Abstract
Rationale:
Daptomycin (DAP) is a key drug for treating severe Staphylococcus infections. The emergence of DAP non-susceptible Staphylococcus aureus has been widely recognized in clinical situations, although the clinical status of DAP non-susceptible coagulase-negative Staphylococcus (CoNS) infections is unclear. We encountered 2 cases of cardiovascular device infections that were associated with DAP non-susceptible CoNS.
Patient concerns:
The first case involved a 60-year-old woman with a pump pocket infection in a left ventricular assist device. DAP non-susceptible Staphylococcus capitis subsp. ureolyticus was isolated from a blood culture after treatment using vancomycin (10 days) and DAP (6 days). The second case involved a 71-year-old man with an aortic graft infection. DAP non-susceptible S capitis subsp. ureolyticus was detected in pus after treatment using vancomycin (2 weeks) and DAP (1 week) without complete removal and debridement.
Diagnosis:
Cardiovascular device infections caused by DAP non-susceptible CoNS.
Interventions and outcomes:
Whole genome sequencing of these strains revealed multiple mutations in genes that are related to DAP-non-susceptibility in S aureus, which created amino acid substitutions in mprF, dltAB, dltD, rpoC, yycG, cls2, pgsA, and vraSR. To the very best of our knowledge, the substitution patterns were not identical to those previously reported in DAP non-susceptibile S aureus.
Lessons:
Clinicians should be cautious regarding the emergence of DAP non-susceptible CoNS, especially in cases with implanted prosthetic devices, inadequate debridement, and prior usage of vancomycin and DAP. Further studies are needed to understand the relevance of these genetic changes and DAP-non-susceptibility in CoNS strains.
Keywords: amino acid substitution, antimicrobial resistance, daptomycin, prosthetic device, staphylococcus capitis subsp, ureolyticus, whole genome analysis
1. Introduction
Daptomycin (DAP) is part of a new class of natural cyclic lipopeptide antibiotics that are active against Gram-positive organisms. Based on its strong bactericidal effect and good pharmacokinetics, DAP is widely recommended for treating various Staphylococcus aureus infections, including bacteremia and endocarditis.[1] However, the emergence of DAP resistance in S aureus has been described in laboratory studies,[2] clinical trials,[3] and post-marketing surveillance.[4] Exposure to DAP causes S aureus strains to develop an altered membrane potential and a more positive membrane surface charge, which leads to DAP resistance.[5]
In contrast, coagulase-negative Staphylococcus (CoNS) rarely exhibits decreased susceptibility to DAP, as a previous study[6] has indicated that DAP was active for 99.8% of CoNS isolates at a susceptibility breakpoint of ≤ 1 μg/mL. In culture results, CoNS are often considered contaminants, although their roles as real pathogens have been recognized in various clinical situations.[7] Furthermore, CoNS strains can have elevated minimum inhibitory concentration (MIC) values for glycopeptides, which may be related to poor clinical outcomes.[8] However, given the rarity of DAP non-susceptible CoNS infections, its clinical course, incidence, and genetic background remain unclear. We recently encountered 2 cases of DAP non-susceptible CoNS infections that involved patients with implanted cardiovascular devices and used whole genome sequencing to identify amino acid substitutions that could be responsible for the DAP non-susceptibility.
2. Case presentation
2.1. Case 1
A 60-year-old woman with a history of hypertrophic cardiomyopathy had undergone implantation of a left ventricular assist device 2 years ago. Eighteen months later, the device suddenly malfunctioned and the patient underwent an emergent exchange surgery. She subsequently developed a surgical site infection that was treated using intravenous ampicillin/sulbactam.
Three months later, the surgical wound infection recurred and the patient was re-hospitalized with a low-grade fever and tenderness directly around the pump. Her serum level of C-reactive protein (CRP) was slightly elevated (1.39 mg/dL) and gallium-67 scintigraphy revealed inflammation surrounding the device pump (Fig. 1A). A blood culture was positive for methicillin-resistant Staphylococcus capitis subsp. ureolyticus. Intravenous vancomycin (VCM) was empirically initiated, although the patient remained febrile. Debridement surgery was performed for the pump pocket infection on day 7 of that admission, and the organism was also detected in a culture of the purulent tissue. The VCM treatment was switched to DAP (350 mg/day [5.7 mg/kg]) on day 10 because of drug-induced neutropenia. Although the patient's condition seemed favorable, a high fever re-emerged on day 16 and 2 blood cultures were again positive for S capitis subsp. ureolyticus, with drug tests revealing non-susceptibility to DAP (Table 1). Thus, the DAP treatment was discontinued and the patient did not experience recurrence after a 1-month course of combination therapy using clindamycin and rifampicin.
Figure 1.
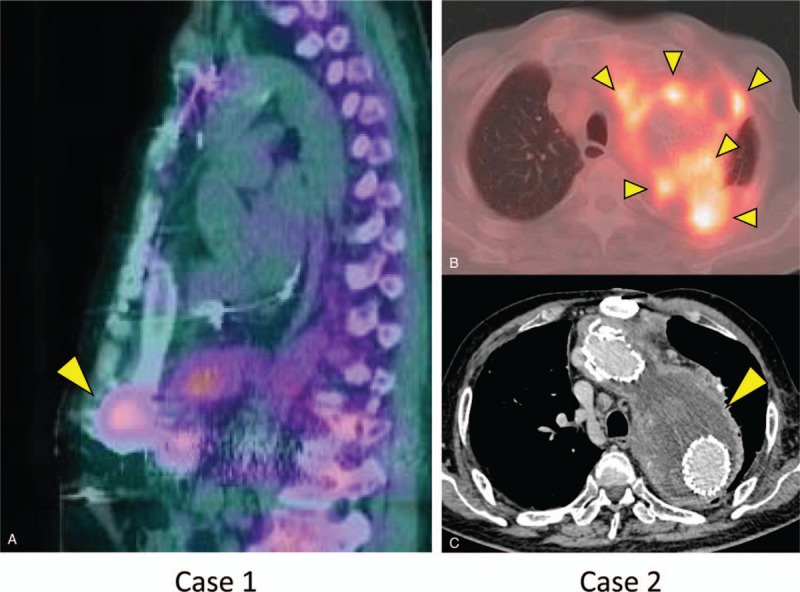
Radiological findings. Gallium-67 scintigraphy revealed uptake at the site of a cardiac pump in Case 1 (A) and in the peri-aortic space in Case 2 (B). Contrast-enhanced computed tomography revealed a massive abscess surrounding the aortic graft in Case 2 (C).
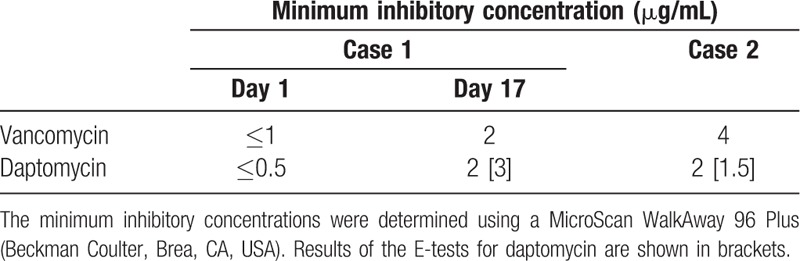
Table 1.
Minimum inhibitory concentrations for Staphylococcus capitis subsp. ureolyticus.
2.2. Case 2
A 71-year-old man who had undergone thoracic endovascular aortic repair 7 years previously was admitted to a hospital with a complaint of fever, back pain, and bloody sputum. Based on the results from positron emission tomography-computed tomography (CT), the patient was diagnosed with an aortic graft infection and underwent CT-guided drainage for a peri-aortic abscess. The pathogen was unclear and he received empirical antimicrobial treatments using meropenem and DAP for 1 week (dose unknown), ampicillin and sulbactam for 3 weeks, VCM for 2 weeks, and oral levofloxacin for 4 weeks. The patient was discharged with a prescription for oral levofloxacin and followed-up at an outpatient clinic. Three months later, his serum CRP level was elevated and radiographic examinations revealed an enlarged peri-aortic abscess (Fig. 1B and C). Based on a diagnosis of a recurrent aortic graft infection, he was referred to our hospital for further treatment.
At admission, the patient had stable vital signs but was febrile and had an elevated white blood cell count (12,220/μL) and CRP level (16.3 mg/dL). The patient underwent CT-guided percutaneous drainage, which removed approximately 200 mL of pus. Gram staining of the discharge revealed Gram-positive cocci and a bacterial culture revealed the presence of DAP non-susceptible S capitis subsp. ureolyticus (Table 1). Linezolid was chosen for treatment, but the patient developed thrombocytopenia after 2 weeks. The linezolid treatment was switched to a combination of VCM and clindamycin. After another 2 weeks, the antibiotic therapy was converted to a combination of oral treatment using trimethoprim/sulfamethoxazole and minocycline, and the patient was discharged.
2.3. Antimicrobial susceptibility testing
The MIC for DAP was initially examined using a MicroScan WalkAway 96 Plus (Beckman Coulter, Brea, CA, USA) at our hospital's clinical microbiology laboratory, which revealed results of 2 μg/mL for both isolates. These results were confirmed using the E-test (bioMe′rieux, Marcy l’Etoile, France) on cation-adjusted Mueller Hinton agar according to the manufacturer's instructions. That test revealed MICs of 3 μg/mL in Case 1 and 1.5 μg/mL in Case 2 (Table 1).
2.4. Whole genome analysis
Whole genome sequencing was performed using the MiSeq system (Illumina, San Diego, CA, USA) to identify mutations that might be associated with the DAP non-susceptibility. The bacterial isolates were cultured overnight in brain heart infusion broth (BD Bacto, Franklin Lakes, NJ, USA) and genomic DNA was then prepared using a PowerSoil DNA isolation kit (Qiagen). The library preparation for the genome analysis has been described in our previous report.[9] The sequence reads were subsequently submitted to the DDBJ/Genbank/EMBL database under accession numbers DRX121940 (Case 1) and DRX121939 (Case 2).
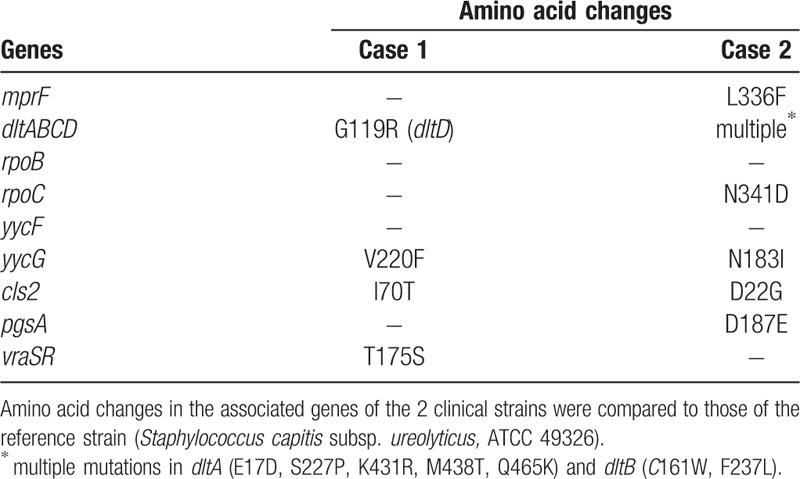
Sequence data assembly revealed genome size and G+C content values of 2,508,206 bp and 32.81% for Case 1 and 2,353,499 bp and 32.78% for Case 2. The sequence reads were assembled de novo on CLC Genomics Workbench and compared to the publicly available data for S capitis subsp. ureolyticus (ATCC 49326). MIC of the reference strain for DAP was confirmed to be ≤ 0.25 μg/mL. Bacterial identification was reconfirmed using the average nucleotide identity (ANI) analysis in EzBioCloud.[10] The result demonstrated that those 2 clinical isolates were S capitis subsp. ureolyticus and not identical each other in their origins (ANI value between the 2 clinical strains; 98.72). To identify amino acid substitutions, we considered the following genes to potentially be related to DAP non-susceptibility based on previous reports:[11–14]mprF, dltABCD, rpoB, rpoC, yycF, yycG, cls2, pgsA, and vraSR. Consequently, multiple amino acids substitutions were detected in dltD, yycG, cls2, and vraSR in Case 1, and mprF, dltAB, rpoC, yycG, cls2, and pgsA in Case 2 (Table 2).
Table 2.
Substitutions of amino acids based on the whole genome sequencing.
3. Discussion
We encountered 2 clinical cases of DAP non-susceptible CoNS infections. Although DAP non-susceptible strains of S aureus have been noted in various clinical settings, these cases rarely involve CoNS. Based on the guidelines from the European Committee on Antimicrobial Susceptibility Testing[15] and the Clinical and Laboratory Standards Institute,[16] DAP susceptibility in Staphylococcus spp. is defined as an MIC of ≤ 1 mg/L and strains with an MIC of > 1 mg/L are considered DAP non-susceptible. We measured the MIC values using a MicroScan WalkAway 96 Plus (Beckman Coulter, Brea, CA, USA) and confirmed the results using the E-test (bioMe′rieux). A recent study from the SENTRY Antimicrobial Surveillance Program (283 hospitals in 42 countries during 2002–2010) revealed that 99.8% of > 22,000 isolates of CoNS strains were susceptible to DAP, with only a few CoNS strains (eg, S sciuri, S auricularis, S warneri, and S capitis) having elevated MIC90 values relative to those of other Staphylococcus spp.[6]
Treatment using VCM or DAP usually precedes the development of DAP resistance in S aureus.[17] and both of our patients had received these drugs before the emergence of DAP non-susceptible CoNS (Case 1: 10 days of VCM and 6 days of DAP, Case 2: 2 weeks of VCM and 1 week of DAP). A previous study has indicated that DAP non-susceptible S aureus emergence could be predicted by a low dosage of DAP, persistent infection, and high bacterial loads,[18] which indicates that an adequate dose of DAP should be administered to avoid resistance. The safety of high-dose DAP (> 6 mg/kg) has been widely recognized,[19] and the administration of a high DAP dose is recommended, especially in refractory cases. Moreover, both of our cases involved prosthetic devices (a left ventricular assist device in Case 1 and aortic graft in Case 2), and the difficulty that is associated with debridement and removal of these foreign bodies might also have contributed to the emergence of DAP non-susceptible isolates.
There are some possible mechanisms for the increased MIC of DAP in our isolates. As have reported in S aureus, a positive charge at the membrane surface[20] and a thickening of the cell wall[21] could be associated with DAP-non-susceptibility, with major mutations thought to involve mprF, dltABCD, rpoB/rpoC, and yycF/yycG.[11] To the best of our knowledge, only 1 report has described whole genome analysis of multidrug-resistant S capitis subsp. ureolyticus, although that report did not describe any genetic changes that were potentially responsible for the DAP non-susceptibility.[22] In this context, our molecular analysis detected multiple amino acids substitutions in these possibly responsible genes for both isolates (vs the publicly available isolate). Interestingly, both of the S capitis subsp. ureolyticus isolates had amino acid changes commonly in dltABCD, yycG, and cls2, which thus is speculated to be majorly related to DAP non-susceptibility in CoNS. Of note, these substitution patterns were not completely identical to the previously reported patterns for DAP non-susceptible S aureus strains.[11–14] Thus, they would be novel to be reported in the literature and also might be characteristic to CoNS strains. However, we are unable to conclude an association between the DAP non-susceptibility and these genetic changes, given the lack of available isolates before the DAP exposure. Further molecular analysis is needed to identify the genetic variant(s) that are responsible for DAP non-susceptibility in CoNS.
In conclusion, we encountered 2 cases of DAP non-susceptible CoNS infections. Both cases were associated with artificial device infections, and emergences of DAP non-susceptible strains were preceded by the administration of VCM and DAP. In general, CoNS infrequently causes refractory infections and thus has limited opportunity to become non-susceptible to DAP. The present cases demonstrated that CoNS can also develop DAP non-susceptibility in the specific situations described above. Our whole genome sequencing detected several amino acid substitutions in proteins that may be responsible for DAP non-susceptibility in S aureus. Resistance in VCM emerged after 4 decades of its clinical use, while DAP resistance occurred shortly after its debut. In this era of antibiotic shortage, emergence of DAP-resistant strain is of great concern to clinicians and should be closely monitored in various bacterial species.
Author contributions
Conceptualization: Hideharu Hagiya.
Investigation: Yo Sugawara, Keigo Kimura, Isao Nishi, and Masahiro Hayashi.
Resources: Masahiro Hayashi.
Supervision: Shigeto Hamaguchi, Yukihiro Akeda, and Kazunori Tomono.
Writing – original draft: Hideharu Hagiya.
Writing – review and editing: Shigeto Hamaguchi, Yukihiro Akeda, and Kazunori Tomono.
Footnotes
Abbreviations: ANI = average nucleotide identity, CoNS = coagulase-negative Staphylococcus, CRP = C-reactive protein, CT = computed tomography, DAP = daptomycin, MIC = minimum inhibitory concentration, VCM = vancomycin.
Patient consent: Informed consents were obtained from the patients for the publication.
The authors have no conflicts of interests to declare.
References
- [1].Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011;52:e18–55. [DOI] [PubMed] [Google Scholar]
- [2].Rose WE, Rybak MJ, Tsuji BT, et al. Correlation of vancomycin and daptomycin susceptibility in Staphylococcus aureus in reference to accessory gene regulator (agr) polymorphism and function. J Antimicrob Chemother 2007;59:1190–3. [DOI] [PubMed] [Google Scholar]
- [3].Fowler VG, Jr, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006;355:653–65. [DOI] [PubMed] [Google Scholar]
- [4].Sader HS, Moet GJ, Farrell DJ, et al. Antimicrobial susceptibility of daptomycin and comparator agents tested against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: trend analysis of a 6-year period in US medical centers (2005-2010). Diagn Microbiol Infect Dis 2011;70:412–6. [DOI] [PubMed] [Google Scholar]
- [5].Kaatz GW, Lundstrom TS, Seo SM. Mechanisms of daptomycin resistance in Staphylococcus aureus. Int J Antimicrob Agents 2006;28:280–7. [DOI] [PubMed] [Google Scholar]
- [6].Sader HS, Jones RN. Antimicrobial activity of daptomycin in comparison to glycopeptides and other antimicrobials when tested against numerous species of coagulase-negative Staphylococcus. Diagn Microbiol Infect Dis 2012;73:212–4. [DOI] [PubMed] [Google Scholar]
- [7].von Eiff C, Peters G, Heilmann C. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect Dis 2002;2:677–85. [DOI] [PubMed] [Google Scholar]
- [8].Tacconelli E, Tumbarello M, Donati KG, et al. Glycopeptide resistance among coagulase-negative staphylococci that cause bacteremia: epidemiological and clinical findings from a case-control study. Clin Infect Dis 2001;33:1628–35. [DOI] [PubMed] [Google Scholar]
- [9].Sugawara Y, Akeda Y, Sakamoto N, et al. Genetic characterization of blaNDM-harboring plasmids in carbapenem-resistant Escherichia coli from Myanmar. PLoS One 2017;12:e0184720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yoon SH, Ha SM, Kwon S, et al. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol 2017;67:1613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Steed ME, Hall AD, Salimnia H, et al. Evaluation of daptomycin non-susceptible Staphylococcus aureus for stability, population profiles, mprF mutations, and daptomycin activity. Infect Dis Ther 2013;2:187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mehta S, Cuirolo AX, Plata KB, et al. VraSR two-component regulatory system contributes to mprF-mediated decreased susceptibility to daptomycin in in vivo-selected clinical strains of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2012;56:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Friedman L, Alder JD, Silverman JA. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob Agents Chemother 2006;50:2137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Peleg AY, Miyakis S, Ward DV, et al. Whole genome characterization of the mechanisms of daptomycin resistance in clinical and laboratory derived isolates of Staphylococcus aureus. PLoS One 2012;7:e28316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters. Version 6.0. 2018. Available at: http://www.eucast.org/clinical_breakpoints/ Accessed May 5, 2018 [Google Scholar]
- [16].Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. CLSI document M100-S26. 2016. CLSI, Wayne, PA. [Google Scholar]
- [17].Pillai SK, Gold HS, Sakoulas G, et al. Daptomycin nonsusceptibility in Staphylococcus aureus with reduced vancomycin susceptibility is independent of alterations in MprF. Antimicrob Agents Chemother 2007;51:2223–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moise PA, North D, Steenbergen JN, et al. Susceptibility relationship between vancomycin and daptomycin in Staphylococcus aureus: facts and assumptions. Lancet Infect Dis 2009;9:617–24. [DOI] [PubMed] [Google Scholar]
- [19].Falcone M, Russo A, Venditti M, et al. Considerations for higher doses of daptomycin in critically ill patients with methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2013;57:1568–76. [DOI] [PubMed] [Google Scholar]
- [20].Rubio A, Moore J, Varoglu M, et al. LC-MS/MS characterization of phospholipid content in daptomycin-susceptible and-resistant isolates of Staphylococcus aureus with mutations in mprF. Mol Membr Biol 2012;29:1–8. [DOI] [PubMed] [Google Scholar]
- [21].Cui L, Tominaga E, Neoh H-m, et al. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 2006;50:1079–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li X, Lei M, Song Y, et al. Whole genome sequence and comparative genomic analysis of multidrug-resistant Staphylococcus capitis subsp. urealyticus strain LNZR-1. Gut Pathog 2014;6:45. [DOI] [PMC free article] [PubMed] [Google Scholar]


