Abstract
Liquid chromatography–mass spectrometry (LC–MS)-based metabolomics can have a major impact in multiple research fields, especially when combined with other technologies, such as stable isotope tracers and genetically modified mice. This review highlights recent applications of metabolomic technology in the study of xenobiotic metabolism and toxicity, and the understanding of disease pathogenesis and therapeutics. Metabolomics has been employed to study metabolism of noscapine, an aryl hydro-carbon receptor antagonist, and to determine the mechanisms of liver toxicities of rifampicin and isoniazid, trichloroethylene, and gemfibrozil. Metabolomics-based insights into the pathogenesis of inflammatory bowel disease, alco-hol-induced liver diseases, non-alcoholic steatohepatitis, and farnesoid X receptor signaling pathway-based thera-peutic target discovery will also be discussed. Limitations in metabolomics technology such as sample preparation and lack of LC–MS databases and metabolite standards, need to be resolved in order to improve and broaden the application of metabolomic studies.
Keywords: Metabolomics, Xenobiotic metabolism, Toxicology, Pathogenesis
Introduction
Metabolomics is an area that studies low Mr chemical fingerprints through an unbiased analysis of all the metabolites in biofluids (serum, urine), and extracts of cells, tissues, and organs (Johnson and Gonzalez 2012; Johnson et al. 2012a). In combination with other technologies in systems biology (e.g., genomics, transcriptomics, proteomics), the full cellular pathways can be dissected and understood. Metabolomics can give a comprehensive view of the combinatorial results of genetic factors, and xenobiotic exposure through the diet, drug administration and environment (Johnson and Gonzalez 2012). Metabolomics has found broad application in studies of xenobiotic metabolism (Chen et al. 2007) and toxicity (Robertson 2005), and in disease diagnostic biomarker discovery (Dumas et al. 2014).
The most common platforms used for metabolomics are gas chromatography–mass spectrometry (GC–MS), liquid chromatography–mass spectrometry (LC–MS), and pro-ton nuclear magnetic resonance (1H NMR). Each platform has advantages and disadvantages that have been detailed in previous reviews (Patterson et al. 2010). Compared with the limited sensitivity of 1H NMR and extensive sample preparation (e.g., derivatization) and low throughput for GC–MS, high sensitivity and ease of sample preparation make LC–MS among the most widely used platforms in metabolomics.
The combination of metabolomics with some other technologies such as stable isotope tracers and genetically modified mouse models can further broaden the scope of application. Metabolomics using steady-state concentration-based metabolite profiling sometimes does not reflect the contribution of each biochemical pathway. However, the introduction of isotopes into metabolomics can be successfully used to both monitor metabolite flux and study the metabolism of xenobiotics. Stable isotope tracers can reveal the metabolic networks. For example, LC–MS-based metabolomics with D4-cholic acid (CA) as a metabolic tracer, was used to successfully determine the metabolism and reconjugation of bile acids and demonstrated that silencing of 90 % of Slc27a5 expression significantly decreases the reconjugation of D(4)-CA to D(4)-taurocholic acid (D(4)-TCA) (Castro-Perez et al. 2011). Stable isotope resolved metabolomics (SIRM) was also successfully applied for pathway tracing to obtain metabolic parameters in human cancer subjects in situ (Lane et al. 2011). Stable isotope tracers have also been used to profile the metabolic fate of therapeutic drugs. After introduction of labeled drugs into the system, LC–MS was used to probe their metabolites in biological fluids. For example, three new metabolites of acetaminophen (APAP) were found through principle components analysis (PCA) of urine obtained from the mice dosed with unlabeled APAP and [acetyl-2H3] APAP (Chen et al. 2008a). A more detailed review of the application of isotope-labeled compounds in metabolism was published earlier (Mutlib 2008). Genetically modified mice are also valuable tools to understand the role of specific genes in the xenobiotic metabolism, toxicology mechanisms, or diseases pathogenesis. A metabolomics study was performed to compare the in vivo metabolism of procainamide in wild-type mice, CYP2D6-humanized mice, and human liver microsomes to demonstrate that the major metabolite N-acetylprocainamide in human urine was approximately 25-fold higher in humans than wild-type mice (Li et al. 2012), providing a potential clue to understanding the species difference in procainamide-induced lupus. LC–MS-based metabolomics studies have been applied in multiple research fields (Table 1), a few of which are detailed below.
Table 1.
List of metabolomics application in the study of xenobiotic metabolism, toxicology mechanisms, and the pathogenesis and of diseases
| Xenobiotic/diseases | Model | Key finding | References |
|---|---|---|---|
| Isoniazid | Humans | 7 Novel metabolites | (Li et al. 2011) |
| Ethanol | Wild-type and Cyp2e1-null mice | A novel metabolite N-acetyltaurine | (Shi et al. 2012) |
| α-Tocopherol | Wild-type mice | 3 Novel metabolites | (Johnson et al. 2012b) |
| Cocaine | Wild-type mice and rats | Species-dependent metabolism of cocaine | (Yao et al. 2013) |
| Tempol | Wild-type mice | 8 Novel metabolites | (Li et al. 2013c) |
| Saquinavir |
In vitro system, wild-type and Cyp3a- null mice |
20 Novel metabolites and one reactive metabolite α-hydroxyaldehyde |
(Li et al. 2014a) |
| 2,3,7,8-Tetrachlorod- ibenzo-p-dioxin (TCDD) |
Wild-type, Ahr−/−, and Arnt Liv mice | The role of CES3, TGFβ in TCDD-induced steato- hepatitis |
(Matsubara et al. 2012) |
| Rifaximin | Wild-type, Pxr-null, and hPXR mice | Long-term exposure of rifaximin-induced hepatic steatosis in hPXR mice |
(Cheng et al. 2012) |
| Acetaminophen | Wild-type, Ppara-null, Ucp2-null Mice |
The protection of APAP-induced hepatotoxicity by PPARα-induced expression of UCP2 |
(Patterson et al. 2012) |
| CCl4 | Rats | Oxidative stresses to multiple rat organs | (Jiang et al. 2012) |
| Acetaminophen | Wild-type, and Txnrd1 ΔLiv mice | Important role of Txnrd1 in APAP-induced hepato- toxicity |
(Patterson et al. 2013) |
| Bupleurotoxin | Wild-type mice | Key role of GABA receptor signaling pathway in cerebral lesion |
(Zhang et al. 2013b) |
| Isoniazid | Wild-type and Cyp2e1-null mice | The involvement of bile acids accumulation and mito- chondria β-oxidation in isoniazid toxicity |
(Cheng et al. 2014) |
| Lithocholic acid | Wild-type, Tg-3A4, Vdr
IEpC and Vdr IEpC/3A4 mice |
Protection role of intestinal CYP3A4 in LCA-induced hepatotoxicity |
(Cheng et al. 2013) |
| 3-Chloropropane-1,2-di- palmitate |
Rats | The alteration of indoxyl sulfate, xanthurenic acid, phenylacetylglycine, non-anedioic acid, and taurine |
(Li et al. 2013e) |
| Aflatoxin B1 | Rats | Gluconeogenesis and lipid metabolism disorder in aflatoxin B1-induced acute hepatotoxicity |
(Lu et al. 2013) |
| Bisphenol a | Rats | DNA methylation damage, disrupted choline pathway | (Chen et al. 2014) |
| Type 2 diabetes mellitus | Monkey, mice | The important role of SLC6A20 kidney transporter in type 2 diabetes mellitus |
(Patterson et al. 2011a) |
| Hepatocellular carcinoma | Human | Aberrant lipid metabolism in hepatocellular carcinoma | (Patterson et al. 2011b) |
| Autosomal dominant poly- cystic kidney disease |
Wild-type and Pkd1cko mice | The important role of Pkd1 in autosomal dominant polycystic kidney disease |
(Menezes et al. 2012) |
| Cholestatic liver disease | Wild-type and Abcb11-null mice | Impaired mitochondrial fatty acid β-oxidation in Abcb11-null mice precedes cholestasis |
(Zhang et al. 2012) |
| Squamous cell carcinoma | SCCVII-tumor-bearing mice | Liver dysfunction induced by tumor growth imposed inflammatory response |
(Li et al. 2013d) |
| Breast cancer | Human | Altered FA β-oxidation in patients with breast cancer | (Shen et al. 2013) |
| Pseudoxanthoma elasticum |
Wild-type and ABCC6-null mice | The important role of circulated pyrophosphate via ABCC6-dependent mechanism |
(Jansen et al. 2013) |
| Type 2 diabetes mellitus | Human | The key role of 2-aminoadipic acid in type 2 diabetes mellitus |
(Wang et al. 2013) |
| Prostate cancer | Human | Biochemical alterations associated with cell growth, energetics, stress |
(McDunn et al. 2013) |
| Pancreatic tumor | Pdx-Cre KrasLSL-G12D/+ mice, p53
fl/fl, Atg7 fl/fl, Atg5 fl/fl |
p53 status determines the role of autophagy in pancre- atic tumor development |
(Rosenfeldt et al. 2013) |
| Alzheimer’s disease | Human | Disturbance of phospholipids metabolism | (Gonzalez-Dominguez et al. 2014) |
| Acute respiratory distress syndrome (ARDS) |
Human | The alteration of amino acid metabolism, glycolysis and gluconeogenesis, fatty acid biosynthesis, phos- pholipids, and purine metabolism |
(Evans et al. 2013) |
| Gastric ulcer | Rats | The disruption of sphingophospholipid and fatty acid metabolism pathway |
(Tianjiao et al. 2014) |
| Graft-versus-host disease (GVHD) |
Mice | Early dysregulation of host hepatic GSH metabolism and oxidative stress |
(Suh et al. 2014) |
| Rhegmatogenous retinal detachment |
Human | The disruption of histidine metabolism and citrate cycle |
(Li et al. 2014b) |
| Cardiovascular diseases (CVD) |
Human | The important role of mitochondrial damage and dysfunction in CVD |
(Rizza et al. 2014) |
| Polycystic ovary syndrome (PCOS) |
Human | Abnormalities of lipid- and androgen-metabolism in PCOS patients |
(Zhao et al. 2014) |
| Pulmonary embolism | Pigs | Involvement of many metabolic pathways (e.g., glyco- lysis, TCA cycle) |
(Bujak et al. 2014) |
LC–MS‑based metabolomics study in the study of xenobiotic metabolism
Humans are frequently exposed to various xenobiotics (e.g., drugs, herbs, food, environmental pollutants), and different metabolic fates exist for xenobiotics from all these sources. Elucidation of their metabolic pathways will help to understand the beneficial (therapeutic roles) and deleterious (toxic) effects of chemicals. LC–MS-based metabolomics was first used to study drug metabolism (Plumb et al. 2003), and this method has been successfully applied to the metabolism of numerous xenobiotics.
The metabolic profile and bioactivation of noscapine
Noscapine, a clinically effective cough suppressant drug, has drawn much attention due to its anti-tumor activities toward multiple tumor types, including lymphoma, breast cancer, melanoma, ovarian carcinoma, glioblastoma, colon cancer and non-small cell lung cancer (Mahmoudian and Rahimi-Moghaddam 2009). However, safety concerns exist for noscapine, including its potential carcinogenic properties (Schuler et al. 1999), decreased glutathione and enhanced lipid peroxidation (Aneja et al. 2004), and drug– drug interaction with warfarin (Fang et al. 2010; Zhang et al. 2013a). The metabolism of noscapine was investigated and some first-pass metabolic pathways detected, including C–C cleavage, O-demethylation, and cleavage of methylenedioxy group (Tsunoda and Yoshimura 1981). Yet, limitation exists on the methodology to obtain a more complete metabolic profile of this agent. Recently, the complete metabolic profile of noscapine was elucidated using ultra-performance chromatography electrospray ionization quadrupole time-of-flight mass spectrometry (UPLC-ESI-QTOFMS)-based metabolomics (Fang et al. 2012). Besides the metabolites previously observed, novel metabolites were found after oral gavage of noscapine (300 mg/kg), including an N-demethylated metabolite, two hydroxylated metabolites, one metabolite undergoing both demethylation and cleavage of the methylenedioxy group, and a bis-dem-ethylated metabolite. Additionally, new glucuronide conjugates were detected (Fig. 1). One reactive ortho-quinone metabolite was identified that might be involved in the clinical noscapine–warfarin interaction and the potential toxicity risk of noscapine.
Fig. 1.
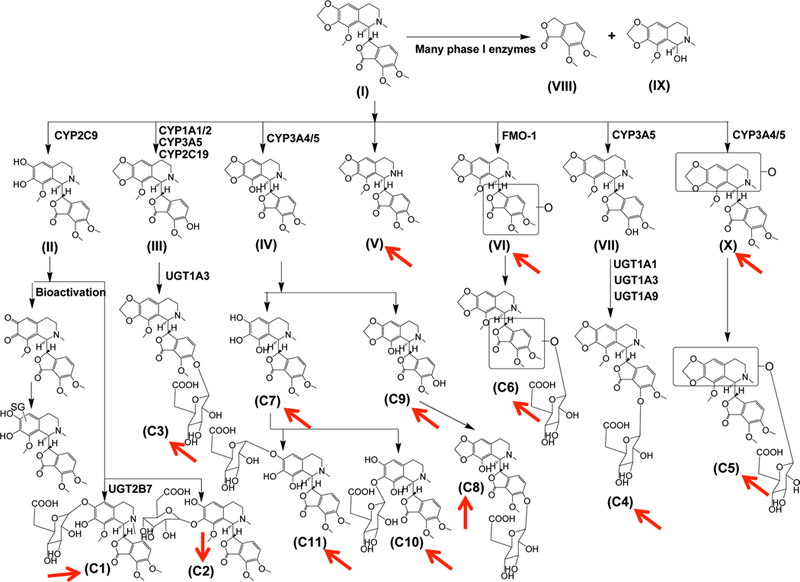
Metabolic mapping of noscapine by use of metabolomics. Novel metabolites discovered by UPLC-ESI-QTOFMS-based metab-olomics (e.g., metabolomics based on ultra-performance liquid chromatography electrospray ionization quadrupole time-of-flight mass spectrometry) are shown by red arrows
In vivo application of the pure aryl hydrocarbon receptor antagonist GNF‑351 is limited to the gastrointestinal track
Development of aryl hydrocarbon receptor (AHR) antagonist is an area of great interest given the role of this receptor in toxicology and physiology. GNF-351 is a recently developed AHR antagonist with the capacity to inhibit dioxin response element (DRE)-dependent and independent activity (Smith et al. 2011). Given the important role of pharmacokinetic properties toward the development of the therapeutic agents, UPLC-ESI-QTOFMS-based metabolomics study was employed to evaluate the absorption and metabolic behavior of GNF-351. GNF-351 was poorly absorbed into the blood after oral administration with nearly all of the parent compound and its metabolites appearing in the faces and not in blood and urine (Fig. 2). Several phase I metabolites were produced by human and mouse microsomes, including two oxidized (VI and VII) and one tri-demethylated (VIII) metabolite of GNF-351. UGT1A4-catalyzed glucuronidation of GNF-351 also exhibited species differences. Additionally, profiling the metabolic enzymes indicated that the major enzymes involved in metabolism of GNF-351 were in the CYP1A family that are encoded by target genes regulated by AHR, which serve to accelerate metabolism of GNF-351 and further weaken the antagonism activity of GNF-351. In this study, metabolomics was successfully applied to demonstrate the limitation of a potential drug candidate by its poor metabolism and absorption behavior (Fang et al. 2014).
Fig. 2.
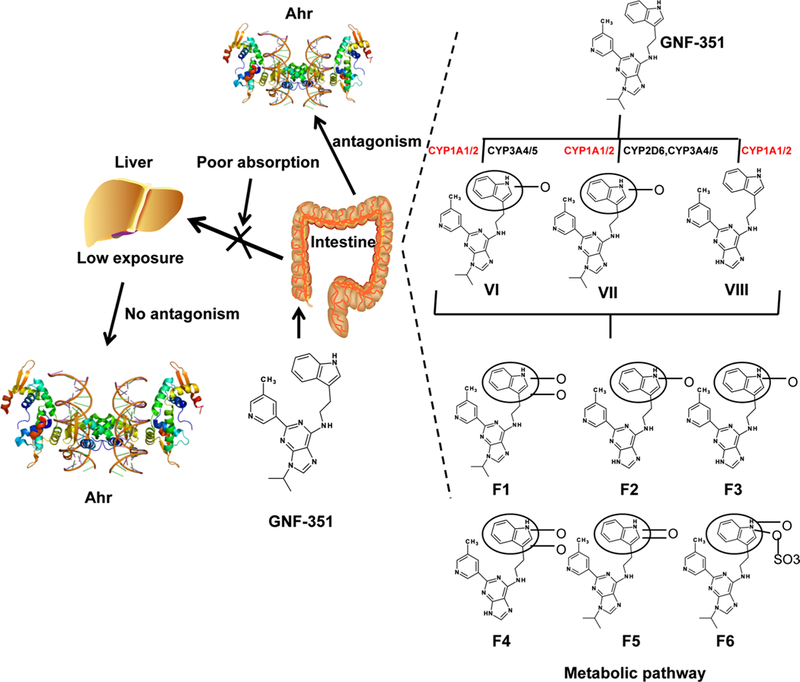
Metabolomics reveals the limitations for the in vivo application of GNF-351 due to poor absorption and high metabolism. The metabolic pathway was determined using UPLC-ESI-QTOFMS-based metabolomics. Red color highlights the drug-metabolizing enzymes regulated by Ahr
LC–MS‑based metabolomics study mechanisms of xenobiotic toxicity
Metabolomic approaches have been increasingly employed as an important tool in toxicology. Through a comprehensive analysis of metabolomes, the alterations in response to toxicity stressors can be detected, and clues to the mechanism of toxicity can be determined through correlating the relationship between the chemical perturbations and the influenced biochemical pathways. Compared with the application of other omics technologies, genomics, transcriptom-ics, and proteomics, in toxicity studies, metabolomics-based study results reflect the final results induced by a cascade of toxic events, which are closer with the toxic phenotype.
Mechanism of liver toxicity induced by co‑therapy with rifampicin and isoniazid
Both rifampicin (RIF) and isoniazid (INH) are first-line anti-tuberculosis drugs, and the co-therapy with these two drugs in humans can result in the liver toxicity. This toxicity is difficult to reproduce in rats and mice, and the mechanism of hepatoxicity has remained elusive. In a recent study, pregnane X receptor (PXR) humanized (hPXR) were engaged to successfully reproduce the liver toxicity induced by co-treatment with rifampicin (RIF) and isoniazid (INH); toxicity was not observed in wild-type mice and Pxr-null mice. This is due to the fact that mouse PXR is not activated by pharmacological concentrations of rifampicin in mice, while this drug readily activates human PXR. Additionally, liver toxicity was not observed in hPXR mice treated with either RIF or INH; both drugs need to be administered simultaneously. To provide mechanistic clues on the RIF, INH-co-therapy-induced liver toxicity, bile metabolomics was adopted revealing extremely high concentrations of an important intermediate in porphyrin biosynthesis protoporphyrin IX (PPIX) in hPXR mice treated with RIF and INH, which might be caused by PXR-regulated over-expression of aminolevulinic acid synthase (ALAS).
The potential mechanism of toxicity is RIF activation of PXR and induction of the PXR target gene Alas which leads to an increase in PPIX levels. In a mechanism that has yet to be determined, INH could inhibit the downstream enzyme lead-ing to heme production that causes a backup in the pathway and a massive toxic increase in PPIX (Fig. 3). This study is an example of the use of metabolomics and genetically modified mice to provide a new paradigm to understand hepatotoxicity mechanisms associated with drug therapy in humans (Li et al. 2013b).
Fig. 3.
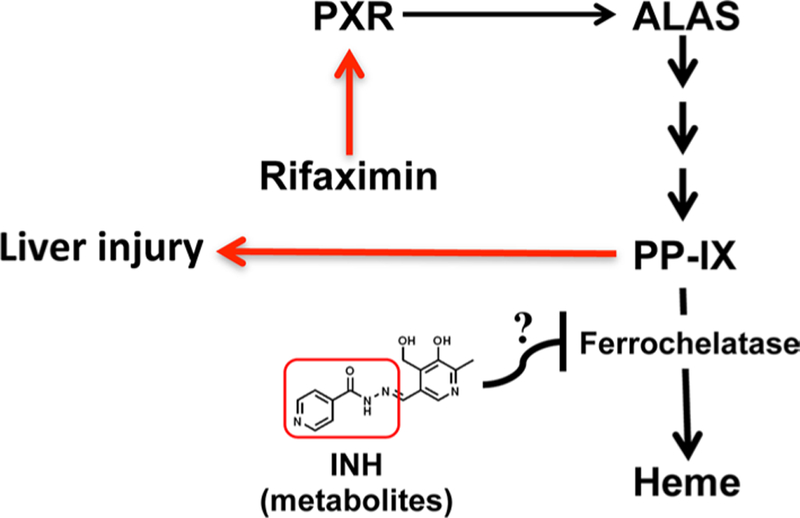
Metabolomics reveals the mechanism of liver toxicity induced by co-therapy with rifampicin and isoniazid. RIF activates human PXR and induction of the PXR target gene Alas leading to increase in protoporphyrin IX (PP-IX) levels. In a mechanism that has yet to be determined, INH could inhibit the downstream enzyme ferroche-latase leading to less heme production and a massive toxic increase in PP-IX
Major contribution of trichloroacetate (TCA) toward trichloroethylene (TCE)‑induced biomarkers alteration
Trichloroethylene (TCE), a chlorinated solvent, has been widely used for degreasing metals and dry cleaning (Bakke et al. 2007). Exposure to TCE can induce severe health problems, due to multiple organs damage, including liver, kidney and the immune system toxicities, and carcinogenesis toward multiple types of cancers (e.g., renal cell carcinoma, non-Hodgkin’s lymphoma, liver or biliary cancer). Toxicity has been attributed to the metabolism of TCE to its major metabolites dichloroacetate (DCA) and trichloroacetate (TCA). However, the mechanism of toxicity and the contribution of DCA and TCA toward TCE toxicity remain unclear. Metabolomics revealed that TCE treatment resulted in a decrease in urine of endogenous metabolites related to fatty acid metabolism, possibly a result of TCE-induced expression of fatty acid metabolism-associated peroxisome proliferator-activated receptor α (PPARα) tar-get genes (Fig. 4). TCE treatment also disrupted the home-ostasis of serum phospholipids as revealed by increased serum lysophosphatidylcholine (LPC) 18:0, LPC 18:1 (9Z), and phosphatidylcholine (PC) metabolites. Additionally, through comparison of metabolomics alteration induced by TCA and DCA, the more comparable metabolites profile alteration was observed for TCA than DCA, indicating more contribution of TCA toward TCE-induced toxicity (Fang et al. 2013).
Fig. 4.
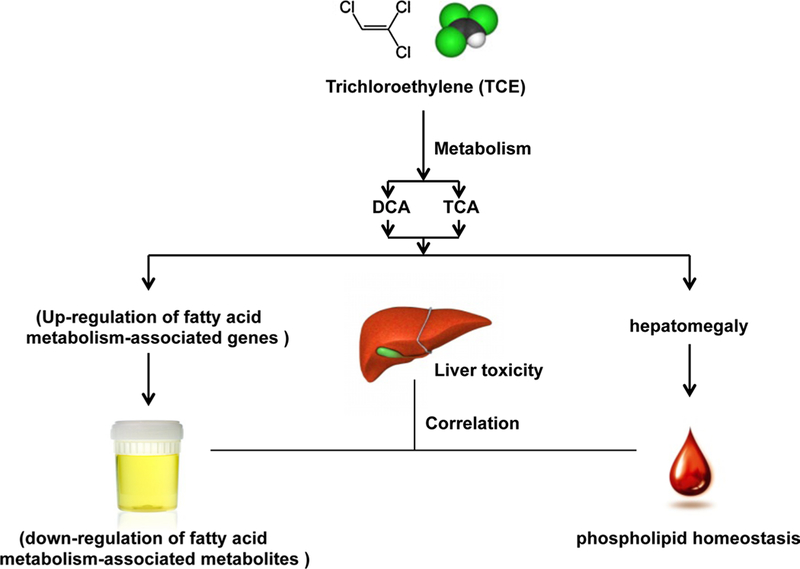
Major contribution of trichloroacetate (TCA) in trichloroethylene (TCE)-induced biomarkers alteration. TCE can be metabolized to form two PPARα agonists dichloroacetate (DCA) and TCA. The up-regulated expres-sion genes by TCA activation of PPARα results in increased metabolism of fatty acids that results in lower levels of urinary metabolites that reflect fatty acid. TCE-induced hepatomegaly induces altered phospholipid homeostasis in serum
PPARα‑dependent disruption of homeostasis of lysophosphatidylcholine and bile acid induced by gemfibrozil
Gemfibrozil, one of the most widely prescribed anti-dyslip-idemia fibrate drugs, was reported to induce many adverse effects, including alterations of liver function, cholestatic jaundice, and cholelithiasis in some patients. However, the mechanisms of these toxicities remain unclear. A combination of UPLC–MS-based metabolomics and Ppara-null mice was used to elucidate the mechanism of gemfibrozil-induced hepatotoxicity. Disrupted homeostasis of bile acids and phospholipids was detected, and this alteration was PPARα-dependent (Liu et al. 2014), which is closely correlated with the alteration of the genes involved in the metabolism and transport of LPC and bile acids com-pounds (Fig. 5). This study provides a new insight into the mechanism of gemfibrozil-induced hepatotoxicity using the combination of LC–MS-based metabolomics and traditional toxicological strategies.
Fig. 5.
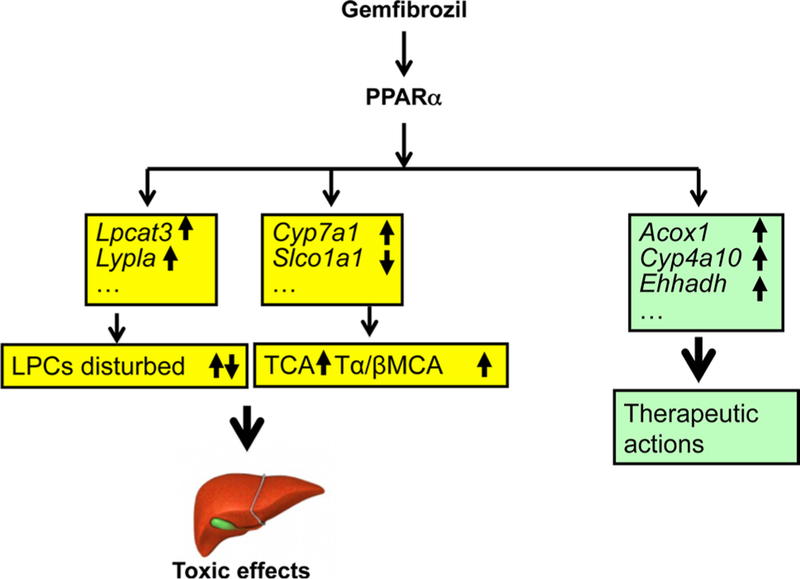
Metabolomics reveals that PPARα mediated gemfibrozil-induced hepatotoxicity. The therapeutic role of gemfibrozil can be explained by PPARα-induced expression of target genes involved in fatty acid β-oxidation. PPARα-dependent regulation of the metabolic genes involved in the LPC and bile acid metabolism and/or transport result in alteration of downstream metabolic pathways
Mechanism identification of cadmium (Cd) toxicity through analysis of integrated redox proteomics and metabolomics of mitochondria
Cd exposure leads to hepatotoxicity, nephrotoxicity, pulmonotoxicity, neurotoxicity, bone toxicity, and even carcinogenesis. Metabolomics showed that Cd exposure can result in alteration of levels of branched-chain amino acid (BCAA) and carnitine metabolites, indicating disruption of amino acid and fatty acid metabolism. Furthermore, redox proteomics using isotope-coded affinity tag (ICAT) combined mass spectrometry was adopted to analyze the redox states of liver mitochondrial proteins, revealing alterations in 24 Cys in proteins functioning in branched-chain amino acid (BCAA) metabolism and 14 Cys in pro-teins functioning in fatty acid (acylcarnitine/carnitine) metabolism, which closely correlated with the metabo-lomics results. Based on the above results, the conclusion was drawn that Cd-induced hepatotoxicity was due in part to alterations in mitochondrial protein redox state and metabolites (Go et al. 2014). This study is a good example of elucidating toxicity mechanism using a combination of proteomics and metabolomics.
Metabolomics to understand the pathogenesis and related therapeutic targets of diseases
Metabolomics can give an accurate description of the bio-chemical profiles of blood and tissues, which can facilitate an understanding of the alterations in complex biological networks involved in diseases.
Metabolomics to elucidate the disease mechanism of inflammatory bowel disease (IBD) and colon cancer
Inflammatory bowel disease (IBD) is a chronic disorder of the gastrointestinal tract and includes ulcerative colitis and Crohn’s disease. To date, although multiple factors such as environmental stress, microbial insults, and auto-immunity, have been reported to contribute to the etiology of IBD (Schreiber and Hampe 2000; Xavier and Podol-sky 2007), the mechanism of IBD is considered diverse and inadequately described. LC–MS-based metabolomic analysis of deproteinized serum from control and dextran sulfate sodium (DSS)-treated mice was carried out revealing that experimental IBD significantly shifted the balance between saturated LPC (LPC 18:0) and unsaturated LPCs (LPC 18:1, LPC 18:2), which results from the inhibition of stearoyl-CoA desaturase 1 (SCD1) activity in liver. This alteration exacerbates pro-inflammatory responses, which is considered to be a key event in the DSS-induced IBD model; this was supported by the evidence that Scd1-null mice are more susceptible to DSS treatment than wild-type mice (Chen et al. 2008b). A combination of metabolomics and a corresponding mouse model was further employed to explore the role of nuclear receptors in IBD. Treatment with rifaximin, a human intestine-specific PXR activator, significantly protects against DSS-induced and trinitrobenzene sulfonic acid (TNBS)-induced IBD in hPXR mice but not in wild-type and Pxr-null mice. Serum metabolomics revealed a similar metabolomics profile among rifaximin-treated wild-type, Pxr-null and hPXR mice, indicating that rifaximin may be acting locally in the colon and not systemically to decrease the severity of DSS-induced colitis (Cheng et al. 2010). An intestine-specific vitamin D receptor (VDR) knockout mouse and showed that disruption of the intestinal Vdr gene exacerbated the IBD, as indicated by high score of IBD symptoms, severe damage of the intestine mucosal layer and increased expression of genes encoding pro-inflammatory cytokines (Kim et al. 2013). Furthermore, feces metabolomics showed decreased con-centrations of taurine and taurine-conjugated bile acids in intestine-specific vitamin D receptor knockout mice, indicating disruption of taurine metabolism by IBD. Given that taurine plays an important role in anti-inflammation and taurine treatment can ameliorate the symptoms of IBD, the metabolomics comparison between wild-type mice and intestine-specific vitamin D receptor knockout mice demonstrated the mechanism by which loss of VDR expression in the intestine exacerbated IBD.
Colon cancer remains a leading cause of cancer mortality worldwide. Metabolomics was used to analyze the serum metabolic profiles between healthy volunteers and colon cancer patients, revealing a significant alteration of amino acid profiles (Leichtle et al. 2012). Urine metabolomics analysis of patients with colon cancer indicated increased tryptophan metabolism and altered tricarboxylic acid cycle and gut microflora metabolism in patients (Qiu et al. 2010). In another study, metabolomics revealed differences in urine metabolites between wild-type and Apc-min/+ mice (mice bearing heterozygous mutation in adeno-matous polyposis coli gene that develops multiple intestinal neoplasia) and found alteration of metabolites related to polyamine metabolism, nucleic acid metabolism, and methylation. The changes in these metabolites were also found in mice with azoxymethane-induced tumors and in tumor tissues of colon cancer patients, indicating the involvement of these metabolic pathways in the pathogenesis of colon cancer (Manna et al. 2014).
Metabolomics to reveal the pathogenesis of liver diseases
Since various liver diseases have become a leading reason for deaths in the USA and the world, metabolomics was applied to explore the pathogenesis of experimentally induced liver disorders. Metabolomics profiling was carried out on urine obtained from alcohol-treated wild-type and Ppara-null mice. Besides some common metabolites (e.g., ethyl sulfate, ethyl-β-D-glucuronide, 4-hydroxy-phenylacetic acid, 4-hydroxyphenylacetic acid sulfate, 2-hydroxyphenylacetic acid, adipic acid, pimelic acid) identified in alcohol-treated wild-type and Ppara-null mice, one specific metabolite, indole-3-lactic acid, was observed to only increase in alcohol-treated Ppara-null mice (Manna et al. 2010). The proposed mechanism is that the loss of PPARα impairs conversion of tryptophan to NAD+ through quinolinic acid and fatty acid β-oxidation. The oxidation of alcohol to acetaldehyde and acetic acid resulted in a reduction in the ratio of NAD+/NADH, followed by accumulation of free fatty acid in the liver. The increased indole-3-lactic acid in urine was due to increased reduction of indole-3-pyruvic acid to indole-3-lactic acid driven by the buildup of NADH.
Non-alcoholic steatohepatitis (NASH), a progressive form of non-alcoholic fatty liver disease, can develop into cirrhosis, hepatic failure, and hepatocellular carcinoma. Serum metabolomics showed significant decreases in pal-mitoyl-, stearoyl-, and oleoyl-lysophosphatidylcholine (LPC), and increase in tauro-β-muricholate, taurocholate, and 12-hydroxyeicosatetraenoic acid (12-HETE) in mice treated with a methionine and choline-deficient (MCD) diet that produces a liver pathology mimicking NASH. The relevant mechanism is the pro-inflammation cytokine regulation of genes involved in LPC degradation and the synthesis and transport of bile acids (Tanaka et al. 2012). Others also demonstrated an increase in azelaic acid-mono ester in serum that resulted from attenuation of hepatic carboxy-lesterase 3 (CES3) expression in the MCD-treated NASH model (Matsubara et al. 2012). Monitoring metabolites related to energy production in both wild-type mice and estrogen-related receptor α (ERRα) knockout mice showed that loss of orphan nuclear receptor estrogen-related recep-tor α (ERRα) promoted the necrosis of hepatocytes in response to carcinogen due to a deficiency of energy pro-duction, which also helps the development of agents (e.g., rapamycin) for chemoprevention of hepatocarcinogenesis through decreasing the level of ERRα (Hong et al. 2013).
Microbiome remodeling results in the inhibition of intestinal farnesoid X receptor (FXR) signaling and decreased obesity
Metabolomics was applied to find a new therapeutic target of obesity (Li et al. 2013a). The influence of antioxidant tempol on the genus Lactobacillus-mediated bile salt hydrolase (BSH) activity resulted in the accumulation of bile acids including tauro-β-muricholic acid (TβMCA) in intestine. TβMCA was found to be an antagonist of FXR, and inhibition of FXR signaling was thought to mediate the anti-obesity effects of tempol (Fig. 6). The role of FXR signaling in obesity was demonstrated by use of mice lack-ing this receptor in the intestine; tempol treatment does not have an anti-obesity effect in intestine-specific Fxr-null mice and suggest that targeting intestinal FXR with an FXR antagonist could be a promising anti-obesity therapy.
Fig. 6.
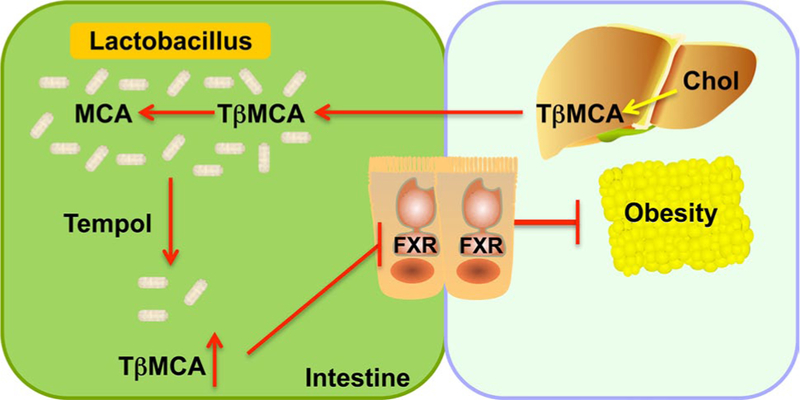
Metabolomics reveals the importance of intestinal farnesoid X receptor (FXR) signaling pathway in obesity. Tempol alters the gut microbiome by preferentially killing Lactobacillus and which decreased bile salt hydrolase activity (BSH), which hydrolyzes tauro-β-muricholic acid (TβMCA) that is formed in liver as a result of cholesterol (Chol) metabolism. Lactobacillus BSH efficiently deconju-gates TβMCA to MCA. Lower intestinal Lactobacillus BSH results in increased TβMCA that inhibits intestinal FXR signaling resulting in decreased obesity in high-fat diet-fed mice
Challenges for the utilization of metabolomics in xenobiotic metabolism, toxicology mechanism, and diseases pathogenesis
Sample preparation in metabolomics studies
Proper sample preparation is vital for a successful metabolomics study. Inadequate sample handling and storage can compromise the reliability of metabolomics data. Different extraction protocols were reported to result in different metabolite profiles and the corresponding interpretation of metabolic pathways (Duportet et al. 2012). Other stud-ies evaluated the pre-analytical aspects and sample quality assessment in metabolomics studies of human blood, and showed that many factors were important for the final results, including even placement of samples in ice water, the utilization of EDTA plasma, and the preferred use of non-refrozen biobank samples (Yin et al. 2013). Sample preparation is particularly important for the ability to compare data between laboratories. Therefore, development of unselective, reproductive, simple, and fast sample preparation methods should be an area of immediate concern.
Impact of the lack of metabolite standards and LC–MS databases
In order to accurately identify and quantify ions discovered by metabolomics, standards should be available. Metabolomics-based xenobiotic metabolism study often identifies metabolites with low abundance, and the MS/MS data are further employed to aid in the prediction of metabolite structures through the analysis of MS/MS fragmentation patterns (Chen et al. 2007). However, this method can-not be used for some compounds in which it is difficult to obtain many MS/MS fragments. Additionally, some metabolites can produce the same MS/MS fragmentation pattern, which can be used in the prediction of metabolite structures that are difficult to decipher. Therefore, obtaining metabolite standards is vital for proper structural identification. In the absence of a commercially available standard, chemical synthesis and purification of the predicted structure based on the accurate mass and MS/MS fragmentation pattern of an ion must be carried out where feasible. However, in xenobiotic metabolism studies, this is not practical nor is it necessary. For example, as shown above, 22 metabolites of noscapine were found in the urine of treated mice, many of which are of low abundance. However, knowing the structure of the parent compound and the conjugates makes prediction possible, but precise stereochemistry cannot be determined. Thus, it is not necessary to synthesize all possible metabolites in order to produce a metabolic map of the drug. Indeed, synthesis of all the metabolites of a particular xenobiotic is nearly impossible and costs consider-able time and money. In addition, for some metabolites, the synthesis is especially difficult, such as glucuronides, glutathiones, and oxidates, and the introduction of chirality (Walker et al. 2011). Another method to obtain the metabolite standards is to isolate the compound from an in vitro metabolic incubation system or from in vivo biological flu-ids (e.g., urine, bile). However, this method cannot be used to prepare metabolite standards present at low abundance (Espina et al. 2009).
It is vitally important to determine the structures of in vivo biomarkers associated with diseases. Although the structures can be predicted with the combination of accurate masses, MS/MS data, and several databases (e.g., Human Metabolome Database (HMDB), METLIN, ChemDB), standards are ultimately needed for comparison of the retention times and MS/MS fragmentation patterns with the ion in question and for accurate quantitation. Additionally, endogenous metabolites are more complex than xenobiotic metabolites, with over tenfold lower magnitude concentrations found in vivo. Therefore, it is a major challenge to cover a wide concentration range and measure metabolites present at trace levels.
Conclusions
The compilation of recent metabolomic studies in xenobiotic metabolism and toxicity, and biomarkers for diseases pathogenesis and mechanism, demonstrate the power and importance of LC–MS-based metabolomics studies. Metabolomics can give a wealth of information, especially when combined with isotopes tracers and genetically modified mice and integrated with a systems biology analysis. Some limitations such as sample preparation, limited metabolite standards, and more comprehensive development of LC–MS databases are areas that required attention.
Acknowledgments
This work was supported in part by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, and 1R01ES022186–01, National Institutes of Health.
Abbreviations
- APAP
Acetaminophen
- ALAS
Aminolevulinic acid synthase
- AHR
Aryl hydrocarbon receptor
- BSH
Bile salt hydrolase
- CES3
Carboxylesterase 3
- CYP
Cytochrome P450
- CVD
Cardiovascular diseases
- DSS
Dextran sulfate sodium
- DCA
Dichloroacetate
- DRE
Dioxin response elements
- FXR
Farnesoid X receptor
- GC–MS
Gas chromatography–mass spectrometry
- GVHD
Graft-versus-host disease
- IBD
Inflammatory bowel disease
- INH
Isoniazid
- LC–MS
Liquid chromatography–mass spectrometry
- LPC
Lysophosphatidylcholine
- GNF351
N-(2-(1H-Indol-3-yl)ethyl)-9-isopropyl-2-(5-methylpyridin-3-yl)-9H-purin-6-amine
- NASH
Non-alcoholic steatohepatitis
- PPARα
Peroxisome proliferator-activated receptor α
- PC
Phosphatidylcholine
- PCA
Principle components analysis
- PCOS
Polycystic ovary syndrome
- PXR
Pregnane X receptor
- 1H NMR
Proton nuclear magnetic resonance
- PPIX
Protoporphyrin IX
- RIF
Rifampicin
- SCD1
Stearoyl-CoA desaturase 1
- TβMCA
Tauro-β-muricholic acid
- TNBS
Trinitrobenzene sulfonic acid
- TCA
Trichloroacetate
- TCE
Trichloroethylene
- VDR
Vitamin D receptor
Footnotes
Conflict of interest The authors have declared that there are no conflicts of interest.
References
- Aneja R, Katyal A, Chandra R (2004) Modulatory influence of noscapine on the ethanol-altered hepatic biotransformation system enzymes, glutathione content and lipid peroxidation in vivo in rats. Eur J Drug Metab Pharmacokinet 29:157–162 [DOI] [PubMed] [Google Scholar]
- Bakke B, Stewart PA, Waters MA (2007) Uses of and exposure to trichloroethylene in US industry: a systematic literature review. J Occup Environ Hyg 4:375–390 [DOI] [PubMed] [Google Scholar]
- Bujak R, Garcia-Alvarez A, Ruperez FJ et al. (2014) Metabolomics reveals metabolite changes in acute pulmonary embolism. J Proteome Res doi: 10.1021/pr400872j [DOI] [PubMed]
- Castro-Perez JM, Roddy TP, Shah V et al. (2011) Attenuation of Slc27a5 gene expression followed by LC–MS measurement of bile acid reconjugation using metabolomics and a stable isotope tracer strategy. J Proteome Res 10:4683–4691 [DOI] [PubMed] [Google Scholar]
- Chen C, Gonzalez FJ, Idle JR (2007) LC–MS-based metabolomics in drug metabolism. Drug Metab Rev 39(2–3):581–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Krausz KW, Idle JR, Gonzalez FJ (2008a) Identification of novel toxicity-associated metabolites by metabolomics and mass isotopomer analysis of acetaminophen metabolism in wild-type and Cyp2e1-null mice. J Biol Chem 283:4543–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Shah YM, Morimura K et al. (2008b) Metabolomics reveals that hepatic stearoyl-CoA desaturase 1 downregulation exacerbates inflammation and acute colitis. Cell Metab 7:135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Zhou K, Chen X et al. (2014) Metabolomic analysis reveals metabolic changes caused by bisphenol A in rats. Toxicol Sci doi: 10.1093/toxsci/kfu016 [DOI] [PubMed]
- Cheng J, Shah YM, Ma XC et al. (2010) Therapeutic role of rifaximin in inflammatory bowel disease: clinical implication of human pregnane X Receptor activation. J Pharmacol Exp Ther 335:32–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Krausz KW, Tanaka N, Gonzalez FJ (2012) Chronic exposure to rifaximin causes hepatic steatosis in pregnane X receptor-humanized mice. Toxicol Sci 129:456–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Krausz KW, Li F, Ma XC, Gonzalez FJ (2013) CYP2E1-dependent elevation of serum cholesterol, triglycerides, and hepatic bile acids by isoniazid. Toxicol Appl Pharm 266:245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Fang ZZ, Kim JH, Krausz KW, Tanaka N, Chiang JY, Gonzalez FJ (2014) Intestinal CYP3A4 protects against lithocholic acid-induced hepatotoxicity in vitamin D receptor intestine-deficient mice. J Lipid Res 55:455–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas ME, Kinross J, Nicholson JK (2014) Metabolic phenotyping and systems biology approaches to understanding metabolic syndrome and fatty liver disease. Gastroenterology 146:46–62 [DOI] [PubMed] [Google Scholar]
- Duportet X, Aggio RBM, Carneiro S, Villas-Boas SG (2012) The biological interpretation of metabolomic data can be misled by the extraction method used. Metabolomics 8:410–421 [Google Scholar]
- Espina R, Yu LN, Wang JY et al. (2009) Nuclear magnetic resonance spectroscopy as a quantitative tool to determine the concentrations of biologically produced metabolites: implications in metabolites in safety testing. Chem Res Toxicol 22:299–310 [DOI] [PubMed] [Google Scholar]
- Evans CR, Karnovsky A, Kovach MA, Standiford TJ, Burant CF, Stringer KA (2013) Untargeted LC-MS metabolomics of bronchoalveolar lavage fluid differentiates acute respiratory distress syndrome from health. J Proteome Res doi: 10.1021/pr4007624 [DOI] [PMC free article] [PubMed]
- Fang ZZ, Zhang YY, Ge GB, Huo H, Liang SC, Yang L (2010) Time-dependent inhibition (TDI) of CYP3A4 and CYP2C9 by noscapine potentially explains clinical noscapine-warfarin interaction. Br J Clin Pharmacol 69:193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang ZZ, Krausz KW, Li F, Cheng J, Tanaka N, Gonzalez FJ (2012) Metabolic map and bioactivation of the antitumour drug noscapine. Br J Pharmacol 167:1271–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang ZZ, Krausz KW, Tanaka N et al. (2013) Metabolomics reveals trichloroacetate as a major contributor to trichloroethylene-induced metabolic alterations in mouse urine and serum. Arch Toxicol 87:1975–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang ZZ, Krausz KW, Nagaoka K et al. (2014) In vivo application of the pure aryl hydrocarbon receptor antagonist GNF-351 is limited to the gastrointestinal track. Br J Pharmacol 171:1735–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Roede JR, Orr M, Liang Y, Jones DP (2014) Integrated redox proteomics and metabolomics of mitochondria to identify mechanisms of Cd toxicity. Toxicol Sci 171:1735–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Dominguez R, Garcia-Barrera T, Gomez-Ariza JL (2014) Combination of metabolomic and phospholipid-profiling approaches for the study of Alzheimer’s disease. J Proteomics doi: 10.1016/j.jprot.2014.01.014 [DOI] [PubMed]
- Hong EJ, Levasseur MP, Dufour CR, Perry MC, Giguere V (2013) Loss of estrogen-related receptor a promotes hepatocarcinogenesis development via metabolic and inflammatory disturbances. Proc Natl Acad Sci USA 110:17975–17980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RS, Kucukosmanoglu A, de Haas M et al. (2013) ABCC6 pre-vents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc Natl Acad Sci USA 110:20206–20211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LM, Huang J, Wang YL, Tang HR (2012) Metabonomic analysis reveals the CCl4-Induced systems alterations for multiple rat organs. J Proteome Res 11:3848–3859 [DOI] [PubMed] [Google Scholar]
- Johnson CH, Gonzalez FJ (2012) Challenges and opportunities of metabolomics. J Cell Phys 227:2975–2981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH, Patterson AD, Idle JR, Gonzalez FJ (2012a) Xenobiotic metabolomics: major impact on the metabolome. Annu Rev Pharmacol 52:37–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH, Slanar O, Krausz KW et al. (2012b) Novel metabolites and roles for alpha-tocopherol in humans and mice discovered by mass spectrometry-based metabolomics. Am J Clin Nutr 96:818–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Yamaori S, Tanabe T et al. (2013) Implication of intestinal VDR deficiency in inflammatory bowel disease. Bba-Gen Subj 1830:2118–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane AN, Fan TWM, Bousamra M, Higashi RM, Yan J, Miller DM (2011) Stable isotope-resolved metabolomics (SIRM) in cancer research with clinical application to non-small cell lung cancer. OMICS 15:173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichtle AB, Nuoffer JM, Ceglarek U et al. (2012) Serum amino acid profiles and their alterations in colorectal cancer. Metabolomics 8:643–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Miao Y, Zhang LR, Neuenswander SA, Douglas JT, Ma XC (2011) Metabolomic analysis reveals novel isoniazid metabo-lites and hydrazones in human urine. Drug Metab Pharmacok 26:569–576 [DOI] [PubMed] [Google Scholar]
- Li F, Patterson AD, Krausz KW et al. (2012) Metabolomics reveals the metabolic map of procainamide in humans and mice. Biochem Pharmacol 83:1435–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Jiang CT, Krausz KW et al. (2013a) Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun 4:2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Lu J, Cheng J et al. (2013b) Human PXR modulates hepatotoxic-ity associated with rifampicin and isoniazid co-therapy. Nat Med 19:418–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Pang XY, Krausz KW et al. (2013c) Stable isotope- and mass spectrometry-based metabolomics as tools in drug metabolism: a study expanding tempol pharmacology. J Proteome Res 12:1369–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Patterson AD, Krausz KW et al. (2013d) Metabolomics reveals that tumor xenografts induce liver dysfunction. Mol Cell Proteomics 12:2126–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JS, Wang S, Wang MQ, Shi WX, Du XY, Sun CH (2013e) The toxicity of 3-chloropropane-1,2-dipalmitate in Wistar rats and a metabonomics analysis of rat urine by ultra-performance liquid chromatography-mass spectrometry. Chem-Biol Interact 206:337–345 [DOI] [PubMed] [Google Scholar]
- Li F, Lu J, Ma XC (2014a) CPY3A4-mediated α-hydroxyaldehyde formation in saquinavir metabolism. Drug Metab Dispos 42:213–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Li H, Jiang P, Liu X, Xu D, Wang F (2014b) Investigating the pathological processes of rhegmatogenous retinal detachment and proliferative vitreoretinopathy with metabolomics analysis. Mol BioSyst doi: 10.1039/c3mb70386j [DOI] [PubMed]
- Liu A, Krausz KW, Fang ZZ, Brocker C, Qu A, Gonzalez FJ (2014) Gemfibrozil disrupts lysophosphatidylcholine and bile acid homeostasis via PPARα and its relevance to hepatotoxicity. Arch Toxicol doi: 10.1007/s00204-013-1188-0 [DOI] [PMC free article] [PubMed]
- Lu XY, Hu B, Shao L et al. (2013) Integrated analysis of transcriptom-ics and metabonomics profiles in aflatoxin B1-induced hepato-toxicity in rat. Food Chem Toxicol 55:444–455 [DOI] [PubMed] [Google Scholar]
- Mahmoudian M, Rahimi-Moghaddam P (2009) The anti-cancer activity of noscapine: a review. Recent Pat Anti-Cancer 4:92–97 [DOI] [PubMed] [Google Scholar]
- Manna SK, Patterson AD, Yang QA et al. (2010) Identification of non-invasive biomarkers for alcohol-induced liver disease using uri-nary metabolomics and the Ppara-null mouse. J Proteome Res 9:4176–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna SK, Tanaka N, Krausz KW et al. (2014) Biomarkers of coordinate metabolic reprogramming in colorectal tumors in mice and humans. Gastroenterology doi: 10.1053/j.gastro.2014.01.017 [DOI] [PMC free article] [PubMed]
- Matsubara T, Tanaka N, Krausz KW et al. (2012) Metabolomics identifies an inflammatory cascade involved in dioxin- and diet-induced steatohepatitis. Cell Metab 16(5):634–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDunn JE, Li Z, Adam KP et al. (2013) Metabolomic signatures of aggressive prostate cancer. Prostate 73(14):1547–1560 [DOI] [PubMed] [Google Scholar]
- Menezes LF, Zhou F, Patterson AD et al. (2012) Network analysis of a Pkd1-mouse model of autosomal dominant polycystic kidney disease identifies HNF4α as a disease Modifier. PLoS Genet 8:e1003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlib AE (2008) Application of stable isotope-labeled compounds in metabolism and in metabolism-mediated toxicity studies. Chem Res Toxicol 21:1672–1689 [DOI] [PubMed] [Google Scholar]
- Patterson AD, Gonzalez FJ, Idle JR (2010) Xenobiotic metabolism: a view through the metabolometer. Chem Res Toxicol 23: 851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AD, Bonzo JA, Li F et al. (2011a) Metabolomics reveals attenuation of the SLC6A20 kidney transporter in nonhuman pri-mate and mouse models of type 2 diabetes mellitus. J Biol Chem 286(22):19511–19522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AD, Maurhofer O, Beyoglu D et al. (2011b) Aberrant lipid metabolism in hepatocellular carcinoma revealed by plasma metabolomics and lipid profiling. Cancer Res 71:6590–6600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AD, Shah YM, Matsubara T, Krausz KW, Gonzalez FJ (2012) Peroxisome proliferator-activated receptor α induction of uncoupling protein 2 protects against acetaminophen-induced liver toxicity. Hepatology 56:281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AD, Carlson BA, Li F et al. (2013) Disruption of thioredoxin reductase 1 protects Mice from acute acetaminophen-induced hepatotoxicity through enhanced NRF2 activity. Chem Res Toxi-col 26:1088–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb RS, Stumpf CL, Granger JH, Castro-Perez J, Haselden JN, Dear GJ (2003) Use of liquid chromatography/time-of-flight mass spectrometry and multivariate statistical analysis shows promise for the detection of drug metabolites in biological fluids. Rapid Commun Mass Spectrom 17:2632–2638 [DOI] [PubMed] [Google Scholar]
- Qiu YP, Cai GX, Su MM et al. (2010) Urinary metabonomic study on colorectal cancer. J Proteome Res 9:1627–1634 [DOI] [PubMed] [Google Scholar]
- Rizza S, Copetti M, Rossi C et al. (2014) Metabolomics signature improves the prediction of cardiovascular events in elderly sub-jects. Atherosclerosis 232:260–264 [DOI] [PubMed] [Google Scholar]
- Robertson DG (2005) Metabonomics in toxicology: a review. Toxicol Sci 85:809–822 [DOI] [PubMed] [Google Scholar]
- Rosenfeldt MT, O’Prey J, Morton JP et al. (2013) p53 status deter-mines the role of autophagy in pancreatic tumour development. Nature 504:296–300 [DOI] [PubMed] [Google Scholar]
- Schreiber S, Hampe J (2000) Genomics and inflammatory bowel dis-ease. Curr Opin Gastroenterol 16:297–305 [DOI] [PubMed] [Google Scholar]
- Schuler M, Muehlbauer P, Guzzie P, Eastmond DA (1999) Noscap-ine hydrochloride disrupts the mitotic spindle in mammalian cells and induces aneuploidy as well as polyploidy in cultured human lymphocytes. Mutagenesis 14:51–56 [DOI] [PubMed] [Google Scholar]
- Shen J, Yan L, Liu S, Ambrosone CB, Zhao H (2013) Plasma metabo-lomic profiles in breast cancer patients and healthy controls: by race and tumor receptor subtypes. Transl Oncol 6:757–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi XL, Yao D, Chen C (2012) Identification of N-acetyltaurine as a novel metabolite of ethanol through metabolomics-guided bio-chemical analysis. J Biol Chem 287:6336–6349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Murray IA, Tanos R et al. (2011) Identification of a high-affinity ligand that exhibits complete aryl hydrocarbon receptor antagonism. J Pharmacol Exp Ther 338:318–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JH, Kanathezhath B, Shenvi S et al. (2014) Thiol/redox metabo-lomic profiling implicates GSH dysregulation in early experimen-tal graft versus host disease. PLoS ONE 9(2):e88868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Matsubara T, Krausz KW, Patterson AD, Gonzalez FJ (2012) Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology 56:118–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tianjiao L, Shuai W, Xiansheng M et al. (2014) Metabolomics cou-pled with multivariate data and pathway analysis on potential biomarkers in gastric ulcer and intervention effects of Corydalis yanhusuo Alkaloid. PLoS ONE 9:e82499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda N, Yoshimura H (1981) Metabolic-fate of noscapine 3 fur-ther-studies on identification and determination of the metabo-lites. Xenobiotica 11:23–32 [DOI] [PubMed] [Google Scholar]
- Walker GS, Ryder TF, Sharma R, Smith EB, Freund A (2011) Vali-dation of isolated metabolites from drug metabolism studies as analytical standards by quantitative NMR. Drug Metab Dispos 39:433–440 [DOI] [PubMed] [Google Scholar]
- Wang TJ, Ngo D, Psychogios N et al. (2013) 2-Aminoadipic acid is a biomarker for diabetes risk. J Clin Invest 123:4309–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier RJ, Podolsky DK (2007) Unravelling the pathogenesis of inflammatory bowel disease. Nature 448:427–434 [DOI] [PubMed] [Google Scholar]
- Yao D, Shi XL, Wang L, Gosnell BA, Chen C (2013) Characteriza-tion of differential cocaine metabolism in mouse and rat through metabolomics-guided metabolite profiling. Drug Metab Dispos 41:79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin PY, Peter A, Franken H et al. (2013) Preanalytical aspects and sample quality assessment in metabolomics studies of human blood. Clin Chem 59:833–845 [DOI] [PubMed] [Google Scholar]
- Zhang YY, Li F, Patterson AD et al. (2012) Abcb11 deficiency induces cholestasis coupled to impaired β-fatty acid oxidation in mice. J Biol Chem 287:24784–24794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Seguin RP, Kunze KL, Zhang YY, Jeong H (2013a) Charac-terization of inhibition kinetics of (S)-warfarin hydroxylation by noscapine: implications in warfarin therapy. Drug Metab Dispos 41:2114–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lu C, Liu X et al. (2013b) Global and targeted metabolomics reveal that bupleurotoxin, a toxic type of polyacetylene, induces cerebral lesion by inhibiting GABA receptor in mice. J Proteome Res doi: 10.1021/pr400968c [DOI] [PubMed]
- Zhao X, Xu F, Qi B et al. (2014) Serum metabolomics study of polycystic ovary syndrome based on liquid chromatography-mass spectrometry. J Proteome Res 13:1101–1111 [DOI] [PubMed] [Google Scholar]


