Abstract
Objectives:
Growing evidence suggests dietary factors influence cognition, but the effects of nutrient intake on cerebral metabolism in adults are currently unknown. The present study investigated the relationship between major macronutrient intake (fat, carbohydrate, and protein) and cerebral neurochemical profiles in middle-aged adults.
Methods:
Thirty-six adults recorded dietary intake for three days prior to completing cognitive testing and a proton magnetic resonance spectroscopy (1H MRS) scan. 1H MRS of occipitoparietal gray matter was used to assess glutamate (Glu), N-acetyl aspartate (NAA), choline (Cho), and myo-inositol (mI) relative to creatine (Cr) levels.
Results:
Regression analyses revealed that high intake of polyunsaturated fatty acids (PUFA) was associated with lower cerebral Glu/Cr (p = .005), and high intake of saturated fat (SFA) was associated with poorer memory function (p = .030) independent of age, sex, education, estimated intelligence, total caloric intake, and body mass index.
Discussion:
In midlife, greater PUFA intake (ω−3 and ω−6) may be associated with lower cerebral glutamate, potentially indicating more efficient cellular reuptake of glutamate. SFA intake, on the other hand, was linked with poorer memory performance. These results suggest that dietary fat intake modification may be an important intervention target for the prevention of cognitive decline.
Keywords: Diet, Polyunsaturated fat, Saturated fat, Proton magnetic resonance spectroscopy, Executive function, Memory, Aging
As life expectancy increases, the burden of age-related cognitive decline and dementia continues to grow, and is currently estimated to cost the global population $600 billion per year (1). Effective therapies for dementia are currently limited (2) and identification of modifiable risk factors is imperative for preserving cognitive function throughout the lifespan. Accumulating evidence suggests an important role for diet in healthy brain function. High carbohydrate intake has been linked with increased risk for cognitive impairment (3), while high polyunsaturated fat (PUFA) intake may be protective against dementia (4, 5). High omega-3 (ω−3) PUFA consumption in particular is seen as especially beneficial, associated with better cognitive performance (6) and higher brain integrity (7, 8) including better regulation of glutamatergic synapses, which play a crucial role in memory (9).
Given existing evidence supporting the effects of diet on cognition, it is logical to suppose that nutrition affects cerebral metabolites of neurobiological significance. Cerebral neurochemical profiles provide sensitive indicators of brain health before the onset of cognitive impairment; yet to date, no known studies have examined nutrient intake in relation to cerebral metabolism in middle-aged adults. In the current study, proton magnetic resonance spectroscopy (1H MRS) was used to quantify several neurometabolites relevant to cognitive function (10) including N-acetyl-aspartate (NAA), an index of neuronal viability, glutamate (Glu), a major excitatory neurotransmitter critical for memory function (11) but neurotoxic at high concentrations (12), choline containing compounds (Cho) such as phosphocholine and glycerophosphocholine, an indicator of phospholipid composition, myo-inositol (mI), an organic osmolite and putative marker of gliosis, and creatine (Cr), a marker of energy metabolism (10, 13). Therefore the aims of this project were to explore the relationship between major dietary macronutrients (carbohydrates, protein, PUFA, and saturated fat) and cerebral neurochemical profiles in healthy, middle-aged adults. As dietary ω−3 PUFAs are believed to play a role in the regulation of glutamatergic synapses (9), it was hypothesized that greater dietary PUFA intake would be associated with lower, free cerebral Glu concentrations, potentially indicating more efficient cerebral glutamate reuptake in our sample of cognitively intact middle-aged adults. Furthermore, based on previous work indicating opposite effects of carbohydrate and protein intake on risk for cognitive impairment (3), similar effects of nutrient intake on neuronal viability were expected, in that greater carbohydrate intake would be related to lower NAA, while greater protein intake would be associated with higher NAA.
Methods
Participants
Participants were recruited through flyers and newspaper advertisements as part of a larger fitness and brain function study. Individuals were excluded if they reported a history of coronary artery disease, metabolic dysfunction (e.g., hypertension, dyslipidemia, insulin resistance), smoking, or use of vasoactive medications in order to control for any sources of bias in the participant sample that may relate to brain functioning or nutritional intake. Additionally, participants with reported neurological disease (e.g., stroke, Parkinson’s disease, clinically significant traumatic brain injury), major psychiatric illness (e.g., schizophrenia, bipolar disorder), or substance abuse were not eligible for the study. Body mass in kilograms and height in centimeters were determined for all participants using a physician’s beam balance scale in order to calculate body mass index (BMI). BMI was calculated by dividing body mass in kilograms by height in meters squared and was included as a covariate in statistical analyses.
Forty individuals were asked to record dietary intake for three consecutive days in addition to cognitive testing and MRI scanning as part of a larger fitness and brain function study. Two participants were excluded from analyses due to poor quality 1H MRS data (Cramer-Rao Lower Bounds for NAA/Cr, Glu/Cr, mI/Cr, or Cho/Cr >12%). Two additional participants were excluded from analyses based on reported energy intakes below or above three standard deviations from the sample mean. The final sample consisted of 36 participants.
Procedure
The study was conducted in accordance with the guidelines of the Helsinki Declaration of 1975 and with approval from the University of Texas at Austin Institutional Review Board. The funding institutions did not play any role in data collection, analysis, or manuscript preparation. Data were collected from September 2010 to March 2012. All volunteers provided written informed consent before participating in the study. Participants first completed a medical history questionnaire to determine whether any exclusionary medical conditions or treatments were present. A general health assessment, cardiorespiratory fitness assessment, and neuropsychological/brain imaging assessment were administered to all eligible participants. Additionally, three-day dietary intake was determined for all participants. The assessments were conducted on separate days within two months of the initial assessment. Participants did not report any major lifestyle or dietary changes during the data collection period.
Diet Assessment
Participants were asked to complete a 3-day food record for three consecutive days of their choice. Forty-four percent of the records were completed over three consecutive weekdays, while the remaining food records were completed over a three-day period spanning both weekday and weekend days. Characterization of nutrient intake using 3-day food records has shown high agreement with both 9-day food records and the food frequency questionnaire (14). Dietary intake entries for all participants were clarified by a trained research assistant and analyzed by a Registered Dietitian using Nutritionist Pro Software (Axxya Systems, Stafford, Texas, USA). Average energy and macronutrient intake per day, including a detailed breakdown of carbohydrate, protein, PUFA (ω−3 and ω−6 fatty acids), and saturated fat (SFA) intake in grams, was calculated and provided by the software.
Neuropsychological Evaluation
Participants completed a battery of standard clinical neuropsychological instruments with established reliability and validity (15). To reduce the number of statistical comparisons, the measures were grouped according to two cognitive domains: memory and executive function. In order to compute domain scores, raw scores were converted to z-scores using the participant sample’s mean and standard deviation. Timed-test scores were adjusted by multiplying by −1 so that higher scores indicated better performance. The memory domain score was determined by averaging the following tests: California Verbal Learning Test-II immediate recall and delayed recall, and recognition discrimination (16). The attention-executive function domain score was calculated by averaging the following tests: Trail making A and B time to completion (17), WAIS-III Digit Span Subtest (18), and a verbal 2-Back task accuracy and reaction time (administered during the MRI scan session (19). Global cognitive functioning was assessed by administration of the Mini Mental Status Exam (MMSE; (20), and a measure of estimated intelligence was calculated based on participants scores on the Weschler Test of Adult Reading (21). A trained research assistant administered and scored the neuropsychological test battery using standard administration and scoring criteria.
1H-magnetic resonance spectroscopy
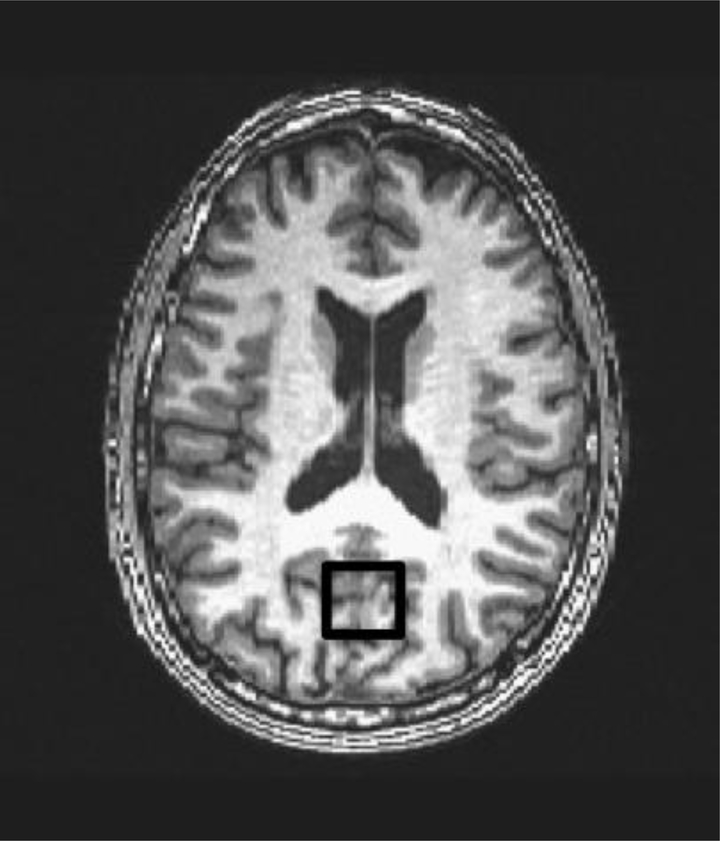
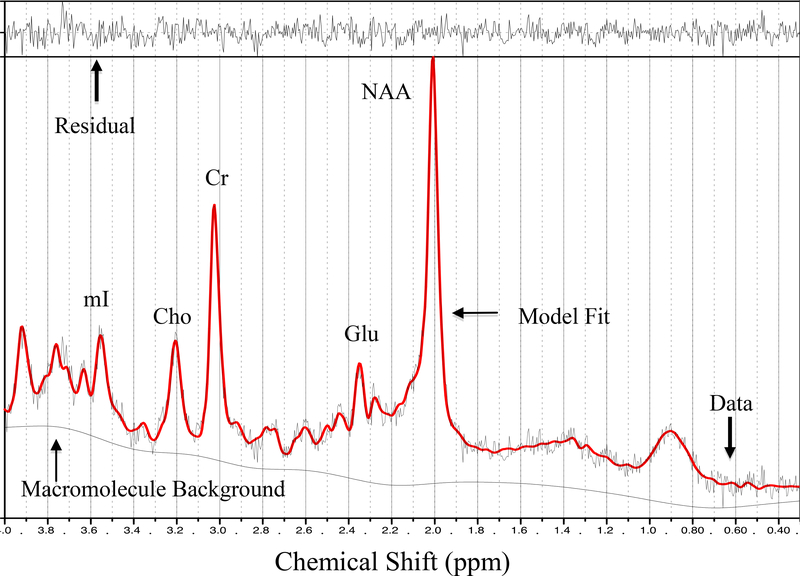
1H MRS data for participants were obtained from a single session using a 3T GE Signa Excite MRI scanner equipped with a standard head coil. Single voxel proton MRS was performed using the GE pulse sequence PROBE-P, an automated point resolved spectroscopy sequence with chemical shift selected water suppression. Each spectroscopic voxel was prescribed from 3D high-resolution spoiled gradient echo sagittal images (256 × 256 matrix, FOV = 24 × 24 cm2, 1 mm slice thickness, 0 gap) of the entire brain. 1H-MRS parameters were as follows: echo time/repetition time (TE/TR)=35/3000 ms, 128 excitations, 5,000 Hz spectral width, volume ~6 cm3 from occipitoparietal gray matter including the posterior cingulate gyrus (Figure 1). The posterior cingulate gyrus is an important region of interest in relation to cognition. It is responsible for efficiency of cognitive processing and attention (22), and metabolic changes in that region have been implicated in early stages of dementia (23). In addition, alterations in the concentrations of MRS-visible metabolites in the posterior cingulate have demonstrated sensitivity to aging (24), neurodegenerative diseases (25), sedentary lifestyle (26), and obesity (27). A single experimenter localized the voxel placement on all subjects in order to ensure the quality of the data. A digital archive of the voxel placement was saved and reviewed for consistency. The concentrations of Glu, NAA, Cho, and mI were reported as ratios relative to Cr, which is considered to be the most stable metabolite available for use as an internal reference (10, 25). Although absolute concentrations of neurometabolites would be ideal, the present methods were limited to assessing relative neurometabolites concentrations to Cr. The average Cramer-Rao lower bound values for all metabolites were below 8%, thus suggesting that the concentrations were reliably estimated. LCModel, a commercially available software, was used to quantify and separate the metabolite resonances from the macromolecule background (29) (Figure 2). The metabolites were quantified at the following resonance frequencies: Glu, 2.34 ppm; NAA, 2.02 ppm; total Cho, 3.25 ppm; Cr, 3.03 ppm; and mI, 3.56 ppm.
Figure 1.
Anatomical image with superimposed voxel outline of 1H MRS volume in the occipitoparietal gray matter.
Figure 2.
Representative 1H MRS spectrum. mI = myo-inositol; Cho = choline + phosphocholine; Cr = creatine + phosphocreatine; Glu = glutamate; NAA = N-acetyl-aspartate
Statistical Analyses
Regression analyses were used to further assess the relationships between nutrient intake and neurochemical concentrations (Glu/Cr, NAA/Cr, Cho/Cr, and mI/Cr in the occipitoparietal gray matter). A priori covariates, including total caloric intake, age, sex, education level, estimated full-scale intellectual quotient (IQ), and BMI were controlled for in analyses to minimize sources of bias in the data. All statistical analyses were performed using SPSS 21.0 (SPSS Inc., Chicago, IL). Because multiple comparisons were made, an adjusted Bonferroni corrected alpha level of 0.0062 was used as the statistical significance criterion.
Further regression analyses were conducted to explore the significant association between dietary fat and cerebral metabolism in relation to cognitive performance. A two-tailed alpha level of 0.05 was used as the criterion for significance because of the exploratory nature of the analyses.
Results
Descriptive Statistics
Demographic and nutritional intake variables for the participant sample (N = 36) are summarized in Table 1. Twenty-five participants were men (69%) and 11 were women (31%). All participants were healthy, middle-aged adults (M = 54.11 years, SD = 5.66).
Table 1.
Selected demographic characteristics and average nutrient intake per day from 3-day food record.
| N = 36 | M | SD |
|---|---|---|
| Demographic and Physiological Characteristics | ||
| Age | 54.11 | 5.66 |
| Education (years) | 16.69 | 2.25 |
| BMI (kg/m2) | 25.05 | 4.47 |
| Average Nutrient Intake | ||
| Total caloric intake (kcal/day) | 1838.46 | 460.62 |
| Carbohydrate (g) | 220.83 | 81.51 |
| Protein (g) | 82.15 | 27.75 |
| Total fat (g) | 69.90 | 29.72 |
| Saturated fat (g) | 20.87 | 9.24 |
| Polyunsaturated fat (PUFA) (g) | 11.38 | 6.33 |
| ω−3 PUFA (g) | 1.24 | 0.82 |
| ω−6 PUFA (g) | 9.17 | 6.00 |
Nutrient intake and cerebral metabolism
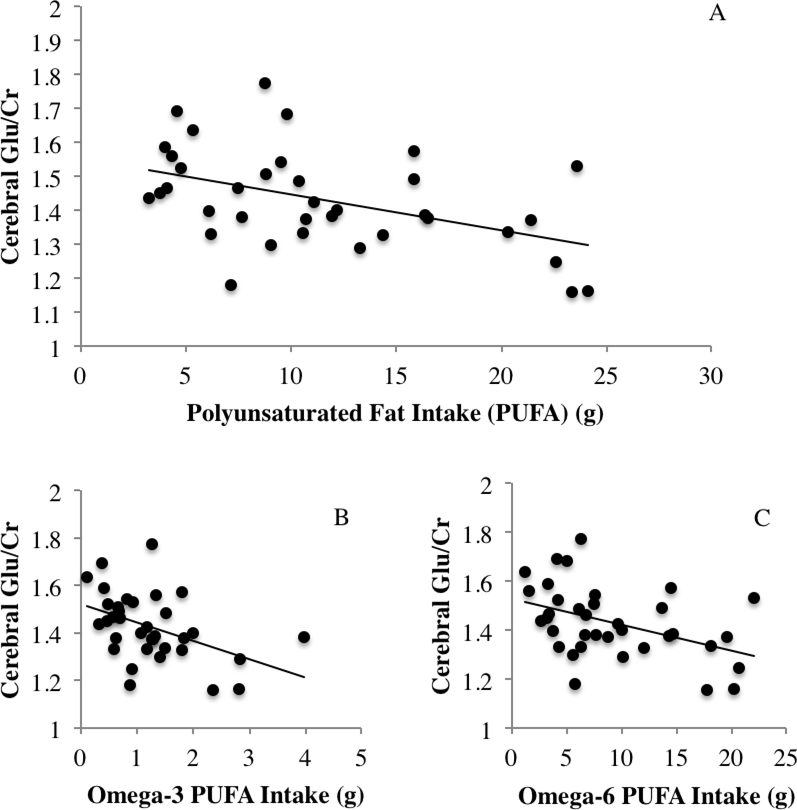
The association between total dietary PUFA intake and cerebral Glu/Cr was the only relationship that met criteria for statistical significance (adjusted Bonferroni corrected alpha of 0.0062) in the initial linear regression analyses after controlling for age, sex, education, estimated IQ, total caloric intake, saturated fat intake, and BMI, (β = −.542, t = −3.025, p = 0.005; Figure 3a). Contrary to our hypotheses, there were no significant effects of carbohydrate (β = .384, t = 1.578, p = 0.126) or protein intake (β = .062, t = .255, p = 0.824) on cerebral NAA/Cr. However, there was a statistical trend suggesting greater carbohydrate intake was associated with higher cerebral Cho/Cr (β = .661, t = 2.252, p = 0.037). All other relationships between macronutrient intake and cerebral Glu/Cr, mI/Cr, NAA/Cr, or Cho/Cr concentrations were non-significant (ps > .10). The concentrations and standard deviations for metabolites are listed in Table 2.
Figure 3.
A-C. Inverse association between cerebral glutamate and total PUFA (A), omega-3 (B), and omega-6 PUFA (C).
Table 2.
Neuropsychological assessment and cerebral metabolite concentrations.
| N = 36 | M | SD |
|---|---|---|
| Global cognitive functioning | ||
| MMSE | 28.75 | 1.36 |
| Estimated Intelligence | ||
| FSIQ | 112.11 | 7.53 |
| Memory (z score) | −0.06 | 0.93 |
| CVLT-II immediate recall | 10.78 | 3.37 |
| CVLT-II delayed recall | 11.17 | 3.15 |
| CVLT-II discriminability index | 2.97 | 0.76 |
| Attention-executive function (z score) | −0.06 | 0.71 |
| Trail Making Test A time, seconds | 29.33 | 7.49 |
| Trail Making Test B time, seconds | 62.06 | 15.99 |
| 2-Back accuracy, % correct | 74.33 | 11.61 |
| 2-Back reaction time, ms | 1166.59 | 237.38 |
| WAIS-III Digit Span Subtest | 18.75 | 4.48 |
| Neurochemical concentration | ||
| Glu/Cr (Cramer Rao Lower Bound) | 1.43 (7.36) | 0.15 (0.99) |
| mI/Cr (Cramer Rao Lower Bound) | 0.67 (6.92) | 0.07 (1.30) |
| NAA/Cr (Cramer Rao Lower Bound) | 1.41 (3.22) | 0.10 (0.59) |
| Cho/Cr (Cramer Rao Lower Bound) | 0.17 (5.00) | 0.02 (0.72) |
Based on the significant association between PUFA and cerebral Glu/Cr, further exploratory regression analyses using an alpha level of 0.05 revealed that both ω−3 PUFA (β = −.404, t = −2.731, p = 0.011) and ω−6 PUFA (β = −.470, t = −2.601, p = 0.015) were associated with lower Glu/Cr concentration (Figure 3b-c). The effect of SFA was non-significant in all of the models (ps > 0.10).
Dietary fat intake and cognitive function
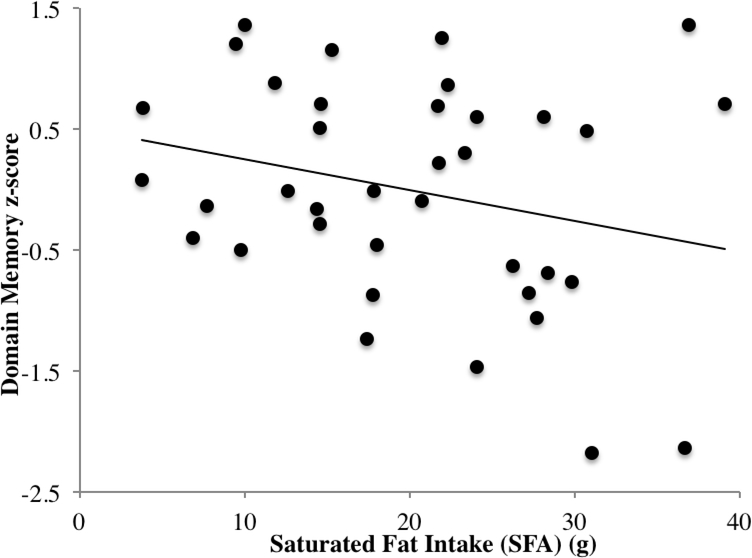
Cognitive test scores are reported in Table 2. Dietary PUFAs were not significantly related to indices of global cognitive status, the domain memory score, or domain attention executive function score. However, the effect of SFA intake was significant, suggesting that greater intake of SFA was associated with poorer performance in the composite memory domain (β = −.469, t = −2.286, p = 0.030; Figure 4). There were no other significant associations between dietary fat components and cognitive function in memory or attention-executive domains (ps > 0.16).
Figure 4.
Inverse relationship between saturated fat intake (g) and domain score memory performance.
Discussion
The present study is the first to investigate the relationship between nutrient intake and cerebral metabolism in middle-aged adults. The results provide evidence that dietary habits may influence cerebral metabolism and cognitive performance in healthy, middle-aged adults. Major findings for the current study were that total PUFA intake (both ω−3 and ω−6 PUFA), was associated with lower cerebral Glu in occipitoparietal gray matter. Additionally, intake of SFA was associated with poorer overall memory performance. The results were significant after adjusting for total caloric intake, age, sex, education, estimated intelligence, and BMI, suggesting that variation in dietary fat intake may independently account for cerebral metabolism alterations and cognitive functioning.
Maintaining low cerebral Glu in midlife may be protective against future cognitive decline, as accumulation of extracellular Glu is neurotoxic and may increase brain vulnerability to neurodegenerative diseases (12, 30). Glu functioning is critical for learning and memory (11) and excessive Glu due to alterations in Glu uptake and transport may be partly responsible for cognitive decline in the aging brain (31). In fact, recent work in aged rats demonstrated that administration of a pharmacological Glu modulator was associated with increased hippocampal spine density that correlated with memory performance (32). One potential mechanism through which dietary ω−3 PUFA may exert cognitive benefits is through regulation of glutamatergic synapses (9). The membranes of astrocytes contain high concentrations of the ω−3 PUFA, docosahexaenoic acid (DHA), and previous work has suggested that astroglial functioning is related to dietary ω−3 PUFA (33, 34). Surrounding astrocytes of neuronal compartments are thought to maintain efficient transportation of Glu in the neuronal environment (35), and the degeneration of astroglia that occurs progressively with age may compromise regulatory functions and disrupt Glu homeostasis (36). Therefore, we speculate that a diet in support of efficient astroglial functioning may also promote healthy brain aging. However, future studies that employ direct methods of measuring astroglial functioning are needed to discern whether lowered Glu concentrations associated with ω−3 PUFA intake are a result of greater uptake by astrocytes, or rather an effect of some other mechanism such as reduced biosynthesis of Glu.
Interestingly, dietary ω−6 PUFA was also related to lower cerebral Glu in the present study. Available research describing the relationship between dietary ω−6 PUFA and the brain have shown mixed results. Some studies have suggested protective effects of ω−6 PUFA against Alzheimer’s disease (37), while others have shown opposite effects of ω−6 PUFA on cognitive functioning (38). Dietary ω−6 PUFAs have also been linked with pro-inflammatory (39) and atherogenic processes (40). However, it is possible these detrimental effects may vary depending on relative ω−3 PUFA in the diet or apolipoprotein E genotype (41, 42). Given the presence of ω−6 PUFA in certain foods associated with Mediterranean style diets (i.e., walnuts, almonds, olive oil), known for their beneficial effects on brain and cardiovascular function (43), further investigation of dietary ω−6 PUFA and the brain appears warranted.
Previous work has established that individuals with metabolic syndrome, a condition associated with both current cognitive dysfunction and future cognitive decline (44, 45), show elevated cerebral Glu (46), which points toward the regulation of Glu transmission as a potential area of early intervention for brain aging. In particular, Haley et al. (2012) noted that peripheral atherosclerosis moderated elevated cerebral Glu concentrations, suggesting that arterial wall thickening may be instrumental in the neurotoxic accrual of Glu in the brain (46). Previous studies have demonstrated a link between ω−3 PUFA intake and lowered prevalence of atherosclerosis (47), which proposes a vascular mechanism by which dietary ω−3 PUFA may support brain health. Individuals with evidence of metabolic dysfunction were excluded from the current study; however, future investigation of dietary fat intake in adults with atherosclerosis may be informative of the pathogenesis of cerebral Glu accumulation.
Carbohydrate, protein, and SFA intake were not significantly related to neuronal viability or other cerebral metabolites in the current study. However, excessive carbohydrate and SFA intake have been implicated in obesity and metabolic dysfunction (48, 49), both of which have been linked with the development of cognitive impairment (44, 45). Although unrelated to measures of cerebral metabolism, dietary SFA was significantly associated with poorer overall memory performance. Diets high in SFA have been linked with detriments in cognitive performance and later development of cognitive impairment (50, 51). SFA intake has also been associated with components of metabolic syndrome, such as elevated cholesterol (52) and insulin resistance (53), as well as β-amyloid production and deposition in the brain (54). Furthermore, several other reports have shown deficits in hippocampal-dependent learning and memory along with reductions in brain-derived neurotropic factor, increased blood-brain barrier permeability, and increased pro-inflammatory cytokines related to SFA-rich diets (55). The current study sample consisted of healthy adults, suggesting that dietary SFA may potentially influence cognition independently of clinically significant metabolic changes. However, future studies may wish to investigate the relationship between dietary intake and cerebral metabolites in populations with impaired vascular and metabolic functioning.
Although the present study is the first report of dietary intake and cerebral metabolism in middle-aged adults, prior studies investigating the role of dietary ω−3 using 1H MRS during early stages of development and childhood have reported conflicting results. Previous work in rats suggests that perinatal deprivation of dietary ω−3 is associated with reduced cerebral mI in the prefrontal cortex of adult rats, but no effects were observed on combined concentration of Glu and glutamine (Glx; (56). Furthermore, other work has suggested that children maintaining low dietary DHA intake exhibit lower mI, NAA, Cho, and Cr in the anterior cingulate cortex, but show no significant differences in Glx concentration, relative to high dietary DHA consumers (57). Taken together along with the results of the present study, these findings may suggest that the effects of dietary ω−3 on cerebral metabolism vary throughout the lifespan. Dietary ω−3 is believed to play a critical role in brain development during early stages of life and may influence brain functioning into adulthood (58). Although all participants in the current study were healthy adults, perinatal and childhood intake of ω−3 for participants is unknown, and therefore, we cannot rule out long-term nutritional effects that result from early stages of development. Yet, other explanations for these conflicting results may be that previous studies did not distinguish Glu from glutamine, and therefore could only assess the combination of these two metabolites. Furthermore, the current study measured cerebral metabolism in the posterior cingulate cortex, whereas the previous studies assessed neurometabolites in the prefrontal cortex and anterior cingulate. The posterior cingulate cortex is a region known to be sensitive to early stages of dementia as well as other factors such as body weight and sedentary status (23–26, 59), and therefore may exhibit greater sensitivity to dietary changes compared to other brain regions.
Limitations for the current study include the cross-sectional study design and small sample size. The relatively small and healthy sample suggests that these findings should be considered preliminary and may not generalize to a broader population. Yet, a significant strength of the current study is the novel investigation of dietary intake in relation to cerebral metabolism and cognition. The use of a healthy sample allows for the assessment of cerebral metabolites independent of impaired metabolic or vascular functioning, which have been previously shown to impact cerebral metabolism. Although a validated dietary record was used to characterize nutritional intake, limitations associated with the use of food records should also be addressed in that they reflect self-report of the participant and rely on participant motivation for accuracy. Although food records may have higher validity and reliability than food questionnaires (14), one weakness may be the inability to capture longer-term, weekly, or seasonal variance in diet. Furthermore, examination of dietary fat intake in the present study was limited to dietary PUFA and saturated fat, which does not account for potential effects of other dietary fat components such as monounsaturated fat and trans fat that may affect cognition and brain functioning. Lastly, while it is plausible that dietary fat intake may be driving the observed effects, it is also possible that cerebral metabolite concentrations may be related to genetic influences or other lifestyle factors.
In summary, greater intake of total PUFA (both ω−3 and ω−6 PUFA) was associated with lower cerebral Glu in the occipitoparietal gray matter. Additionally, dietary SFA was related to poorer overall memory performance. To the authors’ knowledge, this is the first study to examine the relationship between nutrient intake and brain metabolites in middle-aged adults. Glu has previously been identified as a sensitive marker for conditions of brain vulnerability, and therefore may aid in the identification of dietary nutrients or development of nutritional interventions that support brain health. Future studies may wish to investigate the relationship between diet and cerebral metabolism in populations at risk for cognitive decline, as well as implement specific dietary interventions aimed at the preservation of brain functioning.
Acknowledgements
APH, HT, MMG, and TT designed research; MMG, TT, and CKC conducted research; APH, JND, and HT provided essential materials and instruments; SO performed statistical analyses and wrote paper; SO and APH had primary responsibility for final content. All authors read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Supported in part by American Federation for Aging Research grant 8A0024 to APH and National Institutes of Health grant R01 NS075565 to APH
References
- 1.Wimo AP M World Alzheimer report: The global economic impact of dementia.. United Kingdom, London: Alzheimer’s Disease International, 2010. [Google Scholar]
- 2.Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, Booker L, Oremus M. Effectiveness of Cholinesterase Inhibitors and Memantine for Treating Dementia: Evidence Review for a Clinical Practice Guideline. Annals of Internal Medicine 2008;148(5):379–97. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- 3.Roberts RO, Roberts LA, Geda YE, Cha RH, Pankratz VS, O’Connor HM, Knopman DS, Petersen RC. Relative Intake of Macronutrients Impacts Risk of Mild Cognitive Impairment or dementia. J Alzheimers Dis 2012;32(2):329–39. doi: 10.3233/JAD-2012-120862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laitinen MH, Ngandu T, Rovio S, Helkala EL, Uusitalo U, Viitanen M, Nissinen A, Tuomilehto J, Soininen H, Kivipelto M. Fat Intake at Midlife and Risk of Dementia and Alzheimer’s Disease: A Population-Based Study. Dementia and geriatric cognitive disorders 2006;22(1):99–107. [DOI] [PubMed] [Google Scholar]
- 5.Vercambre M-N, Grodstein F, Kang JH. Dietary fat intake in relation to cognitive change in high-risk women with cardiovascular disease or vascular factors. European journal of clinical nutrition 2010;64(10):1134–40. doi: 10.1038/ejcn.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontani G, Corradeschi F, Felici A, Alfatti F, Migliorini S, Lodi L. Cognitive and physiological effects of Omega-3 polyunsaturated fatty acid supplementation in healthy subjects. European Journal of Clinical Investigation 2005;35(11):691–9. doi: 10.1111/j.1365-2362.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- 7.Berti V, Murray J, Davies M, Spector N, Tsui WH, Li Y, Williams S, Pirraglia E, Vallabhajosula S, McHugh P, et al. Nutrient patterns and brain biomarkers of Alzheimer’s disease in cognitively normal individuals. J Nutr Health Aging 2015;19(4):413–23. doi: 10.1007/s12603-014-0534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Frontiers in Aging Neuroscience 2015;7:52. doi: 10.3389/fnagi.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latour A, Grintal B, Champeil-Potokar G, Hennebelle M, Lavialle M, Dutar P, Potier B, Billard J-M, Vancassel S, Denis I. Omega-3 fatty acids deficiency aggravates glutamatergic synapse and astroglial aging in the rat hippocampal CA1. Aging Cell 2013;12(1):76–84. doi: 10.1111/acel.12026. [DOI] [PubMed] [Google Scholar]
- 10.Ross AJ, Sachdev PS. Magnetic resonance spectroscopy in cognitive research. Brain Research Reviews 2004;44(2–3):83–102. doi: 10.1016/j.brainresrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Erecińska M, Zaleska MM, Nissim I, Nelson D, Dagani F, Yudkoff M. Glucose and Synaptosomal Glutamate Metabolism: Studies with [15N]Glutamate. Journal of Neurochemistry 1988;51(3):892–902. doi: 10.1111/j.1471-4159.1988.tb01826.x. [DOI] [PubMed] [Google Scholar]
- 12.Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch - Eur J Physiol 2010;460(2):525–42. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 13.Danielsen ERR B Magnetic resonance spectroscopy diagnosis of neurological disease. New York: Marcel Dekker, Inc., 1991. [Google Scholar]
- 14.Yang YJ, Kim MK, Hwang SH, Ahn Y, Shim JE, Kim DH. Relative validities of 3-day food records and the food frequency questionnaire. Nutrition Research and Practice 2010;4(2):142–8. doi: 10.4162/nrp.2010.4.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lezak MD. Neuropsychological assessment. New York: Oxford University Press, 1995. [Google Scholar]
- 16.Delis DCK JH; Kaplan E; Ober BA California verbal learning test: Adult version. San Antonio: The Psychological Corporation, 1987. [Google Scholar]
- 17.Reitan RM. VALIDITY OF THE TRAIL MAKING TEST AS AN INDICATOR OF ORGANIC BRAIN DAMAGE. Perceptual and Motor Skills 1958;8(3):271–6. doi: 10.2466/pms.1958.8.3.271. [DOI] [Google Scholar]
- 18.Wechsler D WAIS-III administration and scoring manual. 3rd ed. ed. San Antonio: The Psychological Corporation, 1997. [Google Scholar]
- 19.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A Parametric Study of Prefrontal Cortex Involvement in Human Working Memory. NeuroImage 1997;5(1):49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Wechsler D Weschler test of adult reading: WTAR. San Antonio: The Psychological Corporation, 2001. [Google Scholar]
- 22.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain : a journal of neurology 2014;137(1):12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herholz K, Salmon E, Perani D, Baron JC, Holthoff V, Frölich L, Schönknecht P, Ito K, Mielke R, Kalbe E, et al. Discrimination between Alzheimer Dementia and Controls by Automated Analysis of Multicenter FDG PET. NeuroImage 2002;17(1):302–16. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]
- 24.Fayed N, Andrés E, Viguera L, Modrego PJ, Garcia-Campayo J. Higher Glutamate + Glutamine and Reduction of N-acetylaspartate in Posterior Cingulate According to Age Range in Patients with Cognitive Impairment and/or Pain. Academic Radiology 2014;21(9):1211–7. doi: 10.1016/j.acra.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Kantarci K, Jack CR, Xu YC, Campeau NG, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG, et al. REGIONAL METABOLIC PATTERNS IN MILD COGNITIVE IMPAIRMENT AND ALZHEIMER’S DISEASE A (1)H MRS STUDY. Neurology 2000;55(2):210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzales MM, Tarumi T, Kaur S, Nualnim N, Fallow BA, Pyron M, Tanaka H, Haley AP. Aerobic Fitness and the Brain: Increased N-Acetyl-Aspartate and Choline Concentrations in Endurance-Trained Middle-Aged Adults. Brain topography 2013;26(1):126–34. doi: 10.1007/s10548-012-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzales MM, Takashi T, Eagan DE, Tanaka H, Vaghasia M, Haley AP. Indirect Effects of Elevated Body Mass Index on Memory Performance Through Altered Cerebral Metabolite Concentrations. Psychosomatic medicine 2012;74(7):691–8. doi: 10.1097/PSY.0b013e31825ff1de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Staffen W, Zauner H, Mair A, Kutzelnigg A, Kapeller P, Stangl H, Raffer E, Niederhofer H, Ladurner G. Magnetic Resonance Spectroscopy of Memory and Frontal Brain Region in Early Multiple Sclerosis. The Journal of Neuropsychiatry and Clinical Neurosciences 2005;17(3):357–63. doi: 10.1176/jnp.17.3.357. [DOI] [PubMed] [Google Scholar]
- 29.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic Resonance in Medicine 1993;30(6):672–9. [DOI] [PubMed] [Google Scholar]
- 30.Lipton SA, Rosenberg PA. Excitatory Amino Acids as a Final Common Pathway for Neurologic Disorders. New England Journal of Medicine 1994;330(9):613–22. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 31.Potier B, Billard J-M, Rivière S, Sinet P-M, Denis I, Champeil-Potokar G, Grintal B, Jouvenceau A, Kollen M, Dutar P. Reduction in glutamate uptake is associated with extrasynaptic NMDA and metabotropic glutamate receptor activation at the hippocampal CA1 synapse of aged rats. Aging Cell 2010;9(5):722–35. doi: 10.1111/j.1474-9726.2010.00593.x. [DOI] [PubMed] [Google Scholar]
- 32.Pereira AC, Lambert HK, Grossman YS, Dumitriu D, Waldman R, Jannetty SK, Calakos K, Janssen WG, McEwen BS, Morrison JH. Glutamatergic regulation prevents hippocampal-dependent age-related cognitive decline through dendritic spine clustering. Proceedings of the National Academy of Sciences 2014;111(52):18733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Champeil-Potokar G, Denis I, Goustard-Langelier B, Alessandri J-M, Guesnet P, Lavialle M. Astrocytes in culture require docosahexaenoic acid to restore the n-3/n-6 polyunsaturated fatty acid balance in their membrane phospholipids. Journal of Neuroscience Research 2004;75(1):96–106. doi: 10.1002/jnr.10817. [DOI] [PubMed] [Google Scholar]
- 34.Grintal B, Champeil-Potokar G, Lavialle M, Vancassel S, Breton S, Denis I. Inhibition of astroglial glutamate transport by polyunsaturated fatty acids: Evidence for a signalling role of docosahexaenoic acid. Neurochemistry International 2009;54(8):535–43. doi: 10.1016/j.neuint.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 35.Sheldon AL, Robinson MB. The Role of Glutamate Transporters in Neurodegenerative Diseases and Potential Opportunities for Intervention. Neurochemistry international 2007;51(6–7):333–55. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cotrina ML, Nedergaard M. Astrocytes in the aging brain. Journal of Neuroscience Research 2002;67(1):1–10. doi: 10.1002/jnr.10121. [DOI] [PubMed] [Google Scholar]
- 37.Morris M, Evans DA, Bienias JL, et al. DIetary fats and the risk of incident alzheimer disease. Archives of neurology 2003;60(2):194–200. doi: 10.1001/archneur.60.2.194. [DOI] [PubMed] [Google Scholar]
- 38.Kalmijn S, Feskens EJM, Launer LJ, Kromhout D. Polyunsaturated Fatty Acids, Antioxidants, and Cognitive Function in Very Old Men. American Journal of Epidemiology 1997;145(1):33–41. [DOI] [PubMed] [Google Scholar]
- 39.Calder PC. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie 2009;91(6):791–5. doi: 10.1016/j.biochi.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Reaven PD, Grasse BJ, Tribble DL. Effects of linoleate-enriched and oleate-enriched diets in combination with alpha-tocopherol on the susceptibility of LDL and LDL subfractions to oxidative modification in humans. Arteriosclerosis, Thrombosis, and Vascular Biology 1994;14(4):557–66. [DOI] [PubMed] [Google Scholar]
- 41.Barberger-Gateau P, Letenneur L, Deschamps V, Pérès K, Dartigues J-F, Renaud S. Fish, meat, and risk of dementia: cohort study. BMJ : British Medical Journal 2002;325(7370):932–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denis I, Potier B, Vancassel S, Heberden C, Lavialle M. Omega-3 fatty acids and brain resistance to ageing and stress: Body of evidence and possible mechanisms. Ageing Research Reviews 2013;12(2):579–94. doi: 10.1016/j.arr.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Tangney CC. DASH and Mediterranean-type Dietary Patterns to Maintain Cognitive Health. Current nutrition reports 2014;3(1):51–61. doi: 10.1007/s13668-013-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crichton GE, Elias MF, Buckley JD, Murphy KJ, Bryan J, Frisardi V. Metabolic syndrome, cognitive performance, and dementia. J Alzheimers Dis 2012;30 Suppl 2:S77–87. doi: 10.3233/jad-2011-111022. [DOI] [PubMed] [Google Scholar]
- 45.Elias MFG MF; Waldstein SR Obesity, cognitive functioning, and dementia: Back to the future. Journal of Alzheimer’s Disease 2012;30(2):S113–S25. [DOI] [PubMed] [Google Scholar]
- 46.Haley A, Gonzales M, Tarumi T, Tanaka H. Subclinical vascular disease and cerebral glutamate elevation in metabolic syndrome. Metab Brain Dis 2012;27(4):513–20. doi: 10.1007/s11011-012-9306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He K, Liu K, Daviglus ML, Mayer-Davis E, Jenny NS, Jiang R, Ouyang P, Steffen LM, Siscovick D, Wu C, et al. Intakes of long-chain n-3 polyunsaturated fatty acids and fish in relation to measurements of subclinical atherosclerosis. The American journal of clinical nutrition 2008;88(4):1111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. The American Journal of Clinical Nutrition 2010;91(3):502–9. doi: 10.3945/ajcn.2008.26285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the metabolic syndrome. Clinical Nutrition 2004;23(4):447–56. doi: 10.1016/j.clnu.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Okereke OI, Rosner BA, Kim DH, Kang JH, Cook NR, Manson JE, Buring JE, Willett WC, Grodstein F. Dietary fat types and 4-year cognitive change in community-dwelling older women. Annals of Neurology 2012;72(1):124–34. doi: 10.1002/ana.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalmijn S, Launer LJ, Ott A, Witteman JCM, Hofman A, Breteler MMB. Dietary fat intake and the risk of incident dementia in the Rotterdam study. Annals of Neurology 1997;42(5):776–82. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 52.Keys A, Anderson JT, Grande F. Serum cholesterol response to changes in the diet: IV. Particular saturated fatty acids in the diet. Metabolism 1965;14(7):776–87. doi: 10.1016/0026-0495(65)90004-1. [DOI] [PubMed] [Google Scholar]
- 53.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet 2010;375(9733):2267–77. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puglielli L, Konopka G, Pack-Chung E, Ingano LAM, Berezovska O, Hyman BT, Chang TY, Tanzi RE, Kovacs DM. Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid [beta]-peptide. Nat Cell Biol 2001;3(10):905–12. [DOI] [PubMed] [Google Scholar]
- 55.Kanoski SE, Davidson TL. Western Diet Consumption and Cognitive Impairment: Links to Hippocampal Dysfunction and Obesity. Physiology & behavior 2011;103(1):59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McNamara RK, Able J, Jandacek R, Rider T, Tso P, Lindquist DM. Perinatal n-3 fatty acid deficiency selectively reduces myo-inositol levels in the adult rat PFC: an in vivo (1)H-MRS study. Journal of lipid research 2009;50(3):405–11. doi: 10.1194/jlr.M800382-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McNamara RK, Jandacek R, Tso P, Weber W, Chu W-J, Strakowski SM, Adler CM, DelBello MP. Low docosahexaenoic acid status is associated with reduced indices in cortical integrity in the anterior cingulate of healthy male children: A (1)H MRS Study. Nutritional neuroscience 2013;16(4):183–90. doi: 10.1179/1476830512Y.0000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiser MJ, Butt CM, Mohajeri MH. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients 2016;8(2):99. doi: 10.3390/nu8020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Haley AP, Gonzales MM, Tarumi T, Miles SC, Goudarzi K, Tanaka H. Elevated cerebral glutamate and myo-inositol levels in cognitively normal middle-aged adults with metabolic syndrome. Metab Brain Dis 2010;25(4):397–405. doi: 10.1007/s11011-010-9221-y. [DOI] [PubMed] [Google Scholar]