Abstract
Transcription of protein-coding genes by RNA polymerase II can be regulated at multiple points during the process of RNA synthesis, including initiation, elongation, and termination. In vivo data suggests that elongating polymerases exhibit heterogeneity throughout the gene body, suggestive of changes in elongation rate and/or pausing. Here, we review evidence from a variety of different experimental approaches for understanding regulation of transcription elongation. We compare steady-state measurements of nascent RNA density and polymerase occupancy to time-resolved measurements and point out areas of disagreement. Finally, we discuss future avenues of investigation for understanding this critically important step in gene regulation. This article is part of a Special Issue entitled: Chromatin in time and space.
Keywords: Transcription, Elongation, Single-molecule, Splicing, Imaging, Polymerase
1. Introduction
The process of transcription can be broadly divided into three phases namely, initiation, elongation and termination. These steps are mechanistically distinct: 1) initiation consists of promoter recognition, opening of the DNA helix, and possible abortive synthesis of the first few bases of RNA; 2) elongation begins with promoter clearance after synthesis of 8–9 bases; 3) and termination results in the dissociation of the RNA–DNA hybrid and release of the nascent transcript. Historical efforts to understand gene regulation have focused on the biochemical steps of initiation: understanding how, when, and where DNA is unwound to permit RNA polymerase to access the template strand. Although post-initiation gene regulation has been known for decades [1], the prevalence of post-initiation control in vivo has come into sharper focus through the use of genome-wide approaches [2]. Thus, the straightforward biochemical distinction of elongation as a uniform enzymatic activity state fails to capture the variety of polymerase behaviors observed in cells. In fact, both the rate of elongation and efficiency of elongation are proposed as regulatory mechanisms in cells, allowing one to ask both “when” and also “if” an initiated polymerase reaches the end of the gene. In this review, we address the first question: is modulation of transcription elongation rate a regulatory mechanism in vivo? Moreover, for simplicity, we restrict ourselves to transcription elongation which occurs in the body of the gene, far from the 5′ end and the observed promoter proximal pausing, which is treated by Adelman and co-authors in this issue.
In recent years, the question of modulation of transcript elongation has been addressed using a variety of techniques, including deep sequencing, chromatin immunoprecipitation, optical force microscopy, and single-molecule fluorescence imaging. The results have been illuminating, and new developments indicate a range of unanticipated behavior for an elongating polymerase. For example, polymerase speeds have been observed from a low of 0.8 kb/min [3] to over 50 kb/min in living cells [4]. Nucleosomes can function as barriers to elongation [5], but the elongation rate can also change the composition of transcribed nucleosomes [6]. These measurements and others reviewed here indicate the field is at an exciting juncture: it is now possible to do in vivo enzymology with RNA polymerase for direct comparison to in vitro models. In this review, we focus primarily on eukaryotic RNA polymerase II (RNAPII), provide a brief motivation based on in vitro work, examine the evidence for changes and/or heterogeneity of elongation in vivo, and finally address the implications for gene expression regulation. Our survey of published results suggests that polymerase elongation heterogeneity inferred from steady-state measurements is only partially recapitulated by time-resolved measurements, and we discuss several hypotheses for reconciling these differences.
2. Transcript elongation by RNA polymerase II in vitro
All protein coding genes are transcribed by RNAPII, and transcript elongation by RNAPII has been the subject of numerous studies in vitro using ensemble and single-molecule assays to understand the biochemical properties, mechanistic details and the regulatory processes that control elongation properties of this enzyme (for a comprehensive review, see [7]). RNAPII incorporates nucleotides to the 3′-end of a growing RNA chain by a Brownian-Ratchet mechanism that allows for forward and backward movement of the polymerase along the DNA template [8–10]. Transcript elongation is often punctuated by reversible transitions of RNAPII from an “on-line” pathway to short lived “off-line” states of temporary inactivity defined as pausing [11] or prolonged and irreversible inactive states called transcriptional arrest [12]. Such transitions and extension times define how long it takes RNAPII to transcribe a gene.
Mammalian RNAPII transcribes naked DNA in vitro with an average elongation rate of 300–400 nucleotides a minute. The overall rate of transcript elongation by RNAPII is a function of pause recognition (frequency of pausing), the time spent by the polymerase at the pause (pause duration), and the rate of elongation between pause events (pause free velocity). Several of the general transcription factors such as TFIIF, Elongin and ELL enhance the rate of elongation by RNAPII principally by suppressing pausing [13–16], the primary effect being either on pause duration or enhancing the pause free velocity. There is little evidence to indicate that elongation factors can reduce the frequency of pausing in vitro.
Although RNAPII escapes from most pause states where the 3′-end of the transcript has dislodged from the active center of the enzyme, or has traveled backwards (backtracking) along the nucleic acid chain by one or two nucleotides relative to the 3′-end of the transcript [17], extensive backtracking by several nucleotides results in long-lived arrested state. RNAPII can resume elongation from a backtracked arrest state in the presence of the transcription factor TFIIS that induces the intrinsic transcript cleavage activity of RNAPII, cleaving the RNA internally to create a new 3′-end [18–21]. The ability of TFIIS to help RNA polymerase transcribe at high opposing forces is further indication of the active role of TFIIS in rescuing backtracked polymerases [22]. This role is further highlighted by the fact that TFIIF and TFIIS function cooperatively to reduce backtracking by enhancing transcript cleavage and repositioning the polymerase back on the “on-line” pathway [23]. Although TFIIS cooperates with TFIIF to enhance the elongation properties of RNAPII, binding of TFIIS to RNA polymerase is also controlled by the negative elongation factor NELF, which might play a crucial role in NELF induced pausing by RNAPII [24]. In support of this idea, phosphorylation of Ser2 residue of the heptapeptide repeat of the CTD of RNAPII by the cyclin dependent kinase of PTEFb is essential to overcome the inhibitory effect of NELF and promoting elongation [25–27]. Thus, the fine-tuned interplay of both positive and negative elongation factors controls the elongation properties of RNAPII to regulate transcription in vitro.
In addition to pausing and arrest, ensemble studies of transcript elongation have also found that nucleosomes reduce the elongation rate by presenting a significant barrier to transcript elongation by RNAPII in vitro [28–32]. It is evident from modeling studies combining nucleosomal structure with that of an elongating polymerase that this barrier arises from the strong DNA binding of nucleosomal dyad downstream of the transcription bubble [33]. Once this barrier is crossed, the polymerase can transcribe to the end of the nucleosome with relatively little difficulty. This structural modeling also suggests a model for traversal of the transcription complex through nucleosomes in which the DNA upstream of the polymerase loops back, allowing the deposition of the displaced downstream nucleosome behind the elongating polymerase.
Several protein factors (TFIIS, TFIIF and FACT) mediate elongation of RNAPII through nucleosomes in vitro [32,34]. Although the general transcription factors TFIIF and TFIIS individually do not support robust transcription through nucleosomes in vitro, they do so in combination; TFIIS suppresses the backtracked pause 45 bp into the nucleosome while TFIIF simultaneously enhances the elongation of the polymerase [32]. FACT functions to destabilize the nucleosome core by removing the H2A/H2B dimer and re-depositing it in the wake of the elongating polymerase after transcribing past the nucleosome core [35]. In this way, elongation factors can function as histone chaperones. The connection between transcript elongation and histone chaperone activity has recently been visualized using atomic force microscopy [6]. By simultaneously observing RNAPII and the nucleosome on a single segment of DNA, the authors use a quantitative kinetic model to argue in favor of the DNA looping model [33] with nucleosomes re-deposited behind the polymerase. Moreover, this study suggests that efficient traversal through a nucleosome and the ability to redeposit the hexosome in its wake might be directly related to the elongation rate of the polymerase, with a slow polymerase better able to cover its tracks.
3. Transcription elongation in vivo
Although nucleosomes present a clear elongation barrier in vitro, the extent to which chromatin might modify elongation rates in vivo is indeterminate. Two approaches have been taken to investigate this topic. First, the position of active RNAPII relative to nucleosomes has been measured for all genes in budding yeast [36]. A genome-wide RNAPII footprinting approach coupled with deep sequencing revealed an increased number of reads near the nucleosome dyad. This increase in read density was interpreted as RNAPII pausing at the nucleosome boundaries. Second, an orthogonal approach attempts to position nucleosomes in vivo in order to directly observe the effect on RNAPII elongation. These experiments attempt to establish a direct comparison to in vitro results obtained from the high affinity 601 nucleosome positioning sequence [37]. Gracey and coworkers were able to position nucleosomes on the 601 sequence of an episomal vector injected into the mouse and taken up by hepatocytes, but this positioning was only transient [38]. In a related study in mammalian cell lines, this same sequence led to a~3 fold enrichment of nucleosome occupancy and resulted in an increase in RNAPII density immediately upstream of the sequence [39]. Conversely in yeast, the 601 sequence had no effect on RNAPII distribution on the gene and in fact resulted in a decrease in nucleosome occupancy [40].
One area where RNAPII elongation has attracted considerable attention is in relation to splicing (Oberdoerffer and colleagues, this issue). Changes in velocity or pausing in the body of the gene have been invoked as a mechanism for controlling the fidelity and/or choice of exon inclusion. A mutant polymerase which has a slower elongation rate in vitro has been shown to effect alternative splicing in vivo in human cell lines [41] and budding yeast [42]. Furthermore, changes in velocity are triggered by UV-induced hyperphosphorylation of the RNAPII CTD, suggesting a regulatory role for RNAPII elongation speeds [43]. Increased RNAPII density on variant exons correlates with a RNAPII CTD phosphorylation state (Ser5-P) which is similar to that of promoter-proximal polymerases [44]. Likewise, alternative splicing of CD45 correlates with an increased density of RNAPII in a CTCF (CCCTC-binding factor)-dependent manner [45]. In yeast, a peak of RNAPII occupancy at the 3′ splice site motivated a model that transcriptional pausing at this position may be a checkpoint associated with co-transcriptional splicing [46]. In this same study, the cyclical time-dependent accumulation of RNAPII occupancy suggested bursts of splicing activity. Finally, in a complementary study which directly quantified nascent pre-mRNA distribution across the genome, increased density of transcribing RNAPII was observed at the 3′ end, which was attributed to a slower elongation rate of polymerases in that region of the gene [47] as was suggested by earlier single-molecule RNA FISH experiments [48,49]. In summary, the density of RNAPII and nascent RNA appears heterogeneous over the gene body, suggesting kinetic variability in the rate of elongation.
However, these measurements are frequently done under steady-state conditions. Time-resolved measurements done both at the single-cell and population level suggest that elongation rates are remarkably constant over the gene, leading to an apparent contradiction between steady-state and time-resolved approaches. In a comprehensive analysis done by Singh and Padgett which used release from a transcriptional block followed by qRT-PCR, the speed of RNAPII was measured at 3.8 kb/min [50]. Elongation rates were highly uniform for all genes and over different parts of the gene, regardless of the number of introns. The authors concluded that there were few or no pausing events over hundreds of kilobases of chromatin. Single-cell microscopy studies which directly observe the production of nascent pre-mRNA at an active locus using the MS2 stem loop system [51] (Fig. 1) reached a similar conclusion. Brody et al. created reporter genes with a variable number of introns preceding the stem-loop cassette and determined by fluorescence recovery after photobleaching (FRAP) that elongation kinetics are not modulated by splicing events [52]. Moreover, by measuring the dynamics of both RNAPII and the nascent RNA, the authors were able to tease apart the relative contributions of elongation and post-elongation to the observed kinetics. They observed splicing-dependent changes in the recovery of the MS2-RNA fluorescence but not in the RNAPII fluorescence or in RNAPII distribution as measured by ChIP. These results suggest that the transcripts were retained for processing after the polymerases have departed. In some cases, this processing time lasted~10 min. A different single-cell study measured retention of the transcript after the poly-adenylation site, linking proof-reading to a step occurring post-elongation [53]. Again, different dynamics were observed for RNAPII and the nascent transcript, indicating that the occupancy of the RNA was not an indicator of active transcription dynamics. These analyses draw attention to the multiple kinetic steps involved in RNA synthesis and to the difficulty in unambiguously assigning an observed change to one particular step such as elongation, or pausing, or termination (Fig. 1B) [54,55].
Fig. 1.
Live cell kinetic measurements of transcription elongation. A) Pre-mRNA can be visualized using the MS2 bacteriophage capsid protein. DNA which encodes for RNA stem loops is inserted into the reporter gene of interest [51]. Once transcribed, the stem loops bind the constitutively-expressed fluorescently-labeled capsid protein. Each RNA stem loop binds a dimer of the capsid protein. The nascent RNA is then visible at the active locus in the living cell. B) Fluorescence recovery after photobleaching (FRAP) of nascent RNA. The fluorescence recovers on a time scale determined by both elongation and termination [55]. The slope of the curve depends on the length of the MS2 array, the length of the gene after the MS2 array, the velocity of the polymerase, and the termination time. The FRAP curves display the recovery dependence for different termination times (T) at a fixed velocity (v). C) Fluctuation analysis on single yeast genes [56]. Using time-lapse imaging coupled with automated tracking enables the determination of the transcription autocorrelation (red circles). The decay depends only on the dwell time of the nascent RNA (τ), and the amplitude is the product of the initiation rate (c) and the dwell time. The original data is from [55,56].
Recently, several groups have achieved single gene transcription imaging in both mammalian cell lines and yeast [3,4,56]. In yeast, we observed that the dwell time of nascent transcripts was narrowly distributed, implying that the elongation rate was highly uniform over the gene at a rate of 1.2 kb/min (Fig. 1C). Interestingly, the elongation rate increased during G2, but this increase may have been due to global changes in chromatin approaching mitosis. Yunger et al. observed a rate of 0.3–0.8 kb/min in HEK293 cells and were unable to detect pausing in their assay. On the opposite end of the spectrum, polymerase velocities over 50 kb/min were measured on a viral reporter gene, reflecting highly efficient initiation and elongation from a single integration site [4]. Oddly, this same gene exhibited a drastically slower velocity of 1.6 kb/ min when integrated as an array. Integration of transgene repeats can cause changes to the chromatin structure [57], so the difference between arrays and single integrations will need to be considered when comparing different reporter systems. However, these measurements raise the possibility that the measured elongation rate is the result of a continuous progression of fits and starts by the polymerase, but that under favorable conditions the polymerase can move at extremely fast speeds down a gene. In that sense, current time-resolved measurements may simply not have the resolution to capture the heterogeneity. Conversely, with the exception of yeast cell cycle variable elongation which occurs on the same gene [56], the extreme range of measured elongation rates may primarily reflect different experimental approaches and analyses. Regulated changes in elongation have yet to be observed using time-resolved measurements. Thus, steady-state and time-resolved measurements are presently at odds, necessitating a systematic comparison of these two approaches.
4. Comparing steady-state and time-resolved measurements
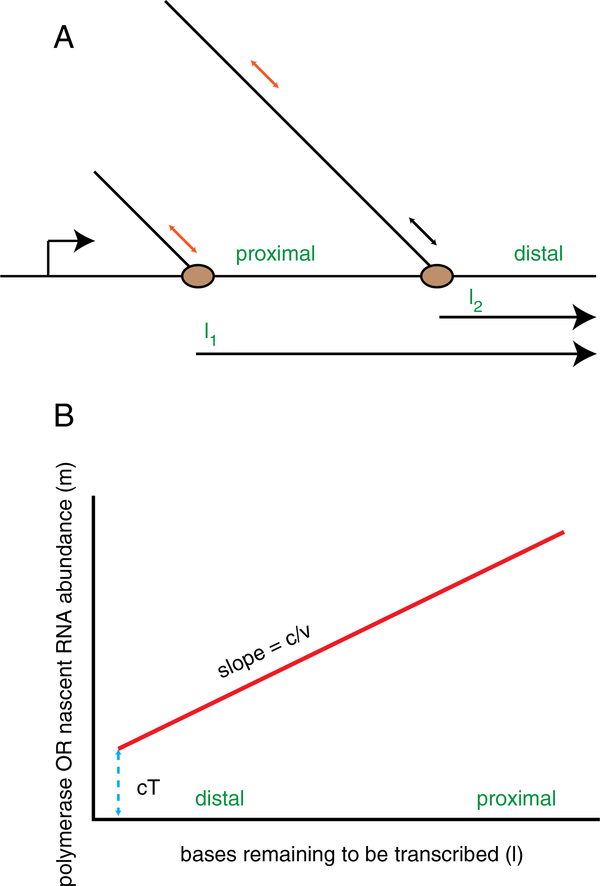
While most in vitro measurements are based on direct observations of the kinetics of RNA synthesis using time-resolved measurements, in vivo measurements of elongation are based on both steady-state and time-resolved measurements. The distinction between these two approaches is important. Steady-state measurements report the density of some quantity, which is the ratio of kinetic rate constants. Time-resolved measurements report the kinetic rate constants directly. An example is nascent RNA quantification, which is a measure of the density of elongating polymerases on a particular gene (Fig. 2A). Steady-state occupancy of RNAPII is determined by the kinetic balance of the frequency of initiation and the time that RNAPII spends on the gene:
| (1) |
where m is the number of polymerases or nascent RNA, c is the initiation rate, and τ is the time the polymerase spends on the gene. At a minimum, the time τ depends on the velocity of the polymerase (v), the distance in bases the polymerase must transcribe (l), and the termination time (T) which includes the time for cleavage, poly-adenylation, and processing:
| (2) |
Fig. 2.
Steady-state measurements for determining the relative balance of initiation, elongation, and termination. A) Cartoon of a nascent RNA abundance measurement. The position of a PCR amplicon or FISH probe used to quantify abundance (m) is shown by the red and gray bars. In this instance, m1=2; m2=1. The distance from the position of the polymerase to the end of the gene is shown by l1,2. B) Length dependence of nascent RNA abundance. As the distance to the 3′ end increases, the time that a nascent RNA is present increases (red line). The slope is the ratio of initiation rate (c) to velocity (v); the offset is the product of the initiation rate (c) and the termination time (T).
Experimentally, by measuring the amount of nascent RNA (m) as a function of position from the 3′ end of the gene (l), one can determine the slope (c/v) and the offset (cT) (Fig. 2B).
The critical point in this analysis is that neither quantity (v, T) can be separated from the initiation rate c using this approach. For example, a change in the slope can be due either to a change in velocity or a change in initiation rate. By using a ratiometric measure (for example the ratio of proximal nascent RNA to distal nascent RNA) one can remove the initiation rate, but it is still impossible to separate the effects of elongation and termination. Eliminating the initiation rate and expressing the result in terms of the measured parameters give:
| (3) |
where l1,2 is the distance to the end of the gene from the proximal and distal positions; m1,2 is the abundance of RNA with the proximal and distal sequence, respectively. Alternatively, to eliminate the initiation rate, one could simply take the ratio of the offset to slope to obtain vT. Again, it is impossible to separate effects on velocity and termination using this steady-state measurement. Intuitively, this result can be understood by considering the case where the termination rate is very slow compared to the velocity: polymerases pile up at the 3′ end, and the ratio m1/m2~1.
This analysis points to the inherent difficulty in separating the contributions of initiation, elongation, and termination to the occupancy of polymerases and nascent RNA on a particular gene [54–56]. Moreover, under steady-state conditions, a number of assumptions are necessary to infer changes in elongation rate and/or pausing, and these assumptions may not be valid under all circumstances [58]. For example, a local increase in RNAPII density, say over a particular region of the gene body, can be caused by a change in the initiation rate into the region or by the elongation rate out of the region (Eq. (1)). Therefore, cryptic initiation–initiation at positions other than the annotated transcription start site–would be indistinguishable from pausing. It is becoming clear that most of the eukaryotic genome is transcribed (85% in yeast [59,60], 98% in humans [61]), and it may be that: 1) the resulting RNAs are functional, 2) the process of transcription itself is functional (independent of the synthesized RNAs), or 3) that pervasive transcription is simply spurious and results from RNAPII initiating from nucleosome-depleted DNA [62]. Regardless of the functional role, the existence of pervasive transcription must be considered when inferring elongation changes from steady-state data. Likewise, the emerging ubiquity of anti-sense transcription which often initiates near the terminators of annotated genes urges caution when interpreting RNAPII pausing at the 3′ end [2,63].
Complicating the analysis even further is the fact that the same factors which facilitate elongation (and which therefore might be involved in modulating elongation rates) also play an essential role in repressing cryptic initiation. For example, Spt6 plays a critical role in maintaining chromatin structure in yeast, and Spt6 mutants lead to cryptic initiation across the genome [64,65]. In addition, the disruption of nucleosome structure is dependent on active transcription, suggesting that many elongation factors function as histone chaperones which help the removal and replacement of nucleosomes during elongation [66]. Based on several studies in yeast, a general model of chromatin organization by elongating polymerases has emerged [67–71]. RNAPII recruits the histone methyltransferase Set2 through interactions with the phosphorylated RNAPII CTD, resulting in transcription-dependent H3 methylation. The histone deacetylase Rpd3S recognizes methylated histones and deacetylates these histones within transcribed regions, thereby erasing the transcriptional memory of the active gene and restoring the chromatin to a hypoacetylated state that is repressive for transcription. Thus, the coordinated regulation between Rpd3S and Set2 is essential for repressing transcription initiation from intragenic cryptic promoters [68]. Also, because this coordination is in part mediated by the RNAPII CTD, mutations in this region may have pleiotropic effects which alter not only RNAPII elongation but also transcriptional fidelity and the ability to suppress cryptic initiation during elongation.
5. Conclusion and outlook
The complexity of post-initiation steps in transcription provides numerous opportunities for regulation of RNA processing and gene expression [72]. However, the interdependent nature of initiation, elongation, termination, and chromatin modifications makes it difficult to precisely attribute observed changes in RNAPII occupancy or nascent RNA distribution to one process or the other. Steady-state population measurements utilizing PCR-based approaches have exquisite sensitivity and may be able to detect very small changes in elongation heterogeneity. However, density measurements are always measurements of the relative balance of initiation and elongation, just as an equilibrium constant is the ratio of “on” and “off” rates. On the other hand, kinetic measurements are difficult to make and lower throughput, usually relying on chemical inhibition or genetic modification. Although there is not a definitive consensus from these time-resolved measurements, the preponderance of evidence suggests highly uniform elongation, in contrast to the peaks and variability seen from steady-state measurements. These data then beg the question: what is the minimum length of a detectable pause and what sort of kinetic pause is necessary to be biologically meaningful? In other words, if the cell is regulating elongation, how much modulation is necessary to change the fate of an RNA? Recent work suggests that for a multi-step kinetic process such as splicing, the distribution of splicing time has lower variability, which also means that small changes in elongation (2-fold) might have a large effect on splicing efficiency [73,74]. Detecting such changes in vivo and correlating them with a biological outcome will be a significant experimental challenge.
Acknowledgements
Thanks to M. Ferguson and A. Coulon for discussion regarding the manuscript. T. Lionnet kindly provided data for Fig. 1. DRL and MP are supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
This article is part of a Special Issue entitled: Chromatin in time and space.
References
- [1].Sekimizu K, Kobayashi N, Mizuno D, Natori S, Purification of a factor from Ehrlich ascites tumor cells specifically stimulating RNA polymerase II, Biochemistry 15 (1976) 5064–5070. [DOI] [PubMed] [Google Scholar]
- [2].Core LJ, Waterfall JJ, Lis JT, Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters, Science 322 (2008) 1845–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yunger S, Rosenfeld L, Garini Y, Shav-Tal Y, Single-allele analysis of transcription kinetics in living mammalian cells, Nat. Methods 7 (2010) 631–633. [DOI] [PubMed] [Google Scholar]
- [4].Maiuri P, Knezevich A, De Marco A, Mazza D, Kula A, McNally JG, Marcello A, Fast transcription rates of RNA polymerase II in human cells, EMBO Rep. 12 (2011) 1280–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hodges C, Bintu L, Lubkowska L, Kashlev M, Bustamante C, Nucleosomal fluctuations govern the transcription dynamics of RNA polymerase II, Science 325 (2009) 626–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bintu L, Kopaczynska M, Hodges C, Lubkowska L, Kashlev M, Bustamante C, The elongation rate of RNA polymerase determines the fate of transcribed nucleosomes, Nat. Struct. Mol. Biol 18 (2011) 1394–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Selth LA, Sigurdsson S, Svejstrup JQ, Transcript elongation by RNA polymerase II, Annu. Rev. Biochem 79 (2010) 271–293. [DOI] [PubMed] [Google Scholar]
- [8].Bai L, Shundrovsky A, Wang MD, Sequence-dependent kinetic model for transcription elongation by RNA polymerase, J. Mol. Biol 344 (2004) 335–349. [DOI] [PubMed] [Google Scholar]
- [9].Bar-Nahum G, Epshtein V, Ruckenstein AE, Rafikov R, Mustaev A, Nudler E, A ratchet mechanism of transcription elongation and its control, Cell 120 (2005) 183–193. [DOI] [PubMed] [Google Scholar]
- [10].Guajardo R, Sousa R, A model for the mechanism of polymerase translocation, J. Mol. Biol 265 (1997) 8–19. [DOI] [PubMed] [Google Scholar]
- [11].Palangat M, Meier TI, Keene RG, Landick R, Transcriptional pausing at +62 of the HIV-1 nascent RNA modulates formation of the TAR RNA structure, Mol. Cell 1 (1998) 1033–1042. [DOI] [PubMed] [Google Scholar]
- [12].Hawley DK, Wiest DK, Holtz MS, Wang D, Transcriptional pausing, arrest, and readthrough at the adenovirus major late attenuation site, Cell. Mol. Biol. Res 39 (1993) 339–348. [PubMed] [Google Scholar]
- [13].Bradsher JN, Tan S, McLaury HJ, Conaway JW, RC Conaway RNA polymerase II transcription factor SIII. II. Functional properties and role in RNA chain elongation, J. Biol. Chem 268 (1993) 25594–25603. [PubMed] [Google Scholar]
- [14].Shilatifard A, Haque D, Conaway RC, Conaway JW, Structure and function of RNA polymerase II elongation factor ELL. Identification of two overlapping ELL functional domains that govern its interaction with polymerase and the ternary elongation complex, J. Biol. Chem 272 (1997) 22355–22363. [DOI] [PubMed] [Google Scholar]
- [15].Shilatifard A, Lane WS, Jackson KW, Conaway RC, Conaway JW, An RNA polymerase II elongation factor encoded by the human ELL gene, Science 271 (1996) 1873–1876. [DOI] [PubMed] [Google Scholar]
- [16].Tan S, Aso T, Conaway RC, Conaway JW, Roles for both the RAP30 and RAP74 subunits of transcription factor IIF in transcription initiation and elongation by RNA polymerase II, J. Biol. Chem 269 (1994) 25684–25691. [PubMed] [Google Scholar]
- [17].Palangat M, Landick R, Roles of RNA:DNA hybrid stability, RNA structure, and active site conformation in pausing by human RNA polymerase II, J. Mol. Biol 311 (2001) 265–282. [DOI] [PubMed] [Google Scholar]
- [18].Izban MG, Luse DS, The RNA polymerase II ternary complex cleaves the nascent transcript in a 3′–5′ direction in the presence of elongation factor SII, Genes Dev. 6 (1992) 1342–1356. [DOI] [PubMed] [Google Scholar]
- [19].Reines D, Elongation factor-dependent transcript shortening by templateengaged RNA polymerase II, J. Biol. Chem 267 (1992) 3795–3800. [PMC free article] [PubMed] [Google Scholar]
- [20].Wiest DK, Wang D, Hawley DK, Mechanistic studies of transcription arrest at the adenovirus major late attenuation site. Comparison of purified RNA polymerase II and washed elongation complexes, J. Biol. Chem 267 (1992) 7733–7744. [PubMed] [Google Scholar]
- [21].Yoo OJ, Yoon HS, Baek KH, Jeon CJ, Miyamoto K, Ueno A, Agarwal K, Cloning, expression and characterization of the human transcription elongation factor, TFIIS, Nucleic Acids Res. 19 (1991) 1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Galburt EA, Grill SW, Wiedmann A, Lubkowska L, Choy J, Nogales E, Kashlev M, Bustamante C, Backtracking determines the force sensitivity of RNAP II in a factor-dependent manner, Nature 446 (2007) 820–823. [DOI] [PubMed] [Google Scholar]
- [23].Zhang C, Yan H, Burton ZF, Combinatorial control of human RNA polymerase II (RNAP II) pausing and transcript cleavage by transcription factor IIF, hepatitis delta antigen, and stimulatory factor II, J. Biol. Chem 278 (2003) 50101–50111. [DOI] [PubMed] [Google Scholar]
- [24].Palangat M, Renner DB, Price DH, Landick R, A negative elongation factor for human RNA polymerase II inhibits the anti-arrest transcript-cleavage factor TFIIS, Proc. Natl. Acad. Sci. U. S. A 102 (2005) 15036–15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Marshall NF, Peng J, Xie Z, Price DH, Control of RNA polymerase II elongation potential by a novel carboxyl-terminal domain kinase, J. Biol. Chem 271 (1996) 27176–27183. [DOI] [PubMed] [Google Scholar]
- [26].Renner DB, Yamaguchi Y, Wada T, Handa H, Price DH, A highly purified RNA polymerase II elongation control system, J. Biol. Chem 276 (2001) 42601–42609. [DOI] [PubMed] [Google Scholar]
- [27].Yamaguchi Y, Takagi T, Wada T, Yano K, Furuya A, Sugimoto S, Hasegawa J, Handa H, NELF, a multisubunit complex containing RD, cooperates with DSIF to repress RNA polymerase II elongation, Cell 97 (1999) 41–51. [DOI] [PubMed] [Google Scholar]
- [28].Bondarenko VA, Steele LM, Ujvári A, Gaykalova DA, Kulaeva OI, Polikanov YS, Luse DS, Studitsky VM, Nucleosomes can form a polar barrier to transcript elongation by RNA polymerase II, Mol. Cell 24 (2006) 469–479. [DOI] [PubMed] [Google Scholar]
- [29].Izban MG, Luse DS, Transcription on nucleosomal templates by RNA polymerase II in vitro: inhibition of elongation with enhancement of sequence-specific pausing, Genes Dev. 5 (1991) 683–696. [DOI] [PubMed] [Google Scholar]
- [30].Kireeva ML, Hancock B, Cremona GH, Walter W, Studitsky VM, Kashlev M, Nature of the nucleosomal barrier to RNA polymerase II, Mol. Cell 18 (2005) 97–108. [DOI] [PubMed] [Google Scholar]
- [31].Kireeva ML, Walter W, Tchernajenko V, Bondarenko V, Kashlev M, Studitsky VM, Nucleosome remodeling induced by RNA polymerase II: loss of the H2A/H2B dimer during transcription, Mol. Cell 9 (2002) 541–552. [DOI] [PubMed] [Google Scholar]
- [32].Luse DS, Spangler LC, Újvári A, Efficient and rapid nucleosome traversal by RNA polymerase II depends on a combination of transcript elongation factors, J. Biol. Chem 286 (2011) 6040–6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kulaeva OI, Gaykalova DA, Pestov NA, Golovastov VV, Vassylyev DG, Artsimovitch I, Studitsky VM, Mechanism of chromatin remodeling and recovery during passage of RNA polymerase II, Nat. Struct. Mol. Biol 16 (2009) 1272–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Orphanides G, LeRoy G, Chang CH, Luse DS, Reinberg D, FACT, a factor that facilitates transcript elongation through nucleosomes, Cell 92 (1998) 105–116. [DOI] [PubMed] [Google Scholar]
- [35].Belotserkovskaya R, Oh S, Bondarenko VA, Orphanides G, Studitsky VM, Reinberg D, FACT facilitates transcription-dependent nucleosome alteration, Science 301 (2003) 1090–1093. [DOI] [PubMed] [Google Scholar]
- [36].Churchman LS, Weissman JS, Nascent transcript sequencing visualizes transcription at nucleotide resolution, Nature 469 (2011) 368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lowary PT, Widom J, New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning, J. Mol. Biol 276 (1998) 19–42. [DOI] [PubMed] [Google Scholar]
- [38].Gracey L, Chen Z-Y, Maniar J, Valouev A, Sidow A, Kay M, Fire A, An in vitro-identified high-affinity nucleosome-positioning signal is capable of transiently positioning a nucleosome in vivo, Epigenetics Chromatin 3 (2010) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Subtil-Rodriguez A, Reyes JC, BRG1 helps RNA polymerase II to overcome a nucleosomal barrier during elongation, in vivo, EMBO Rep. 11 (2010) 751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Perales R, Zhang L, Bentley D, Histone occupancy in vivo at the 601 nucleosome binding element is determined by transcriptional history, Mol. Cell. Biol 31 (2011) 3485–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].de la Mata M, Alonso CR, Kadener S, Fededa JP, Blaustein M, Pelisch F, Cramer P, Bentley D, Kornblihtt AR, A slow RNA polymerase II affects alternative splicing in vivo, Mol. Cell 12 (2003) 525–532. [DOI] [PubMed] [Google Scholar]
- [42].Howe KJ, Kane CM, Ares M, Perturbation of transcription elongation influences the fidelity of internal exon inclusion in Saccharomyces cerevisiae, RNA 9 (2003) 993–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Muñoz MJ, Santangelo MSP, Paronetto MP, de la Mata M, Pelisch F, Boireau S, Glover-Cutter K, Ben-Dov C, Blaustein M, Lozano JJ, Bird G, Bentley D, Bertrand E, Kornblihtt AR, DNA damage regulates alternative splicing through inhibition of RNA polymerase II elongation, Cell 137 (2009) 708–720. [DOI] [PubMed] [Google Scholar]
- [44].Batsche E, Yaniv M, Muchardt C, The human SWI/SNF subunit Brm is a regulator of alternative splicing, Nat. Struct. Mol. Biol 13 (2006) 22–29. [DOI] [PubMed] [Google Scholar]
- [45].Shukla S, Kavak E, Gregory M, Imashimizu M, Shutinoski B, Kashlev M, Oberdoerffer P, Sandberg R, Oberdoerffer S, CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing, Nature 479 (2011) 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Alexander RD, Innocente SA, Barrass JD, Beggs JD, Splicing-dependent RNA polymerase pausing in yeast, Mol. Cell 40 (2010) 582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Carrillo Oesterreich F, Preibisch S, Neugebauer KM, Global analysis of nascent RNA reveals transcriptional pausing in terminal exons, Mol. Cell 40 (2010) 571–581. [DOI] [PubMed] [Google Scholar]
- [48].Femino AM, Fay FS, Fogarty K, Singer RH, Visualization of single RNA transcripts in situ, Science 280 (1998) 585–590. [DOI] [PubMed] [Google Scholar]
- [49].Wada Y, Ohta Y, Xu M, Tsutsumi S, Minami T, Inoue K, Komura D, Kitakami J, Oshida N, Papantonis A, Izumi A, Kobayashi M, Meguro H, Kanki Y, Mimura I, Yamamoto K, Mataki C, Hamakubo T, Shirahige K, Aburatani H, Kimura H, Kodama T, Cook PR, Ihara S, A wave of nascent transcription on activated human genes, Proc. Natl. Acad. Sci 106 (2009) 18357–18361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Singh J, Padgett RA, Rates of in situ transcription and splicing in large human genes, Nat. Struct. Mol. Biol 16 (2009) 1128–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bertrand E, Chartrand P, Schaefer M, Shenoy SM, Singer RH, Long RM, Localization of ASH1 mRNA particles in living yeast, Mol. Cell 2 (1998) 437–445. [DOI] [PubMed] [Google Scholar]
- [52].Brody Y, Neufeld N, Bieberstein N, Causse SZ, Böhnlein E-M, Neugebauer KM, Darzacq X, Shav-Tal Y, The in vivo kinetics of RNA polymerase II elongation during co-transcriptional splicing, PLoS Biol. 9 (2011) e1000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Martins SB, Rino J, Carvalho T, Carvalho C, Yoshida M, Klose JM, de Almeida SF, Carmo-Fonseca M, Spliceosome assembly is coupled to RNA polymerase II dynamics at the 3′ end of human genes, Nat. Struct. Mol. Biol 18 (2011) 1115–1123. [DOI] [PubMed] [Google Scholar]
- [54].Boireau S, Maiuri P, Basyuk E, de la Mata M, Knezevich A, Pradet-Balade B, Bäcker V, Kornblihtt A, Marcello A, Bertrand E, The transcriptional cycle of HIV-1 in real-time and live cells, J. Cell Biol 179 (2007) 291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lionnet T, Wu B, Grünwald D, Singer RH, Larson DR, Nuclear physics: quantitative single-cell approaches to nuclear organization and gene expression, Cold Spring Harb. Symp. Quant. Biol 75 (2010) 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Larson DR, Zenklusen D, Wu B, Chao JA, Singer RH, Real-time observation of transcription initiation and elongation on an endogenous yeast gene, Science 332 (2011) 475–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wang F, Koyama N, Nishida H, Haraguchi T, Reith W, Tsukamoto T, The assembly and maintenance of heterochromatin initiated by transgene repeats are independent of the RNA interference pathway in mammalian cells, Mol. Cell. Biol 26 (2006) 4028–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Peil K, Värv S, Lõoke M, Kristjuhan K, Kristjuhan A, Uniform distribution of elongating RNA polymerase II complexes in transcribed gene locus, J. Biol. Chem 286 (2011) 23817–23822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].David L, Huber W, Granovskaia M, Toedling J, Palm CJ, Bofkin L, Jones T, Davis RW, Steinmetz LM, A high-resolution map of transcription in the yeast genome, Proc. Natl. Acad. Sci 103 (2006) 5320–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Xu Z, Wei W, Gagneur J, Perocchi F, Clauder-Munster S, Camblong J, Guffanti E, Stutz F, Huber W, Steinmetz LM, Bidirectional promoters generate pervasive transcription in yeast, Nature 457 (2009) 1033–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermüller J, Hofacker IL, Bell I, Cheung E, Drenkow J, Dumais E, Patel S, Helt G, Ganesh M, Ghosh S, Piccolboni A, Sementchenko V, Tammana H, Gingeras TR, RNA maps reveal new RNA classes and a possible function for pervasive transcription, Science 316 (2007) 1484–1488. [DOI] [PubMed] [Google Scholar]
- [62].Struhl K, Transcriptional noise and the fidelity of initiation by RNA polymerase II, Nat. Struct. Mol. Biol 14 (2007) 103–105. [DOI] [PubMed] [Google Scholar]
- [63].He Y, Vogelstein B, Velculescu VE, Papadopoulos N, Kinzler KW, The antisense transcriptomes of human cells, Science 322 (2008) 1855–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Cheung V, Chua G, Batada NN, Landry CR, Michnick SW, Hughes TR, Winston F, Chromatin- and transcription-related factors repress transcription from within coding regions throughout the Saccharomyces cerevisiae genome, PLoS Biol. 6 (2008) e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kaplan CD, Laprade L, Winston F, Transcription elongation factors repress transcription initiation from cryptic sites, Science 301 (2003) 1096–1099. [DOI] [PubMed] [Google Scholar]
- [66].Mason PB, Struhl K, The FACT complex travels with elongating RNA polymerase II and is important for the fidelity of transcriptional initiation in vivo, Mol. Cell. Biol 23 (2003) 8323–8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Keogh M-C, Kurdistani SK, Morris SA, Ahn SH, Podolny V, Collins SR, Schuldiner M, Chin K, Punna T, Thompson NJ, Boone C, Emili A, Weissman JS, Hughes TR, Strahl BD, Grunstein M, Greenblatt JF, Buratowski S, Krogan NJ, Cotranscriptional Set2 methylation of histone H3 lysine 36 recruits a repressive Rpd3 complex, Cell 123 (2005) 593–605. [DOI] [PubMed] [Google Scholar]
- [68].Carrozza MJ, Li B, Florens L, Suganuma T, Swanson SK, Lee KK, Shia W-J, Anderson S, Yates J, Washburn MP, Workman JL, Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription, Cell 123 (2005) 581–592. [DOI] [PubMed] [Google Scholar]
- [69].Joshi AA, Struhl K, Eaf3 chromodomain interaction with methylated H3-K36 links histone deacetylation to pol II elongation, Mol. Cell 20 (2005) 971–978. [DOI] [PubMed] [Google Scholar]
- [70].Li B, Gogol M, Carey M, Pattenden SG, Seidel C, Workman JL, Infrequently transcribed long genes depend on the Set2/Rpd3S pathway for accurate transcription, Genes Dev. 21 (2007) 1422–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Youdell ML, Kizer KO, Kisseleva-Romanova E, Fuchs SM, Duro E, Strahl BD, Mellor J, Roles for Ctk1 and Spt6 in regulating the different methylation states of Histone H3 lysine 36, Mol. Cell. Biol 28 (2008) 4915–4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Larschan E, Bishop EP, Kharchenko PV, Core LJ, Lis JT, Park PJ, Kuroda MI, X chromosome dosage compensation via enhanced transcriptional elongation in Drosophila, Nature 471 (2011) 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Aitken S, Alexander RD, Beggs JD, Modelling reveals kinetic advantages of cotranscriptional splicing, PLoS Comput. Biol 7 (2011) e1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Schmidt U, Basyuk E, Robert M-C, Yoshida M, Villemin J-P, Auboeuf D, Aitken S, Bertrand E, Real-time imaging of cotranscriptional splicing reveals a kinetic model that reduces noise: implications for alternative splicing regulation, J. Cell Biol 193 (2011) 819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]