Abstract
A recent study reported that kidney transplant recipients of offspring living donors had higher graft loss and mortality. This seemed counterintuitive, given the excellent HLA matching and younger age of offspring donors; we were concerned about residual confounding and other study design issues. We used SRTR data 2001–2016 to evaluate death-censored graft failure (DCGF) and mortality for recipients of offspring versus non-offspring living donor kidneys, using Cox regression models with interaction terms. Recipients of offspring kidneys had lower DCGF than recipients of non-offspring kidneys (15-year cumulative incidence 21.2% vs 26.1%, p<0.001). This association remained after adjustment for recipient and transplant factors (aHR=0.73 0.77 0.82, p<0.001), and was attenuated among African American donors (aHR 0.77 0.85 0.95; interaction: p=0.01) and female recipients (aHR 0.77 0.84 0.91, p<0.001). Although offspring kidney recipients had higher mortality (15-year mortality 56.4% vs 37.2%, p<0.001), this largely disappeared with adjustment for recipient age alone (aHR=1.02 1.06 1.10, p=0.002) and was non-significant after further adjustment for other recipient characteristics (aHR=0.93 0.97 1.01, p=0.1). Kidneys from offspring donors provided lower graft failure and comparable mortality. An otherwise eligible donor should not be dismissed because they are the offspring of the recipient, and we encourage continued individualized counseling for potential donors.
INTRODUCTION
As the kidney recipient pool ages, it is increasingly common to find living kidney donors who are the adult offspring of recipients (1). A recent publication in the American Journal of Transplantation by Cohen et al. reported that recipients of offspring donors had higher mortality and graft loss (2). These results seemed counterintuitive for several reasons. First, the recipients of offspring kidneys are generally older, and age needs to be properly accounted for when comparing to recipients of non-offspring kidneys; however, we were concerned that the use of a coarsely categorized recipient age variable might have introduced residual confounding. Beyond this methodologic concern, we worried that Cohen’s study did not account for the advantages of an offspring donor.
In our philosophical framework, the age and human leukocyte antigen (HLA) matching of an offspring donor should be considered mediators of the offspring relationship when examining their recipient’s outcomes. That is, the excellent HLA matching of parent-offspring pairs should confer an advantage in graft survival (3), and offspring donors should generally be younger, and thus confer lower risk of graft loss (4). Cohen et al. initially sought to examine whether donor-specific alloimmunization during female recipients’ pregnancies with their offspring donors conferred worse outcomes, and thus adjusted for donor age and number of HLA matches in order to isolate specifically the impact of offspring donor relationship. However, by making conclusions regarding the effect of donor age, HLA matches, and offspring relationship separately, they have analyzed factors independently that, instead, are related as mediators of the offspring relationship (5).
To understand better the impact of offspring donors, we used SRTR data to quantify the association of offspring kidneys and graft failure and mortality, accounting for recipient, transplant, and donor characteristics in several iterations of models to isolate methodologic issues with recipient age as well as mediators of HLA matching and donor age. Additionally, we explored donor race and recipient gender as effect modifiers. We hypothesized that donor race might amplify the effect of shared parent-offspring risk factors such as risk variants in the apolipoprotein L1 gene (APOL1) which contribute to kidney failure in African Americans (6, 7) or shared environmental or social risk factors which can lead to worse post-transplant outcomes among minorities (8, 9).
METHODS
Data source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere (10). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
Study population
We studied 91,665 adult living donor kidney-only recipients between 2001–2016. We excluded situations where donors were labeled as offspring but the age difference between recipient and donor was less than 10 years (N=36). The youngest age of offspring kidney recipients was 36 years; accordingly, we excluded non-offspring kidney recipients younger than 36 years (N=17,508). We further excluded recipients with missing body mass index (BMI) or BMI outside the range of 15–45 (N=1,301).
Post-transplant outcomes
We compared cumulative incidence of death-censored graft failure (DCGF) and mortality of offspring kidney recipients versus non-offspring kidney recipients. We used Cox proportional hazards regression to describe the adjusted hazard ratio of each outcome, adjusting for recipient covariates and donor covariates described below. We graphed cumulative incidence of DCGF and mortality for offspring kidney recipients and non-offspring kidney recipients using Kaplan-Meier methods.
Recipient factors
Because we hypothesized that categorization of recipient age confounded the mortality analysis reported by Cohen et al., we sought to replicate their analysis with a categorical recipient age, then to revise the analysis using a continuous recipient age. For the analysis with recipient age as a categorical variable, we categorized age similarly to Cohen et al., with three categories: below 40 years, 40–54 years, and 55 years and older. For the analysis with recipient age as a continuous variable, we scaled age by 10 years and created a spline for age over 50 years, based on Martingale residual plots which demonstrated a different linear association between age and each outcome above versus below age 50 years.
Analysis was also adjusted for gender, race/ethnicity (white/other, African American, Hispanic), dialysis vintage by year, diabetes, BMI by 5 units with a spline for BMI>25 based on Martingale residual plots, previous solid organ transplant, insurance (private, non-private), education (high school or less, more than high school, unknown using a missingness indicator), depleting induction therapy (thymoglobulin or alemtuzumab), non-depleting induction therapy (daclizumab or basiliximab), maintenance therapy of tacrolimus, maintenance therapy of cyclosporine, ABO incompatibility, and transplant year.
Donor factors
We considered donor characteristics of age and number of HLA matches as mediators of the association between offspring status and post-transplant outcomes; that is, recipients of offspring kidneys would, by the nature of their donor being their child, have a younger donor than recipients of non-offspring kidneys whose donor is not their child. We performed formal mediation analysis using the methods of Baron and Kenny (11) as well as Sobel tests to determine whether this mediation was statistically significant (12). Thus, we report characteristics of offspring and non-offspring donors but did not adjust for these characteristics in our regression models. In comparison, Cohen et al. limited their primary outcome to a subset of transplants with 3 HLA matches and adjusted for number of HLA matches in their sensitivity analyses. They also adjusted for donor age in all analyses.
Effect modification by donor race/ethnicity and recipient gender
We repeated our analysis incorporating interaction terms between offspring donor relationship and African American race as well as offspring donor relationship and Hispanic ethnicity in order to explore whether the association differs by race/ethnicity. We also incorporated interaction terms between offspring donor relationship and recipient gender in order to explore whether graft loss varied between mothers versus fathers of offspring donors.
Statistical analysis
We used the Wilcoxon rank sum test for continuous variables, χ2 test for categorical variables, and log-rank test for cumulative incidence. An α of 0.05 was considered significant. Confidence intervals are reported as per the method of Louis and Zeger (13). All analyses were performed using Stata 14.0/MP for Linux (College Station, Texas).
RESULTS
Study Population
Of 72,820 living donor recipients, 15,601 (21.4%) received kidneys from offspring donors (Table 1). Offspring kidney recipients were older (median age 60 vs 50 years, p<0.001) but their donors were younger (median age 33 vs 45 years, p<0.001) compared to non-offspring kidney recipients and their donors. Recipients of offspring kidneys were more likely to be female (42.9% vs 37.0%, p<0.001), African American (19.3% vs 12.3%, p<0.001), Hispanic (15.5% vs 11.1%, p<0.001), and have diabetes (43.1% vs 30.5%, p<0.001), but less likely to have had a previous transplant (9.1% vs 12.4%, p<0.001), private insurance (45.3% vs 62.2%, p<0.001), and more than high school education (41.5% vs 56.7%, p<0.001). Offspring and non-offspring kidney recipients had clinically similar BMI (median 27.9 vs 27.1) and time on dialysis (median 0.8 vs 0.7 years). Offspring donors were more likely to be African American (19.3% vs 9.9%, p<0.001) and Hispanic (15.7% vs 10.8%, p<0.001), but less likely to be female (55.5% vs 63.1%, p<0.001).
Table 1. Characteristics of offspring kidney transplants vs non-offspring kidney transplants.
Recipients of offspring donor kidneys were older, more likely to be female, more likely to be African American or Hispanic, and more likely to be diabetic than recipients of non-offspring donor kidneys.
| Offspring kidney transplants (N=15,601) |
Non-offspring kidney transplants (N=57,219) |
p | |
|---|---|---|---|
| Recipient characteristics | |||
| Age, median (IQR) | 60 (54–66) | 50 (42–58) | <0.001 |
| Female (%) | 42.9 | 37.0 | <0.001 |
| Race/ethnicity (%) | <0.001 | ||
| White/other | 65.2 | 76.6 | |
| African American | 19.3 | 12.3 | |
| Hispanic | 15.5 | 11.1 | |
| Diabetes (%) | 43.1 | 30.5 | <0.001 |
| Previous solid organ transplant | 9.1 | 12.4 | <0.001 |
| BMI, median (IQR) | 27.9 (24.5–31.7) | 27.1 (23.7–31.2) | <0.001 |
| Dialysis years, median (IQR) | 0.8 (0–1.9) | 0.7 (0–1.8) | <0.001 |
| Private insurance (%) | 45.3 | 62.2 | <0.001 |
| Education (%) | <0.001 | ||
| High school or less | 46.3 | 33.5 | |
| More than high school | 41.5 | 56.7 | |
| Unknown | 12.2 | 9.9 | |
| Transplant characteristics | |||
| Depleting induction (%) | 46.8 | 52.1 | <0.001 |
| Non-depleting induction (%) | 32.6 | 29.6 | <0.001 |
| Tacrolimus (maintenance) (%) | 79.5 | 82.1 | <0.001 |
| Cyclosporine (maintenance) (%) | 14.7 | 11.6 | <0.001 |
| ABO incompatibility (%) | 1.0 | 1.5 | <0.001 |
| HLA mismatch (%) | <0.001 | ||
| 0 | 3.1 | 9.2 | |
| 1 | 10.4 | 3.3 | |
| 2 | 34.5 | 10.0 | |
| 3 | 48.7 | 19.5 | |
| 4 | 1.4 | 20.1 | |
| 5 | 0.8 | 24.1 | |
| 6 | 0.4 | 13.0 | |
| Donor characteristics | |||
| Age, median (IQR) | 33 (27–39) | 45 (37–53) | <0.001 |
| Female (%) | 55.5 | 63.1 | <0.001 |
| Race/ethnicity (%) | <0.001 | ||
| White/other | 65.0 | 79.3 | |
| African American | 19.3 | 9.9 | |
| Hispanic | 15.7 | 10.8 |
More than 97% of offspring transplants had ≤3 HLA mismatches (Figure 1), with 3.1% that had 0 HLA mismatches, 10.4% with 1 HLA mismatch, 34.5% with 2 HLA mismatches, and 48.7% with 3 HLA mismatches. In comparison, non-offspring transplants had HLA mismatches that were more uniformly distributed, with only 42.0% of non-offspring transplants with ≤3 HLA mismatches. Of non-offspring transplants, 9.2% had 0 HLA mismatches, 3.3% had 1 HLA mismatch, 10.0% had 2 HLA mismatches, 19.5% had 3 HLA mismatches, 20.1% had 4 HLA mismatches, 24.1% had 5 HLA mismatches, and 13.0% had 6 HLA mismatches.
Figure 1. Distribution of HLA mismatches for offspring living donor transplants and non-offspring living donor transplants.
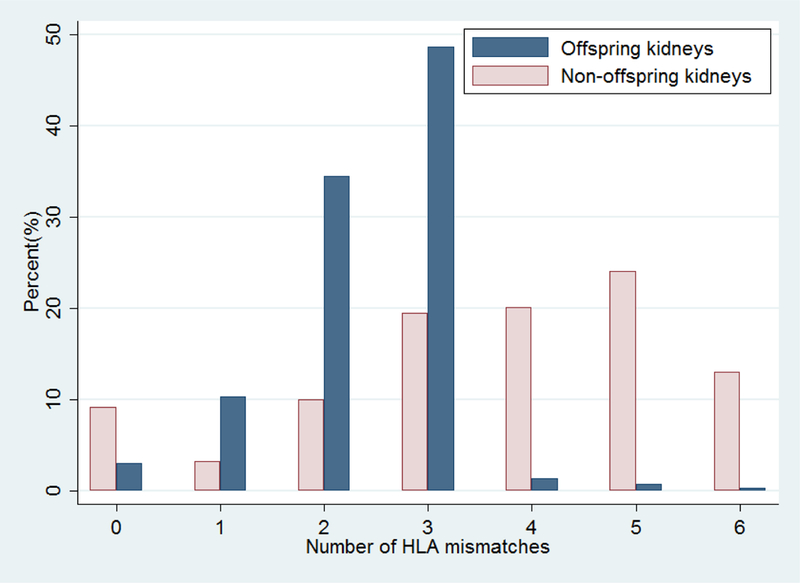
More than 97% offspring living donor transplants had ≤3 HLA mismatches, while 42.0% of non-offspring living donor transplants had ≤3 HLA mismatches.
Confounding in previous study
Adjusting for recipient factors including a categorized recipient age, HLA mismatches, and donor age, the method used by Cohen et al., we reproduced their finding of a 33% higher risk of mortality with offspring kidneys (adjusted hazard ratio [aHR] 1.271.331.40, p<0.001). However, if we used a continuous recipient age rather than categorized, we found only a 10% higher risk of mortality with offspring kidneys (aHR 1.051.101.16, p<0.001). Bias was introduced by categorization of recipient age. Adjusting for recipient factors, donor age, and number of HLA mismatches, we reproduced their finding of higher risk of DCGF with offspring kidneys (aHR 1.041.121.21, p=0.002).
Mortality
Recipients of offspring kidneys had higher mortality than recipients of non-offspring kidneys (crude 15-year mortality: 56.4% vs 37.2%, p<0.001) (Figure 2). Adjusting for recipient age alone, this association largely disappeared (per 10 years, aHR 1.021.061.10, p=0.002), and became non-significant with further adjustment for other recipient and transplant characteristics (aHR 0.930.971.01, p=0.1) (Table 2).
Figure 2. Cumulative incidence of mortality after offspring living donor transplants and non-offspring living donor transplants, adjusted for recipient and donor factors.
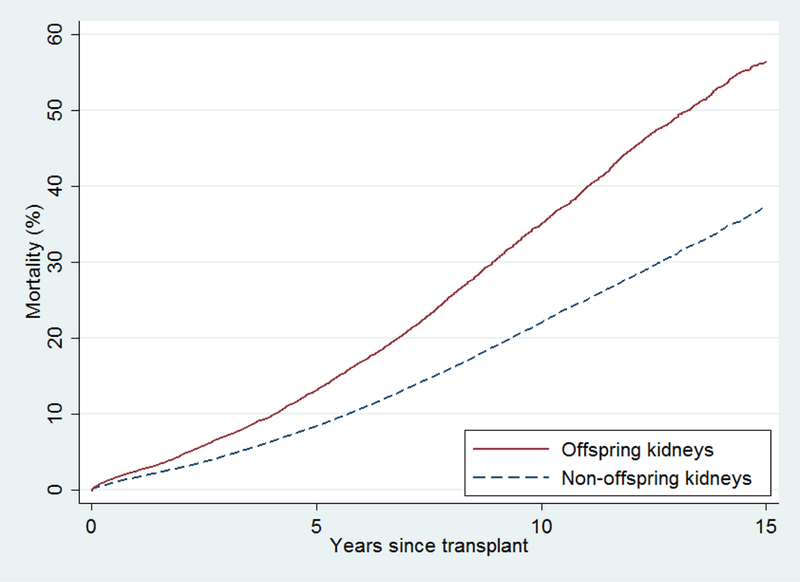
Recipients of offspring kidneys had higher unadjusted mortality than recipients of non-offspring kidneys (crude 15-year mortality: 56.4% vs 37.2%, p<0.001). Adjusting for recipient age alone as a continuous variable, this association largely disappeared (per 10 years, aHR=1.021.061.10, p=0.002), and became non-significant after further adjustment for other recipient and transplant characteristics (aHR=0.930.971.01, p=0.1).
Table 2. Association of recipient characteristics and post-transplant outcomes (death-censored graft failure and mortality).
After adjustment, offspring kidney was associated with lower risk of graft failure and comparable risk of mortality.
| aHR of mortality | p | aHR of graft failure | p | |
|---|---|---|---|---|
| Offspring donor | 0.93 0.97 1.01 | 0.1 | 0.73 0.77 0.82 | <0.001 |
| Age (per 10 years), <50 | 1.53 1.61 1.69 | <0.001 | 0.77 0.81 0.85 | <0.001 |
| Age (per 10 years), >50 | 1.68 1.72 1.77 | <0.001 | 0.95 0.99 1.03 | 0.6 |
| African American | 0.92 0.96 1.01 | 0.1 | 1.69 1.78 1.88 | <0.001 |
| Hispanic | 0.64 0.68 0.72 | <0.001 | 0.85 0.91 0.98 | 0.01 |
| Female | 0.86 0.89 0.93 | <0.001 | 1.05 1.10 1.15 | <0.001 |
| Dialysis vintage (per year) | 1.033 1.038 1.042 | <0.001 | 1.02 1.03 1.04 | <0.001 |
| Diabetes | 1.92 1.99 2.06 | <0.001 | 1.14 1.20 1.26 | <0.001 |
| Previous solid organ transplant |
1.37 1.44 1.52 | <0.001 | 1.16 1.23 1.32 | <0.001 |
| BMI (per 5 units), <25 | 0.76 0.80 0.84 | <0.001 | 0.96 1.03 1.11 | 0.3 |
| BMI (per 5 units), >25 | 1.09 1.11 1.13 | <0.001 | 1.13 1.16 1.19 | <0.001 |
| Depleting induction | 1.00 1.04 1.08 | 0.06 | 1.08 1.14 1.20 | <0.001 |
| Non-depleting induction | 1.00 1.04 1.08 | 0.04 | 0.95 1.00 1.06 | 0.9 |
| Cyclosporine (maintenance) |
0.78 0.84 0.89 | <0.001 | 0.48 0.51 0.56 | <0.001 |
| Tacrolimus (maintenance) | 0.68 0.73 0.77 | <0.001 | 0.41 0.44 0.47 | <0.001 |
| ABO incompatibility | 1.07 1.24 1.43 | 0.004 | 1.38 1.62 1.92 | <0.001 |
| Private insurance | 0.72 0.74 0.77 | <0.001 | 0.87 0.91 0.95 | <0.001 |
| Education | ||||
| High school or less | Reference | Reference | ||
| More than high school | 0.84 0.87 0.90 | <0.001 | 0.86 0.90 0.95 | <0.001 |
| Unknown | 0.82 0.86 0.91 | <0.001 | 0.82 0.87 0.93 | <0.001 |
| Calendar year of transplant |
0.940 0.946 0.952 | <0.001 | 0.93 0.94 0.95 | <0.001 |
Death-censored graft failure
Recipients of offspring kidneys had lower DCGF than recipients of non-offspring kidneys (crude 15-year DCGF: 21.2% vs 26.1%, p<0.001) (Figure 3). After adjustment for recipient factors, offspring kidneys were associated with 23% lower risk of DCGF (total effect of offspring relationship: aHR 0.730.770.82, p<0.001) (Table 2).
Figure 3. Cumulative incidence of death-censored graft failure after offspring living donor transplants and non-offspring living donor transplants.
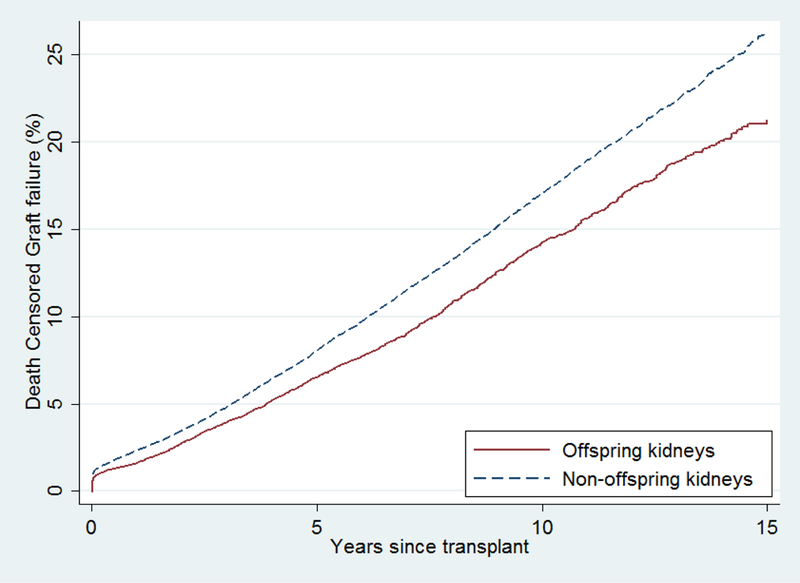
Recipients of offspring kidneys had lower DCGF than recipients of non-offspring kidneys (crude 15-year DCGF: 21.2% vs 26.1%, p<0.001). After adjustment for recipient factors, offspring kidneys were associated with 23% lower risk of DCGF (aHR 0.730.770.82, p<0.001).
Examining how donor age and HLA mismatches are mediators in the association between offspring relationship and DCGF, the indirect effect of offspring relationship through donor age was a 17% higher risk of DCGF (per 10 years donor age, aHR 1.141.171.20, p<0.001). The indirect effect of offspring relationship through HLA mismatches was an 11% higher risk of DCGF (per HLA mismatch, aHR 1.091.111.13, p<0.001). The direct effect of offspring relationship after adjusting for donor age and HLA mismatches was a 13% higher risk of DCGF (aHR 1.051.131.22, p=0.002). Sobel test estimates confirmed statistically significant mediation of offspring relationship through donor age (p<0.001) and HLA mismatches (p<0.001). That is, the lower risk of DCGF of offspring kidneys is mediated by the younger age and better HLA matching of offspring donors.
Effect modification by donor race/ethnicity and recipient gender
The association of having an offspring donor with a lower risk of DCGF was attenuated for African American donors (p value of interaction term: 0.01) (Table 3). Recipients of white/other race offspring donor kidneys had a 28% lower risk of DCGF (aHR 0.670.720.78, p<0.001), recipients of African American offspring donor kidneys had a 15% lower risk of DCGF (aHR 0.770.850.95, p=0.004), and recipients of Hispanic offspring donor kidneys had a 22% lower risk of DCGF (aHR 0.670.780.92, p=0.003). The association between having an offspring donor and mortality did not vary by donor race/ethnicity (Table 3).
Table 3. Association of offspring kidneys and post-transplant outcomes, modified by donor race/ethnicity.
Risk associated with offspring donors varied statistically significantly by race for graft failure but non-significantly for mortality. Among African American donors, the association of offspring kidneys and graft failure was attenuated.
| aHR of mortality | p value of interaction |
aHR of DCGF | p value of interaction |
|
|---|---|---|---|---|
| White/other | 0.95 0.99 1.04 | 0.67 0.72 0.78 | ||
| African American | 0.83 0.92 1.01 | 0.07 | 0.77 0.85 0.95 | 0.01 |
| Hispanic | 0.80 0.90 1.01 | 0.08 | 0.67 0.78 0.92 | 0.4 |
The association of having an offspring donor with a lower risk of DCGF was attenuated for female recipients (p value of interaction term: 0.005). Mothers who received an offspring kidney had a 16% lower risk of DCGF (aHR 0.770.840.91, p<0.001), while fathers who received an offspring kidney had a 28% lower risk of DCGF (aHR 0.660.720.78, p<0.001) compared to receiving non-offspring kidneys. The association between offspring donors and mortality did not differ by recipient gender (interaction p=0.2).
DISCUSSION
Using national registry data, we studied the association between offspring donor kidney transplantation and graft loss and mortality among living donor recipients between 2001 and 2016. Offspring kidney recipients were older than non-offspring kidney recipients, but their donors were younger than the donors of non-offspring kidneys. While more than 97% of offspring transplants had three or fewer HLA mismatches, only 42% of non-offspring transplants had three or fewer HLA mismatches. After adjustment for recipient characteristics, recipients of offspring kidneys had 23% lower risk of DCGF compared to recipients of non-offspring kidneys (aHR 0.730.770.82, p<0.001), and a similar risk of mortality (aHR 0.930.971.01, p=0.1). The lower risk of DCGF was attenuated among African American donors and among mothers who received offspring donor kidneys. However even among recipients of kidneys from African American donors, graft failure risk of offspring kidneys was still 15% lower than non-offspring kidneys, and among mothers who received offspring donor kidneys, graft failure risk was 16% lower than non-offspring kidneys.
Our findings of a comparable mortality risk between offspring kidneys and non-offspring kidneys are in contrast to recent suggestions that offspring donor kidneys had higher mortality risk (2). We believe this is due to residual confounding by categorization of a continuous variable (14). Cohen et al. adjusted their analysis for recipient age, but used a categorical variable for age with all recipients over 55 years old as one category. Within the category of recipients over 55 years old, offspring kidney recipients were still much older than non-offspring kidney recipients. We found that the higher mortality risk largely disappeared after adjusting for continuous recipient age alone (aHR 1.021.061.10, p=0.002), and become non-significant after further adjustment for other characteristics.
Our findings of a lower risk of graft failure from offspring donor kidneys also counter the recent report that offspring kidneys had higher risk of graft failure than non-offspring kidneys (2). There were two reasons for this difference. First, Cohen et al. limited their primary analysis to recipients with exactly three HLA mismatches and adjusted for number of HLA matches in sensitivity analyses. Almost half of parent-offspring pairs actually had fewer mismatches and might be expected to have a lower risk of allograft failure. On the other hand, only one in five non-offspring kidney transplants had three HLA mismatches. In addition, we did not control for donor age, because the nature of the donor-recipient relationship for offspring donors leads to their younger age relative to their parent recipient. That is, we considered the HLA matching and younger donor age of offspring kidney donors to be mediators of the parent-offspring relationship, and we found that better HLA mismatch and younger age of donors are where the benefit of an offspring donor lies. If a given recipient has two donors present who have the same age and number of HLA matches, and only differ by one being the offspring of the recipient, there is lower risk of DCGF with the non-offspring donor (2).
Another issue worthy of consideration is that, despite these excellent recipient outcomes, the use of offspring as kidney donors is not without risk to the donor. Evaluation of all potential donors requires risk assessment as described in the Kidney Disease: Improving Global Outcomes (KDIGO) guideline on living kidney donors (15). Especially for donors related to their potential recipient, consideration of genetic factors in kidney disease requires that the donor evaluation team know the cause of the potential recipient’s kidney failure. In the case of APOL1-related kidney disease in the donor’s family or sub-Saharan African ancestry, KDIGO guidelines recommend testing for APOL1 risk alleles be offered as part of donor evaluation (15, 16).
In addition to the potential presence of APOL1-related kidney disease, which is more common in African Americans (7, 17), prior work also suggests that other clinical, social, and environmental risk factors may disproportionately impact outcomes from offspring kidneys in African American transplant recipients. For example, clustering of ESRD and associated risk factors has been noted within African American families, and first- or second-degree relatives of ESRD patients are at increased risk for developing ESRD (18). A disproportionately higher burden of cardiovascular disease risk factors (e.g., diabetes and hypertension) within African American families (18–21) may also influence graft survival from offspring kidneys (22). In our study, we found that African American race attenuated the graft survival benefit of offspring kidneys, and recommend that donor evaluation and informed consent continue to be a personalized process respecting the autonomy of the potential donor. It should be noted that nearly one in five recipients of offspring kidneys was African American, and that there has been worsening disparity in living kidney donation for African American transplant candidates in the US (23).
Our study has a few limitations. Like any study using national registry data, we are limited by the granularity of data. We do not have information regarding APOL1 risk variants or individual-level income, which have been previously associated with transplant outcomes. Thus, we used race/ethnicity, health insurance type, and education level as proxies to assess potential genetic and socioeconomic factors that may influence offspring living donor kidney transplantation outcomes. Next, we chose not to control for donor factors in consideration of graft loss and mortality in recipients. Though this limits isolation of the consideration of inheritable risk of kidney disease, we believe the benefit of offspring donors is not in comparison to the counterfactual potential non-offspring donor. That is, if a recipient has two potential donors present for evaluation, they will be different ages, have different BMIs, have different compatibility with the recipient, and carry different risks of future kidney disease themselves. As such, transplant providers should continue to provide individualized counseling for transplant candidates and their potential donors.
In conclusion, willing adult offspring remain excellent potential donors for patients in need of kidney transplantation. Although the magnitude of decreased risk of graft loss varies by donor race/ethnicity and recipient gender, the younger donor age and HLA matching of offspring donors confer better graft survival than non-offspring donors. We recommend that donor evaluation continue to include both personalized counseling regarding the potential donor’s risk of future kidney disease as well as respect for autonomy in the informed consent process.
ACKNOWLEDGMENTS
This work was supported by grants number F32DK109662 (Holscher), K01DK101677 (Massie), K01DK114388 (Henderson), and K24DK101828 (Segev) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) by grant number K01HS024600 (Purnell) from the Agency for Healthcare Research and Quality (AHRQ), and an American College of Surgeons Resident Research Scholarship (Holscher). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Abbreviations:
- aHR
adjusted hazard ratio
- APOL1
apolipoprotein L1
- BMI
body mass index
- DCGF
death censored graft failure
- ESRD
end-stage renal disease
- HLA
human leukocyte antigen
- HRSA
Health Resources and Services Administration
- IQR
interquartile range
- KDIGO
Kidney Disease: Improving Global Outcomes
- OPTN
Organ Procurement and Transplantation Network
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Rowe TAHJ, McElroy L, Ladner DP, Lindquist LA. The Evolution of Living Kidney Donation and Transplantation in Older Adults. J Am Geriatr Soc 2015;63(12):2616–2620. [DOI] [PubMed] [Google Scholar]
- 2.Cohen JB, Owei L, Sawinski DL, Porrett PM. Inferior long-term allograft and patient outcomes among recipients of offspring living donor kidneys. Am J Transplant 2018;18(7):1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orandi BJ, Garonzik-Wang JM, Massie AB, Zachary AA, Montgomery JR, Van Arendonk KJ et al. Quantifying the risk of incompatible kidney transplantation: a multicenter study. Am J Transplant 2014;14(7):1573–1580. [DOI] [PubMed] [Google Scholar]
- 4.Massie AB, Leanza J, Fahmy LM, Chow EK, Desai NM, Luo X et al. A Risk Index for Living Donor Kidney Transplantation. Am J Transplant 2016;16(7):2077–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richiardi L, Bellocco R, Zugna D. Mediation analysis in epidemiology: methods, interpretation and bias. International journal of epidemiology 2013;42(5):1511–1519. [DOI] [PubMed] [Google Scholar]
- 6.Freedman BI, Langefeld CD, Turner J, Nunez M, High KP, Spainhour M et al. Association of APOL1 variants with mild kidney disease in the first-degree relatives of African American patients with non-diabetic end-stage renal disease. Kidney international 2012;82(7):805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reeves-Daniel AM, DePalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant 2011;11(5):1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purnell TS, Luo X, Kucirka LM, Cooper LA, Crews DC, Massie AB et al. Reduced Racial Disparity in Kidney Transplant Outcomes in the United States from 1990 to 2012. J Am Soc Nephrol 2016;27(8):2511–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taber DJ, Hamedi M, Rodrigue JR, Gebregziabher MG, Srinivas TR, Baliga PK et al. Quantifying the Race Stratified Impact of Socioeconomics on Graft Outcomes in Kidney Transplant Recipients. Transplantation 2016;100(7):1550–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant 2014;14(8):1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of personality and social psychology 1986;51(6):1173–1182. [DOI] [PubMed] [Google Scholar]
- 12.Sobel ME. Asymptotic Confidence Intervals for Indirect Effects in Structural Equation Models. Sociological Methodology 1982;13:290–312. [Google Scholar]
- 13.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics 2009(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothman KJ. Six persistent research misconceptions. J Gen Intern Med 2014;29(7):1060–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lentine KL, Kasiske BL, Levey AS, Adams PL, Alberu J, Bakr MA et al. Summary of Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation 2017;101(8):1783–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massie AB, Muzaale AD, Luo X, Chow EKH, Locke JE, Nguyen AQ et al. Quantifying Postdonation Risk of ESRD in Living Kidney Donors. Journal of the American Society of Nephrology : JASN 2017;28(9):2749–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locke JE, Sawinski D, Reed RD, Shelton B, MacLennan PA, Kumar V et al. Apolipoprotein L1 and Chronic Kidney Disease Risk in Young Potential Living Kidney Donors. Annals of surgery 2017. [DOI] [PMC free article] [PubMed]
- 18.Freedman BI, Spray BJ, Tuttle AB, Buckalew VM Jr. The familial risk of end-stage renal disease in African Americans. American journal of kidney diseases : the official journal of the National Kidney Foundation 1993;21(4):387–393. [DOI] [PubMed] [Google Scholar]
- 19.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. Jama 2003;290(2):199–206. [DOI] [PubMed] [Google Scholar]
- 20.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Annals of internal medicine 2014;160(8):517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morenoff JD, House JS, Hansen BB, Williams DR, Kaplan GA, Hunte HE. Understanding social disparities in hypertension prevalence, awareness, treatment, and control: the role of neighborhood context. Soc Sci Med 2007;65(9):1853–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taber DJ, Hunt KJ, Fominaya CE, Payne EH, Gebregziabher M, Srinivas TR et al. Impact of Cardiovascular Risk Factors on Graft Outcome Disparities in Black Kidney Transplant Recipients. Hypertension (Dallas, Tex : 1979) 2016;68(3):715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purnell TS, Luo X, Cooper LA, Massie AB, Kucirka LM, Henderson ML et al. Association of Race and Ethnicity With Live Donor Kidney Transplantation in the United States From 1995 to 2014. Jama 2018;319(1):49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


