Abstract
Rationale:
Most patients with hepatocellular carcinoma (HCC) have lost the chance of radical treatment at the time of their visit, and the prognosis of metastatic HCC is even worse. Sorafenib is currently regarded as a first-line systemic therapy in patients with advanced and metastatic HCC. Apatinib is a new inhibitor of vascular endothelial growth factor receptor 2 tyrosine kinase, which has been reported to be effective in some solid tumors. We herein report a case of apatinib in the treatment of the patient with metastatic HCC who was resistant to sorafenib.
Patient concerns:
A 41-year-old Chinese man with a history of chronic hepatitis B had undergone an emergency partial hepatectomy for tumor ruptured. Despite the treatment with transcatheter arterial chemoembolization and sorafenib, the progression of tumor failed to control.
Diagnoses:
Although the patient had been treated with sorafenib (400 mg, twice daily) for 10 months, computed tomography documented radiological progression.
Interventions:
Due to disease progression, failure of sorafenib and positive expression of vascular endothelial growth factor (VEGF), the drug regimen was changed to apatinib 250 mg once daily. Due to some degree of resistance, the dose was increased up to 425 mg once daily.
Outcomes:
The patient had a disease-free progression of 7 months at 250 mg apatinib. The dosage was adjusted to 425 mg due to drug resistance and the side effects were tolerable. The patient has survived a total of 19 months under apatinib.
Lessons:
Apatinib may be a substitute for the HCC patients with sorafenib resistance in the future, especially for those with high expression of VEGF.
Keywords: apatinib, drug resistance, hepatocellular carcinoma, sorafenib, targeted therapy
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related deaths worldwide.[1] Sorafenib is uniquely recommended as the first line treatment for advanced HCC, but only a modest increase in overall survival. Furthermore, a subgroup of HCC is resistant to sorafenib, and the majority of these patients show disease progression even after an initial satisfactory response.[2] Apatinib is a new inhibitor of vascular endothelial growth factor receptor (VEGFR)-2 tyrosine kinase, which has been reported to be effective in various solid tumors.[3,4] We report a case of apatinib in the treatment of patient with metastatic HCC who was resistant to sorafenib. Written informed consent was provided by the patient to publish the case details and the study was approved by The Ethics Committee of the First People's Hospital of Changzhou.
2. Case report
On July 26, 2015, a 41-year-old Chinese man complained of mild pain in his right upper quadrant for 3 days before he visited our hospital. He had a history of chronic hepatitis B for more than 20 years. Abdominal computed tomography (CT) scan revealed tumors located in segment V (S5) and S6 (10.5 cm × 9.8 cm), S7 (3.2 cm × 2.9 cm) with thrombi in the right branch of portal vein (BCLC stage C). The concentration of serum alpha-fetoprotein (AFP) was >1210 ng/ml (normal range <8 ng/ml). Unfortunately, the patient suffered tumor (S5, S6) ruptured two days later, then an emergency partial hepatectomy (S5, S6 resection, and S7 tumor resection) with removal of portal vein tumor thrombi was performed on July 28, 2015. Postoperative pathology showed HCC with portal vein tumor thrombi but negative margin. Interestingly, the degree of differentiation of HCC was moderate-poor in S5 and S6 while moderate in S7. Immunohistochemical analysis showed that vascular endothelial growth factor (VEGF) was moderate positive (++) in S5 and S6 while weak (+) in S7. The perioperative course was uneventful, and AFP dropped to 3.61 ng/ml on September 01, 2015. From September 11, 2015, to October 13, 2016, the patient suffered from an intrahepatic relapse and metastases in hilar lymph node, peritoneum, abdominal wall, and lung, although he was treated by 3 times of transcatheter arterial chemoembolization (TACE) and sorafenib (400 mg, twice daily). Except for intrahepatic nodules were well controlled by TACE, extrahepatic metastases progressed continuously and showed no response to sorafenib (Table 1). AFP gradually increased to >1210 ng/ml, and never dropped below 1210 ng/ml in the following treatments. At the same time, the patient could touch a hard and ill-defined border mass in abdominal wall and felt the changes of the mass.
Table 1.
Changes in extrahepatic nodules size on CT-scans during treatments.
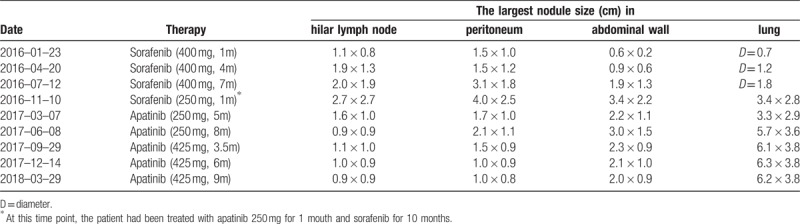
With disease progression, failure of sorafenib and positive expression of VEGF in tumor, the regimen was changed to apatinib at a dosage of 250 mg once daily (BCLC stage C, Child-Pugh A) on October 13, 2016. Regrettably, CT-scan was not given to the patient before taking apatinib, but the patient felt the abdominal wall mass softer than before just a week after taking apatinib. CT-scan showed that the intrahepatic nodule was still stable and non-enhanced areas of necrosis within the metastases in hilar lymph node, peritoneum and abdominal wall 1 month later. It was puzzling that the metastases in lung didn’t show similar changes (Fig. 1A, Table 1). On March 7, 2017, CT showed that the metastases in the hilar lymph nodes, peritoneum and abdominal wall were significantly smaller and less enhanced compared with before, while the lung nodules kept stable (Fig. 1B, Table 1). Meanwhile, the patient felt a shrinkage of the abdominal wall mass. Unfortunately, the patient complained that the abdominal mass began to grow again from May 2017. Later CT-scan (June 08, 2017) showed that the metastases in the peritoneum, abdominal wall, and lung significantly enlarged compared with before except for the hepatic hilar lymph node (Fig. 1C, Table 1). As apatinib was effective on the patient and well tolerated, the patient was recommended to adjust the dosage to 425 mg once daily. The patient felt the mass in abdominal wall shrank again after two weeks of dosage adjustment. On September 29, 2017, CT-scan showed that the hilar lymph node remained steady, and the metastases in peritoneum and abdominal wall shrank again, while lung nodules continued to progress (Table 1). Surprisingly, not only the metastases in the peritoneum and abdominal wall continued to shrink with the hilar lymph nodes remained stable, but also the lung nodules kept stable again on the followed CT-scans On December 14, 2017, and all of them maintained stably in the followed CT-scans on March 29, 2018 (Fig. 1D, Table 1).
Figure 1.
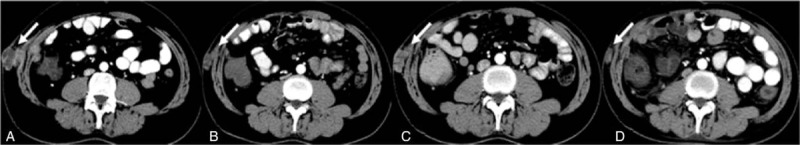
Contrast enhanced CT images of the metastases in abdominal wall treated by apatinib. 1A One month after apatinib 250 mg. 1B Five months after apatinib 250 mg. 1C Eight months after apatinib 250 mg. 1D Nine months after apatinib 425 mg. (white arrow).
Up to now, the patient is still alive and has survived for 19 months since taking apatinib. The adverse events were mild fatigue, hand-foot skin toxicities, and hypertension when taking apatinib 250 mg, which could be tolerated well. When the dose was adjusted to 425 mg, the patient suffered severe diarrhea (7–8 times daily, grade 3) and he had to be relieved by loperamide.
3. Discussion
The therapy options are limited and relapse and metastasis are common in advanced HCC. The patients with metastatic HCC often cannot survive more than 1 year. Sorafenib is a recommended systemic therapy for advanced HCC with a favorable safety profile but only 3 months more of survival gain compared to placebo.[2]
Angiogenesis is today universally considered as a cancer hallmark, as it supplies for the increased request of oxygen and nutrients which is typical of the fast-growing microenvironment of solid tumors. As is well known, angiogenesis is mediated by VEGF, which gives rise to a significantly more robust kinase activity when binding with VEGFR-2. Some studies have shown that high expression of VEGF in HCC is closely related to sorafenib resistance and worse prognosis.[5,6] TACE is an important treatment of HCC, but can potentially cause hypoxic changes in tumors as well as in the surrounding liver tissue due to the anti-cancer effects of chemotherapy infusions and embolization of feeding arteries. These can eventually induce the upregulation of circulating VEGF.[7] However high expression of VEGF makes it an appealing target for novel anti-cancer therapies. Apatinib abrogates the interaction of VEGF with VEGFR-2 and directly inhibits angiogenesis.[3] As to this case, the patient has achieved an unexpected effective result by anti-angiogenic therapy with apatinib as described above, even though his pathogenetic condition had been progressing for 10 months treated by sorafenib. Comparing the targets and their IC50 of apatinib with sorafenib, we can find that the significant advantage of apatinib over sorafenib is its high affinity to VEGFR-2 (IC50 to VEGFR-2 of apatinib and sorafenib is 1 nmol/L and 90 nmol/L respectively),[3,8] so we believe that the difference in affinity to VEGFR-2 would have the different outcomes. Interestingly, the metastases in peritoneum and abdominal wall were sensitive to apatinib, whereas the metastases in lung were relatively insensitive. Gershtein et al [9] demonstrated that an increase in expression of VEGF and VEGFR-2 correlates with the degree of histological differentiation and the stage of the tumor by histological analysis. Therefore, we speculate that the metastases in peritoneum and abdominal wall were caused by the rupture of the tumor in S5 and S6, whereas the lung metastases may originated from the tumor in S7, for the difference in pathological differentiation and expression of VEGF. Some studies have shown that apatinib combined with TACE or alone in treating HCC achieved promising prospects for its anti-angiogenic effect.[4,10] And this is the first case report about apatinib in the treatment of patient with metastatic HCC after failure to sorafenib. It should be noted that apatinib had a certain degree of resistance after a disease-free progression of 7 months at 250 mg, although it could be temporarily overcome by dosage adjusted to 425 mg. The adverse events of the patient were mild fatigue, hand-foot skin toxicities and hypertension which could be tolerated well when the dose was 250 mg. However, the patient suffered severe diarrhea when the dose was adjusted to 425 mg. The adverse events with apatinib could be tolerated and managed as described in previous studies.[4]
As to our case, it is regrettable that there was a lack of pathological differentiation, quantitative detection of VEGF or genetic testing for intraperitoneal and intrapulmonary metastases before and during the treatment with apatinib. Apatinib may be a substitute for the HCC patients with sorafenib resistance or as a first-line treatment for the patients with high expression of VEGF, but further prospective studies are still needed to optimize the treatment.
Author contributions
Conceptualization: Zonghong Han, Zhongming He, and Qi Wang.
Formal analysis: Zonghong Han.
Investigation: Zonghong Han, Zhongming He, and Caoye Wang.
Project administration: Qi Wang.
Supervision: Qi Wang.
Validation: Qi Wang.
Visualization: Zonghong Han and Caoye Wang.
Writing – original draft: Zonghong Han.
Writing – review, and editing: Qi Wang.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, CT = computed tomography, HCC = hepatocellular carcinoma, S5/6/7 = segment V/VI/VII, TACE = transcatheter arterial chemoembolization, VEGF = vascular endothelial growth factor, VEGFR-2 = vascular endothelial growth factor receptor 2.
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Finn RS, Zhu AX, Farah W, et al. Therapies for advanced stage hepatocellular carcinoma with macrovascular invasion or metastatic disease: a systematic review and meta-analysis. Hepatology 2018;67:422–35. [DOI] [PubMed] [Google Scholar]
- [3].Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011;102:1374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yu WC, Zhang KZ, Chen SG, et al. Efficacy and Safety of apatinib in patients with intermediate/advanced hepatocellular carcinoma: a prospective observation study. Medicine (Baltimore) 2018;97:e9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Choi SB, Han HJ, Kim WB, et al. VEGF overexpression predicts poor survival in hepatocellular carcinoma. Open Med (Wars) 2017;12:430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tsuchiya K, Asahina Y, Matsuda S, et al. Changes in plasma vascular endothelial growth factor at 8 weeks after sorafenib administration as predictors of survival for advanced hepatocellular carcinoma. Cancer 2014;120:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ranieri G, Ammendola M, Marech I, et al. Vascular endothelial growth factor and tryptase changes after chemoembolization in hepatocarcinoma patients. World J Gastroenterol 2015;21:6018–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ranieri G, Gadaleta-Caldarola G, Goffredo V, et al. Sorafenib (BAY 43-9006) in hepatocellular carcinoma patients: from discovery to clinical development. Curr Med Chem 2012;19:938–44. [DOI] [PubMed] [Google Scholar]
- [9].Gershtein ES, Dubova EA, Shchegolev AI, et al. Vascular endothelial growth factor and its type 2 receptor in hepatocellular carcinoma. Bull Exp Biol Med 2010;149:749–52. [DOI] [PubMed] [Google Scholar]
- [10].Liu C, Xing W, Si T, et al. Efficacy and safety of apatinib combined with transarterial chemoembolization for hepatocellular carcinoma with portal venous tumor thrombus: a retrospective study. Oncotarget 2017;8:100734–45. [DOI] [PMC free article] [PubMed] [Google Scholar]


