Abstract
There are many theories about the mechanisms of associations between hypothalamic-pituitary-adrenal (HPA) function (indexed by cortisol) and substance use. However, the potential for genes that contribute to both HPA function and substance use to confound the association (e.g., genetic confounding) has largely been ignored. We explore the potential role of genetics in cortisol-substance use associations, build a conceptual framework placing theories and mechanisms for how cortisol and substance use are related into a developmental progression, and develop new hypotheses based on our findings. We conclude that the relationship between cortisol function and substance use is complex, occurs at multiple levels of analysis, and is bidirectional at multiple phases of the substance use progression. Additionally, there is potential for genetic confounding in cortisol-substance use associations, and thus a need for genetically informed designs to investigate how and why cortisol function is associated with substance use phenotypes from initiation through disorder. Gene-environment interplay and developmental context are likely to impact the effectiveness of prevention and intervention efforts to reduce substance use problems.
Keywords: cortisol, substance use, development, genetic, gene enrichment
Substance use costs the American public more than $740 billion/year (National Institute on Drug Abuse [NIDA], 2017). Addiction has its origins in adolescence: 90% of those addicted began using during adolescence, and over $80 billion/year is spent on underage drinking and juvenile justice costs related to adolescent substance use (The National Center on Addiction and Substance Abuse [CASA], 2011). Understanding biological mechanisms underlying the progression of substance use from initiation through disorder is of key importance for prevention and intervention efforts, as substance use disorders are biologically-based (e.g., Conrod and Nikolaou, 2016; Grunberg, 1994; Kreek et al., 2005). One important biological mechanism often studied in relation to substance use is cortisol reactivity to environmental cues. The steroid hormone cortisol has been a major focus of research in biological and psychological sciences because of its important regulatory role in maintaining homeostasis in the face of stressors (Del Giudice et al., 2011; Doom and Gunnar, 2013; Gunnar and Quevedo, 2007). Cortisol reactivity to environmental stressors has been linked with emotional and behavioral problems across the lifespan that are risk factors for developing substance use problems (Alink et al., 2008; Dickerson and Kemeny, 2004; Doom and Gunnar, 2013; Marceau et al., 2015). Generally, decreased stress reactivity has also been directly linked to substance use and related phenotypes at many stages of the progression to substance use disorder (see, e.g., Lovallo, 2006; Sinha, 2011; Stephens and Wand, 2012 for review), with several theoretical explanations for these associations (detailed below). The purpose of this review is to integrate theory and hypotheses about the mechanisms of association between cortisol reactivity to environmental cues and substance use, leveraging known information about the genes associated with each phenotype to clarify potential mechanisms of association.
Cortisol Function
We begin with a brief overview of the physiology and molecular mechanisms of cortisol, focusing on responsivity to stress, as this aspect of cortisol function (as opposed to the diurnal rhythm or awakening response) is among the more frequently examined and theorized aspects of cortisol function in relation to substance use (Koob and Kreek, 2007; Stephens and Wand, 2012). Cortisol is a steroid hormone end-product of the hypothalamic-pituitary-adrenal (HPA) axis, which is a major component of the stress response system (Gunnar and Quevedo, 2007). Cortisol is released at the culmination of a hormonal cascade beginning in the limbic system with the hypothalamus, through several intermediary agents including corticotrophin-releasing hormone (CRH) and binding globulins in the blood (Perogamvros et al., 2012), to the adrenal gland. More specifically, when a stressor is experienced, CRH is released from the hypothalamus, which stimulates the release of adrenocorticotropin hormone (ACTH) from the anterior pituitary. ACTH travels through the bloodstream to bind G-coupled, melanocortin-2 (MC2R) receptors in the adrenal gland, triggering a cascade that releases cortisol into the bloodstream (Gunnar and Quevedo, 2007). Cortisol ultimately crosses the blood-brain barrier and binds to glucocorticoid and mineralocorticoid receptors in the brain, including in the same regions that begin the cascade leading to the release of cortisol (Nelson et al., 2005). This effects the suppression of the release of CRH and ACTH and the subsequent halt to further cortisol production through a negative feedback loop (Sapolsky, 2003; Schmidt-Reinwald et al., 1999). Thus, the brain is a major target organ for cortisol, and through binding to glucocorticoid and mineralocorticoid receptors found in many brain regions, cortisol levels impact a wide variety of brain functions implicated in substance use and related phenotypes (Shirtcliff et al., 2009; Stephens and Wand, 2012; Uchoa et al., 2014). Thus, when we refer to ‘cortisol function’ throughout this theoretical integration, we are referring to the often assumed action of cortisol on relevant brain regions through binding to glucocorticoid receptors (measured most frequently through levels and changes of circulating cortisol in saliva, serum, or blood).
Overview of Conceptual Model
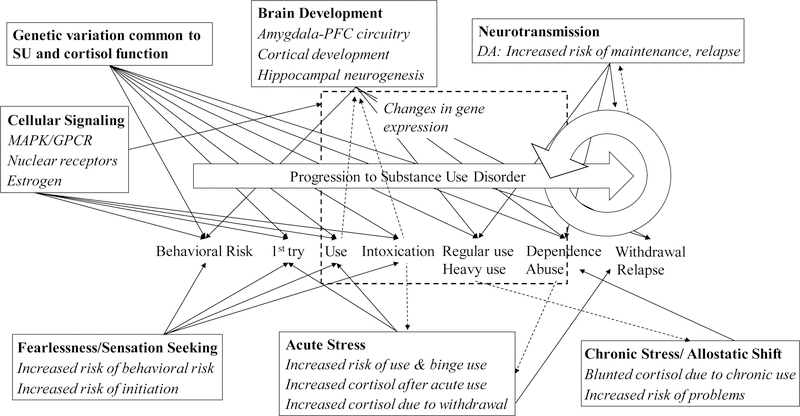
Substance use disorder is defined as the recurrent use of alcohol and/or drugs (e.g., tobacco, cannabis, stimulants, hallucinogens, opioids) that cause clinically and functionally significant impairment (e.g., impaired control, social impairment, risky use), and is classified as mild, moderate, or severe based on the number of diagnostic criteria endorsed by an individual (American Psychiatric Association, 2013). For illustrative purposes, we depict a general progression to substance use disorder in Figure 1. This model is built loosely on literature highlighting milestones in the development of the use of specific drugs (e.g,. cigarettes: Gervais et al., 2006; alcohol: Jackson, 2010). Here, we describe a more general process in order to comment on how and where cortisol function may affect general substance use trajectories. It is important to note that there is wide variation in the timing, speed, and even temporal ordering of milestones highlighted in Figure 1, and that the number and type of milestones vary per specific drug. We use this illustration as the base conceptual model on which we build a more specific theoretical picture of when and how cortisol function may influence an individual’s progression through substance use trajectories, and when and how substance use may influence cortisol functioning. However, associations of cortisol function and substance use may be non-causal. Instead, both cortisol function and substance use phenotypes may be downstream effects of the same earlier influences. Here, we consider the possibility that genetic influences are one such earlier influence. That is, some genes may influence both cortisol function and substance use phenotypes, hereafter referred to as genetic confounding of the associations between cortisol functioning and substance use phenotypes. Thus, before building this conceptual model through the synthesis of extant theories, we examine the potential for genetic confounding in the association of cortisol reactivity and substance use, which could indicate non-causal associations.
Figure 1.
Conceptual model of how cortisol is implicated for substance use progressions.
A generalized progression from behavioral risk to initiation, through increasing use to problematic use, and after quit attempts, withdrawal and relapse is depicted in order to illustrate where key theories and mechanisms are hypothesized to explain cortisol-substance use associations. Solid arrows depict predicted associations whereby cortisol would predict substance use phenotypes. Hashed arrows depict predicted associations whereby substance use would predict cortisol levels or changes. Specific theories or mechanisms are named within each box in bold text. Italiziced text within boxes describe predictions or findings of cortisol unless explicitly about cortisol. Changes in gene expression are depicted in a hashed box, as they are expected to impact the predicted associations depicted as passing through the hashed box. MAPK = mitogen-activated protein kinase pathway; GPCR = G-protein-coupled receptor pathways; DA = dopamine; PFC = prefrontal cortex; SU = substance use.
Potential for genetic confounding
Substance use phenotypes across the developmental course are heritable (Agrawal and Lynskey, 2008; Crabbe and Phillips, 1998; Hicks et al., 2004; Kendler et al., 2014). Heritability differs somewhat for each stage of progression, with some shared, but also some unique genetic influences across milestones (Heath et al., 2002; Kendler et al., 1999; Rhee et al., 2003). Genome-wide association studies (GWAS; e.g., Kalsi et al., 2016; Sherva et al., 2016; Wetherill et al., 2015) and candidate gene studies have indicated that small effects from many genes are related to diverse substance use phenotypes. A Knowledgebase for Addiction Related Genes (referred to here as the KARG addiction gene set) has been published identifying 386 specific genes related to addiction that were empirically supported by multiple sources (list provided in Table S4 of Li et al., 2008). Despite evidence of the important role of genetics for addiction, the possibility of common genetic variance, or normative genetic variation that may contribute both to cortisol levels and profiles of change and to substance use phenotypes is often overlooked in the literature examining associations of cortisol and substance use phenotypes (Kreek et al., 2005).
Cortisol levels and changes in response to stressors are also heritable (Bartels et al., 2003; Gustafsson et al., 2011; Steptoe et al., 2009), and the specific genetic underpinnings of HPA axis activity are theorized and relatively well documented by several reviews (e.g., Chistyakova & Savost’yanov, 2011; Mormede et al. 2011; Redei, 2008; Wust et al., 2004). One of the most comprehensive molecular genetic studies on cortisol secretion to date used a candidate gene and GWAS approach to investigate cortisol secretion and suggested that the FKBP5 gene (variations in which modulate the sensitivity of glucocorticoid receptors, which are the primary receptors to which cortisol binds) was associated with decreased cortisol area under the curve, a measure of overall cortisol secretion (Velders et al., 2012). Notably, this study also found that single nucleotide polymorphisms (SNPs) in the FKBP5 gene were associated with depression, suggesting that genetic influences may at least in part explain the association of cortisol function and depression. Similarly, a recent study using a candidate-based GWAS approach, wherein gene-wide analyses were conducted for a set of 30 genes derived a priori from the literature as being involved in HPA-axis regulation, found that the NR3C2 gene (the gene encoding mineralcorticoid receptors, to which cortisol can also bind) was associated with both cortisol function and depression in the context of early life adversity (Gerritsen et al., 2017). These studies demonstrate the potential for genetic confounding in the association of depression and cortisol function using specific genes.
Regarding substance use, there is evidence of links between cortisol reactivity and substance use, and psychosocial theories (described below) explain how down-regulation of the HPA axis would lead to externalizing problems and substance use (Fries et al., 2005; Raine, 1996; Zuckerman, 1979). Additionally, boys who were at-risk for substance use problems (their father had a substance use disorder) already showed blunted cortisol reactivity compared with boys whose fathers did not have a substance use disorder at age 10 –12 years, suggesting that low cortisol reactivity marks familial risk for substance use disorders even prior to actual substance use disorder onset (Dawes et al., 1999; Moss et al., 1995). This finding could be explained by common genetic influences leading to both cortisol reactivity in middle childhood and early adolescence and later substance use phenotypes. On the other hand, there is careful work primarily in the animal literature showing the importance of glucocorticoid-mediated epigenetic mechanisms for how cortisol levels and responses are implicated in substance use across developmental phenotypes (e.g., Koob and Kreek, 2007; Koob and Le Moal, 2001; Koob and Zorrilla, 2010; Kreek et al., 2005). To date, no published studies have investigated whether associations of cortisol function with externalizing problems and substance use may be associated in part by shared (structural) genetic influences, which could preclude or weaken causal explanations of cortisol reactivity – substance use associations.
Enrichment of Cortisol-related genes for Substance Use
Because of the literature on associations of cortisol reactivity with substance use, the role for genetics in both hormone function and substance use, and the important role steroid hormones play in gene regulation and expression, we hypothesized that a greater proportion of genes would be linked to both cortisol phenotypes and substance use phenotypes than expected by chance. To test this hypothesis, we used the previously published KARG addiction gene set (Li et al., 2008), supplemented with findings from GWAS studies occurring after the curation of the KARG addiction gene, and hand-curated a set of cortisol-related genes from the literature and biosystems databases. Because of the wide variations in cortisol phenotypes, and the relatively few studies that examined genetic influences on specifically cortisol responses to stressors, we chose to cast the widest net for cortisol-related genes in our search. Specifically, we searched the NCBI Gene (https://www.ncbi.nlm.nih.gov/gene) and Biosystems (https://www.ncbi.nlm.nih.gov/BioSystems) databases, the UCSC genome browser (https://genome.ucsc.edu/), and the GWAS catalog (https://www.ebi.ac.uk/gwas) using the keyword “cortisol” in order to select genes related to cortisol function. A review of the literature, using primarily PubMed and Google Scholar were used to supplement the gene sets developed through these searches. We additionally removed genes that appeared incorrectly linked in the databases (e.g., the article supporting inclusion in the database never mentioned that gene), and one non-protein coding gene. These searches yielded a total of 191 genes potentially related to cortisol function. Details on our search strategy and the full gene set, including the gene name, source, and direction/nature of the link to cortisol levels or changes (e.g, in response to stress), are presented in Supplemental Table 1.
A subsequent literature search using primarily PubMed, NCBI Gene (GeneRifs, Bibliography), and Google Scholar was conducted in order to identify two peer-reviewed empirical findings per gene, in the direction of the gene polymorphism being associated with cortisol levels or changes (rather than cortisol influencing gene expression, which we also documented). This “conservative” set included 67 cortisol-related genes (gene names, more detailed functions and relations to cortisol, and references are supplied in Supplemental Table 2). In addition to the 67 genes that showed evidence of being related to cortisol function, for 18 genes, cortisol appeared to influence gene expression, and the majority (106) appeared theoretically or empirically linked but without sufficient supporting (peer-reviewed, empirical) evidence that we could locate. In the literature thus far, candidate genes for cortisol have been curated through theory and expert opinion (e.g., Bolton et al., 2014; Gerritsen et al., 2017; Velders et al., 2011). We included those genes, which often fell into the “insufficient evidence” category. Thus, our search strategy led to a larger candidate set (the full set) as well as a smaller, empirically vetted set (conservative set) of genes potentially linked to cortisol function.
Our addiction gene set was constructed beginning with previously published KARG addiction gene set. We added genes to the KARG addiction gene set identified through searches of the GWAS catalog using the following search terms: addiction, substance use, drug use, alcohol, nicotine, cannabis, cocaine, and opioid. We recorded reported genes for all association findings that reached genome-wide significance (p < 5e-8) in any study for a relevant phenotype (e.g., excluded phenotypes included food addiction, liver enzyme levels, pancreatitis, systolic blood pressure, etc., full list available upon author request). These searches resulted in an additional 85 genes (total = 471). See Supplemental Table 3 for the full addiction list and source articles.
There are some limitations to our gene sets. First, in the addiction gene set, we supplemented the carefully curated KARG set with genes that reached genome-wide significance. Suggestive genes (e.g., that had low p-values, but not reaching genome-wide significance levels) may also contribute to substance use phenotypes in meaningful ways. Second, we did not empirically vet all new GWAS findings to ensure that these effects were replicated, as we did for the cortisol gene sets. This decision was made in part because the GWAS findings are well-powered and likely to reflect real effects (as opposed to smaller candidate gene studies which comprised much of the literature on genetic influences on cortisol) and because the addiction set was empirically-based, whereas the cortisol sets were more theoretically derived. The other key limitation of these genes sets is that the field is ever-changing and by the time this manuscript is published, undoubtedly more genes will already have been identified, and if not, most certainly more genes will be identified in the future. Thus, these gene sets are expected to already be out of date. Nonetheless, we believe these gene sets are good launching pads for future work aiming to explore cortisol and/or addiction-related genes (to build more current gene sets), and that they reflect a good state of the current knowledge of cortisol-related and substance use-related genes.
Next, we examined our full and conservative cortisol-related gene sets, individually, against our addiction gene set for genes that appeared on both sets, in order to determine whether the our addiction gene set was enriched for cortisol-related genes (which could indicate potential genetic confounding of cortisol-substance use associations). We used Nematode bioinformatics analysis tools and data (Lund, n.d.) to calculate the statistical significance of the overlap between two groups of genes.1 According to the NCBI Gene Statistics (updated June 1, 2017), the current number of genes in the human genome is 60,166. ENCODE efforts yielded an estimate of 20,687 protein-coding genes and 18,400 RNA genes, a total of 39,087 genes that are potentially functionally relevant (Pennisi, 2012). As technology has advanced, the estimated number of total genes in the human genome has decreased substantially and is expected to continue to decrease (Ezkurdia et al., 2014; Pennisi, 2012). To be conservative and consistent with current estimates of the total number of genes, we conducted the enrichment analyses based on an estimated total number of 19,000 protein-coding genes (Ezkurdia et al., 2014). Twenty eight genes appeared on both our full cortisol set and the addiction gene set (see Table 1). When we examined the overlap between the conservative gene set and the addiction gene set, 10 genes appeared in both sets. In the analysis of the conservative set, the expected number of overlapping genes was (471*67)/19,000 = 1.66. Given that there were 10 overlapping genes, the representation factor was 10/1.66 = 6.0, p < 5.58e-06. Using the full cortisol gene set, the expected number of overlapping genes was (471*191)/19,000 = 4.73. Given the actual overlap of 28 genes, the representation factor was 28/4.73 = 5.92, p < 3.60e-14. In both cases, there was a highly significant overlap of genes appearing on both sets. Thus, we began building our conceptual model (Figure 1) by adding direct paths from common genetic variance shared for substance use and cortisol function as potential predictors of substance use at various developmental milestones in the basic substance use progression (Figure 1, top left).
Table 1.
Enriched pathways for the 28-gene overlap set.
| Category | Pathway name | set size | candidates contained | p-value | pathway source |
|---|---|---|---|---|---|
| Cell signaling | Glucocorticoid receptor regulatory network | 81 | 6 (7.4%) | 1.92e-08 | PID |
| Estrogen signaling pathway | 100 | 6 (6.0%) | 6.85e-06 | KEGG | |
| Serotonergic synapse | 113 | 6 (5.4%) | 1.35e-07 | KEGG | |
| Brain-Derived Neurotrophic Factor (BDNF) signaling pathway | 144 | 6 (4.2%) | 5.99e-07 | Wikipathways | |
| Neuroactive ligand-receptor interaction1 | 278 | 7 (2.5%) | 1.74e-06 | KEGG | |
| Nuclear Receptors Meta-Pathway1 | 316 | 6 (1.9%) | 5.47e-05 | Wikipathways | |
| Class A/1 (Rhodopsin-like receptors) | 327 | 6 (1.8%) | 6.62e-05 | Reactome | |
| Signal Transduction | 2538 | 15 (0.6%) | 7.96e-05 | Reactome | |
| cAMP signaling pathway | 200 | 6 (3.0%) | 4.08e-06 | KEGG | |
| TNFalpha | 241 | 6 (2.5%) | 1.19e-05 | NetPath | |
| G alpha(i) signaling events | 247 | 6 (2.4%) | 1.37e-05 | Reactome | |
| MAPK Signaling Pathway | 168 | 6 (3.6%) | 1.48e-06 | Wikipathways | |
| MAPK Signaling Pathway | 255 | 6 (2.4%) | 1.64e-05 | KEGG | |
| GPCR signaling-G alpha s PKA and ERK1 | 285 | 7 (2.5%) | 2.15e-06 | INOH | |
| GPCR signaling-G alpha s Epac and ERK | 272 | 6 (2.2%) | 2.36e-05 | INOH | |
| GPCR signaling-G alpha q | 274 | 6 (2.2%) | 2.46e-05 | INOH | |
| GPCR ligand binding | 460 | 6 (1.3%) | 0.00042 | Reactome | |
| Signaling by GPCR | 1310 | 10 (0.8%) | 0.000298 | Reactome | |
| Disease | Sudden Infant Death Syndrome (SIDS) Susceptibility Pathways1 | 159 | 7 (4.4%) | 1.25e-08 | Wikipathways |
| MECP2 and Associated Rett Syndrome | 63 | 6 (9.5%) | 4.13e-09 | PID | |
| Alzheimers Disease | 144 | 6 (4.2%) | 2.22e-07 | Wikipathways | |
| Alzheimers disease | 171 | 6 (3.5%) | 6.13e-07 | KEGG | |
| Integrated Pancreatic Cancer Pathway | 170 | 6 (3.5%) | 5.93e-07 | Wikipathways |
Note.
indicates that the pathways were also overrepresented when using the conservative 10-gene overlap set (See Footnote 2). Genes on the overlap set were: AKR1B1, APOE, APP, BDNF, CHRNA7, CLOCK, CREB1, DRD4, FKBP5, HSPA1A, HTR1A, HTR1B, IGF2, IGFBP2, KLF9, MAPK1, MAPK3, MAPT, NR3C1, OPRK1, OPRM1, PER2, PRKAR1A, RACK1, SGK1, SLC6A3, SLC6A4, and TNF.
What can the overlapping genes tell us about associations of cortisol and substance use?
Overall, the increased representation factors using both the full and conservative cortisol-related gene sets indicate that there is a high degree of known genetic overlap in cortisol phenotypes and addiction phenotypes. The analysis including the conservative set of genes for cortisol phenotypes indicates that the same genes may contribute both to cortisol levels/changes and addiction, and thus may confound cortisol reactivity-substance use associations. However, the actual number of genes and effect sizes of those genes for each phenotype is quite small. Small effects mean that confounding is highly unlikely to explain the entirety of, or even most of cortisol reactivity-substance use associations. The analysis including the full set of genes related to cortisol function, and the presence of genes whose expression is modulated by cortisol on the larger overlap set, indicate that these genes may not simply confound cortisol reactivity-substance use associations. Rather, these genes may point to mechanisms by which cortisol function and substance use are bi-directionally associated.
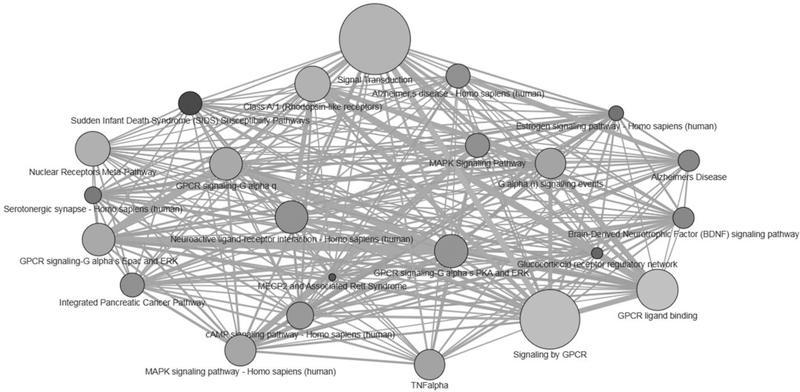
In order to better understand the function of the genes identified as being related both to cortisol phenotypes and to addiction, we conducted an over-representation pathway analysis (Kamburov et al., 2011; Kamburov et al., 2009). Over-representation, or enrichment analysis, is a systems-biology tool that was developed to aid in interpretation of gene sets, transcriptomics, and proteomics data and identifies whether a set of genes are significantly associated with (e.g., are implicated in) certain biological pathways. Biological pathways are defined as cascades of actions and interactions among molecules within cells that lead to changes including, for example, assembly of new molecules like proteins or fats, gene expression, or cell movement (Kamburov et al., 2011; Kamburov et al., 2009). For this analysis, we used the larger overlapping set from the full cortisol gene set, as theory and evidence indicate bidirectional effects of cortisol and substance use. We did not want to exclude the action of cortisol on gene expression as a potential mechanism linking cortisol function to substance use. Specifically, we uploaded our set of 28 overlapping genes into the online over-representation analysis tool provided by Consensus Pathway DataBase, and selected Pathway-based sets, which identifies pathways as defined by multiple pathway databases (13 databases searched, see http://cpdb.molgen.mpg.de/ for details). We selected a minimum overlap with input set of 6, ensuring that we only viewed pathways where at least 20% of the genes on the overlap set were involved. The over-representation analysis yielded 23 enriched pathways.2 These pathways were not independent, often sharing genes that were involved in other enriched pathways. See Figure 2 for a visualization of the relations among enriched pathways. Descriptions of the pathways, grouped into similar mechanistic or theoretical categories, are presented in Table 1. Generally, genes that were associated with both cortisol and addiction were enriched in cellular signaling and some disease pathways (e.g., Alzheimer’s). Unsurprisingly, one enriched pathway was the glucocorticoid receptor regulatory network, which underscores the associations of cortisol function with substance use and suggests that cortisol function itself may be an important biological mechanism in the development of substance use. We also reviewed the literature on the 28 genes found to overlap across the cortisol-related and addiction sets to derive potentially relevant areas for future research. In the next section, we synthesize existing theories of cortisol reactivity-substance use associations, and integrate findings from the gene enrichment analysis where warranted. Additional mechanisms that arose through the literature search and enrichment analysis are also presented.
Figure 2.
Visualization of the inter-connectedness of pathways enriched for genes shared across the full cortisol-related and addiction gene sets.
Predefined gene sets compiled in Consensus Pathway DataBase and their pair-wise overlaps are shown. The size of each node (circle) reflects the size of the gene set. The darker the node, the more highly significantly enriched the pathway is for genes on the 28-gene overlap set (e.g., lower p-values from the over-representation analysis). Edges (lines) denote shared genes in each gene set; thicker edges denote more shared genes, whereas thinner edges denote fewer shared genes.
Building the Conceptual Model: Theory and evidence for cortisol reactivity - substance use links
There are several existing theories explaining observed links between cortisol reactivity and substance use. It is important to note here that the significant associations between cortisol function and substance use in the literature are not always in the same direction, and different theories have been used to explain positive versus inverse associations. The goals of this synthesis are as follows. First, we place major theories and mechanisms of cortisol reactivity-substance use associations into the context of a substance use progression. Second, we visualize how and when the direction of effects are expected to change. Finally, we comment on how and where specific genes may be implicated in cortisol reactivity-substance use associations at various developmental milestones marking the progression of substance use based on the enrichment analysis in order to support existing theory or generate new biological mechanisms for future research to consider.
Acute and chronic stress.
A general stress mechanism is among the more straightforward theories for cortisol reactivity-behavior links. There is evidence showing that increased stress levels (for which cortisol reactivity is a biomarker) are associated with higher consumption of alcohol and other drugs in the moment (e.g., for smoking, Rose et al., 1983; for alcohol, Stephens and Wand, 2012). This association is often interpreted as indicating that individuals choose to use in an attempt to relieve stress. Therefore, under current or acute stress conditions, higher cortisol is expected to be associated with higher substance use, and mark risk for developing substance use problems (depicted by paths from acute stress to 1st try and use in Figure 1). This explanation of substance use as a coping strategy (i.e., self-medication to reduce acute stress) is insufficient to explain the relations between cortisol reactivity and substance use, however. Particularly earlier in the progression to substance use disorder, acute consumption (e.g., of alcohol) can activate the HPA response, functioning as a stressor (e.g., Richardson et al., 2008; Waltman et al., 1993, depicted by the hashed path from intoxication to acute stress in Figure 1). Thus, early in the progression to substance use disorder, there are bidirectional links whereby acute stress and subsequent cortisol reactivity may precipitate the use of alcohol or other drugs, and the effects of those drugs also can induce an acute hormonal stress response.
However, as stressors become chronic, there is a well-documented shift from cortisol reactivity to hypo-reactivity, or a blunting of the cortisol response to stress (Juster, 2010; Peters and McEwen, 2012). This shift is explained by the theory of allostatic load, which posits that cumulative wear and tear on the body leads to altered internal set-points in when various dynamically coordinated physiological regulatory systems (including the HPA axis) activate. Altered set-points (relative to the individuals’ original set-points for constantly adapting to acute challenges) would be adaptive to handle the chronicity of stress but would also leave individuals vulnerable for psychopathology, including substance use problems (Juster, 2010; McEwen, 2005). This vulnerability due to altered set-points from chronic stress (e.g., a low cortisol reactivity – substance use association) is depicted in Figure 1 by the solid arrow from chronic stress/allostatic shift to dependence and abuse). Particularly later in the trajectory of substance use, heavier and chronic use is associated with further hypo-activity of the HPA response (depicted by the hashed arrow from regular and heavy use to chronic stress/allostatic shift in Figure 1), mimicking the cortisol pattern associated with chronic stress and the shift in HPA functioning associated with too high allostatic load (King et al., 2006; Stephens and Wand, 2012). The presence, magnitude, and thresholds of chronicity of stress likely varies across individuals, and whether the individual has experienced an allostatic shift or not has important theoretical repercussions for how cortisol function is likely to be empirically associated with substance use. This challenge provides an important avenue for future research: longitudinal work documenting individual differences in HPA reactivity marking allostatic load and the timing of the shift to hypo-reactivity in relation to substance use phenotypes is needed to fully investigate these ideas.
Fearlessness and sensation seeking.
There are also several theories specific to low cortisol-substance use associations that go beyond cortisol reactivity as a measure of stress. Fearlessness theory (Raine, 1996) suggests that low cortisol levels and responses may be a biomarker indexing low fear. The lack of fear would be associated with substance use and related outcomes because fear (e.g., of punishment) is one of the main deterrents of early initiation of substance use and engagement in related externalizing behaviors (particularly aggression). Alternatively, the sensation seeking hypothesis (Zuckerman, 1979) posits that low cortisol levels and responses may index low arousal, and individuals would therefore seek out experiences (including substance use) that would increase arousal. In either case, these mechanisms may be exacerbated in youth that have been exposed to prolonged stressors and have likely experienced the down-regulation of cortisol in the adrenal gland and brain (Fries et al., 2005; Shirtcliff et al., 2009). As shown in Figure 1 (by paths from fearlessness/sensation seeking to behavioral risk, 1st try, use, and intoxication), we hypothesize that these theoretical mechanisms might be particularly important earlier in the developmental progression, increasing the risk of early initiation.
Indeed, there is some evidence that blunted cortisol reactivity in childhood is associated with concurrent and later externalizing behaviors (e.g., Conradt et al., 2014; Kariyawasam et al., 2002; McBurnett et al., 2003). These findings are relevant because childhood externalizing problems are arguably the strongest predictor of future substance use problems (e.g., Colder et al., 2013; Helstrom et al., 2004; Hicks et al., 2011; Wilens et al., 2011). It is important to note that cortisol reactivity-externalizing associations are not always found, however (Alink et al., 2008). Consistent with the concept of allostatic load, a meta-analysis found that higher basal cortisol was associated with externalizing problems in early childhood, whereas lower basal cortisol was associated with externalizing problems in school-aged children (Alink et al., 2008). There are likely additional moderators to consider that may impact whether youth with lower cortisol reactivity engage in externalizing problems through fearlessness or sensation seeking, including contextual factors like peer groups or restrictiveness/permissiveness of the social environment. For example, in restrictive environments youth may not be able to act in accordance with their biology, whereas in permissive environments youth with lower cortisol reactivity may be free to engage in stimulation-increasing behaviors, like externalizing problems or smoking a cigarette (e.g., gene-environment interaction).
Mechanisms of brain development.
Adolescence is a time of dramatic changes in the HPA axis yielding changes in cortisol levels and reactivity (e.g., generally increasing across adolescence, Gunnar et al., 2009), and is also a sensitive period crucial for brain development (Spear and Silveri, 2016). For example, intracranial and whole brain volume increases and cerebral gray matter decreases from its peak in childhood, and there are increases in cerebral white matter into mid-to-late adolescence followed by a deceleration in the growth of cerebral white matter (Mills et al., 2016). Further, multiple neurotransmitter systems mature, particularly in frontal and limbic regions (Crews et al., 2007). Theoretically, substance use exposure during adolescence, when the brain is undergoing tremendous age- and puberty-related development, may set youth on trajectories that increase the likelihood of progression to substance use disorder (Spear, 2013; depicted in Figure 1 by hashed arrows from use and intoxication to brain development). During adolescence, brain changes have been associated with alcohol and drug use, though the direction of effects remains inconclusive (e.g., whether alcohol and drug use changes trajectories of brain development or whether there is common pre-existing risk leading both to certain neurobiological signatures and substance use; Silveri et al., 2016).
Importantly, adolescent brain development is actively modulated by cortisol and related stress-hormones that pass through the blood-brain barrier and exert effects, for example, on amygdala-prefrontal cortex circuitry (Tottenham and Galván, 2016). Thus, cortisol reactivity could affect substance use phenotypes across the spectrum through associated brain changes. We hypothesize that these effects may in part be genetically mediated, as a recent study found that two genes modulating receptors to which cortisol binds (NR3C2 and FKBP5) also are associated with the volume of the amygdala (Gerritsen et al., 2017). Amygdala-prefrontal cortex circuitry is particularly important for cognitive and emotional processes and decision making, and for the emotional and behavioral problems in adolescence that mark risk for substance use. For example, early life stress led to increased childhood basal cortisol levels which in turn led to less connectivity of amygdala-prefrontal cortex circuitry which was associated with anxiety problems (e.g., Burghy et al., 2012). Amygdala-prefrontal cortex circuitry has also been implicated in the mechanisms maintaining addiction, for example, desire to use despite negative consequences, and relapse (Kalivas and Volkow, 2005). Given these findings, cortisol effects on reduced connectivity of amygdala-prefrontal cortex circuitry is a plausible brain-based mechanism for how cortisol function is implicated in the developmental progression to substance use, both in the early developmental phases and in the later maintenance and relapse phases of the outlined progression (depicted by paths from brain development to behavioral risk, and to regular and heavy use, dependence and abuse, and withdrawal and relapse in Figure 1).
Animal models repeatedly reveal that stress hormones play a key role in how the brain structurally adapts to stressors through epigenetic mechanisms (McEwen et al., 2015). One hypothesis is that glucocorticoid receptor-mediated gene expression may play a role in how exposure to substance use may alter trajectories of brain development. There is evidence of greater cortical expression of glucocorticoid receptors in mRNA in the brain in adolescents and adults than in infancy or late adulthood (Perlman et al., 2007). This finding illustrates that glucocorticoids can act on cells in the cortical regions to influence gene expression (e.g., of NR3C1, the gene that encodes glucocorticoid receptors and which appears on both the cortisol and addiction gene sets), and that increased sensitivity to glucocorticoids (and perhaps increased glucocorticoid-mediated gene expression) begins during adolescence. Adolescent cortical development may provide a critical period of vulnerability for addiction in part because alcohol exposure during critical developmental periods, including adolescence, can disrupt normal development and lead to neurodegeneration and inhibit the formation of new neurons (Crews et al., 2007). It may be that glucocorticoid-mediated gene expression contributes to adolescent vulnerability to addiction, either on its own—for example though effects on neurogenesis—or through interactions of alcohol (or other drugs) with other key systems intertwined with cortisol, like dopamine, serotonin, and cAMP-response element binding protein (CREB) transcription (Crews et al., 2007). Notably, dopamine-related genes (DRD4, SLC6A3 [aka DAT1]), serotonin-related genes (SLC6A4 [aka 5HTTLPR], HTR1B), and CREB-related genes (CREB1) appeared on both the cortisol and addiction gene sets, and serotonergic synapse and Brain-Derived Neurotrophic Factor (BDNF) signaling pathways were enriched for genes on our overlap list, further supporting the notion that cortisol function likely impacts gene expression in a variety of systems in the brain in a way that confers vulnerability for addiction.
In addition to cortical development, another mechanism of epigenetic action of glucocorticoids on the brain is illustrated by neurogenesis in the hippocampus. Adult hippocampal neurogenesis has been linked to addiction bidirectionally, in that drugs of abuse can suppress hippocampal neurogenesis, and low hippocampal neurogenic activity may confer vulnerability to addiction (e.g., psychatric disorders associated with abnormal psychosocial stress responses; Chambers, 2013). This association is likely in part mediated through cortisol function, as increased glucocorticoid action in the brain precipitated by stress has also been linked to adult hippocampal neurogenesis (e.g., Cameron and Gould, 1994; Schoenfeld and Gould, 2012). Increases in glucocorticoids in the brain lead to reduced hippocampal neurogenesis, which can (normatively) be followed by a corresponding increase in hippocampal neurogenesis when the stressor is relieved (Schoenfeld and Gould, 2012), or (abnormally, and particularly in the presence of mental illness) can be followed by impaired hippocampal feedback mechanisms regulating HPA activity (Chambers, 2013). Even in the context of normative increases in hippocampal neurogenesis after a stressor, there can be lasting epigenetic changes (McEwen et al., 2015), and so repeated or high-level stress may have more lasting consequences for hippocampal neurogenesis dysfunction. Further, CYP11A1, CYP17A1 (genes coding members of the cytochrome P450 superfamily of enzymes that are involved in drug metabolism and steroid hormone synthesis) were significantly associated with both cortisol response to dexamethasone suppression test and hippocampal volume in adults (Gerritsen et al., 2017). Because adolescence is a developmental period characterized by increased stress and associated cortisol levels, it is conceivable that abnormally high levels of glucocorticoid action in the brain may impair neurogenesis in the hippocampus at a relatively early age, conferring vulnerability to substance use (e.g., Figure 1, pathway from brain development to behavioral risk). However, this is an empirical question that should be tested in future studies.
Neurotransmission.
Substantial work, particularly in animal models, has been done examining the role of cortisol function in interaction with specific mechanisms of neurotransmission. Of particular salience is the dopaminergic system. Cortisol can bind to dopaminergic neurons, which may confer vulnerability to reinforcing effects of substances. Animal studies show increased HPA hormone levels during acute binge episodes, attenuation during chronic binge conditions, and increased activity during withdrawal (Koob and Kreek, 2007). This work highlights how cortisol reactivity is enmeshed with the mechanisms of substance administration, withdrawal, and relapse, and broadly suggests that cortisol responses may be implicated in reinforcing the addictive cycle, particularly later in the substance use progression (e.g., at the stages of dependence, maintenance, and relapse) in part through its action in the dopaminergic system (Koob and Kreek, 2007; Koob and Le Moal, 2001). Indeed, blunted cortisol responses have been associated with shorter time to alcohol (Breese et al., 2005) and nicotine relapse (al’Absi et al., 2005), and greater cocaine intake after relapse (Junghanns et al., 2003). The relation between cortisol reactivity and later substance use phenotypes is likely bidirectional, with altered cortisol activity after withdrawal (potentially contributing to withdrawal symptoms and relapse susceptibility) and blunted cortisol prospectively predicting relapse (Sinha, 2011; depicted by bidirectional arrows from neurotransmission to the cyclical end of the substance use progression in Figure 1). However, given the role of specific genes in the dopaminergic system both for cortisol phenotypes and substance use phenotypes, future research is needed in order to test whether these bidirectional associations are potentially mechanistic in nature, or whether genetic influences account for the associations.
Given the very small effects of specific genes in these systems with cortisol phenotypes and addiction, any genetic confounding is expected to be small. However, even small effects could mask the true effect sizes of mechanistic bidirectional associations of cortisol reactivity – substance use associations. Misunderstanding true effect sizes has important implications for treatment and intervention, particularly pharmacological intervention seeking to use either the HPA axis or neurotransmitter systems to break the stress-substance use cycle. Notably, other neurotransmitter systems (e.g., opioid, serotonergic) have also been linked to cortisol reactivity and addiction and were highlighted in the enrichment analysis, similarly showing likely bidirectional effects, and the potential for some genetic confounding. For example, OPRK1 and OPRM1, genes encoding kappa and mu opioid receptors, have been associated with both cortisol phenotypes and substance use and appear on both gene sets, as do serotonin-related genes SLC6A4 and HTR1B.
Circadian Rhythm.
Several clock genes appeared on both the full cortisol and KARG addiction gene sets: PER2, CLOCK, and KLF9. Circadian rhythms, including the diurnal pattern of cortisol release (an initial awakening response peaking ~30 minutes after waking, followed by a decline across the day), are driven by the suprachiasmatic nucleus (SCN). Circadian genes, including PER1–3, CLOCK, BMAL, and CRY1/CRY2 are key for the SCN’s circadian timekeeping, and cortisol plays a key role in regulating the circadian expression of PER1 and PER2, among other clock genes, particularly in peripheral tissue (Mavroudis et al., 2012). Mutations in circadian genes are associated with sleep (e.g., CLOCK, Allebrandt et al., 2010), and sleep deprivation can affect the expression of several circadian genes as well as subsequent cortisol production (Ackermann et al., 2013). This is particularly important in light of recent findings that a) poor sleep prospectively predicts adolescent substance use (e.g., Miller et al., 2017; Nguyen‐Louie et al., 2017; Warren et al., 2017; Zhabenko et al., 2016), and b) clock genes (e.g., PER1, PER2) have also been associated with adolescent substance use and alcohol consumption (e.g., alcohol consumption; Comasco et al., 2010; Dong et al., 2011). Testing whether disruptions in circadian rhythms that are moderated or mediated by cortisol function may in part explain how poor sleep is mechanistically linked to adolescent substance use is an important area for future research. It is also important to note that these mechanisms likely occur separately from the brain-mediated cortisol reactivity mechanisms discussed thus far, and rather are peripherally located (e.g., not brain-based) effects of normal (and abnormal) cortisol diurnal rhythms.
There is also evidence that substance use disrupts circadian rhythms including circadian gene expression, and that circadian genes are implicated in how individuals respond to various drugs of abuse (e.g., see Falcon and McClung, 2009; Parekh et al., 2015 for a review). Recent studies have implicated the dopamine system for links between clock genes and addiction, suggesting that disruptions in the circadian rhythm lead to disruptions in neurotransmitter signaling systems (including dopaminergic and other neurotransmitter systems) which then lead to vulnerability to addiction (McClung, 2007; Parekh et al., 2015). This adds another layer to the intertwining of cortisol function and the dopaminergic system with implications for substance use. Together, theory and evidence lead to the following hypotheses: 1) blunted diurnal cortisol patterns (perhaps induced by chronic stress) could act as a disruptor of circadian rhythm because the important role cortisol plays in keeping peripheral tissue ‘synced’ with the SCN via epigenetic mechanisms would be diminished, which could have longer-term downstream effects on addiction. 2) Substance use itself may exacerbate the effects of disrupted circadian rhythm on vulnerability to addiction in part through a combination of cortisol and dopaminergically-mediated mechanisms. At this time, it is unclear whether disruptions in the circadian rhythm are a mechanism of diurnal cortisol -substance use associations, or a separate, correlated outcome. Thus, we elected to exclude circadian rhythm disruptions or sleep in the conceptual framework (which is focused primarily on cortisol reactivity to environmental cues and stressors, rather than diurnal variations).
Cell Signaling.
Many of the genes found to contribute both to cortisol function and addiction are important for cellular signaling, particularly mitogen-activated protein kinases (MAPK) signaling and G-protein coupled receptor (GPCR) signaling and ligand binding, and cAMP signaling, TNFalpha, G alpha(i) signaling events, Class A/1 receptors. This is not surprising, as these genes are involved in all regulatory processes. For example, these cellular signaling pathways (and the underlying genes) are involved in vital cell processes including proliferation, differentiation, regulation, and cell-cycle progression, and thus likely provide scaffolding for epigenetic influences of cortisol on various systems, with far downstream effects on substance use (depicted by paths from cellular signaling to changes in gene expression). Nonetheless, the enriched pathways falling under the umbrella of cell signaling, including nuclear receptors, point to the large pharmacological field of addiction. Importantly, the role of cortisol for expression of genes that are related to how drugs of addiction bind to receptors has (to our knowledge) not yet been explored. If cortisol levels and changes can influence the efficacy of drugs of abuse and the drugs used to treat addiction, there would be large implications for the effectiveness of pharmacological intervention.
The enrichment of the estrogen signaling pathway is particularly interesting. There is evidence of cross-talk between HPA and hypothalamic-pituitary-gonadal axis hormones (Viau, 2002), such that cortisol and estrogen (and androgens) have reciprocal interactions at several levels of each axis, influencing the negative feedback loops of the hormonal end-products of the other axis. Further, animal studies suggest that estrogen enhances drug-seeking behavior (e.g., see Carroll et al., 2004 for discussion). Therefore, we hypothesize that there may be associations of both stress and sex hormones operating together on substance use, and that in conditions of stress (e.g., heightened cortisol reactivity) during particular phases of the menstrual cycle (with increased estrogen), women are particularly vulnerable to internal and external pressures to initiate substances. We hypothesize that this would be particularly salient in adolescence, as estrogen increases dramatically with puberty and so do cortisol levels (depicted by paths from estrogen to behavioral risk, first try, use, and intoxication in Figure 1).
Specific Disease Pathways.
Several specific disease-related pathways were highlighted in our pathway enrichment analysis, including Alzheimer’s, Sudden Infant Death Syndrome, Rett Syndrome, and cancer. It is less likely, in our estimation, that these disease-related pathways will unveil new mechanisms of how cortisol function and substance use are related at various developmental stages of substance use progression (and so they are not depicted in Figure 1). For example, APOE was one of the genes included in both gene sets—related to cortisol and to addiction—and one of the candidates in the Alzheimer’s pathways. Lower cortisol levels and blunted declines in cortisol across the day were prospectively associated with cognitive decline in APOE-e4 carriers (a gene clearly linked to cognitive decline and Alzheimer’s disease) but not APOE-e4 non-carriers (Gerritsen et al., 2011). Further, higher cerebral spinal fluid cortisol levels were associated with an increased likelihood of being an APOE-e4 carrier (Peskind et al., 2001). These findings are consistent with literature suggesting that a variety of cognitive processes (e.g., learning, acquisition, memory, decision-making) are affected by non-optimal (e.g., too much or too little) cortisol (DeKloet et al., 1999), and further suggest a complex and interactive relation between APOE, cortisol and cognitive decline, particularly in patients with Alzheimer’s disease.
Smoking has also been shown to be a risk factor for cognitive decline (Anstey et al., 2009), although there may be a protective effect of moderate alcohol use (Hersi et al., 2017; Panza et al., 2009). This effect may be specific to APOE-e4 non-carriers, whereas for APOE-e4 carriers, light and moderate alcohol consumption may be associated with declines in learning and memory (Downer et al., 2014). Further, APOE was down-regulated in the frontal cortex of alcoholics relative to non-alcoholics (post-mortem), suggesting that APOE may be involved in alcohol-related loss of white matter (Lewohl et al., 2000). Again, the literature paints a complex, interactive picture for the role of APOE in how both cortisol and substance use are implicated for cognitive decline and Alzheimer’s disease (a similar story may be emerging for APP, albeit with much less evidence). However, the literature did not provide any evidence of a mechanism by which APOE may facilitate an association between cortisol and substance use. Thus, based on pleiotropy and the highly comorbid/integrated nature of many diseases, the overlap of genes related to cortisol and addiction and these other disease-related phenotypes are likely downstream effects—that is these diseases are both influenced by cortisol and addiction, and perhaps some of the mechanisms linking the two. Further the majority of the genes implicated in these disease pathways are cell-signaling genes involved broadly in regulatory processes in the body. Thus, disease-based pathways are excluded from the conceptual model because there is little theoretical or empirical evidence for enriched disease pathways playing a mechanistic role in associations of cortisol and substance use.
Summary of the Conceptual Model
The associations of cortisol function and substance use at various developmental milestones in the progression (from behavioral risk to initiation, through increasing use to problematic use, quit attempts, withdrawal, and relapse) is complicated. As reviewed above, psychobiological theories posit both positive and negative associations of cortisol with substance use. Earliest in the progression, fearlessness and sensation seeking hypotheses explain how low cortisol reactivity is associated with the types of behaviors that most put youth at risk for substance use: externalizing problems. Substance use initiation is considered under the broad umbrella of externalizing problems, and thus fearlessness and sensation seeking hypotheses predict that low cortisol reactivity would increase risk not only of externalizing problems but also transitioning to substance use, and beginning the progression.
However, also in the earlier stages of the progression to substance use, general stress/coping mechanisms propose that cortisol, as a biomarker of stress, is positively associated with substance use in part because substance use is a coping strategy individuals may use to attempt to decrease feelings of acute stress (which could be indicated by higher cortisol levels). The associations of cortisol reactivity and substance use in the early- to mid-ranges of the substance use progression are highly bidirectional. Although individuals may decide to pick up a cigarette to reduce their perceived stress, the consequence of smoking that cigarette is increased cortisol levels. The repeated, or regular use as use becomes habitual and/or individuals become addicted, in turn acts as a chronic stressor that can alter the functioning of the hormonal stress response system. Chronic stress, including that from regular and heavy use, can cause an allostatic shift from cortisol increases in response to stressors to a chronic hypo-reactivity of the HPA axis. This blunted cortisol reactivity pattern is associated with increased risk of substance use even later in the progression, for example at the stage of dependence and abuse. At the cyclical end of the substance use progression, there is evidence that withdrawal can also cause increases in cortisol which can in turn increase the likelihood of relapse. It is unclear whether the studies showing these positive cortisol response – withdrawal and relapse associations are found in individuals who have experienced an allostatic shift, or whether they apply only to individuals who have not; this is an important topic for future research. In general, studies testing psychobiological theories of cortisol reactivity-substance use associations have not included the potential of genetic influences or used genetically informed designs, an important limitation of the field.
Some mechanisms are clearly important across the entire progression from behavioral risk through substance use problems, namely genetic variation and brain development. Substance use phenotypes at various milestones in the progression are heritable, and a substantial number of specific genes are associated with both cortisol function and substance use phenotypes. Some genes have been shown to influence both measured levels and changes of cortisol and substance use phenotypes. However, for some genes, cortisol has been shown to modify the expression of genes whose functions have later downstream effects on substance use phenotypes. In general, the molecular mechanisms discussed here are far upstream of actual substance use phenotypes. Cellular signaling, particularly related to MAPK/GPCR and nuclear receptors, is most likely linked to cortisol via epigenetic mechanisms of brain development and neurotransmission that have downstream effects on the substance use progression. Potential cortisol-mediated epigenetic effects are implicated in multiple other known influences on the development of substance use, including brain development, neurotransmission (e.g., of the dopamine and serotonergic systems), and circadian rhythms. The detailed work in animal models and corroborating evidence in humans points to the tightly enmeshed role of cortisol reactivity in the latest phases of reinforcing the addiction cycle. Neurotransmission, however, may be a more ubiquitous mechanism of cortisol reactivity-substance use associations across the entire progression. It was gaps in the literature, rather than null findings, which led us to include neurotransmission as a mechanism relevant particularly to the end of the substance use progression.
These more specific molecular mechanisms highlight that it is unlikely that the association of cortisol reactivity with substance use phenotypes will systematically change from negative to positive to negative to positive across the various milestones marking the developmental progression of substance use, at least using the current classical methods. Instead, this literature highlights the delicate balance of multiple hormonal and neurotransmitter systems, in part through the expression of genes both related to and modified by cortisol, and tells a story of context (including developmental context) – dependent associations and biobehavioral canalization processes. This literature would benefit from dynamical systems approaches (e.g., Boker and Graham, 1998; Dokoumetzidis et al., 2002) that may shed light on nonlinear processes leading to links between cortisol reactivity and the development of substance use.
Implications of the conceptual framework for prevention and treatment
There is substantial evidence that prevention and treatment programs for reducing substance use in adolescents and young adults can be effective, although there is a great deal of heterogeneity in programs’ effectiveness (Sandler et al., 2014). This heterogeneity points to the likelihood of contextual moderators of program effectiveness. Each theory and mechanism discussed in this synthesis has specific implications for prevention or treatment of substance use disorders, and so understanding where in the progression the theory or mechanism is likely to carry the most weight can help time efforts to have the greatest impact. For example, fearlessness/sensation seeking hypotheses suggest that cortisol is a biomarker of low fear or arousal, respectively. Substance use is merely an attempt to increase arousal, or a product of taking chances when feeling little fear of consequences. These hypotheses are geared toward the early end of the progression, and are likely to be more influential in substance use initiation. Thus, prevention efforts based on fearlessness/sensation seeking might include strategies to increase parental monitoring and decrease the opportunity (as opposed to increasing the punishment) to engage in substance use during the developmentally sensitive period for brain development. Given evidence that interactive strategies promoting skills were more effective than programs that were more didactic/knowledge-dissemination based (Sandler et al., 2014), this might be best accomplished by prevention efforts teaching parents and schools strategies to reduce opportunity.
We have highlighted the potential for genetic confounding in several parts of the conceptual framework (Figure 1). This genetic confounding is an inherently non-causal explanation, and precludes any mechanistic association that could be relevant for prevention or treatment efforts. For example, if the same underlying genetic predisposition leads to low cortisol and to substance use, prevention efforts described above based on fearlessness/sensation seeking would not be expected to work. Realistically, it is unlikely that specific measured genes that are related to both cortisol phenotypes and addiction will completely confound observed associations between cortisol reactivity and substance use, given the very small effect sizes of any specific gene on complex phenotypes in the literature. More importantly, we also noted the importance of genetic and epigenetic mechanisms for understanding how cortisol reactivity and various substance use phenotypes are associated along the progression. That is, genetic confounding is not the only possible explanation of these findings. The genes and pathways identified may pinpoint key, potentially causal, biological mechanisms that could be fruitful for future investigations into the mechanisms of cortisol-substance use associations. Or, these specific mechanistic effects may have a bearing on the development of new pharmacological treatments for substance use that work towards, for example, repairing HPA axis functioning in individuals with substance use problems in an attempt to break the cycle of dysregulated HPA function and increased substance use. It is also possible that our findings simply reflect pleiotropy, where genes have multiple, unrelated effects. Future studies designed to examine how and why genes that have been identified as being associated with both cortisol and substance use phenotypes using data that measure genetic influences as well as both phenotypes are needed to test these competing hypotheses (e.g., genetic confounding, pleiotropy, or the identification of potentially causal biological mechanisms underlying the associations). Bi-directional Mendelian Randomization is one such approach that could be leveraged here.
Higher-level theories of gene-environment interplay are also highly valuable for understanding the implications of genetic mechanisms of cortisol reactivity and substance use associations over time. For example in some contexts (e.g., more restrictive environments where genetic predispositions are less often expressed), genetic influences exert less of an influence than in other contexts (e.g., highly permissive environments). This idea, speculatively, could be extended to hypothesize that genes are free to be expressed in contexts that are less structured, and that expression is reigned in when restrictive environments call for it. Because cortisol is highly responsive to environmental cues, cortisol function could theoretically be a mechanism transmitting information about the larger environment to the genome. Taken one step further, the environmental context could have implications for prevention and intervention efforts by exacerbating or diminishing the genetic and epigenetic mechanisms linking cortisol and substance use. For example, if in a more restrictive environment genetic influences are diminished relative to the overwhelming influence of the environment, pharmacological interventions based on gene expression could be less effective. Conversely, such pharmacological interventions may be most effective in permissive environments.
Summary
We built a conceptual framework depicting many (but certainly not all) theories and mechanisms for how cortisol reactivity and substance use are related. We placed these theories in a developmental progression, paid special attention to the possibilities of genetic confounding and genetic and epigenetic mechanisms, and developed new hypotheses to guide future research based on this theoretical synthesis. There are three main conclusions that can be drawn from the literature reviewed here. First, there is potential for genetic confounding in cortisol-substance use associations, and a serious need for genetically informed designs to investigate how and why cortisol phenotypes are associated with substance use at all points in the progression to disorder. This confounding is likely to be small, but existing evidence and work on gene-environment interplay eludes to the importance of considering genetic influences, cortisol function, and environments together in multivariate frameworks to better understand the development of substance use in future research. Second, the relationship between cortisol reactivity and substance use is highly complex, occurs at multiple levels of analysis, and is bidirectional at multiple phases of the substance use progression. In order to forge a complete, coherent, and consistent body of literature, it is key for studies to precisely document the stage of progression to substance use being investigated, test both directions of influence instead of assuming one over the other, and to employ longitudinal methods. Finally, it will be continually important for prevention and intervention scientists to consider the developmental context, in terms of age, pubertal development, brain development, and status in the progression when applying prevention and treatments to individuals for efforts to be most successful.
Supplementary Material
Highlights.
Cortisol is associated with substance use (SU) phenotypes across the SU progression
Associations observed at multiple levels, from intra-cellular action to psychological
Associations of cortisol and SU across the SU progression are often bi-directional
There is potential for some genetic confounding in cortisol-SU associations
Genetically informed designs are critically needed to test cortisol-SU associations
Acknowledgments
Funding. The funding for this study was provided by the National Institute on Drug Abuse: data collection: K01 DA039288 (PI: Marceau). Early work leading to this publication was supported by F31 DA033737 (PI: Marceau).
Footnotes
This website calculates a representation factor and the corresponding probability of finding an overlap of the number of genes in common across two sets. The representation factor is the number of overlapping genes divided by the expected number of overlapping genes, assuming independent groups, based on the number of genes in the human genome. The expected number of overlapping genes is calculated by the number of genes in the first set * the number of genes in the second set, over the total number of genes ((Ngenesset1* Ngenesset2)/ Ngenesgenome). The p-value is calculated using a normal approximation (see http://nemates.org/MA/progs/representation.stats.html).
When repeating this procedure with the smaller overlap set of 10 genes (with a minimum overlap of 3 to attain at least 25% of the genes on the overlap set were involved), 58 pathway-based sets; the vast majority were action pathways of specific drugs which each included SLC6A4, SLC6A3, and OPRM1 (set sizes were each 29 genes; pathway source: SMPDB). Several of the pathways were redundant with those overrepresented in the main analysis (see Table 1 note), and the other two over-represented pathways were the dopaminergic synapse and GPCR signaling-cholera toxin.
References
- Ackermann K, Plomp R, Lao O, Middleton B, Revell VL, Skene DJ, Kayser M, 2013. Effect of sleep deprivation on rhythms of clock gene expression and melatonin in humans. Chronobiology international 30, 901–909. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT, 2008. Are there genetic influences on addiction: Evidence from family, adoption and twin studies. Addiction (Abingdon, England) 103, 1069–1081. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL, 2005. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl) 181, 107–117. [DOI] [PubMed] [Google Scholar]
- Alink LRA, van Ijzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, Koot HM, 2008. Cortisol and externalizing behavior in children and adolescents: Mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Developmental psychobiology 50, 427–450. [DOI] [PubMed] [Google Scholar]
- Allebrandt KV, Teder-Laving M, Akyol M, Pichler I, Müller-Myhsok B, Pramstaller P, Merrow M, Meitinger T, Metspalu A, Roenneberg T, 2010. CLOCK gene variants associate with sleep duration in two independent populations. Biological psychiatry 67, 1040–1047. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub. [Google Scholar]
- Anstey KJ, Mack HA, Cherbuin N, 2009. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. The American journal of Geriatric psychiatry 17, 542–555. [DOI] [PubMed] [Google Scholar]
- Bartels M, Van den Berg M, Sluyter F, Boomsma DI, de Geus EJC, 2003. Heritability of cortisol levels: review and simultaneous analysis of twin studies. Psychoneuroendocrinology 28, 121–137. [DOI] [PubMed] [Google Scholar]
- Boker SM, Graham J, 1998. A dynamical systems analysis of adolescent substance abuse. Multivariate Behavioral Research 33, 479–507. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Hayward C, Direk N, Lewis JG, Hammond GL, Hill LA, Anderson A, Huffman J, Wilson JF, Campbell H, Rudan I, Wright A, Hastie N, Wild SH, Velders FP, Hofman A, Uitterlinden AG, Lahti J, Raikkonen K, Kajantie E, Widen E, Palotie A, Eriksson JG, Kaakinen M, Jarvelin MR, Timpson NJ, Davey Smith G, Ring SM, Evans DM, St Pourcain B, Tanaka T, Milaneschi Y, Bandinelli S, Ferrucci L, van der Harst P, Rosmalen JG, Bakker SJ, Verweij N, Dullaart RP, Mahajan A, Lindgren CM, Morris A, Lind L, Ingelsson E, Anderson LN, Pennell CE, Lye SJ, Matthews SG, Eriksson J, Mellstrom D, Ohlsson C, Price JF, Strachan MW, Reynolds RM, Tiemeier H, Walker BR, 2014. Genome wide association identifies common variants at the SERPINA6/SERPINA1 locus influencing plasma cortisol and corticosteroid binding globulin. PLoS genetics 10, e1004474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, Le DA, O’Dell LE, Overstreet DH, Roberts AJ, Sinha R, Valdez GR, Weiss F, 2005. Stress enhancement of craving during sobriety: a risk for relapse. Alcoholism, clinical and experimental research 29, 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, Fox ME, Hayes AS, Kalin NH, Essex MJ, Davidson RJ, Birn RM, 2012. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature neuroscience 15, 1736–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron H, Gould E, 1994. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience 61, 203–209. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP, 2004. Sex and estrogen influence drug abuse. Trends in pharmacological sciences 25, 273–279. [DOI] [PubMed] [Google Scholar]
- Chambers RA, 2013. Adult hippocampal neurogenesis in the pathogenesis of addiction and dual diagnosis disorders. Drug and alcohol dependence 130, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colder CR, Scalco M, Trucco EM, Read JP, Lengua LJ, Wieczorek WF, Hawk LW Jr., 2013. Prospective associations of internalizing and externalizing problems and their co-occurrence with early adolescent substance use. Journal of abnormal child psychology 41, 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comasco E, Nordquist N, Gokturk C, Aslund C, Hallman J, Oreland L, Nilsson KW, 2010. The clock gene PER2 and sleep problems: association with alcohol consumption among Swedish adolescents. Upsala journal of medical sciences 115, 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt E, Abar B, Lester BM, LaGasse LL, Shankaran S, Bada H, Bauer CR, Whitaker TM, Hammond JA, 2014. Cortisol Reactivity to Social Stress as a Mediator of Early Adversity on Risk and Adaptive Outcomes. Child development 85, 2279–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrod PJ, Nikolaou K, 2016. Annual Research Review: On the developmental neuropsychology of substance use disorders. Journal of child psychology and psychiatry, and allied disciplines 57, 371–394. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, 1998. Genetics of alcohol and other abused drugs. Drug & Alcohol Dependence 51, 61–71. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C, 2007. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacology Biochemistry and Behavior 86, 189–199. [DOI] [PubMed] [Google Scholar]
- Dawes MA, Dorn LD, Moss HB, Yao JK, Kirisci L, Ammerman RT, Tarter RE, 1999. Hormonal and behavioral homeostasis in boys at risk for substance abuse. Drug and alcohol dependence 55, 165–176. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA, 2011. The Adaptive Calibration Model of stress responsivity. Neuroscience & Biobehavioral Reviews 35, 1562–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, 2004. Acute Stressors and Cortisol Responses: A Theoretical Integration and Synthesis of Laboratory Research. Psychological bulletin 130, 355–391. [DOI] [PubMed] [Google Scholar]
- Dokoumetzidis A, Iliadis A, Macheras P, 2002. Nonlinear dynamics in clinical pharmacology: the paradigm of cortisol secretion and suppression. British journal of clinical pharmacology 54, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Bilbao A, Laucht M, Henriksson R, Yakovleva T, Ridinger M, Desrivieres S, Clarke T, Lourdusamy A, Smolka MN, Cichon S, Blomeyer D, Treutlein J, Perreau-Lenz S, Witt S, Leonardi-Essmann F, Wodarz N, Zill P, Soyka M, Albrecht U, Rietschel M, Lathrop M, Bakalkin G, Spanagel R, Schumann G, 2011. Effects of the circadian rhythm gene Period 1 (Per1) on psychosocial stress-induced alcohol drinking. American Journal of Psychiatry, 168, 1090–1098. [DOI] [PubMed] [Google Scholar]
- Doom JR, Gunnar MR, 2013. Stress physiology and developmental psychopathology: past, present, and future. Development and psychopathology 25, 1359–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer B, Zanjani F, Fardo DW, 2014. The relationship between midlife and late life alcohol consumption, APOE e4 and the decline in learning and memory among older adults. Alcohol and alcoholism (Oxford, Oxfordshire) 49, 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezkurdia I, Juan D, Rodriguez JM, Frankish A, Diekhans M, Harrow J, Vazquez J, Valencia A, Tress ML, 2014. Multiple evidence strands suggest that there may be as few as 19 000 human protein-coding genes. Human molecular genetics 23, 5866–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon E, McClung CA, 2009. A role for the circadian genes in drug addiction. Neuropharmacology 56 Suppl 1, 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH, 2005. A new view on hypocortisolism. Psychoneuroendocrinology 30, 1010–1016. [DOI] [PubMed] [Google Scholar]
- Gerritsen L, Comijs HC, Deeg DJ, Penninx BW, Geerlings MI, 2011. Salivary cortisol, APOE-epsilon4 allele and cognitive decline in a prospective study of older persons. Neurobiol Aging 32, 1615–1625. [DOI] [PubMed] [Google Scholar]
- Gerritsen L, Milaneschi Y, Vinkers C, van Hemert B, van Velzen L, Schmaal L, Penninx BW, 2017. HPA Axis Genes, and their Interaction with Childhood Maltreatment, are Related to Cortisol Levels and Stress-Related Phenotypes. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology [DOI] [PMC free article] [PubMed]
- Gervais A, O’loughlin J, Meshefedjian G, Bancej C, Tremblay M, 2006. Milestones in the natural course of onset of cigarette use among adolescents. Canadian Medical Association Journal 175, 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg NE, 1994. Overview: biological processes relevant to drugs of dependence. Addiction (Abingdon, England) 89, 1443–1446. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo K, 2007. The neurobiology of stress and development. Annual review of psychology 58, 145–173. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C, 2009. Developmental changes in hypothalamus?pituitary?adrenal activity over the transition to adolescence: Normative changes and associations with puberty 21, 69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson PA, Gustafsson PE, Anckarsater H, Lichtenstein P, Ljung T, Nelson N, Larsson H, 2011. Heritability of cortisol regulation in children. Twin research and human genetics : the official journal of the International Society for Twin Studies 14, 553–561. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PA, 2002. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Research 5, 113–124. [DOI] [PubMed] [Google Scholar]
- Helstrom A, Bryan A, Hutchison KE, Riggs PD, Blechman EA, 2004. Tobacco and alcohol use as an explanation for the association between externalizing behavior and illicit drug use among delinquent adolescents. Prevention science : the official journal of the Society for Prevention Research 5, 267–277. [DOI] [PubMed] [Google Scholar]
- Hersi M, Irvine B, Gupta P, Gomes J, Birkett N, Krewski D, 2017. Risk factors associated with the onset and progression of Alzheimer’s disease: A systematic review of the evidence. Neurotoxicology [DOI] [PubMed]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ, 2004. Family transmission and heritability of externalizing disorders: a twin-family study. Archives of general psychiatry 61, 922–928. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Schalet BD, Malone SM, Iacono WG, McGue M, 2011. Psychometric and genetic architecture of substance use disorder and behavioral disinhibition measures for gene association studies. Behavior genetics 41, 459–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KM, 2010. Progression through early drinking milestones in an adolescent treatment sample. Addiction (Abingdon, England) 105, 438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, Lange W, Bernzen J, Wetterling T, Rink L, Driessen M, 2003. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol and alcoholism (Oxford, Oxfordshire) 38, 189–193. [DOI] [PubMed] [Google Scholar]
- Juster R, 2010. Allostatic load biomarkers of chronic stress and impact on health and cognition, pp. 2–16. [DOI] [PubMed]
- Kalivas PW, Volkow ND, 2005. The neural basis of addiction: a pathology of motivation and choice. American Journal of Psychiatry 162, 1403–1413. [DOI] [PubMed] [Google Scholar]
- Kalsi G, Euesden J, Coleman JR, Ducci F, Aliev F, Newhouse SJ, Liu X, Ma X, Wang Y, Collier DA, Asherson P, Li T, Breen G, 2016. Genome-Wide Association of Heroin Dependence in Han Chinese. PloS one 11, e0167388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamburov A, Pentchev K, Galicka H, Wierling C, Lehrach H, Herwig R, 2011. ConsensusPathDB: toward a more complete picture of cell biology. Nucleic acids research 39, D712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamburov A, Wierling C, Lehrach H, Herwig R, 2009. ConsensusPathDB--a database for integrating human functional interaction networks. Nucleic acids research 37, D623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariyawasam SH, Zaw F, Handley SL, 2002. Reduced salivary cortisol in children with comorbid Attention deficit hyperactivity disorder and oppositional defiant disorder. Neuro endocrinology letters 23, 45–48. [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Corey LA, Prescott CA, Neale MC, 1999. Genetic and environmental risk factors in the aetiology of illicit drug initiation and subsequent misuse in women. British Journal of Psychiatry 175, 351–356. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Maes HH, Sundquist K, Ohlsson H, Sundquist J, 2014. Genetic and family and community environmental effects on drug abuse in adolescence: a Swedish national twin and sibling study. The American journal of psychiatry 171, 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Munisamy G, de Wit H, Lin S, 2006. Attenuated cortisol response to alcohol in heavy social drinkers. International journal of psychophysiology 59, 203–209. [DOI] [PubMed] [Google Scholar]
- Koob GF, Kreek MJ, 2007. Stress, Dysregulation of Drug Reward Pathways, and the Transition to Drug Dependence. The American journal of psychiatry 164, 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 2001. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 24, 97–129. [DOI] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP, 2010. Neurobiological mechanisms of addiction: focus on corticotropin-releasing factor. Current opinion in investigational drugs (London, England : 2000) 11, 63–71. [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS, 2005. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nature neuroscience 8, 1450–1457. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Wang L, Miles MF, Zhang L, Dodd PR, Harris RA, 2000. Gene expression in human alcoholism: microarray analysis of frontal cortex. Alcoholism, clinical and experimental research 24, 1873–1882. [PubMed] [Google Scholar]
- Li C-Y, Mao X, Wei L, 2008. Genes and (common) pathways underlying drug addiction. PLoS Computational Biology 4, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, 2006. Cortisol secretion patterns in addiction and addiction risk. International Journal of Psychophysiology 59, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J, n.d. Nematode bioinformatics analysis tools and data University of Kentucky. [Google Scholar]
- Marceau K, Ruttle PL, Shirtcliff EA, Essex MJ, Susman EJ, 2015. Developmental and contextual considerations for adrenal and gonadal hormone functioning during adolescence: Implications for adolescent mental health. Developmental psychobiology 57, 742–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavroudis PD, Scheff JD, Calvano SE, Lowry SF, Androulakis IP, 2012. Entrainment of peripheral clock genes by cortisol. Physiological Genomics 44, 607–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurnett K, King J, Scarpa A, 2003. The hypothalamic-pituitary-adrenal system (HPA) and the development of aggressive, antisocial, and substance abuse disorders. Neurodevelopmental mechanisms in psychopathology, 324–344.
- McClung CA, 2007. Circadian rhythms, the mesolimbic dopaminergic circuit, and drug addiction. TheScientificWorldJournal 7, 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, 2005. Stressed or stressed out: what is the difference? Journal of psychiatry & neuroscience : JPN 30, 315–318. [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gray JD, Nasca C, 2015. Recognizing resilience: Learning from the effects of stress on the brain. Neurobiology of Stress 1, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Janssen T, Jackson KM, 2017. The prospective association between sleep and initiation of substance use in young adolescents. Journal of Adolescent Health 60, 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Goddings A-L, Herting MM, Meuwese R, Blakemore S-J, Crone EA, Dahl RE, Güroğlu B, Raznahan A, Sowell ER, Tamnes CK, 2016. Structural brain development between childhood and adulthood: Convergence across four longitudinal samples. NeuroImage 141, 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Vanyukov MM, Martin CS, 1995. Salivary cortisol responses and the risk for substance abuse in prepubertal boys. Biological psychiatry 38, 547–555. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse [NIDA], 2017. Trends & Statistics
- Nelson EE, Leibenluft E, McClure EB, Pine DS, 2005. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological medicine 35, 163–174. [DOI] [PubMed] [Google Scholar]
- Nguyen‐Louie TT, Brumback T, Worley MJ, Colrain IM, Matt GE, Squeglia LM, Tapert SF, 2017. Effects of sleep on substance use in adolescents: a longitudinal perspective. Addiction biology [DOI] [PMC free article] [PubMed]
- Panza F, Capurso C, D’Introno A, Colacicco AM, Frisardi V, Lorusso M, Santamato A, Seripa D, Pilotto A, Scafato E, Vendemiale G, Capurso A, Solfrizzi V, 2009. Alcohol drinking, cognitive functions in older age, predementia, and dementia syndromes. Journal of Alzheimer’s disease : JAD 17, 7–31. [DOI] [PubMed] [Google Scholar]
- Parekh PK, Ozburn AR, McClung CA, 2015. Circadian clock genes: effects on dopamine, reward and addiction. Alcohol 49, 341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi E, 2012. ENCODE Project Writes Eulogy for Junk DNA. Science 337, 1159–1161. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Webster MJ, Herman MM, Kleinman JE, Weickert CS, 2007. Age-related differences in glucocorticoid receptor mRNA levels in the human brain. Neurobiology of Aging 28, 447–458. [DOI] [PubMed] [Google Scholar]
- Perogamvros I, Ray DW, Trainer PJ, 2012. Regulation of cortisol bioavailability--effects on hormone measurement and action. Nature reviews. Endocrinology 8, 717–727. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Wilkinson CW, Petrie EC, Schellenberg GD, Raskind MA, 2001. Increased CSF cortisol in AD is a function of APOE genotype. Neurology 56, 1094–1098. [DOI] [PubMed] [Google Scholar]
- Peters A, McEwen BS, 2012. Introduction for the allostatic load special issue. Physiology & behavior 106, 1–4. [DOI] [PubMed] [Google Scholar]
- Raine A, 1996. Autonomic Nervous System Factors Underlying Disinhibited, Antisocial, and Violent Behavior Biosocial Perspectives and Treatment Implicationsa. Annals of the New York Academy of Sciences 794, 46–59. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC, 2003. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Archives of general psychiatry 60, 1256–1264. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL, 2008. Alcohol self‐administration acutely stimulates the hypothalamic‐pituitary‐adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. European Journal of Neuroscience 28, 1641–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Ananda S, Jarvik ME, 1983. Cigarette smoking during anxiety-provoking and monotonous tasks. Addictive behaviors 8, 353–359. [DOI] [PubMed] [Google Scholar]
- Sandler I, Wolchik SA, Cruden G, Mahrer NE, Ahn S, Brincks A, Brown CH, 2014. Overview of Meta-Analyses of the Prevention of Mental Health, Substance Use and Conduct Problems. Annual review of clinical psychology 10, 243–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, 2003. Stress and Plasticity in the Limbic System. Neurochemical research 28, 1735–1742. [DOI] [PubMed] [Google Scholar]
- Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schurmeyer TH, Kirschbaum C, 1999. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life sciences 64, 1653–1660. [DOI] [PubMed] [Google Scholar]
- Schoenfeld T, Gould E, 2012. Stress, Stress Hormones, and Adult Neurogenesis. Experimental neurology 233, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherva R, Wang Q, Kranzler H, Zhao H, Koesterer R, Herman A, Farrer LA, Gelernter J, 2016. Genome-wide Association Study of Cannabis Dependence Severity, Novel Risk Variants, and Shared Genetic Risks. JAMA psychiatry 73, 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Vitacco MJ, Graf AR, Gostisha AJ, Merz JL, Zahn-Waxler C, 2009. Neurobiology of empathy and callousness: Implications for the development of antisocial behavior. Behavioral Sciences & the Law 27, 137–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Dager AD, Cohen-Gilbert JE, Sneider JT, 2016. Neurobiological signatures associated with alcohol and drug use in the human adolescent brain. Neuroscience and biobehavioral reviews 70, 244–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, 2011. New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep 13, 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, 2013. The role of brain development in drug effect and drug response. Biological Research on Addiction 2, 271–280. [Google Scholar]
- Spear LP, Silveri MM, 2016. Special Issue on the Adolescent Brain. Neuroscience and biobehavioral reviews 70, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens MA, Wand G, 2012. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol research : current reviews 34, 468–483. [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, van Jaarsveld CH, Semmler C, Plomin R, Wardle J, 2009. Heritability of daytime cortisol levels and cortisol reactivity in children. Psychoneuroendocrinology 34, 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Center on Addiction and Substance Abuse [CASA], C.U., 2011. Adolescent substance use: America’s #1 public health problem, New York: CASA, p. 406. [Google Scholar]
- Tottenham N, Galván A, 2016. Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neuroscience & Biobehavioral Reviews 70, 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchoa ET, Aguilera G, Herman JP, Fiedler JL, Deak T, Cordeiro de Sousa MB, 2014. Novel aspects of hypothalamic-pituitary-adrenal axis regulation and glucocorticoid actions. Journal of neuroendocrinology 26, 557–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velders FP, Kuningas M, Kumari M, Dekker MJ, Uitterlinden AG, Kirschbaum C, Hek K, Hofman A, Verhulst FC, Kivimaki M, Van Duijn CM, Walker BR, Tiemeier H, 2011. Genetics of cortisol secretion and depressive symptoms: a candidate gene and genome wide association approach. Psychoneuroendocrinology 36, 1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltman C, Blevins L Jr, Boyd G, Wand GS, 1993. The effects of mild ethanol intoxication on the hypothalamic-pituitary-adrenal axis in nonalcoholic men. The Journal of Clinical Endocrinology & Metabolism 77, 518–522. [DOI] [PubMed] [Google Scholar]
- Warren CM, Riggs NR, Pentz MA, 2017. Longitudinal relationships of sleep and inhibitory control deficits to early adolescent cigarette and alcohol use. J Adolesc 57, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill L, Agrawal A, Kapoor M, Bertelsen S, Bierut LJ, Brooks A, Dick D, Hesselbrock M, Hesselbrock V, Koller DL, Le N, Nurnberger JI, Salvatore JE, Schuckit M, Tischfield JA, Wang J-C, Xuei X, Edenberg HJ, Porjesz B, Bucholz K, Goate AM, Foroud T, 2015. Association of substance dependence phenotypes in the COGA sample. Addiction biology 20, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Martelon M, Joshi G, Bateman C, Fried R, Petty C, Biederman J, 2011. Does ADHD predict substance-use disorders? A 10-year follow-up study of young adults with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 50, 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhabenko O, Austic E, Conroy DA, Ehrlich P, Singh V, Epstein-Ngo Q, Cunningham RM, Walton MA, 2016. Substance Use as a Risk Factor for Sleep Problems Among Adolescents Presenting to the Emergency Department. Journal of Addiction Medicine 10, 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M, 1979. Sensation seeking: Beyond the optimal level of arousal Earlbaum, Hillsdale, NJ. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.