Abstract
BACKGROUND:
Use of long-acting, highly effective contraception has the potential to improve women’s ability to avoid short interpregnancy intervals, which are associated with an increased risk of maternal morbidity and mortality, and preterm delivery. In Uganda, contraceptive implants are not routinely available during the immediate postpartum period.
OBJECTIVE:
The purpose of this study was to compare the proportion of women using levonorgestrel contraceptive implants at 6 months after delivery in women randomized to immediate or delayed insertion.
STUDY DESIGN:
This was a randomized controlled trial among women in Kampala, Uganda. Women who desired contraceptive implants were randomly assigned to insertion of a 2-rod contraceptive implant system containing 75 mg of levonorgestrel immediately following delivery (within 5 days of delivery and before discharge from the hospital) or delayed insertion (6 weeks postpartum). The primary outcome was implant utilization at 6 months postpartum.
RESULTS:
From June to October 2015, 205 women were randomized, 103 to the immediate group and 102 to the delayed group. Ninety-three percent completed the 6 month follow-up visit. At 6 months, implant use was higher in the immediate group compared with the delayed group (97% vs 68%; P < .001), as was the use of any highly effective contraceptive (98% vs 81%; P = .001). Women in the immediate group were more satisfied with the timing of implant placement. If given the choice, 81% of women in the immediate group and 63% of women in the delayed group would choose the same timing of placement again (P = .01). There were no serious adverse events in either group.
CONCLUSION:
Offering women the option of initiating contraceptive implants in the immediate postpartum period has the potential to increase contraceptive utilization, decrease unwanted pregnancies, prevent short interpregnancy intervals, and help women achieve their reproductive goals.
Keywords: contraception, immediate postpartum contraception, le-vonorgestrel contraceptive implants, postpartum
Contraception is rarely offered immediately after delivery in low-resource settings, like Uganda, so many women will not access contraception at all. Uganda has the third highest unmet need for contraception in the world1 and one of the highest excess fertility rates in the world.2
Contraceptive implants are safe, highly effective, long acting, and reversible yet are not routinely available during the immediate postpartum period in Uganda.3 The Ugandan Policy on Family Planning does not currently support immediate postpartum implant use for breast-feeding women within 6 weeks of delivery.4 In a qualitative study among pregnant women in Uganda, participants expressed interest in long-acting, reversible contraceptives and a desire for family planning to space pregnancies.5
Globally, postpartum implant placement has traditionally occurred at a postpartum visit 6 weeks after delivery.6 This timing of the postpartum visit, and initiation of postpartum contraception, is based on historical precedent and does not have a clinical rationale.6 In fact, non—breast-feeding women have been shown to ovulate as early as 25 days postpartum,7 and 30% will have ovulated by 8 weeks.6 Studies in Uganda show that 22—58% of women resume sexual activity by 6 weeks after birth.8-10 The median time to contraceptive use among postpartum women in Uganda is estimated to be 19 months after resumption of sexual intercourse.11
Implants are offered immediately postpartum in many US hospitals.12-15 Waiting 6 weeks to initiate contraception puts women at risk for unintended pregnancy and short interpregnancy intervals. Short interpregnancy intervals of less than 18—24 months are associated with an increased risk of maternal morbidity and mortality,16 preterm delivery, and low-birthweight infants.17
We are not aware of any studies to date evaluating immediate postpartum implant insertion among women in Africa. The purpose of this study was to evaluate the effect of immediate postpartum levonorgestrel contraceptive implant placement compared with contraceptive implant placement at 6 weeks postpartum on 6 month utilization among women in Uganda.
Materials and Methods
This was a randomized controlled trial conducted between June 2015 and May 2016 at Mulago Hospital in Kampala, Uganda. Mulago Hospital is the national teaching and referral hospital. There are approximately 32,000 deliveries per year at Mulago Hospital, of which approximately 22% are cesarean deliveries.18
All women delivering at Mulago hospital are offered comprehensive contraceptive counseling by nurse midwives before discharge. While permanent sterilization is available in the immediate postpartum period, copper intrauterine devices (IUDs) are the only reversible method of contraception available for initiation in the immediate postpartum period. Women are counseled to initiate all other methods of contraception when they return for their routine postpartum visit approximately 6 weeks after delivery.
We recruited women who wanted the contraceptive implant after receiving comprehensive contraceptive counseling, and they were assessed for eligibility. Patients who were 18 years old and older, who spoke English or Luganda, had a vaginal or cesarean delivery at Mulago Hospital within the past 5 days, and could demonstrate that they had a working cellular telephone were eligible for entry into the study. We excluded women who had a medical contraindication to progestin-only contraceptives or were taking Efavirenz medication as part of their HIV antiretroviral treatment.
Women were randomized to immediate insertion (within 5 days of delivery and before leaving the hospital) or delayed insertion (at the routine 6 week postpartum visit, the current standard of care in Uganda).
All devices were placed by nurses with training and experience in contraceptive implant placement. The device used is a commercially available 2 rod contraceptive implant system. Each rod is 2.5 mm × 43 mm and contains 75 mg of the progestin, levonorgestrel. The implants are approved for 5 years of continuous use.19
All women received routine postpartum care according to the standard of care. Participants in both groups were scheduled for a routine 6 week postpartum visit. There was no additional 6 week study visit scheduled, but women who returned for their 6 week visit were asked to complete a brief questionnaire. Clinic attendance records were also used to verify whether a woman returned for her 6 week visit.
Women randomized to delayed insertion had implants placed at the clinic when they came for their postpartum follow-up visit. Prior to implant placement, women in the delayed group were screened for pregnancy with a urine pregnancy test. In addition, they were asked about unprotected intercourse and frequency of breast-feeding. Any woman considered to be at risk for pregnancy was counseled about the possibility and offered a home pregnancy test to take in 2 weeks.
All participants were contacted by telephone at 3 months after delivery. In-person interviews were completed at 6 months after delivery. Multiple attempts were made to assist the participant to come in person. If the participant was unable to return because of extenuating circumstances, then the interview was conducted by phone. Attempts were made to reach the participants until 4 months past the delivery date for the 3 month survey and up to 8 months past the delivery date for the 6 month survey.
We excluded from analysis any surveys inadvertently conducted after these time frames. Both follow-up surveys assessed whether participants were using the implant or another method of contraception. Modern methods of contraception were defined as condoms, combined hormonal contraceptives, progestin-only contraceptives (including implants, injections, pills, and IUDs), copper IUDs, sterilization, and lactational amenorrhea at less than 6 months according to the World Health Organization definition.20
Highly effective methods of contraception were defined as methods with typical-use pregnancy rates of <10% in the first year (World Health Organization tier 1 and 2 methods), which included combined hormonal contraception, progestin-only pills, implants, injections, IUDs, and sterilization.21
Additionally, we assessed bleeding patterns by asking participants how many days after delivery they had bleeding at least once per day (days of lochia), how many days of bleeding in the last month required a pad or a tampon, and how many days participants had bleeding in the last month that did not require a pad or tampon according to the standardized recommendations for defining bleeding in contraceptive trials.22
We measured satisfaction by asking participants how satisfied they were with their current method overall based on a 5 point Likert scale. If they had an implant placed, we asked whether they would choose the same timing of initiation again or earlier or later. To ascertain adverse events, we asked participants whether they have been diagnosed with bleeding or infection at implant site, deep implant placement, pregnancy, blood clots in the lungs or legs, or any other new medical condition. A nurse evaluated the implant site at the follow-up visit.
We estimated 100% of women in the immediate group would have an implant placed. Based on the results of a previous trial of postplacental IUD placement at Mulago Hospital, we estimated that 60% of women in the delayed group would return for a postpartum visit and have an implant placed.23 We opined that a ≥20% difference between the 2 groups is clinically meaningful. To detect this effect with 80% power assuming a 2-sided alpha of 0.05 and assuming a 20% discontinuation rate, we estimated that 184 women (92 women in each group) would be required. We planned to recruit a total of 204 participants to account for 10% loss to follow-up.
Randomization was performed using blocks of 4 and 6, which were varied randomly. Sequential, numbered, opaque envelopes containing a card with computer-generated assignment information were prepared by a statistician not involved in randomization. The envelopes were opened in consecutive order.
During follow-up, attempts were made to blind the research assistant collecting data to the group to which the participant had been randomized. There were no questions on the 3 month or 6 month study instruments that directly asked when the participant received her implant.
The primary outcome was the proportion of women using a contraceptive implant at 6 months after delivery. Secondary outcomes included utilization of the implant at 3 months, postpartum bleeding, side effects and negative outcomes, and satisfaction. Bivariate comparisons were analyzed using a χ2 test or a Fisher exact test for categorical variables and a Student t test or a Mann-Whitney test for continuous variables as appropriate. The primary outcome was evaluated per intent-to-treat analysis.
Sensitivity analyses were carried out to assess the potential impact of loss to follow-up on the primary outcome.
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of California, San Francisco. Data analyses were completed using STATA SE software version 13.1 (Stata, College Station, TX).
The institutional review boards of the University of California, San Francisco, and Mulago Hospital and the Ugandan National Council for Science and Technology approved the protocol. All participants provided written consent prior to enrollment. The Consolidated Standards of Reporting Trials guidelines were used for reporting.24
Results
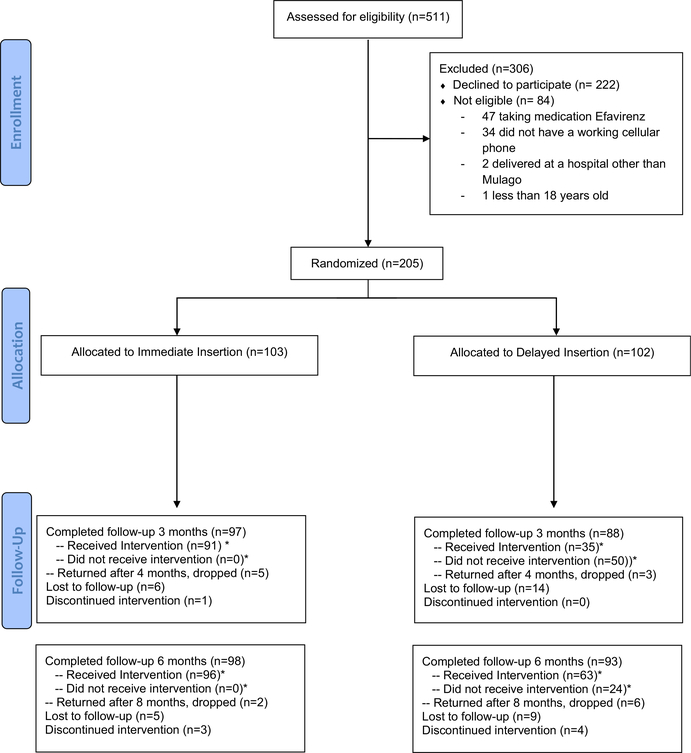
Enrollment occurred between June 2015 and October 2015. During this time, approximately 7500 women delivered at Mulago Hospital. Five hundred eleven women who indicated that they planned to use the contraceptive implant postpartum (7%) were screened for enrollment: 205 were randomized (103 to the immediate group and 102 to the delayed group) (Figure 1). Protocol violations were identified in 3 participants after randomization (1 woman was randomized longer than 5 days since delivery and 2 did not have working cellular phones), but they were included in the analysis per intention to treat.
FIGURE 1:
CONSORT flow diagram
*Included in intention-to-treat analysis
CONSORT, Consolidated Standards of Reporting Trials.
Averbach et al. Immediate initiation of postpartum contraceptive implants. Am J Obstet Gynecol 2017.
By 8 weeks postpartum, 52 women (25%) (23 in the immediate group [22%] and 29 in the delayed group [28%]; P = .44) had returned to Mulago for the scheduled postpartum follow-up visit. One hundred eighty-five women (90%) completed the 3 month follow-up visit and 191 women (93%) completed the 6 month follow-up visit. There was no difference in follow-up between groups at 3 or 6 months.
Women were on average 27 years old (SD, 5.4). Most women were married (48%) or in a relationship (49%) (Table 1). The average age of participants’ male partners was 33 years (SD, 7.9). The majority of women completed some secondary school (56%) and had 2 or more living children (80%). Women reported an average age at first pregnancy of 19 (SD, 3.2). Thirty-three percent of women had a cesarean delivery and 3% of women delivered multiples. Seven percent of women reported previously using the implant for contraception. Women traveled, on average, 72 minutes to seek care at Mulago Hospital (SD, 41.8).
Table 1.
Demographics and baseline characteristics of women randomized to delayed and immediate contraceptive implant initiation
| Variables | Total (n = 205), n, % | Immediate start (n = 103), n, % | Delayed start (n = 102), n, % |
|---|---|---|---|
| Age, y (mean/SD) | 26.9 (5.4) | 26.3 (5.2) | 27.5 (5.5) |
| Marital status | |||
| Single | 4 (2.0) | 3 (2.9) | 1 (1.0) |
| Married | 98 (47.8) | 52 (50.5) | 46(45.1) |
| Divorced/separated/widowed | 3(1.5) | 0(0) | 3 (2.9) |
| In a relationship | 100(48.8) | 48 (4.6) | 52 (51.0) |
| Age of partner, y (mean/SD)a | 32.9 (7.9) | 32.4 (8.3) | 33.4 (7.5) |
| Education completed | |||
| No formal schooling | 6 (2.9) | 2(1.9) | 4 (3.9) |
| Some primary school | 64 (31.2) | 33 (32.0) | 31 (30.4) |
| Some secondary school | 115 (56.1) | 60 (58.3) | 55 (53.9) |
| Some university | 20 (9.8) | 8 (7.8) | 12 (11.8) |
| Number of living children | |||
| 0 | 29 (14.1) | 16(15.5) | 13 (12.7) |
| 1 | 13 (6.3) | 8 (7.8) | 5 (4.9) |
| 2 | 52 (25.4) | 29 (28.2) | 23 (22.5) |
| ≥3 | 111 (54.1) | 50 (48.5) | 61 (59.8) |
| Age at first pregnancy, y (mean/SD) | 18.9(3.2) | 18.7(3.0) | 19.1 (3.3) |
| Cesarean delivery | 68 (33.2) | 37 (35.9) | 31 (30.4) |
| Delivery of multiplesb | 7 (3.4) | 1 (1.0) | 6 (5.9) |
| Prior implant use | 15 (7.3) | 5 (4.9) | 10(9.9) |
| Time traveled to Mulago, min, (mean/SD) | 72.4 (41.8) | 69.5 (38.7) | 75.4 (44.6) |
Seventeen participants reported age of partner was unknown
Six sets of twins and 1 set of triplets.
Averbach et al. Immediate initiation of postpartum contraceptive implants. Am J Obstet Gynecol 2017.
At 3 and 6 months, implant use was higher in the immediate group compared with the delayed group (99% immediate vs 41% delayed [P < .001]; and 97% immediate vs 68% delayed [P < .001] as was the use of any modern contraceptive at 3 and 6 months [100% immediate vs 52%, delayed [P < .001]; and 99% immediate vs 86% delayed [P = .001]) (Table 2).
Table 2.
Utilization after immediate vs delayed postpartum initiation of contraceptive implants at 3 and 6 months (intent to treat)
| Variables | Immediate | Delayed | Risk difference (95% Cl) | P value |
|---|---|---|---|---|
| 3 months postpartum | (n = 91),% | (n = 85, %) | ||
| Using implant at 3 mo | 90 (98.9) | 35 (41.2) | 57.7 (47.05—68.40) | < .001 |
| Using implant or other modern method at 3 monthsa | 91 (100) | 44 (51.8) | 48.2 (37.61—58.86) | < .001 |
| Using implant or other highly effective method at 3 monthsb | 91 (100) | 42 (49.4) | 50.6 (39.96—61.22) | < .001 |
| 6 months postpartum | (n = 96), % | (n = 87), % | ||
| Using implant at 6 mo | 93 (96.9) | 59 (67.8) | 29.1 (18.64—39.47) | < .001 |
| Using implant or other modern method at 6 moa | 95 (99.0) | 75 (86.2) | 12.8 (5.22—20.28) | .001 |
| Using implant or other highly effective method at 6 mob | 94 (97.9) | 70 (80.5) | 17.4 (8.65—26.27) | .001 |
| Pregnancies at 6 mo | 0(0) | 2 (2.3) | 2.3 (0.01—5.58) | .230 |
Data are presented as n (percentage) analyzed with a X2 or Fisher exact test.
CI, confidence interval; IUD, intrauterine device.
Modern methods include implant pill, injection, IUDs, condoms, sterilization (and lactational amenorrhea at 3 months)
Highly effective methods include implant, pill, injection, IUDs, and sterilization.
Averbach et al. Immediate initiation of postpartum contraceptive implants. Am J Obstet Gynecol 2017.
Women in the immediate group were also more likely to be using a highly effective method of contraception at 3 and 6 months (100% immediate vs 50% delayed [P < .001]; and 98% immediate vs 80% delayed [P = .001]). One woman had a single-rod etonogestrel implant placed at an outside clinic. She was considered an implant user for the purposes of the analysis. Twenty-four women (23.5%) in the delayed group returned for implant placement between the 3 month phone call and the 6 month follow-up visit (Figure 1). Women who requested an implant at the 6 month visit were not considered to be implant users at 6 months for the purpose of the analysis.
Sensitivity analyses assuming all participants lost to follow-up were implant users or all were non—implant users did not change the overall findings (data not shown). A last observation carried forward analysis assuming all participants who were observed to be using an implant at the last known visit are still using an implant, and all participants who were not using an implant at last known visit are still not using an implant, did not change the overall findings (data not shown).
Two women became pregnant during study follow-up. Both women presented for delayed insertion and had a negative pregnancy test (one at 84 days postpartum and the other at 46 days postpartum). Both were diagnosed with a pregnancy within a few weeks of insertion and were dated by ultrasound to have pregnancies that predated the contraceptive action of the implant. One woman carried her pregnancy to term and the other had an abortion (she did not disclose whether the abortion was spontaneous or induced).
Among women who received an implant, satisfaction with the method was high overall, with 91% of 90 women in the immediate group reporting being satisfied or very satisfied with the method compared with 88% of 35 women in the delayed group at 3 months (P = .73) and 94% of 93 women in the immediate group compared with 97% of 59 women in the delayed group at 6 months (P = .53) (Table 3). However, women in the immediate group were more satisfied with the timing of implant placement.
Table 3.
Bleeding and satisfaction among implant users among women with immediate vs delayed postpartum initiation of contraceptive implants
| Variables | Immediate | Delayed | P value |
|---|---|---|---|
| Implant users, 3 mo postpartum | (n = 90), % | (n = 35), % | |
| Overall satisfaction with implant | |||
| Very or somewhat satisfied | 82 (91.1) | 30 (88.2) | .730 |
| Satisfaction timing of placement | |||
| Would choose same timing | 81 (90.0) | 18(51.4) | .001 |
| Bleeding days in last month | |||
| None | 71 (78.9) | 25 (71.4) | .260a |
| 1 −6 d/mo | 14(15.6) | 6(17.1) | |
| >6 d/mo | 4 (4.4) | 4 (11.4) | |
| Implant users, 6 mo postpartum | (n = 93), % | (n = 59), % | |
| Overall satisfaction with implant | |||
| Very or somewhat satisfied | 89 (93.7) | 60 (96.7) | .530 |
| Satisfaction timing of placement | |||
| Would choose same timing | 77 (82.8) | 40 (67.8) | .030 |
| Bleeding days in last month | |||
| None | 75 (80.7) | 45 (76.3) | .710a |
| 1 −6 d/mo | 14(15.1) | 12 (20.3) | |
| >6 d/mo | 4 (4.3) | 2 (3.4) | |
Fisher exact test.
Averbach et al. Immediate initiation of postpartum contraceptive implants. Am J Obstet Gynecol 2017.
If given the choice, 90% of women in the immediate group and 51% of women in the delayed group would choose the same timing of placement again at 3 months (P = .001), and 83% of women in the immediate group and 68% of women in the delayed group would choose the same timing of placement again at 6 months (P = .03).
At 6 weeks postpartum, there was no difference in mean days of lochia between women who received the implant immediately after delivery and those who did not receive the implant (13.2 days in both groups, P = .95; data not shown). Among women who had received an implant by 3 months, the majority of women in both groups reported no bleeding days in the month prior to the 3 month visit (79% immediate vs 71% delayed; P = .26) and 6 month visit (81% immediate vs 76% delayed; P = .71) (Table 3).
Seven women had their implants removed during study follow-up (3 [3%] in the immediate group and 4 [4%] in the delayed group, P = .69). The reasons reported for implant removal included arm pain (2 women), early undiagnosed pregnancy at the time the implant was placed (2 women), bothersome bleeding (1 woman), abdominal pain and decreased libido (1 woman), and the recommendation of her primary care provider after a new diagnosis of diabetes (1 woman). There were no serious adverse events during study follow-up.
Comment
We found that contraceptive implant utilization at 6 months was significantly improved by providing implants immediately following delivery among women in Uganda. In addition, women were more satisfied with the timing of implant placement when the implant was placed immediately after delivery.
To the best of our knowledge, this study was the first randomized trial of immediate postpartum implant placement in Africa. The results of this study suggest that increasing access to long-acting reversible contraceptive (LARC) methods immediately postpartum, especially in low-resource settings, will improve utilization. Although Uganda is a geographically restrictive population, this study has application to a larger population of similar low-resource settings across Africa and even globally. Postpartum LARC programs have the potential to allow women to achieve desired birth spacing and decrease maternal and infant morbidity and mortality. Postpartum LARC programs have also been shown to be cost effective in the US setting.25
The effect of immediate postpartum provision of contraceptive implants on postpartum utilization may be even greater than what was seen in our study. We found that 68% of women in the delayed, or standard of care group, were using implants at 6 months compared with 97% in the immediate group. This is likely a conservative estimate of effect because of the clinical trial setting, which enrolled only women willing to follow up, provided reimbursement for travel, and provided the opportunity for participants to talk on the phone to a study nurse about their contraceptive plan at 3 months postpartum.
At 8 weeks postpartum, only 25% of women had returned to Mulago for a routine scheduled postpartum visit, and by 3 months postpartum, only 41% of women in the delayed group were using an implant. Soon after the 3 month study call, a number of participants in the delayed group returned for implant placement. In observational studies of women in Uganda, only 25—28% report using contraception between 3 and 12 months postpartum.26,27
Our findings are consistent with observational studies in the United States where continuation rates after immediate postpartum placement of contraceptive implants have been shown to be <95% after 6 months.12 Our findings are similar to a small randomized trial conducted in the United States among adolescents, which showed that 70% of women in the delayed group were using implants at 3 months compared with 92% in the immediate group (P = .02).28
Our study has several strengths. While another observational study has shown an association between immediate postpartum insertion of contraceptive implants and implant utilization,12 this is a randomized trial demonstrating that association. It can be difficult to know whether the differential use of implants in the observational study was due to the intervention itself or other participant characteristics associated with choosing immediate insertion. Because of the randomization, our study highlights the importance of the intervention itself. Another strength of our study was the low loss to follow-up, which minimizes the risk of bias in our findings.
Our study has several limitations. First, it was not feasible to design this study to evaluate the effect of immediate postpartum provision of contraceptive implants on interpregnancy interval: the outcome with clear, demonstrated public health benefits. There were 2 pregnancies in our delayed group and none in the immediate group. Still our study was not powered to detect meaningful differences in pregnancy and our follow-up was not long enough to do so. We would expect the majority of early repeat pregnancies to occur after 6 months,12 especially among a population of women known to have high exclusive breast-feeding rates in the first few months.2 Utilization of effective postpartum contraception has been shown to decrease short interpregnancy in-tervals,29 which are associated with increased maternal and infant morbidity and mortality.16,30 Implant utilization is likely a reasonable surrogate outcome for avoidance of early repeat pregnancy.
In summary, offering women the option of initiating contraceptive implants in the immediate postpartum period in low-resource settings like Uganda has the potential to decrease unwanted pregnancies, prevent short interpregnancy intervals, and help women achieve their reproductive goals. Health ministries should focus on expanding policies and protocols that facilitate immediate postpartum insertion of LARC.
Acknowledgment
This study had a clinical trial registration number of NCT02341027 (ClinicalTrials.gov, www.clinicaltrials.gov).
This study was supported by the Society of Family Planning Research Fund.
Footnotes
The authors report no conflict of interest.
Contributor Information
Sarah Averbach, Department of Obstetrics, Gynecology, and Reproductive Sciences, University of California, San Francisco, San Francisco, CA.
Othman Kakaire, Department of Obstetrics and Gynecology, Makerere University College of Health Sciences, Kampala, Uganda.
Herbert Kayiga, Department of Obstetrics and Gynecology, Makerere University College of Health Sciences, Kampala, Uganda.
Felicia Lester, Department of Obstetrics, Gynecology, and Reproductive Sciences, University of California, San Francisco, San Francisco, CA.
Abby Sokoloff, Department of Obstetrics, Gynecology, and Reproductive Sciences, University of California, San Francisco, San Francisco, CA.
Josaphat Byamugisha, Department of Obstetrics and Gynecology, Makerere University College of Health Sciences, Kampala, Uganda.
Christine Dehlendorf, Department of Obstetrics, Gynecology, and Reproductive Sciences, University of California, San Francisco, San Francisco, CA.
Jody Steinauer, Department of Obstetrics, Gynecology, and Reproductive Sciences, University of California, San Francisco, San Francisco, CA.
References
- 1.Alkema L, Kantorova V, Menozzi C, Biddlecom A. National, regional, and global rates and trends in contraceptive prevalence and unmet need for family planning between 1990 and 2015: a systematic and comprehensive analysis. Lancet 2013;381:1642–52. [DOI] [PubMed] [Google Scholar]
- 2.Uganda Bureau of Statistics and ICF International. Uganda Demographic and health survey, 2013. Kampala, Uganda: Uganda Bureau of Statistics; and Calverton, MD: ICF International. [Google Scholar]
- 3.Jacobstein R, Polis CB. Progestin-only contraception: injectables and implants. Best Pract Res Clin Obstet Gynaecol 2014;28: 795–806. [DOI] [PubMed] [Google Scholar]
- 4.Ministry of Health, Uganda. The national policy guidelines and service standards for sexual and reproductive health and rights, 4th ed. Kampala, Uganda: Reproductive Health Division, Ministry of Health, Uganda; 2016. [Google Scholar]
- 5.Morse JE, Rowen TS, Steinauer J, Byamugisha J, Kakaire O. A qualitative assessment of Ugandan women’s perceptions and knowledge of contraception. Int J Gynaecol Obstet 2014;124:30–3. [DOI] [PubMed] [Google Scholar]
- 6.Speroff L, Mishell DR Jr. The postpartum visit: it’s time for a change in order to optimally initiate contraception. Contraception 2008;78:90–8. [DOI] [PubMed] [Google Scholar]
- 7.Gray RH, Campbell OM, Zacur HA, Labbok MH, MacRae SL. Postpartum return of ovarian activity in nonbreastfeeding women monitored by urinary assays. J Clin Endocrinol Metab 1987;64:645–50. [DOI] [PubMed] [Google Scholar]
- 8.Alum AC, Kizza IB, Osingada CP, Katende G, Kaye DK. Factors associated with early resumption of sexual intercourse among postnatal women in Uganda. Reprod Health 2015;12:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osinde MO, Kaye DK, Kakaire O. Influence of HIV infection on women’s resumption of sexual intercourse and use of contraception in the postpartum period in rural Uganda. Int J Gynaecol Obstet 2012;116:171–2. [DOI] [PubMed] [Google Scholar]
- 10.Odar E, Wandabwa J, Kiondo P. Sexual practices of women within six months of childbirth in Mulago Hospital, Uganda. Afr Health Sci 2003;3:117–23. [PMC free article] [PubMed] [Google Scholar]
- 11.Wamala R, Kabagenyi A, Kasasa S. Predictors of time-to-contraceptive use from resumption of sexual intercourse after birth among women in Uganda. Int J of Pop Rese 2017;2017:1–12. [Google Scholar]
- 12.Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol 2012;206:481 e1–7. [DOI] [PubMed] [Google Scholar]
- 13.Ireland LD, Goyal V, Raker CA, Murray A, Allen RH. The effect of immediate postpartum compared to delayed postpartum and interval etonogestrel contraceptive implant insertion on removal rates for bleeding. Contraception 2014;90:253–8. [DOI] [PubMed] [Google Scholar]
- 14.Wilson S, Tennant C, Sammel MD, Schreiber C. Immediate postpartum etonogestrel implant: a contraception option with long-term continuation. Contraception 2014;90:259–64. [DOI] [PubMed] [Google Scholar]
- 15.Gurtcheff SE, Turok DK, Stoddard G, Murphy PA, Gibson M, Jones KP. Lactogenesis after early postpartum use of the contraceptive implant: a randomized controlled trial. Obstet Gynecol 2011;117:1114–21. [DOI] [PubMed] [Google Scholar]
- 16.Conde-Agudelo A, Belizan JM. Maternal morbidity and mortality associated with interpregnancy interval: cross sectional study. BMJ 2000;321:1255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFranco E, Ehrlich S, Muglia L. Influence of interpregnancy interval on birth timing. BJOG 2014;121:1633–40. [DOI] [PubMed] [Google Scholar]
- 18.Autry AM, Knight S, Lester F, et al. Teaching surgical skills using video internet communication in a resource-limited setting. Obstet Gynecol 2013;122:127–31. [DOI] [PubMed] [Google Scholar]
- 19.Jadelle [package insert]. Bayer; December 2016. Available at: accessdata.fda.gov. Accessed May 11,2017.
- 20.World Health Organization. Fact Sheet 1—Family planning/contraception. 2015. Available at: http://who.int/mediacentre/factsheets/fs351/en/. Accessed May 5, 2016.
- 21.Hatcher R, Trussell J, Nelson A, et al. Contraceptive technology, 20th ed. New York: Ardent Media; 2011. [Google Scholar]
- 22.Mishell DR Jr, Guillebaud J, Westhoff C, et al. Recommendations for standardization of data collection and analysis of bleeding in combined hormone contraceptive trials. Contraception 2007;75:11–5. [DOI] [PubMed] [Google Scholar]
- 23.Lester F, Kakaire O, Byamugisha J, Averbach S, Fortin J, Goldberg A. Intracesarean insertion of the Copper T380A versus 6 weeks post-cesarean: a randomized clinical trial. Contraception 2014;91:198–203. [DOI] [PubMed] [Google Scholar]
- 24.Schulz KF, Altman DG, Moher D; CONSORT Gtoup. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han L, Teal SB, Sheeder J, Tocce K. Preventing repeat pregnancy in adolescents: is immediate postpartum insertion of the contraceptive implant cost effective? Am J Obstet Gynecol 2014;211:24.e1–7. [DOI] [PubMed] [Google Scholar]
- 26.Sileo KM, Wanyenze RK, Lule H, Kiene SM. Determinants of family planning service uptake and use of contraceptives among postpartum women in rural Uganda. Int J Public Health 2015;60:987–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutaremwa G, Kabagenyi A, Wandera SO, Jhamba T, Akiror E, Nviiri HL. Predictors of modern contraceptive use during the postpartum period among women in Uganda: a population-based cross sectional study. BMC Public Health 2015;15:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryant AG, Bauer AE, Stuart GS, et al. Eto-nogestrel-releasing contraceptive implant for postpartum adolescents: a randomized controlled trial. J Pediatr Adolesc Gynecol 2016;16:30135–8. [DOI] [PubMed] [Google Scholar]
- 29.Thiel de Bocanegra H, Chang R, Howell M, Darney P. Interpregnancy intervals: impact of postpartum contraceptive effectiveness and coverage. Am J Obstet Gynecol 2014;210:311 e1–8. [DOI] [PubMed] [Google Scholar]
- 30.Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA 2006;295:1809–23. [DOI] [PubMed] [Google Scholar]