Abstract
Parainfluenza virus (PIV) infection can progress from upper respiratory tract infection (URTI) to lower respiratory tract disease (LRTD) in immunocompromised hosts. Risk factors for progression to LRTD and presentation with LRTD without prior URTI are poorly defined. Hematopoietic cell transplant (HCT) recipients with PIV infection were retrospectively analyzed using standardized definitions of LRTD. PIV was detected in 540 HCT recipients; 343 had URTI alone and 197 (36%) had LRTD (possible, 76; probable, 19; proven, 102). Among 476 patients with positive nasopharyngeal samples, the cumulative incidence of progression to probable/proven LRTD by day 40 was 12%, with a median time to progression of 7 days (range, 2–40). In multivariable analysis, monocytopenia (HR 2.22; p=0.011), steroid use ≥1mg/kg prior to diagnosis (HR 1.89; p=0.018), co-pathogen detection in blood (HR 3.21; p=0.027), and PIV-3 type (HR 3.57; p=0.032) were associated with increased progression risk. In the absence of all the 4 risk factors, no patients progressed to LRTD, while progression risk increased to >30% if 3 or more risk factors were present. Viral load or ribavirin use appeared to have no effect on progression. Among 121 patients with probable/proven LRTD, 64 (53%) presented LRTD without prior URTI, and decreased lung function before infection and lower respiratory co-pathogens were risk factors for this presentation; mortality was unaffected by the absence of prior URTI. We conclude that the risk of progression to probable/proven LRTD exceeded 30% with ≥ 3 risk factors. To detect all cases of LRTD, virologic testing of lower respiratory samples is required regardless of URTI symptoms.
Keywords: Parainfluenza virus, Hematopoietic cell transplantation, Lower respiratory tract disease, Progression, Ribavirin
INTRODUCTION
Parainfluenza virus (PIV) is a respiratory virus commonly detected after hematopoietic cell transplantation (HCT) [1–3]. Human PIV has four subtypes, numbered 1 through 4 [4, 5]. PIV type 3 (PIV-3) is the most common subtype and a frequent cause of outbreaks among HCT recipients [6–8]. PIV can progress from upper respiratory tract infection (URTI) to lower respiratory tract disease (LRTD), resulting in mortality as high as 40–50% [3, 9–11]. Identification of risk factors for progression to LRTD and their relative importance is critical in clinical practice and designing clinical trials of novel drugs and immunotherapies.
Progression to LRTD in respiratory viral infections generally indicates progression from URTI to LRTD. However, PIV LRTD is occasionally diagnosed without evidence of prior URTI in HCT recipients [9, 11–13]. Although better understanding of this phenomenon is critical to manage viral infections after HCT and to develop comprehensive prevention strategies, little is known about the biological mechanism of or risk factors for PIV LRTD without prior URTI.
We previously proposed a classification of LRTD into three groups: possible, probable, and proven, using PIV infection [14]. Survival after probable or proven LRTD was significantly worse than that after possible LRTD, which was similar to survival after URTI.
The aim of this study was to evaluate the progression rate from URTI to LRTD in HCT recipients with PIV infection, the risk factors for progression to LRTD, and the effect of ribavirin on progression based on stringent definitions. Moreover, since PIV LRTD frequently occurred without prior URTI, we identified factors associated with LRTD as an initial manifestation of infection.
METHODS
Study design
This retrospective cohort study evaluated patients who were transplanted between 1990 and 2011 at Fred Hutchinson Cancer Research Center (Fred Hutch) and were demonstrated to have laboratory-documented PIV infection after transplantation; a subset of patients were analyzed in a previously published paper on characteristics of PIV LRTD [14]. Only an individual’s first episode of PIV infection was analyzed. Patients’ demographic data and transplant information were retrieved from the Fred Hutch database, and other data related to the clinical course of PIV infections were collected by medical chart reviews. The study was approved by the Institutional Review Board at the Fred Hutch.
Laboratory testing
PIV was examined in HCT recipients with upper respiratory tract symptoms or bronchoscopic examination using conventional culture, direct fluorescent antibody (DFA) staining, and/or reverse transcription-polymerase chain reaction (RT-PCR) assay in respiratory samples. The routine test for 17 respiratory viruses using RT-PCR started in 2007 at our center. We generally performed examination for respiratory viruses using a nasopharyngeal sample when patients had URTI symptoms and bronchoscopy when patients had lower respiratory tract symptoms and radiographic abnormalities. Viral load was determined by quantitative RT-PCR using nasopharyngeal samples at diagnosis of URTI [15].
Definitions
PIV detection in a nasopharyngeal sample was defined as URTI; virological evaluation was performed when patients presented with URTI symptoms [14]. Patients with radiographic signs of LRTD with or without clinical symptoms were evaluated by bronchoalveolar lavage (BAL) according to the attending physician’s discretion. PIV LRTD categories were designated as previously described [14]: possible referring to PIV detection in the upper respiratory tract with new pulmonary infiltrates; probable as PIV detection in the lower respiratory tract (e.g. a bronchoalveolar lavage [BAL] or lung biopsy sample) with lower respiratory tract symptoms and no new pulmonary infiltrates; and proven LRTD based on PIV detection in the lower respiratory tract with new pulmonary infiltrates [14]. Progression to LRTD occurred when patients were diagnosed with LRTD ≥ 2 days after URTI diagnosis [16]. A co-pathogen was defined as a significant pathogen (bacterium, fungus, virus) detected in a concurrent respiratory sample or in a blood sample obtained within two days of diagnosis of PIV infection. Coagulase-negative staphylococcal bacteremia and asymptomatic cytomegalovirus DNAemia or antigenemia were not considered significant co-pathogens. Cell counts closest to the day of infection diagnosis within 1 week were recorded. Peak steroid doses at PIV diagnosis and post diagnosis were recorded during a period of 2 weeks before and after diagnosis, respectively [14].
Statistical analysis
The probabilities of progression to LRTD among patients who presented with URTI were estimated by cumulative incidence curves, treating death as a competing risk. Cox proportional hazards models were used to evaluate unadjusted and adjusted hazard ratios (aHR) for progression from URTI or possible LRTD to probable or proven LRTD. Logistic regression models were used to evaluate cross-sectional associations between each risk factor and occurrence of LRTD without prior PIV detection in upper respiratory tract samples among patients with probable/proven LRTD. Several models were evaluated for each endpoint when the number of events did not permit inclusion of all variables into one model. The log-rank test was used to compare univariate hazards of time-to-event outcomes. Two-sided p-values < 0.05 were considered statistically significant. All statistical analyses were performed using SAS 9.4 (TS1M3) for Windows (SAS Institute, Inc., Cary, NC).
RESULTS
Incidence of PIV infections and patient characteristics
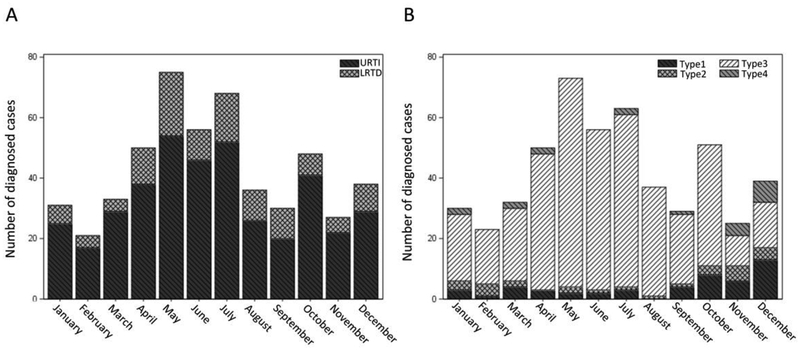
PIV was detected in 540 HCT recipients altogether; 343 (64%) had URTI alone and 197 (36%) had LRTD. The median time to PIV infection after HCT was 70.5 days (range, 0 – 3140) and 80% of the PIV infections were due to PIV-3. The number of PIV infections by month for 20 years between January 1992 and December 2011 is shown in Figure 1A. PIV infection was more frequently detected between April and July than throughout the remainder of the year. The incidence by each PIV subtype is shown in Figure 1B. PIV-3 infections commonly occurred in spring-summer, while the other types were detected more in autumn and winter.
Figure 1. Monthly distribution of PIV infection.
(A) The number of URTI or LRTD cases with PIV infection by month between January 1992 and December 2011. (B) The number of PIV infection cases by each type between January 1992 and December 2011. PCR testing for PIV-4 started in January 2007 and the cases with unknown PIV type were excluded.
Characteristics of patients with PIV infection are shown in Table 1. Among 540 patients with PIV infection, median age at diagnosis was 42.3 (range, 0–73). Among 197 LRTD cases, possible, probable, and proven LRTD cases occurred in 76 (39%), 19 (10%), and 102 (52%), respectively. Nosocomial infection, co-pathogen in blood, and low white blood cell counts at diagnosis were more frequently observed in LRTD cases than in URTI alone cases. The median viral load in nasal wash samples at diagnosis was 6.5 log10 copies/ml (range, 2.8–9.7 log10 copies/ml).
Table 1.
Characteristics of all patients with PIV infection
| Characteristics | Total | URTI alone | LRTD | ||
|---|---|---|---|---|---|
| possible | probable | proven | |||
| (N=540) | (N=343) | (N=76) | (N=19) | (N=102) | |
| Sex | |||||
| Male | 318 (59) | 200 (58) | 39(51) | 15 (79) | 64 (63) |
| Female | 222 (41) | 143 (42) | 37 (49) | 4 (21) | 38 (37) |
| Age at PIV infection, year | |||||
| ≤ 20 | 101 (19) | 71 (21) | 17 (22) | 2 (11) | 11 (11) |
| 21–60 | 360 (67) | 222 (65) | 44 (58) | 17 (89) | 77 (75) |
| > 60 | 79 (15) | 50 (15) | 15 (20) | 0 (0) | 14 (14) |
| Disease risk | |||||
| Standard | 344 (64) | 223 (65) | 51 (67) | 14 (74) | 56 (55) |
| High | 196 (36) | 120 (35) | 25 (33) | 5 (26) | 46 (45) |
| Transplant year | |||||
| 1990–2000 | 270 (50) | 176 (51) | 25 (33) | 15 (79) | 54 (53) |
| 2001–2011 | 270 (50) | 167 (49) | 51 (67) | 4 (21) | 48 (47) |
| Number of transplantation | |||||
| 1st | 489(91) | 312 (91) | 68 (89) | 19 (100) | 90 (88) |
| 2nd | 49 (9) | 29 (8) | 8 (11) | 0 (0) | 12 (12) |
| 3rd | 2 (0) | 2 (1) | 0 (0) | 0 (0) | 0 (0) |
| Cell source | |||||
| Bone marrow | 252 (47) | 169 (49) | 22 (29) | 16 (84) | 45 (44) |
| Peripheral blood stem cell | 264 (49) | 164 (48) | 45 (59) | 3 (16) | 52 (51) |
| Cord blood | 24 (4) | 10 (3) | 9 (12) | 0 (0) | 5 (5) |
| Donor type | |||||
| Autologous | 103 (19) | 70 (20) | 16 (21) | 0 (0) | 17 (17) |
| Related | 200 (37) | 135 (39) | 22 (29) | 8 (42) | 35 (34) |
| Unrelated | 237 (44) | 138 (40) | 38 (50) | 11 (58) | 50 (49) |
| Conditioning regimen | |||||
| MA including high-dose TBI (≥12Gy) | 209 (39) | 127 (37) | 28 (37) | 11 (58) | 43 (42) |
| MA ± low-dose TBI (≤ 2Gy) | 240 (44) | 155 (45) | 34 (45) | 6 (62) | 45 (44) |
| Reduced intensity | 91 (17) | 61 (18) | 14 (18) | 2 (11) | 14 (14) |
| GVHD prophylaxis | |||||
| CNI+MTX | 276 (51) | 127 (37) | 33 (43) | 15 (79) | 43 (42) |
| CNI+MMF | 110 (20) | 155 (45) | 24 (32) | 2 (11) | 45 (44) |
| Others | 51 (9) | 61 (18) | 3 (4) | 2 (11) | 14 (14) |
| ATG use | |||||
| No | 474 (88) | 306 (89) | 67 (88) | 13 (68) | 88 (86) |
| ≥ 6 months before diagnosis | 12 (2) | 7 (2) | 4 (5) | 0 (0) | 1 (1) |
| Within 6 months before diagnosis | 46 (9) | 25 (7) | 5 (7) | 5 (26) | 11 (11) |
| After diagnosis | 8 (1) | 5 (1) | 0 (0) | 1 (5) | 2 (2) |
| %FEV1/FVC pre PIV infection | |||||
| ≥ 70 | 377 (70) | 247 (72) | 49 (64) | 13 (68) | 68 (67) |
| < 70 | 93 (17) | 53 (15) | 14 (18) | 4 (21) | 22 (22) |
| Missing | 70 (13) | 43 (13) | 13 (17) | 2 (11) | 12 (12) |
| %TLC pre PIV infection | |||||
| ≥ 80 | 386(71) | 251 (73) | 53 (70) | 14 (74) | 68 (67) |
| < 80 | 61 (11) | 33 (10) | 9 (12) | 2 (11) | 17 (17) |
| Missing | 93 (17) | 59 (17) | 14 (18) | 3 (16) | 17 (17) |
| Days between transplantation and PIV infection | |||||
| ≤ 30 | 119 (22) | 66 (19) | 22 (28) | 3 (16) | 27 (26) |
| 31–100 | 349 (64) | 238 (69) | 40 (51) | 10 (53) | 39 (38) |
| > 100 | 76 (14) | 41 (12) | 16 (21) | 6 (32) | 36 (35) |
| Nosocomial infection | |||||
| No | 405 (75) | 280 (82) | 51 (67) | 13 (68) | 61 (60) |
| Yes | 132 (24) | 60 (17) | 25 (33) | 6 (32) | 41 (40) |
| Missing | 3 (1) | 3 (1) | 0 (0) | 0 (0) | 0 (0) |
| PIV type | |||||
| PIV-1 | 51 (9) | 25 (7) | 16 (21) | 1 (5) | 9 (9) |
| PIV-2 | 29 (5) | 23 (7) | 3 (4) | 0 (0) | 3 (3) |
| PIV-3 | 432 (80) | 275 (80) | 51 (67) | 17 (89) | 89 (87) |
| PIV-4 | 22 (4) | 16 (5) | 5 (7) | 1 (5) | 0 (0) |
| Unclassified | 6 (1) | 4 (1) | 1 (1) | 0 (0) | 1 (1) |
| Diagnostic test of URTI | |||||
| CU/DFA | 367 (68) | 258 (75) | 50 (66) | 12 (63) | 47 (46) |
| PCR | 112 (21) | 76 (22) | 20 (26) | 1 (5) | 15 (15) |
| Both | 13 (2) | 4 (1) | 6 (8) | 0 (0) | 3 (3) |
| Missing | 48 (9) | 5 (1) | 0 (0) | – | – |
| Co-pathogen | |||||
| No | 477 (88) | 308 (90) | 65 (86) | 18 (95) | 86 (84) |
| Upper resoiratory tract | 43 (8) | 29 (8) | 8 (11) | 1 (5) | 5 (5) |
| Blood | 20 (4) | 6 (2) | 3 (4) | 0 (0) | 11 (11) |
| White blood cell count | |||||
| > 1000/μL | 444 (82) | 297 (87) | 57 (75) | 19 (10) | 71 (70) |
| ≤ 1000/μL | 81 (15) | 32 (9) | 19 (25) | 0 (0) | 30 (29) |
| Missing | 15 (3) | 14 (4) | 0 (0) | 0 (0) | 1 (1) |
| Neutorophil count | |||||
| > 500/μL | 441 (82) | 296 (86) | 56 (74) | 18 (95) | 71 (70) |
| ≤ 500/μL | 81 (15) | 33 (10) | 19 (25) | 1 (5) | 29 (28) |
| Missing | 15 (3) | 14 (4) | 1 (1) | 0 (0) | 2 (2) |
| Lymphocyte count | |||||
| > 300/μL | 328 (61) | 221 (64) | 42 (55) | 11 (58) | 54 (53) |
| 100–300/μL | 124 (23) | 79 (23) | 21 (28) | 1 (5) | 23 (23) |
| < 100/μL | 67 (12) | 26 (8) | 11 (14) | 7 (37) | 23 (23) |
| Missing | 21 (4) | 17 (5) | 2 (3) | 0 (0) | 2 (2) |
| Monocyte count | |||||
| > 300/μL | 237 (44) | 167 (49) | 34 (45) | 4 (21) | 32 (31) |
| 100–300/μL | 147 (27) | 97 (28) | 18 (24) | 9 (47) | 23 (23) |
| < 100/μL | 134 (25) | 61 (18) | 22 (29) | 6 (32) | 45 (44) |
| Missing | 22 (4) | 18 (5) | 2 (3) | 0 (0) | 2 (2) |
| Steroid dose before diagnosis | |||||
| No | 238 (44) | 157 (46) | 40 (53) | 5 (26) | 36 (35) |
| < 1 mg/kg | 146 (27) | 88 (26) | 25 (33) | 4 (21) | 29 (28) |
| 1–2 mg/kg | 143 (26) | 93 (27) | 10 (13) | 7 (37) | 33 (32) |
| > 2 mg/kg | 13 (2) | 5 (1) | 1 (1) | 3 (16) | 4 (4) |
All values are indicated as the number (percentage).
Abbreviations: MA, myeloablative; TBI, toral body irradiation; GVHD, graft versus host disease; CNI, calcineurin inhibitor; MTX, methotrexate; MMF, mycophenolate mofetil; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ATG, anti-thymoglobulin; CU, culture
Progression to LRTD and risk stratification
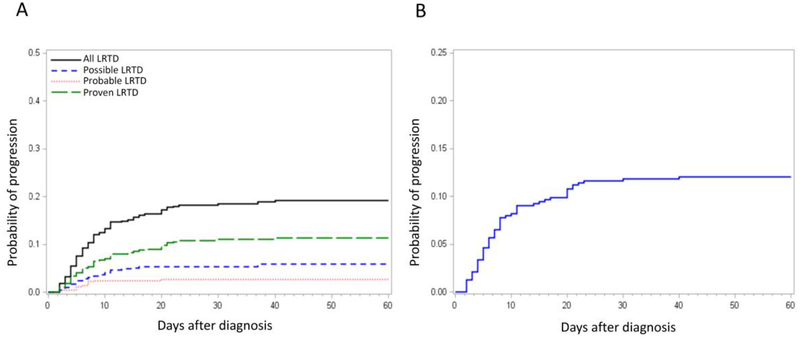
Among 540 patients with PIV infection, 424 (79%) patients originally presented with URTI as an initial manifestation of their PIV infection. Among these 424 patients, 81 (19%) progressed to LRTD in a median of 7 days (range, 2–40) following the diagnosis of URTI (black line in Figure 2A). The probability of progression from URTI to each LRTD group is also shown in Figure 2A. Among 476 patients with positive nasopharyngeal samples (i.e. URTI and possible LRTD [N=52] as an initial manifestation), the probability of progression to probable/proven LRTD with PIV detection in the lung within 40 days was 12% (Figure 2B).
Figure 2. Probability of progression from URTI to LRTD.
(A) The probability of progression from URTI to final LRTD status. Black line, progression from URTI to any type of LRTD; Blue line, progression from URTI to possible LRTD; Red line, progression from URTI to probable LRTD; Green line, progression from URTI to proven LRTD. (B) The probability of progression from URTI or possible LRTD to probable or proven LRTD.
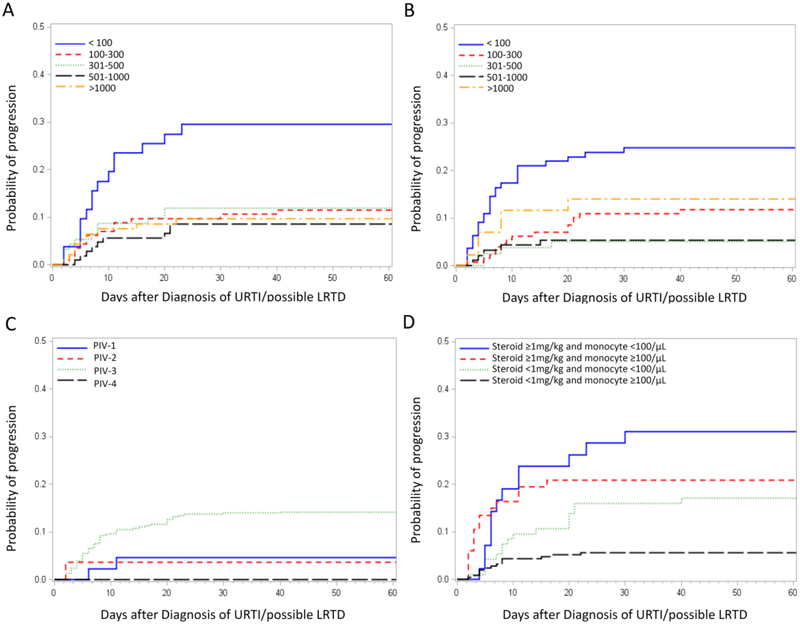
Since survival after possible LRTD has been documented to be similar to that after URTI and much higher than that after probable or proven LRTD [14], we analyzed the risk factors for progression from URTI/possible LRTD to probable/proven LRTD. In univariable Cox models, low blood cell counts were selected as important risk factors (Table 2). Patients with a lymphocyte count of less than 100 cells/μL had a high progression rate of approximately 30%, while patients with lymphocyte counts of greater or equal to 100 cells/μL had similar progression rates of 9–12%, independent of the lymphocyte level (Figure 3A). Similar patterns were seen for monocyte counts with levels less than 100 cells/μL having higher progression rates (Figure 3B). No completely protective level could be identified for either lymphocyte or monocyte counts. The diagnostic methods had no correlation with the progression rate (PCR alone vs conventional culture and/or DFA; HR 0.71; 95% CI [confidence interval] 0.36–1.40; p=0.32). In a multivariable model of risk factors for progression, PIV-3, co-pathogen in blood, low monocyte counts, and steroid use of ≥1mg/kg before PIV infection were significantly associated with a high progression rate to probable/proven LRTD (Table 2). Additional multivariable models were formed with different combinations of significant risk factors in the univariable analysis including transplant year, but did not yield additional factors that remained significant in the models (data not shown). The probability of progression by each PIV type was shown in Figure 3C. Co-pathogens in the blood were mainly bacteria (S. aureus in 5 cases, P. aeruginosa in 4 cases), with candidemia and EBV viremia observed in two cases each. The probability of progression to probable/proven LRTD by a combination of pairs of the most important factors (steroid use and monocyte count) is shown in Figure 3D, resulting in progression rates of approximately 30%.
Table 2.
Risk factors for progression from URTI/possible LRTD to probable/proven LRTD (N=476)
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Transplant year | ||||||
| 1990–2000 | 1.00 | |||||
| 2001–2011 | 0.55 | 0.3–0.9 | 0.028 | |||
| Cell source | ||||||
| PBSC | 1.00 | |||||
| BM or CB | 1.93 | 1.1–3.4 | 0.019 | |||
| ATG use before PIV infection | ||||||
| No/More than 6 months | 1.00 | 1.00 | ||||
| Within 6 months | 2.27 | 1.1–4.5 | 0.025 | 1.89 | 0.9–3.8 | 0.09 |
| PIV type | ||||||
| PV-1, 2, 4 | 1.00 | 1.00 | ||||
| PV-3 | 4.55 | 0.3–14.3 | 0.011 | 3.57 | 1.1–11.1 | 0.032 |
| Co-pathogen | ||||||
| No | 1.00 | 1.00 | ||||
| Upper respiratory tract | 0.85 | 0.3–2.4 | 0.76 | |||
| Blood | 3.00 | 1.1–8.3 | 0.035 | 3.21 | 1.1–9.0 | 0.027 |
| White blood cell count | ||||||
| > 1000/μL | 1.00 | |||||
| ≤ 1000/μL | 2.29 | 1.3–4.1 | 0.006 | |||
| Neutrophil count | ||||||
| > 500/μL | 1.00 | |||||
| ≤ 500/μL | 2.05 | 1.1–3.8 | 0.02 | |||
| Lymphocyte count | ||||||
| > 300/μL | 1.00 | 1.00 | ||||
| 100–300/μL | 1.16 | 1.7–5.6 | 0.66 | |||
| < 100/μL | 3.25 | 1.7–6.1 | <.001 | 1.79 | 0.9–3.6 | 0.10 |
| Monocyte count | ||||||
| > 300/μL | 1.00 | 1.00 | ||||
| 100–300/μL | 1.68 | 0.8–3.4 | 0.16 | |||
| < 100/μL | 3.96 | 2.1–7.5 | <.001 | 2.22 | 1.2–4.2 | 0.011 |
| Steroid dose before diagnosis | ||||||
| No | 1.00 | 1.00 | ||||
| < 1 mg/kg | 0.94 | 0.4–2.1 | 0.89 | |||
| 1–2 mg/kg | 2.53 | 1.4–4.6 | 0.002 | 1.89 | 1.1–3.2 | 0.018 |
| > 2 mg/kg | 4.20 | 1.0–18.1 | 0.054 | |||
| Steroid dose after diagnosis* | ||||||
| No | 1.00 | |||||
| < 0.5 mg/kg | 0.60 | 0.3–1.4 | 0.24 | |||
| 0.5–1 mg/kg | 0.67 | 0.3–1.6 | 0.36 | |||
| > 1 mg/kg | 2.35 | 0.7–7.6 | 0.15 | |||
| Ribavirin use* | ||||||
| No | 1.00 | |||||
| Yes | 2.29 | 0.7–7.4 | 0.16 | |||
| Viral load in NP sample at diagnosis** | 1.00 | 1.0–1.0 | 0.46 | |||
These variables were analyzed as time-dependent.
Viral load was analyzed as a continuous variable.
All variables in Table 1 were used for the univariate analysis. Co-pathogen and ribavirin use at diagnosis of URTI/possible LRTD were included in the analysis. Only variables with p < 0.05 in any analysis are shown in this table. The following parameters were also shown regardless of p values; steroid dose after diagnosis, ribabirin use.
Abbreviations: PBSC, periperal blood stem cell; BM, bone marrow; CB, cord blood; ATG, anti-thymocyte globulin; NP, nasopharyngeal
Figure 3. Probability of progression from URTI/possible LRTD to probable/proven LRTD.
(A) Cumulative incidence of progression by lymphocyte levels. (B) Cumulative incidence of progression by monocyte levels. (C) Cumulative incidence of progression by PIV type. (D) Cumulative incidence of progression to LRTD by steroid dose before diagnosis and PIV type.
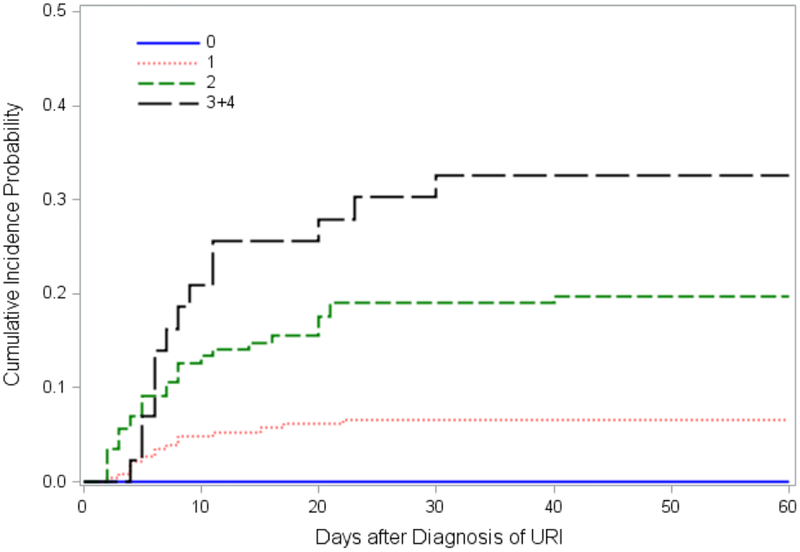
We then analyzed progression rates relative to the number of risk factors that are present at the detection of URTI. No patient progressed to LRTD in the absence of all four risk factors, while there was an escalating risk with increasing number of risk factors (Figure 4). We also performed a similar analysis among patients who presented with URTI alone, as generally done in the published literature. Probabilities of progression from URTI to all LRTD including possible LRTD (Supplemental Figure 1A) or to probable/proven LRTD only (Supplemental Figure 1B) by the number of risk factors showed similar patterns. Overall, the risk of progression among patients with URTI alone resulted in somewhat higher rates of than those observed among patients with URTI/possible.
Figure 4. Probability of progression from URTI/possible LRTD to probable/proven LRTD by the number of risk factors.
Cumulative incidence of progression by the number of risk factors (PIV type, co-pathogen, monocyte count, and steroid dose; n=64 in 0, n=227 in 1, n=142 in 2, and n=43 in ≥3 risk factors).
Among 136 patients with quantitated viral loads in nasal wash samples at diagnosis, an association between viral load and progression was not observed (Table 2).
Effect of ribavirin use at URTI diagnosis
Eighteen patients received ribavirin therapy (inhalation, N=13; systemic, N=3; both, N=1; unknown, N=1) after the diagnosis of URTI or possible LRTD, and three of these patients progressed to probable/proven LRTD. In a univariable Cox regression model of risk factors for progression to probable/proven LRTD, ribavirin use at URTI or possible LRTD was not significantly associated with progression (Table 2). In bivariate analyses adjusting for each important risk factor, ribavirin use also had no preventive effect on progression to probable/proven LRTD (adjustment variable, PIV type; aHR, 2.17; 95% CI, 0.67–6.96; p = 0.19; adjustment variable, co-pathogen; aHR, 2.32; 95% CI, 0.72–7.46; p = 0.16; adjustment variable, monocyte count; aHR, 1.39; 95% CI, 0.43–4.53; p = 0.59; adjustment variable, steroid use; aHR, 2.29; 95% CI, 0.71–7.35; p = 0.16)
Risk factors for LRTD without prior URTI among probable/proven LRTD cases
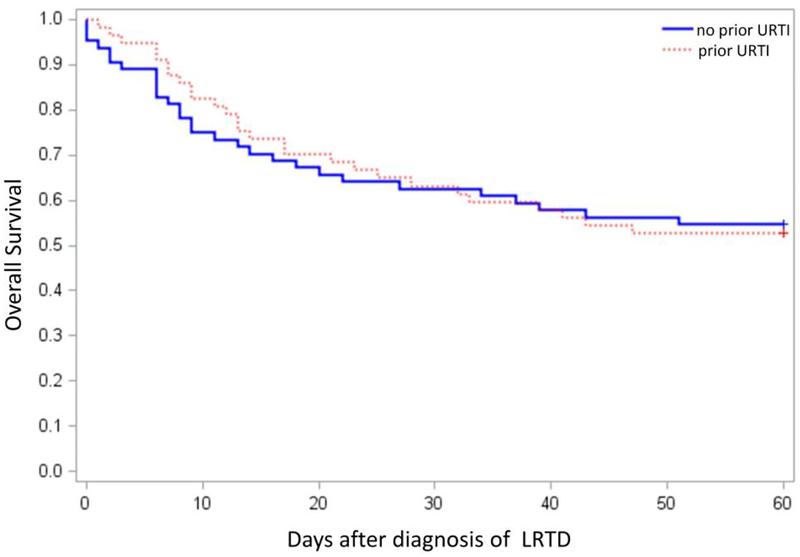
Among 121 probable/proven LRTD cases, 64 (53%) had LRTD without first presenting as URTI. The median time to PIV LRTD after HCT was 76 days (range, 3–2343) and 81 days (range, 5–2862) in LRTD cases with and without prior URTI, respectively (p=0.51). Demographics of each patient group are shown in Supplemental Table 1. Of the 64 patients initially presenting with probable/proven LRTD, 13 (20%) had a nasopharyngeal test performed using DFA or culture in 7/13 (54%) and PCR in 6/13 (46%), and all the results were negative. In multivariable logistic regression models, factors associated with the development of LRTD without prior URTI versus LRTD that progressed from URTI were low percentage of total lung capacity (TLC) (< 80%) before PIV infection, absence of documented URTI symptoms, oxygen requirement at diagnosis, and co-pathogen detection in the lower respiratory tract (Table 3). The main co-pathogens were Aspergillus spp. [N=9], cytomegalovirus [N=6], and P. aeruginosa [N=5]. Among 48 cases with calculated viral loads in BAL samples, the viral loads were not significantly different between LRTD cases with and without prior URTI. The mortality rate after LRTD without prior URTI was similar to that after LRTD with prior URTI (Figure 5; p=0.98)
Table 3.
Risk factors for LRTD without prior URTI (N=121)
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age at PIV infection | ||||||
| ≤ 20 | 0.25 | 0.1–1.3 | 0.09 | |||
| 21–60 | 0.44 | 0.1–1.5 | 0.19 | |||
| > 60 | 1.00 | |||||
| Cell source | ||||||
| PBSC | 1.00 | 1.00 | ||||
| BM or CB | 0.39 | 0.2–0.8 | 0.012 | 0.47 | 0.1–1.7 | 0.25 |
| %FEV1/FVC pre PIV infection | ||||||
| ≥ 70 | 1.00 | |||||
| < 70 | 2.25 | 0.9–5.6 | 0.08 | |||
| %TLC pre PIV infection | ||||||
| ≥ 80 | 1.00 | 1.00 | ||||
| < 80 | 3.41 | 1.1–10.3 | 0.030 | 5.56 | 1.1–25.0 | 0.036 |
| Days between transplantation and PIV infection | ||||||
| ≤ 30 | 0.92 | 0.4–2.4 | 0.87 | |||
| 31–100 | 0.42 | 0.2–1.0 | 0.047 | |||
| > 100 | 1.00 | |||||
| Inpatient at diagnosis | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 3.44 | 1.6–7.3 | 0.001 | 1.61 | 0.4–6.0 | 0.48 |
| Diagnosis on weekday | ||||||
| No | 1.00 | |||||
| Yes | 0.51 | 0.2–1.5 | 0.21 | |||
| Documented URTI symptom at diagnosis | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.19 | 0.1–0.4 | <.001 | 0.17 | 0.0–0.7 | 0.017 |
| LRTD symptom at diagnosis | ||||||
| No | 1.00 | |||||
| Yes | 3.37 | 1.1–10.3 | 0.033 | |||
| PIV type | ||||||
| PIV-1, 2, 4 | 1.00 | |||||
| PIV-3 | 0.26 | 0.1–1.0 | 0.049 | |||
| Co-pathogen | ||||||
| No / Blood alone | 1.00 | 1.00 | ||||
| Upper respi ratory tract | 0.38 | 0.0–3.5 | 0.40 | 0.41 | 0.0–40.1 | 0.24 |
| Lower respi ratory tract | 14.60 | 4.1–52.0 | <.001 | 43.70 | 6.0–321 | 0.006 |
| Oxygen use at diagnosis | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 10.30 | 4.4–24.5 | <.001 | 9.51 | 2.2–41.2 | 0.003 |
| Steroid dose before diagnosis | ||||||
| No | 1.00 | |||||
| < 1 mg/kg | 1.80 | 0.7–4.7 | 0.23 | |||
| 1–2 mg/kg | 0.38 | 0.2–0.9 | 0.034 | |||
| > 2 mg/kg | 1.96 | 0.3–11.3 | 0.45 | |||
All variables in Supplemental Table 1 were used for the univariate analysis. Only variables with p < 0.1 in any analysis except diagnosis on weekday are shown in this table. The most informative multivariable model is shown here and additional models with different combinations of risk factors did not yield other factors that remained after adjustment.
Abbreviations: PBSC, periperal blood stem cell; BM, bone marrow; CB, cord blood; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity
Figure 5. Probability of overall survival comparing LRTD with and without prior URTI.
Kaplan-Meier estimate of overall survival among 121 probable/proven LRTD cases. (P value 1.00).
DISCUSSION
This study demonstrated that the progression rate of PIV-associated URTI to probable/proven PIV LRTD was approximately 12% and that the total number of risk factors (presence of PIV-3, co-pathogen in the blood, low monocyte counts, and steroid use of ≥ 1mg/kg at the time of diagnosis) that are present at the time of URTI diagnosis predicts the probability of progression. Probable/proven LRTD without prior URTI was observed in about half of LRTD cases and occurred more frequently in patients with restrictive lung function who had a co-infection in their lungs. These findings provide new information for those caring for HCT recipients, particularly those with respiratory symptoms during the post-HCT period.
Several studies on the incidence or outcomes of PIV infections in patients with hematological malignancies or transplant recipients have reported highly variable progression rates from URTI to LRTD ranging from 0 to 74% [3, 9, 11, 12, 17], with an average rate of 36%. This wide range could be due to varying cohort sizes (median subjects, N=13; range, 4–213) or because the definition of LRTD was inconsistent among the studies. We reported previously that the survival after possible LRTD (PIV detection only in upper respiratory tract) is similar to that after URTI and much better than that after probable/proven LRTD (PIV detection in lower respiratory tract) [14]. In this current cohort study of 540 subjects, the progression rate was approximately 50% higher when proven/probable and possible LRTD cases were combined (19% vs. 12% for proven/probable only) (Figure 2A–B). Considering the differential outcomes after LRTD [14], we recommend reporting probable and proven LRTD as among LRTD cases in future studies which would permit the consistent interpretation of study endpoints and results.
Using Cox regression models, we identified four risk factors for progression from URTI/possible LRTD to probable/proven LRTD. Low monocyte count and PIV-3 type were newly identified risk factors. Previous studies including studies of other respiratory viruses showed the importance of lymphocyte count [9, 16, 18, 19]. In our study, however, low monocyte count appeared to be more important in progression to LRTD than lymphopenia, which is similar to the results of risk factors for mortality after PIV LRTD [14]. Previous reports have shown that monocytes are important to protect viral infection [20, 21]. However, the role of monocytopenia in the progression from PIV URTI to LRTD should be considered for further investigation. Importantly, the four factors we identified can stratify the risk for progression in HCT recipients with a positive nasopharyngeal sample. Indeed, the absence of any of the four risk factors was associated with protection from progression while increasing numbers of risk factors correlated with increased risk. Nevertheless, monocytopenia and corticosteroid dose, a surrogate marker of graft-versus-host disease, appear to be the most relevant of these four factors. Of interest, viral load in nasopharyngeal specimen as a possible predictor for progression was analyzed in a subset of patients and was not predictive of progression to LRTD. This is consistent with a lack of a viral load as a risk for progression after HCT with human metapneumovirus infection [16].
Administration of an antiviral drug may prevent progression to LRTD for other viral infections such as RSV or influenza, based on data from non-randomized studies [19, 22, 23]. Although there is a small study that suggested a preventive effect of ribavirin [22, 23], other studies and a recent meta-analysis failed to show the efficacy of ribavirin both in disease progression and mortality from PIV LRTD [3, 14, 17, 5]. In our study, adjusted analyses did not suggest a preventive effect of preemptive ribavirin. Steroid use has been consistently shown to be important for progression in this and other studies [11, 17]. Based on our study, we recommend reducing the steroid dose whenever feasible to less than 1 mg/kg when PIV is detected until a new anti-viral drug such as DAS181 or virus-specific T cell therapy are shown to be effective and available for use [26–30].
Although we could not evaluate difference between each PIV subtype because of the insufficient number of cases, PIV-3 had a significantly higher progression rate than the other types in the multivariable analysis. Since the incidence of PIV-1, 2, and 4 infections are low, less is known about the pathogenesis of these PIV types, especially in PIV-4. Further studies are needed to examine the role of strain differences in pathogenicity.
In immunocompromised patients, LRTDs are occasionally documented without manifestation of URTI. In our study, 53% of patients with probable/proven LRTD had no prior presentation of URTI, while previous reports showed frequencies between 13 and 58% [9, 11–13]. The differences in the observed frequency rates may be due to how diagnostic work-ups proceed for PIV using BAL or biopsy samples. We generally test for respiratory viruses including PIV by PCR when we perform bronchoscopic examination regardless of the presence or absence of URTI symptoms. We confirmed that the absence of URTI symptoms was associated with LRTD without URTI, which is plausible and would argue against the possibility that we missed earlier testing in patients who should have been tested. One possible reason for the lack of URTI symptoms may be the inability to mount an inflammatory response following viral acquisition. However, the maximum steroid dose in the 2 weeks prior to diagnosis did not appear to be an important factor, whereas oxygen requirement at diagnosis and a pulmonary co-pathogen were other significant factors. We hypothesize that inpatients have a higher likelihood of diagnostic imaging, possibly in the context of preexisting restrictive lung function and/or hypoxia, which may have led to more bronchoscopic procedures. Meanwhile, early bronchoscopic examination for LRTD with a broad diagnostic panel that includes respiratory viruses regardless of upper respiratory symptoms may maximize diagnostic yield for pulmonary disease.
This study has the advantage of including the largest number of HCT recipients with PIV infections, which permitted the performance of multivariable Cox regression analyses of risk factors for progression to LRTD that can clearly stratify the risk groups. Moreover, we applied the stringent definition of LRTD based on a previous outcome study [14], and examined for the first time risk factors that might explain the phenomenon of LRTD without URTI. Limitations of this study include the inclusion of cases over a relatively long period of time and insufficient cases to analyze the effect of preemptive ribavirin, viral load on progression, or the differences by each subtype despite of data collection for 20 years. To decrease the effect of a long time period on the analyzed results, we included transplant year and the factors that have changed during 20 years such as diagnostic methods or cell source in multivariable model. Moreover, because of the retrospective nature of our study, we might have missed information of URTI symptoms from medical charts due to inconsistent documentation practices or failure of patients to report mild URTI symptoms, especially late after HCT. We generally performed examination for respiratory viruses using a nasopharyngeal sample when patients had URTI symptoms and bronchoscopy when patients had lower respiratory tract symptoms and radiographic abnormalities. Nevertheless the final decision was left to the physicians who managed the patients. Therefore, an aggressive work-up was declined in some cases.
In conclusion, the progression rate of PIV to probable/proven LRTD in our large retrospective series was approximately 12% and it exceeded 30% in patients who had at least three risk factors, with low monocyte counts and steroid use of ≥ 1mg/kg prior to URTI being the most important predictors. Our finding that monocytes rather than lymphocytes appear to play an important role in progressive disease suggests that further studies to evaluate host defense pathways for PIV are needed. Ribavirin did not appear to have a protective effect on progression. These findings define populations that appear to be protected from progression and those at high risk. This should facilitate the rational development of preventive and therapeutic strategies in immunocompromised hosts. Considering that approximately half of HCT recipients with LRTD showed absence of URTI symptoms, intensive virologic testing is recommended in patients with pneumonia or evidence of lower respiratory tract disease.
Supplementary Material
Highlights.
Progression rate to PIV lower respiratory tract disease (LRTD) by day 40 was 12%.
Progression to probable/proven LRTD was associated with four risk factors.
No patients progressed to LRTD in the absence of all four risk factors.
ACKNOWLEDGMENTS
This work was partially supported by grants from the National Institutes of Health (K24HL093294 and CA15704). S.S. is a recipient of a fellowship from the Joel Meyers Endowment Scholarship. The authors would like to thank Zachary Stednick for database services and Terry Stevens-Ayers for laboratory assistance. Specimens were provided by the Vaccine and Infectious Disease Division Biospecimen Repository at Fred Hutch.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: S.S. and M.B. received research support from Ansun Biopharma, Inc. M.B. also serves as a consultant to Ansun Biopharma, Inc. All other authors declare no competing financial interests.
References
- 1.Renaud C, Campbell AP. Changing epidemiology of respiratory viral infections in hematopoietic cell transplant recipients and solid organ transplant recipients. Current opinion in infectious diseases 2011; 24: 333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schiffer JT, Kirby K, Sandmaier B, Storb R, Corey L, Boeckh M. Timing and severity of community acquired respiratory virus infections after myeloablative versus non-myeloablative hematopoietic stem cell transplantation. Haematologica 2009; 94: 1101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah DP, Shah PK, Azzi JM, Chemaly RF. Parainfluenza virus infections in hematopoietic cell transplant recipients and hematologic malignancy patients: A systematic review. Cancer letters 2016; 370: 358–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henrickson KJ. Parainfluenza viruses. Clinical microbiology reviews 2003; 16: 242–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fry AM, Curns AT, Harbour K, Hutwagner L, Holman RC, Anderson LJ. Seasonal trends of human parainfluenza viral infections: United States, 1990–2004. Clinical infectious diseases 2006; 43: 1016–22. [DOI] [PubMed] [Google Scholar]
- 6.Hodson A, Kasliwal M, Streetly M, MacMahon E, Raj K. A parainfluenza-3 outbreak in a SCT unit: sepsis with multi-organ failure and multiple co-pathogens are associated with increased mortality. Bone marrow transplantation 2011; 46: 1545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maziarz RT, Sridharan P, Slater S, et al. Control of an outbreak of human parainfluenza virus 3 in hematopoietic stem cell transplant recipients. Biology of blood and marrow transplantation 2010; 16: 192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortez KJ, Erdman DD, Peret TC, et al. Outbreak of human parainfluenza virus 3 infections in a hematopoietic stem cell transplant population. The Journal of infectious diseases 2001; 184: 1093–7. [DOI] [PubMed] [Google Scholar]
- 9.Chemaly RF, Hanmod SS, Rathod DB, et al. The characteristics and outcomes of parainfluenza virus infections in 200 patients with leukemia or recipients of hematopoietic stem cell transplantation. Blood 2012; 119: 2738–45; quiz 969. [DOI] [PubMed] [Google Scholar]
- 10.Ustun C, Slaby J, Shanley RM, et al. Human parainfluenza virus infection after hematopoietic stem cell transplantation: risk factors, management, mortality, and changes over time. Biology of blood and marrow transplantation 2012; 18: 1580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood 2001; 98: 573–8. [DOI] [PubMed] [Google Scholar]
- 12.Lewis VA, Champlin R, Englund J, et al. Respiratory disease due to parainfluenza virus in adult bone marrow transplant recipients. Clinical infectious diseases 1996; 23: 1033–7. [DOI] [PubMed] [Google Scholar]
- 13.Wendt CH, Weisdorf DJ, Jordan MC, Balfour HH Jr., Hertz MI. Parainfluenza virus respiratory infection after bone marrow transplantation. The New England journal of medicine 1992; 326: 921–6. [DOI] [PubMed] [Google Scholar]
- 14.Seo S, Xie H, Campbell AP, et al. Parainfluenza virus lower respiratory tract disease after hematopoietic cell transplant: viral detection in the lung predicts outcome. Clinical infectious diseases 2014; 58: 1357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuypers J, Wright N, Ferrenberg J, et al. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol 2006; 44: 2382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo S, Gooley TA, Kuypers JM, et al. Human Metapneumovirus Infections Following Hematopoietic Cell Transplantation: Factors Associated With Disease Progression. Clinical infectious diseases 2016; 63: 178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivasan A, Wang C, Yang J, Shenep JL, Leung WH, Hayden RT. Symptomatic parainfluenza virus infections in children undergoing hematopoietic stem cell transplantation. Biology of blood and marrow transplantation 2011; 17: 1520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim YJ, Guthrie KA, Waghmare A, et al. Respiratory syncytial virus in hematopoietic cell transplant recipients: factors determining progression to lower respiratory tract disease. The Journal of infectious diseases 2014; 209: 1195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah DP, Ghantoji SS, Shah JN, et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. The Journal of antimicrobial chemotherapy 2013; 68: 1872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol 2009; 10: 1200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cros J, Cagnard N, Woollard K et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 recetors. Immunity 2010; 33: 375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah DP, Ghantoji SS, Ariza-Heredia EJ, et al. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood 2014; 123: 3263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kmeid J, Vanichanan J, Shah DP, et al. Outcomes of Influenza Infections in Hematopoietic Cell Transplant Recipients: Application of an Immunodeficiency Scoring Index. Biology of blood and marrow transplantation 2016; 22: 542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakrabarti S, Collingham KE, Holder K, Oyaide S, Pillay D, Milligan DW. Parainfluenza virus type 3 infections in hematopoetic stem cell transplant recipients: response to ribavirin therapy. Clinical infectious diseases 2000; 31: 1516–8. [DOI] [PubMed] [Google Scholar]
- 25.Elizaga J, Olavarria E, Apperley J, Goldman J, Ward K. Parainfluenza virus 3 infection after stem cell transplant: relevance to outcome of rapid diagnosis and ribavirin treatment. Clinical infectious diseases 2001; 32: 413–8. [DOI] [PubMed] [Google Scholar]
- 26.Guzman-Suarez BB, Buckley MW, Gilmore ET, et al. Clinical potential of DAS181 for treatment of parainfluenza-3 infections in transplant recipients. Transplant infectious disease 2012; 14: 427–33. [DOI] [PubMed] [Google Scholar]
- 27.Drozd DR, Limaye AP, Moss RB, et al. DAS181 treatment of severe parainfluenza type 3 pneumonia in a lung transplant recipient. Transplant infectious disease 2013; 15: E28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waghmare A, Wagner T, Andrews R, et al. Successful Treatment of Parainfluenza Virus Respiratory Tract Infection With DAS181 in 4 Immunocompromised Children. Journal of the Pediatric Infectious Diseases Society 2015; 4: 114–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salvatore M, Satlin MJ, Jacobs SE, et al. DAS181 for Treatment of Parainfluenza Virus Infections in Hematopoietic Stem Cell Transplant Recipients at a Single Center. Biology of blood and marrow transplantation 2016; 22: 965–70. [DOI] [PubMed] [Google Scholar]
- 30.Aguayo-Hiraldo PI, Arasaratnam RJ, Tzannou I, et al. Characterizing the cellular immune response to Parainfluenza virus 3. The Journal of infectious diseases 2017; 216: 153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.