authors reviewed the paper, read and approved the final manuscript.
Data curation: Rongfang Feng, Peng Wang, Zixiao Chen, Yaoyao Yang, Jiawei Jiao, Mengmeng Li, Bo Fu.
Funding acquisition: Peng Wang, Zhenxiang Zhang.
Methodology: Rongfang Feng, Peng Wang, Chenhao Gao.
Resources: Rongfang Feng, Zixiao Chen, Yaoyao Yang, Jiawei Jiao, Mengmeng Li, Ling Li, Zhenxiang Zhang.
Software: Rongfang Feng.
Supervision: Rongfang Feng, Peng Wang, Chenhao Gao, Jianbo Yang.
Writing – original draft: Rongfang Feng.
Writing – review & editing: Rongfang Feng, Peng Wang, Chenhao Gao, Jianbo Yang, Shiguang Wang.
Supplementary Material
Footnotes
Abbreviations: ADL = activities of daily living, BI = Barthel Index, CIs = confidence intervals, FIM = Functional Independence Measure, HAMD = Hamilton Depression Scale, NIHSS = National Institutes of Health Stroke Scale, PSD = poststroke depression, RCTs = randomized controlled trials, RR = risk ratios, SCTs = self-controlled trials, SF-36 = 36-Item Short Form Health Survey, SSRIs = selective serotonin reuptake inhibitors, SSS = Scandinavian Stroke Scale, TESS = Treatment Emergent Symptom Scale, UKU Scale = Udvalg for Kliniske Undersøgelser Side Effect Rating Scale, WMD = weighted mean difference.
This work was supported by the National Natural Science Foundation, China (No. 81500433); the Henan Educational Committee Foundation (No. 2018-ZZJH-547; 13A320686;16A310003; 12A310010); the Science and Technology Planning Project of Henan (No. 134200510018; 132102310108; 162102410057; 132102310138; 122300410338); the Science and Technology Planning Project of Zhengzhou (No. 121PPTGG508-11; 10PTGS484-7; 121PPTGG507-9); the Science and Technology Planning Project of Zhengzhou University (No. 2017cxcy610), the research foundations of Nursing school of Zhengzhou University (HLSXYJG2016007,2018HLXYXK03, HLXYYJS201806).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke 2015;9:1017–25. [DOI] [PubMed] [Google Scholar]
- [2].Ayerbe L, Ayis S, Wolfe CDA, et al. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Brit J Psychiatry 2013;202:14–21. [DOI] [PubMed] [Google Scholar]
- [3].Arseniou S, Arvaniti A, Samakouri M. Post-stroke depression: recognition and treatment interventions. Psychiatrike 2011;22:240–8. [PubMed] [Google Scholar]
- [4].De RA, Fransen E, Brouns R, et al. Poststroke depression and its multifactorial nature: results from a prospective longitudinal study. J Neurol Sci 2014;347:159–66. [DOI] [PubMed] [Google Scholar]
- [5].Hackett ML, Yapa C, Parag V, et al. Frequency of depression after stroke: a systematic review of observational studies. Stroke 2005;36:1330–40. [DOI] [PubMed] [Google Scholar]
- [6].Murray V, Arbin MV, Bartfai A, et al. Double-blind comparison of sertraline and placebo in stroke patients with minor depression and less severe major depression. J Clin Psychiatry 2005;66:708–16. [DOI] [PubMed] [Google Scholar]
- [7].Spalletta G, Caltagirone C. Sertraline treatment of post-stroke major depression: an open study in patients with moderate to severe symptoms. Funct Neurol 2003;18:227–32. [PubMed] [Google Scholar]
- [8].Guo R-Y, Su L, Liu L-A, et al. Effects of Linggui Bafa on the therapeutic effect and quality of life in patients of post-stroke depression. Chin Acupunct Moxibust 2009;29:785–90. [PubMed] [Google Scholar]
- [9].Rasmussen A, Lunde M, Poulsen DL, et al. A double-blind, placebo-controlled study of sertraline in the prevention of depression in stroke patients. Psychosomatics 2003;44:216–21. [DOI] [PubMed] [Google Scholar]
- [10].Burns A, Russell E, Stratton-Powell H, et al. Sertraline in stroke-associated lability of mood. Int J Geriatr Psychiatry 1999;14:681–5. [PubMed] [Google Scholar]
- [11].Hackett ML, Anderson CS, House AO. Interventions for preventing depression after stroke. Cochrane Database Syst Rev 2004;2:CD003437. [DOI] [PubMed] [Google Scholar]
- [12].Bhogal SK, Teasell R, Foley N, et al. Heterocyclics and selective serotonin reuptake inhibitors in the treatment and prevention of poststroke depression. J Am Geriatr Soc 2010;53:1051–7. [DOI] [PubMed] [Google Scholar]
- [13].Xu X, Zou D, Shen L, et al. Efficacy and feasibility of antidepressant treatment in patients with poststroke depression. Medicine 2016;95:e5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psychiatry 2016;173:221–31. [DOI] [PubMed] [Google Scholar]
- [15].Yi ZM, Liu F, Zhai SD. Fluoxetine for the prophylaxis of poststroke depression in patients with stroke: a meta-analysis. Int J Clin Pract 2010;64:1310–7. [DOI] [PubMed] [Google Scholar]
- [16].Salter KL, Foley NC, Zhu L, et al. Prevention of poststroke depression: does prophylactic pharmacotherapy work? J Stroke Cerebrovasc Dis 2013;22:1243–51. [DOI] [PubMed] [Google Scholar]
- [17].Hackett ML, Anderson CS, House AO. Management of depression after stroke: a systematic review of pharmacological therapies. Stroke 2005;36:1092–71098. [DOI] [PubMed] [Google Scholar]
- [18].Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md StateMed J 1965;14:61–5. [PubMed] [Google Scholar]
- [19].Mcpherson KM, Pentland B, Cudmore SF, et al. An inter-rater reliability study of the Functional Assessment Measure (FIM + FAM). Int Rehab Med 1996;18:341–7. [DOI] [PubMed] [Google Scholar]
- [20].Heun R, Burkart M, Mater W, et al. Internal and external validity of the WHO Well-Being Scale in the elderly general population. Acta Psychiatrica Scandinavica 2010;99:171–8. [DOI] [PubMed] [Google Scholar]
- [21].Ware JE, Jr, Snow KK, Kosinski M, et al. SF-36 Health Survey Manual and Interpretation Guide. 1993;Boston: New England Medical Center the Health Institute, 1–12. [Google Scholar]
- [22].Aberg E, Adielsson G, Almqvist A. Multicenter trial of hemodilution in ischemic stroke-background and study protocol. Scand Stroke Study Group Stroke 1985;16:885–90. [DOI] [PubMed] [Google Scholar]
- [23].Brott T, Adams HP, Olinger CO, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–70. [DOI] [PubMed] [Google Scholar]
- [24].Scales for assessment of diagnosis and severity of mental disorders. Acta Psychiatr Scand 1993;87:1–87. [PubMed] [Google Scholar]
- [25].Joanna Briggs Institute Reviewers Manual: 2008 Edition [M]. Joanna Briggs Institute, 2007. [Google Scholar]
- [26].Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. London, UK: The Cochrane Collaboration; 2011. [Google Scholar]
- [27].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;316:469–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cao H-J, Zhang J, You H-F, et al. Effect of zoloft on cerebral apoplexy patients with depressive symptoms. J Xinxiang Med Coll 2003;20:53–4. [Google Scholar]
- [29].Tian G-P. Sertraline hydrochloride treatment the clinical effect of the treatment of depression after stroke. Primary Med Forum 2016;20:1606–7. [Google Scholar]
- [30].Zifko UA, Rupp M, Schwarz S. Sertraline in der behandlung der post-stroke depression epergebnisse einer offenen multicenterstudie. Wiener Medizinische Wochenschrift 2002;152:343–8. [DOI] [PubMed] [Google Scholar]
- [31].Xie R, Liu J, Quan H. A prospective random clinical contrast study of treatment with sertraline in elderly patients with post-stroke depression. Chin J Clin Neurosci 2005;13:294–7. [Google Scholar]
- [32].Gao G-X, Wang J-R, Sun S. The effect of early application of sertraline in the treatment of post-stroke anxiety and depression and cognitive function. Mod J Integrated Trad Chin West Med 2014;23:3027–9. [Google Scholar]
- [33].Almeida OP, Waterreus A, Hankey GJ. Preventing depression after stroke: results from a randomized placebo-controlled trial. J Clin Psychiatry 2006;67:1104–9. [DOI] [PubMed] [Google Scholar]
- [34].De RA, Brouns R, Geurden M, et al. Risk factors for poststroke depression: identification of inconsistencies based on a systematic review. J Geriatric Psychiatry Neurol 2014;27:147–58. [DOI] [PubMed] [Google Scholar]
- [35].Kutlubaev MA, Hackett ML. Part II: predictors of depression after stroke and impact of depression on stroke outcome: an updated systematic review of observational studies. Int J Stroke 2015;9:1026–36. [DOI] [PubMed] [Google Scholar]
- [36].Chen Y, Guo JJ. Meta-analysis of antidepressant treatment for patients with poststroke depression. Stroke 2006;37:1365–6. [DOI] [PubMed] [Google Scholar]
- [37].Chen Y, Guo JJ, Zhan S, et al. Treatment effects of antidepressants in patients with post-stroke depression: a meta-analysis. Ann Pharmacother 2006;40:2115–22. [DOI] [PubMed] [Google Scholar]
- [38].Wickenberg-Bolin U, Goransson H, Fryknas M, et al. Improved variance estimation of classification performance via reduction of bias caused by small sample size. BMC Bioinformatics 2006;7:127–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hackett ML, Köhler S, O’Brien JT, et al. Neuropsychiatric outcomes of stroke. Lancet Neurol 2014;13:525–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


