Abstract
Neuroinflammation is a common pathological correlate of HIV-associated neurocognitive disorders (HAND) in individuals on antiretroviral therapy (ART). Triggering receptor-expressed on myeloid cells 2 (TREM2) regulates neuroinflammation, clears extracellular Amyloid (A)-β, surveys for damaged neurons, and orchestrates microglial differentiation. TREM2 has not been studied in HIV+ brain tissues. In this retrospective study we investigated TREM2 expression levels and localization to microglia, Aβ protein levels, and tumor necrosis factor (TNF)-α transcript levels in the frontal cortices of 52 HIV+ decedents. All donors had been on ART; 14 were cognitively normal (CN), 17 had asymptomatic neurocognitive impairment (ANI), and 21 had minor neurocognitive disorder (MND). Total TREM2 protein levels were increased in the soluble and decreased in the membrane-enriched fractions of MND brain tissues compared to CN; however, brains from MND Hispanics showed the most robust alterations in TREM2 as well as significantly increased TNF-α mRNA and Aβ levels when compared to CN Hispanics. Significant alterations in the expression of total TREM2 protein and transcripts for TNF-α were not observed in non-Hispanics, despite higher levels of Aβ in the non-Hispanic CN group compared to the non-Hispanic ANI and MND groups. These findings show that decreased and increased TREM2 in membrane-bound fractions and in soluble-enriched fractions, respectively, is associated with increased Aβ and neuroinflammation in this cohort of HIV+ brains, particularly those identifying as Hispanics. These findings suggest a role for TREM2 in the brain of HIV+ individuals may deserve more investigation as a biomarker for HAND and as a possible therapeutic target.
Keywords: HIV-associated neurocognitive disorders, triggering receptor expressed on myeloid cells 2, microglia, amyloid-β, neuroinflammation
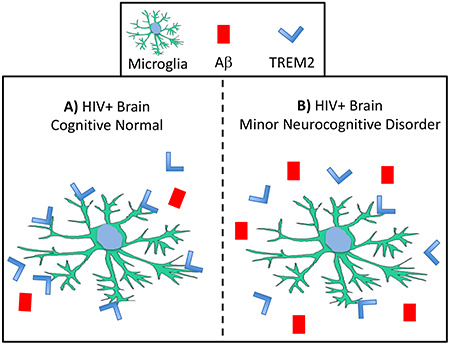
HIV-associated neurocognitive disorders (HAND) remain a significant problem despite effective antiretroviral therapy; however, the mechanisms underlying the neuropathogeneisis of HAND are not well understood. In this manuscript we describe alterations in the levels of TREM2 and Aβ in brains of cases diagnosed with minor neurocognitive disorders (MND) compared to brains from cognitively normal (CN) HIV+ cases. (A) In HIV+ cases that were diagnosed as CN, we identified high levels of TREM2 in membrane-enriched fractions, increased TREM2 colocalization with the microglia marker IBA1, and lower levels of Aβ when compared to (B) MND cases, in which, the levels of TREM2 were increased in soluble-enriched fractions and decreased in membrane-enriched fractions, levels of Aβ were increased, and TREM2 signal associated with IBA1 positive cells was reduced compared to CN cases. These data may indicate that TREM2 can be a useful biomarker for HAND progression in some individuals and that altered levels of TREM2 may play a role in the neuropathogenesis of HAND.
Introduction
HIV-associated neurocognitive disorders (HAND) and other HIV-induced neurological disorders persist despite the ability to control HIV replication in the periphery with combined antiretroviral therapy (ART) (Heaton et al. 2010, Heaton et al. 2011). Since the advent of ART, the most severe forms of HAND have decreased, however, the occurrence of asymptomatic neurocognitive impairment (ANI), and minor neurocognitive disorder (MND) has increased(Saylor et al. 2016). Neuroinflammation and accumulation of amyloid-β (Aβ) persist as neuropathological correlates of HAND in brain tissues of HIV+ individuals that were on ART (Levine et al. 2016, Ortega & Ances 2014, Andras & Toborek 2013, Gisslen et al. 2009, Tavazzi et al. 2014). Furthermore, HIV infection in the ART era is associated with premature aging, as indicated by epigenetic markers, T-cell senescent phenotypes, and other age-related comorbidities (Pathai et al. 2014, Guaraldi et al. 2011, Gross et al. 2016, Fulop et al. 2017). Novel therapeutic targets are needed to reduce inflammation and slow the neurodegenerative process in HIV-infected persons on ART.
Multiple neurotoxic mechanisms may underlie the neurodegenerative process in individuals with HAND, including alterations in autophagic flux(Fields et al. 2013, Fields et al. 2015a), mitochondrial dysfunction(Fields et al. 2015b), vascular damage(Fulop et al. 2017, Mishra & Singh 2014), and oxidative stress(Andras & Toborek 2013). Neuroinflammation and accumulation of extracellular debris is associated with multiple neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and HAND. Recent discoveries have linked dysfunctional triggering receptor expressed on myeloid cells (TREM) 2 to neuroinflammation and accumulation of Aβ (Ulrich et al. 2014) in mouse models (Wang et al. 2015, Ren et al. 2018, Jiang et al. 2018). TREM2 was originally identified as a receptor expressed on monocyte-derived dendritic cells (DCs) that modulate DC maturation and responses to pathogenic stimuli(Bouchon et al. 2001). More recent studies have shown that TREM2 is expressed by numerous myeloid lineage cells including monocyte-derived macrophages and the resident brain macrophages, microglia(Jay et al. 2017, Zhao et al. 2018, Tang & Le 2016). Microglia survey the environment for cues on the disease state, interacting with extracellular milieu, other glial cells, and neurons(Tang & Le 2016). Although recently recognized as an overly simplified classification, microglia may assume one of two phenotypes: M1 or M2(Zhang et al. 2018). M1 microglia are associated with inflammatory cytokines, such as TNF-α, and increased neurodegeneration. In contrast, the M2 phenotype microglia express anti-inflammatory cytokines IL-10 and IL-4 and receptors, such as TREM2, that efficiently phagocytose extracellular debris and dying cells(Orihuela et al. 2016, Tang & Le 2016, Zhao et al. 2018). Although, the M1/M2 distinction is recognized as overly simplified, macrophages and microglia expressing TREM2 have a more anti-inflammatory phenotype(Wang et al. 2015, Painter et al. 2015). High levels of soluble TREM2 in the cerebrospinal fluid (CSF) are associated with worse inflammation(Zhong et al. 2017), worse neurocognitive outcomes(Henjum et al. 2016), and age(Byrne et al. 2018). Loss of function TREM2 mutants are linked to neurodegenerative disorders, including AD, PD, and Fronto-temporal dementias(Jonsson et al. 2013) (Painter et al. 2015). Therefore, much research is now focused on the role of TREM2 in neurogenerative diseases.
HIV infection of the brain is associated with increased expression of inflammatory cytokines, such as tumor necrosis factor (TNF)-α(Ortega & Ances 2014), and increased levels of Aβ(Esiri et al. 1998, Giometto et al. 1997, Green et al. 2005), including intraneuronal Aβ (Achim et al. 2009). Brain macrophages, resident microglia or perivascular macrophages, play a major role in regulating the inflammatory milieu in the brains of HIV+ individuals. Damaged neurons with reduced spine density are also a common neuropathologic feature of HAND decedents in the ART era. Alterations in the ability of TREM2 to recognize and clear Aβ and injured neurons may contribute to the neurodegenerative process underlying HAND (Rivest 2015, Atagi et al. 2015, Zhao et al. 2018); however, the role of TREM2 in HAND has yet to be explored.
In this study, we hypothesized that alterations in TREM2 levels and cellular localization in the brain may be altered in HIV+ individuals that were diagnosed with HAND. To test this, we analyzed brain tissues from a well-characterized cohort of HIV+ individuals who were on ART for expression TREM2 as well as a target of TREM2-mediated phagocytosis, Aβ. We also analyzed cellular localization of TREM2 in the frontal cortices of these patients. To determine the neuroinflammatory status in these tissues, we assessed mRNA levels for TREM2 and TNF-α. Interestingly, alterations in TREM2 levels were most robust when stratified by ethnicity: Hispanic versus non-Hispanic. The results from these studies may lead to therapeutic strategies in some of the HIV+ population.
Methods
Study population
For the present study, we evaluated brain tissues from a total of 52 HIV+ donors (Tables 1 and 2), from the National NeuroAIDS Tissue Consortium (NNTC) (Institutional Review Board [IRB] #080323). All studies adhered to the ethical guidelines of the National Institutes of Health and the University of California, San Diego. These cases had neuromedical and neuropsychological examinations within a median of 12 months before death. The associated pathology was most frequently due to systemic cytomegalovirus (CMV), Kaposi sarcoma, and liver disease. Subjects were excluded if they had a history of CNS opportunistic infections or non-HIV-related developmental, neurologic, psychiatric, or metabolic conditions that might affect CNS functioning (e.g., loss of consciousness exceeding 30 minutes, psychosis, etc). HAND diagnoses were determined from a comprehensive neuropsychological test battery administered according to standardized protocols(Woods et al. 2004).
Table 1.
Clinical and demographic characteristics by HAND diagnoses.
| Variables | CN (n=14) | HAND (n=38) | CN vs HAND | CN vs ANI vs MND | |||
|---|---|---|---|---|---|---|---|
| ANI (n=17) | MND (n=21) | p-value (CN vs HAND; t-test) | Effect Size - Cohen’s d (CN vs HAND) | p-value (ANOVA) | Effect Size: overall, (CN vs ANI, CN vs MND, ANI vs MND) | ||
| Demographics | |||||||
| Hispanic ethnicity, n (%) | 57.1 | 56.3 | 66.7 | N/A | N/A | N/A | N/A |
| Sex (f/m) | 0/14 | 7/10 | 2/19 | N/A | N/A | N/A | N/A |
| Years of Education | 12.0 ± 2.09 | 12.36 ± 2.42 | 11.53 ± 3.02 | 0.79 | 0.03 | 0.75 | 0.25 (0.17, 0.00, 0.23) |
| Years of Age | 37.3 ± 5.5 | 44 ± 9.0 | 45.9 ± 8.3 | 0.005** | 1.31 | 0.009** | 0.44 (0.32, 0.43, 0.10) |
| HIV Disease Characteristics | |||||||
| Duration on ART regimen (months) | 68.83 ± 48.05 | 75.74 ± 83.68 | 68.7 ± 57.93 | 0.95 | 0.25 | 0.82 | 0.05 (0.04, 0.004, 0.05) |
| Plasma VL (log) | 4.3 ± 1.61 | 3.8 ± 1.55 | 4.2 ± 1.62 | 0.76 | 0.2 | 0.81 | 0.13 (0.12, 0.03, 0.10) |
| CD4 count | 147.7 ± 239.01 | 142.8 ± 198.21 | 30.1 ± 35.81 | 0.33 | 0.32 | 0.12 | 0.31 (0.01, 0.26, 0.27) |
| PMI (hours) | 6.5 ± 3.75 | 20.3 ± 21.1 | 9.6 ± 8.1 | 0.09 | 0.68 | 0.08 | 0.43 (0.40, 0.09, 0.34) |
Table 2.
Clinical and demographic characteristics by self-identified ethnicity.
| Variables | Hispanic (n=32) | Non-Hispanic (n=20) | p-value (Hispanic vs non-Hispanic; t-test) | Effect Size - Cohen’s d (Hispanic vs non-Hispanic) |
|---|---|---|---|---|
| Demographics | ||||
| Sex (f/m) | 9/23 | 3/17 | N/A | N/A |
| Years of Education | 11.8 ± 2.69 | 13.88 ± 3.64 | 0.1 | 0.65 |
| Years of Age | 43.3 ± 8.79 | 45.5 ± 8.14 | 0.5 | 0.26 |
| HIV Disease Characteristics | ||||
| HAND (%) | 75 | 70 | N/A | N/A |
| Duration on ART | 91.4 ± 68.39 | 48.4 ± 41.73 | 0.26 | 0.76 |
| Plasma VL (log) | 3.77 ± 1.55 | 5.19 ± 0.94 | 0.02 | 1.11 |
| CD4 count | 89.9 ± 159.4 | 41.1 ± 73.2 | 0.26 | 0.39 |
| PMI (hours) | 11.5 ± 14.4 | 16.3 ± 16.04 | 0.5 | 0.31 |
Neuromedical and Neuropsychological evaluation
Participants underwent a comprehensive neuromedical evaluation that included assessment of medical history, structured medical and neurological examinations, and the collection of blood, cerebrospinal fluid (CSF), and urine samples, as previously described (Woods et al. 2004, Heaton et al. 2010). Clinical data (plasma viral load [VL], postmortem interval, CD4 count, global, learning and motor deficit scores [GDS, LDS, and MDS]) were collected for the HAND donor cohorts. In order to investigate TREM2 neuropathology in cases most relevant to current HIV+ patients on ART regimens, participants who received a HAD diagnosis were excluded.
Neuropsychological evaluation was performed, and HAND diagnoses were determined via a comprehensive neuropsychological test battery, which was constructed to maximize sensitivity to neurocognitive deficits associated with HIV infection [see (Woods et al. 2004) for a list of tests]. Raw tests scores were transformed into demographically adjusted T-scores, including adjustments for age, education, gender and race. These demographically adjusted T-scores were converted to clinical ratings to determine presence and degree of neurocognitive impairment on seven neurocognitive domains, as previously described (Woods et al., 2004). As part of the neuropsychological battery, participants also completed self-report questionnaires of everyday functioning (i.e., Lawton and Brody Activities of Daily Living questionnaire;(Lawton & Brody 1969), and/or Patient’s Assessment of Own Functioning; PAOFI; (Chelune & Baer 1986, Chelune 1986). Participant’s performance on the neuropsychological test battery and their responses to the everyday functioning questionnaires were utilized to assign HAND diagnoses following established criteria (Antinori et al. 2007), i.e., HIV-associated asymptomatic neurocognitive impairment (ANI), and HIV-associated mild neurocognitive disorder (MND).
Demographic characteristics
Demographic information (age, years of education, gender, ethnicity/race) was obtained via self-report. Self-reported ethnicity was ascertained following NIH guidelines, which define “Hispanic/Latino” as a person of Mexican, Puerto Rican, Cuban, South or Central American, or other Spanish-speaking culture of origin regardless of race (Office of Management and Budget, 1997).
Immunoblot
Frontal cortex tissues from human brains were homogenized and fractionated and then processed for immunoblotting. In brief, as previously described (Fields et al. 2013), tissues from human brain samples (0.1 g) were homogenized in 0.7 mL of fractionation buffer (1.0 mM HEPES, 5.0 mM Benzaidine, 2.0 mM b-mercaptoethanol, 3.0 mM EDTA, 0.5 mM magnesium sulfate, 0.05% sodium azide, pH 8.8) containing phosphatase (Millipore cat# 524624) and protease inhibitor (Millipore cat# 539131) cocktails. Supernatants were centrifuged to generate soluble- and membrane-enriched fractions as follows. Samples were precleared by centrifugation at 5000 × g for 5 minutes at 4°C. Supernatants were retained and placed into appropriate ultracentrifuge tubes and were centrifuged at 436,000 × g for 1 hour at 4°C in a TL-100 rotor (Beckman Coulter, Brea, CA). This supernatant was collected, as representing the soluble enriched fraction, and the pellets were resuspended in 0.2 mL of buffer and homogenized for the membrane-enriched fraction. After determination of the protein content, soluble- and membrane-enriched fractions were resolved by SDS-PAGE and transferred onto PVDF membrane using the iBlot transfer system (Invitrogen). The membranes were incubated for one hour in blocking solution containing 5% bovine serum albumin and phosphate buffered saline + .05% tween-20 (PBST). Membranes were then then incubated overnight at 4°C with TREM2 antibody (1:400; R&D Systems; Cat# AF1828; RRID:AB_2208689). Following visualization, blots were stripped (Millipore ReBlot Plus, cat# MILL-2504) and probed with a mouse monoclonal antibody against β-actin (ACTB; 1:2000; Sigma-Aldrich; Cat# A5316; RRID:AB_476743) as a loading control. The membranes were incubated for 1 hour at room temperature in PBS containing secondary antibodies conjugated to horse radish peroxidase for visualization (1:5000 Bio-Rad anti-rabbit [cat# 170–6515] and anti-mouse [cat# 170–6516]) specific for the corresponding primary antibody. Bands were visualized by adding enhanced chemiluminescent substrate (Thermo SCIENTIFIC; SuperSignal ® West Femto; cat# 34095) to the membranes. Images were obtained, and semi-quantitative analysis was performed with the VersaDoc gel imaging system and Quantity One software (Bio-Rad).
Immunohistochemistry and double immunolabeling
Double immunolabeling studies were performed to determine the cellular localization of TREM2. For this purpose, vibratome sections of human brains were washed with PBS, pre-treated in 3% H2O2, and blocked with 10% serum (Vector Laboratories, cat# s-1000), 3% bovine serum albumin (Sigma) in PBST. Sections were next incubated at 4 C overnight with the primary antibodies against TREM2 (red) and antibodies against GFAP (green) (1:200; Dako Cat# Z0334; RRID:AB_10013382), IBA1 (green) (1:1000; WAKO cat# 019–19741; RRID:AB_839504), or MAP2 (green) (1:200; Sigma-Aldrich cat# M4403; RRID:AB_477193). Sections were then incubated with secondary antibodies tagged with tetramethylrhodamine (1:200; ThermoFisher cat# A-21447) to detect TREM2 and with antibodies tagged with fluorescein isothiocyanate (1:500; ThermoFisher FI2000) to detect GFAP, IBA1, or MAP2. Sections were mounted on superfrost slides (ThermoFisher) and cover-slipped with media containing DAPI (ThermoFisher cat# D3571; RRID:AB_2307445). Sections were imaged with a Zeiss 63X (N.A. 1.4) objective on an Axiovert 35 microscope (Zeiss) with an attached MRC1024 laser scanning confocal microscope system. All image analysis experiments were performed by Mary Swinton and conducted blind-coded, code was broken after analysis was performed. Not all specimens were amenable to double immunolabeling, however, 6 specimens from the HIV CN group and 6 specimens from the HAND group were analyzed.
Materials
The following antibodies were used in immunoblot, immunohistochemistry or both: TREM2 (1:400; R&D Systems; Cat# AF1828; RRID:AB_2208689), ACTB (1:2000; Sigma-Aldrich; Cat# A5316; RRID:AB_476743), MAP2 (1:200; Sigma-Aldrich Cat#M4403; RRID:AB_477193), GFAP (1:200; Dako Cat# Z0334; RRID:AB_10013382), and IBA1 (1:1000; WAKO Cat#019–19741; RRID:AB_839504). Information on validation of antibodies is available on company websites.
Real-time PCR
Total RNA was isolated from 50 mg of postmortem brain tissues from Broddmann Area 46 using the Qiagen RNeasy Lipid Tissue Kit per manufacturer instructions (Qiagen; cat# 74804). Total RNA was quantified and then reverse transcribed to cDNA using the High Capacity cDNA (Applied Biosystems; cat# 4368814) kit per manufacturer instructions. To quantify relative expression of mRNA targets, Taqman (ThermoFisher; cat# 4331182) probes specific for TREM2 (Hs00219132_m1), TNF (Hs00174128_m1), and ACTB (4310881E) were incubated with the cDNA and PCR amplified using the Quantstudio 3 Real-time PCR machine. Fold changes were calculated against controls using the comparative Ct method, as previously described (Schmittgen & Livak 2008). All cases with available RNA were analyzed by Real-time PCR (rt2PCR) using technical triplicates; data points were removed if the mean was three standard deviations outside the group mean.
Aβ quantification
Levels of two different species of Aβ peptide (Aβ40, and Aβ42) were quantified using a MesoScale electro chemiluminescent assay (MesoScale Discovery, Rockville, MD; cat# K15200G, V-Plex). The assay is designed to also detect Aβ38, however, the signal was undetectable in all samples. Brain homogenates were fractionated by ultra-high-speed centrifugation at 436,000 × g for 1 hour at 4 Celsius. Soluble-enriched fractions were diluted in diluent provided (Biedler et al. 1978) by the manufacturer and then protein content was quantified using BCA assay. Aβ38, Aβ40, and Aβ42 levels in the soluble fractions were quantified using the “Human (6E10) Aβ 3-Plex” sandwich ELISA immune assay kit according to the manufacturer’s instructions. Light emission following electrochemical stimulation was quantified using the Sector Imager 2400 reader (MSD). All cases were analyzed for Aβ using technical triplicates; data points were removed if the mean was three standard deviations outside the group mean.
Statistical analysis
All the analyses of images were conducted on coded samples blinded to the examiner. After the results were obtained, the code was broken, and data were analyzed with the StatView program (SAS Institute, Inc., Cary, NC). The studies analyzed postmortem samples that were stratified according to the last available antemortem cognitive diagnoses; hence, no randomization process was performed as the groups were predetermined. Due to small sample size, data were analyzed as non-parametric. No sample size calculations were performed prior to analyses and the study was not pre-registered. Outliers were defined as 1.5 times the interquartile range above Q3 or below Q1. Comparisons among groups were performed with Kruskal-Wallis test followed by Dunn’s multiple comparisons test, two-way ANOVA, or unpaired Student’s t test where appropriate. All results were expressed as mean ± SEM. The differences were significant if p values were <0.05 and the actual p value was reported if near, but over the 0.05 cutoff. All sample sizes (n= number of individual HIV+ tissues examined) are included in figure legends.
Results
Clinical and neuropathological characteristics of cognitively normal compared to HAND decedents that were on ART
All 52 NNTC autopsy cases were analyzed to assess relevant differences between postmortem brain samples from HIV+ decedents. We first categorized cohorts by HAND status and then compared sex, age, viral load (VL), and nadir CD4+ cell count (Table 1). The HAND cohort was predominantly male (88%) and the average age of HAND decedents was significantly higher than the cognitively normal (CN) group (Table 1). Plasma VL was similar among the different cognitive groups (Table 1). The CN group had the highest CD4 counts (Table 1), while the “MND” group had the lowest CD4 count (Table 1). Post-mortem interval (PMI) averaged 6.5, 20.3, and 9.6 hours for the CN, ANI, and MND groups, respectively (Table 1). However, despite the higher PMI for the ANI samples, there were no significant differences between the groups based on cognitive or ethnic stratifications (Tables 1 and 2).
Next, we compared clinical characteristics of our cohort when categorized by ethnicity, Hispanic versus non-Hispanic (Table 2). The non-Hispanic cohort consisted of decedents of multiple self-identified ethnicities: four Black, two Native Alaskan, one Hawaiian, and 13 white. There was no difference in the age of the groups. Plasma VL was significantly higher in the non-Hispanic group compared to the Hispanic cohort, however, there was no significant difference plasma VL quantity between the cognitive groups in the Hispanic or non-Hispanic samples. VL was detectable in all cases. No other differences were revealed by analyses of the available clinical data for the Hispanic and non-Hispanic groups. PMI averaged 11.5 and 16.3 hours for the Hispanic and non-Hispanic cohorts, respectively, however, these differences did not reach significance (Table 2).
Total TREM2 levels are increased in soluble-enriched fractions and decreased in membrane-enriched fractions of brain lysates from HIV+ decedents diagnosed with neurocognitive impairment
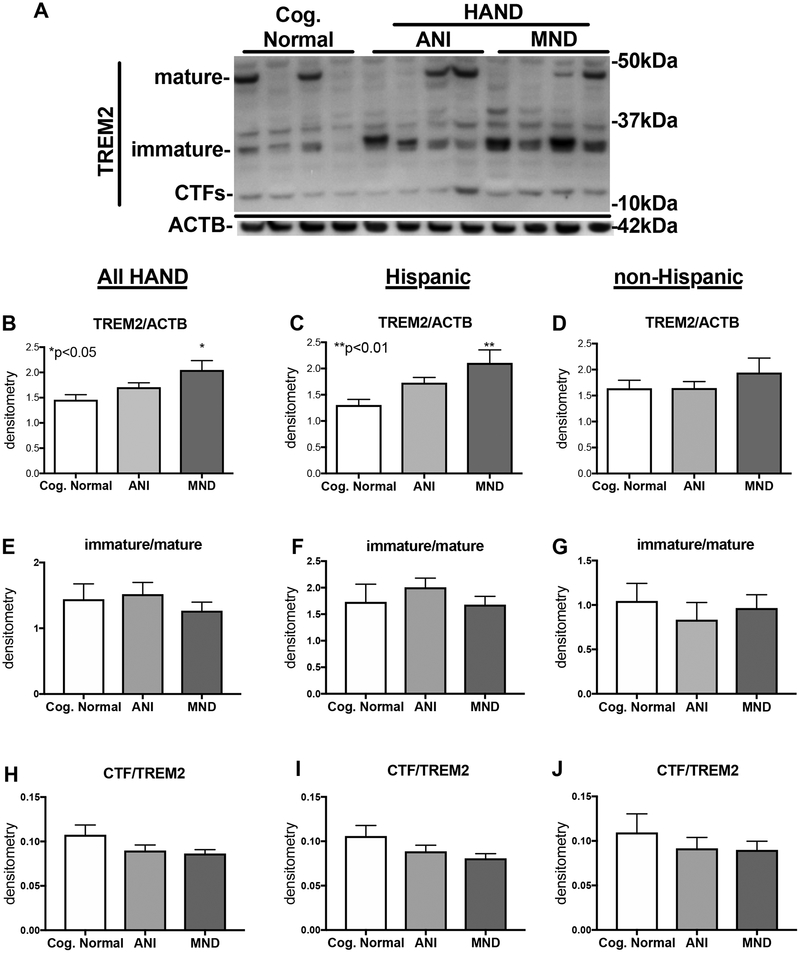
TREM2 protein is detected in three isoforms: the mature (~50kDa), the immature (~28 kDa), and the carboxy terminal fragment (CTF; ~13 kDa). To determine the expression levels of TREM2 in brains of HIV+ decedents (Table 1), we analyzed soluble and membrane-enriched fractions of frontal cortex lysates by immunoblot using a commercially available antibody generated toward TREM2 (R&D Systems, Cat#AF1828). (Hispanic cases are in the left two lanes and non-Hispanic in the right two lanes of each cognitive group) Immunoblot analyses revealed three TREM2 isoforms corresponding to the mature, the immature, and the CTF (Fig. 1A). In the soluble fractions, the immature isoform TREM2 presented as a doublet in some samples and showed the strongest intensity of the three TREM2 isoforms (Fig. 1A). The intensity of immature band appeared increased in samples from HIV+ decedents diagnosed with HAND, compared to CN (Fig. 1A). The band corresponding to the mature fragment was clearly present in the soluble enriched fractions but was not strongly detected in all cases (Fig. 1A). The band corresponding to CTF, however, was detected in all cases (Fig. 1A). To quantify the intensity of the signal of total TREM2, we performed densitometry analyses of all three bands and normalized to ACTB. Densitometry analyses of the signal for TREM2, and normalization to ACTB levels revealed that total TREM2 levels in soluble fractions of brain lysates were significantly (p<0.05) increased in MND cases compared to the CN cases (Fig. 1B). However, when analyzed by ethnicity, Hispanic decedents with MND diagnoses had the highest levels of total TREM2 levels in the soluble fractions; in which TREM2 levels were significantly increased (p<0.05) compared to CN decedents (Fig. 1C). In soluble fractions from non-Hispanic donors, TREM2 levels were unchanged in CN compared to ANI or MND brains (Fig. 1D). When analyzing in the soluble-enriched fractions the ratio of intensity of the immature:mature bands for TREM2, no difference was detected between CN, ANI, or MND groups in all HAND cases, nor when stratified by ethnicity (Fig. 1E–G). Regarding CTF to TREM2 (immature+mature isoforms) ratio, no significant differences were detected when analyzing the entire cohort together (Fig. 1H) or when stratified as Hispanic or non-Hispanic (Fig. 1I and J).
Figure 1. TREM2 is increased in soluble-enriched fractions of brain homogenates from HAND decedents that were on ART.
(A) Soluble-enriched fractions of frontal cortex brain lysates from HIV+ decedents were resolved by SDS-PAGE, and then analyzed by immunoblot for TREM2 protein levels. (B and C) Immature TREM2 band densitometry analyses in all HIV+ cases, stratified by NCI (CN, ANI, and MND). (D and E) Immature TREM2 band densitometry analyses in Hispanic HIV+ cases, stratified by NCI. (F and G) Immature TREM2 band densitometry analyses in all non-Hispanic HIV+ cases, stratified by NCI. Statistical significance was determined by one-way ANOVA when comparing more than two groups, or student’s T-test when comparing two groups. For each cognitive group, the left two lanes are from Hispanic and the right two lanes are from non-Hispanic cases (n=52, where n= the number of human specimens analyzed).
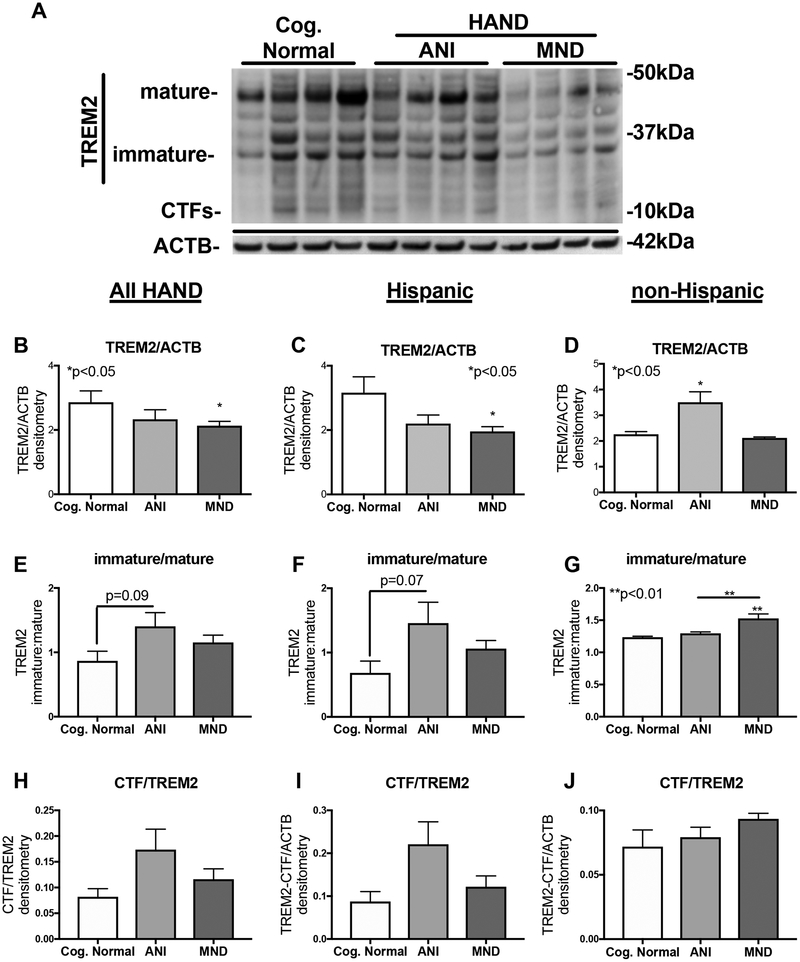
In membrane-enriched fractions, the band corresponding to the mature TREM2 isoform was of the strongest intensity (Fig. 2A). The immature fragment was detected strongly in CN and ANI samples, but less intense in the MND samples; the CTF isoform was not prominent in these images (Fig. 2A). The intensity of total TREM2 bands in membrane-enriched fractions was less visible among HIV+ cases diagnosed with HAND, particularly MND cases (Fig. 2A). Similar to the soluble-enriched fractions, TREM2 alterations were more robust in the Hispanics (left two lanes of each cognitive group) compared to the non-Hispanics in this cohort (Fig. 2A). Densitometry analysis of total TREM2 band intensity revealed significant decreases in HIV+ MND cases compared to CN (Fig. 2B). Interestingly, in Hispanic donors total TREM2 signal detected in brains from MND donors was significantly reduced compared to TREM2 levels in HIV+ Hispanic donors that were diagnosed as CN (Fig. 2C). In brain lysates from non-Hispanic donors, total TREM2 levels were significantly elevated in the ANI cases compared to CN cases, but TREM2 levels in MND cases were not altered compared to CN cases (Fig. 2D). The ratio of the intensity of the immature:mature TREM2 signal trends toward increasing in ANI compared to CN (p=0.09) when all cases were analyzed together (Fig. 2E). In Hispanic cases, the ratio of the intensity of the immature:mature TREM2 signal trends toward increasing in ANI compared to CN (p=0.07) (Fig. 2F). In the non-Hispanic cases, however, the ratio of the intensity of the immature:mature TREM2 signal is significantly (p<0.01) increased in MND compared to CN (Fig. 2G). In all HAND cases, the intensity of the CTF is not changed between the cognitive groups (Fig. 2H–J).
Figure 2. TREM2 is decreased in membrane-enriched fractions of brain homogenates from HAND decedents that were on ART.
(A) Membrane-enriched fractions of frontal cortex brain lysates from HIV+ decedents were resolved by SDS-PAGE, and then analyzed by immunoblot for TREM2 protein levels. (B and C) Immature TREM2 band densitometry analyses in all HIV+ cases, stratified by NCI. (D and E) Immature TREM2 band densitometry analyses in Hispanic HIV+ cases, stratified by NCI. (F and G) Immature TREM2 band densitometry analyses in all non-Hispanic HIV+ cases, stratified by NCI. Statistical significance was determined by one-way ANOVA when comparing more than two groups, or student’s T-test when comparing two groups. For each cognitive group, the left two lanes are from Hispanic and the right two lanes are from non-Hispanic decedents (n=52, where n= the number of human specimens analyzed).
TREM2 and TNF-α mRNA levels are altered in brains of HAND decedents on ART
Microglial phenotypes, M1 versus M2, are characterized by increased expression of inflammatory cytokines (ie. TNF-α) and immunomodulatory molecules (ie. TREM2), respectively. To determine the relative gene expression levels of TREM2 and TNF-α, we quantified mRNA by rt2PCR. Analysis of the relative expression of mRNA for TREM2 by two-way ANOVA revealed a significant difference in the expression of mRNA for TREM2 (p<0.05) between the Hispanic and non-Hispanic groups (Fig. 3A). TNF-α mRNA levels were significantly increased in brains from Hispanics diagnosed with MND, compared to CN controls (Fig. 3B. *p<0.05). Analysis of the levels of mRNA for TNF-α by ethnicity, revealed a significant difference in the levels of mRNA for TNF-α between the Hispanic and non-Hispanic samples (**p<0.01).
Figure 3. TREM2 and TNF-α mRNA levels are altered in brains of HAND decedents on ART.
Total RNA was extracted from frontal cortex tissues, reverse transcribed to cDNA, and analyzed for (A) TREM2, (B) TNF-a, which was normalized to ACTB levels by rt2PCR. Fold-change compared to RNA extracted from an age-matched HIV- sample was calculated using the comparative CT method. Analyzed by two-way ANOVA; *p<0.05. (n=42, where n= the number of human specimens analyzed).
Microglial expression of TREM2 is variable among HAND donors
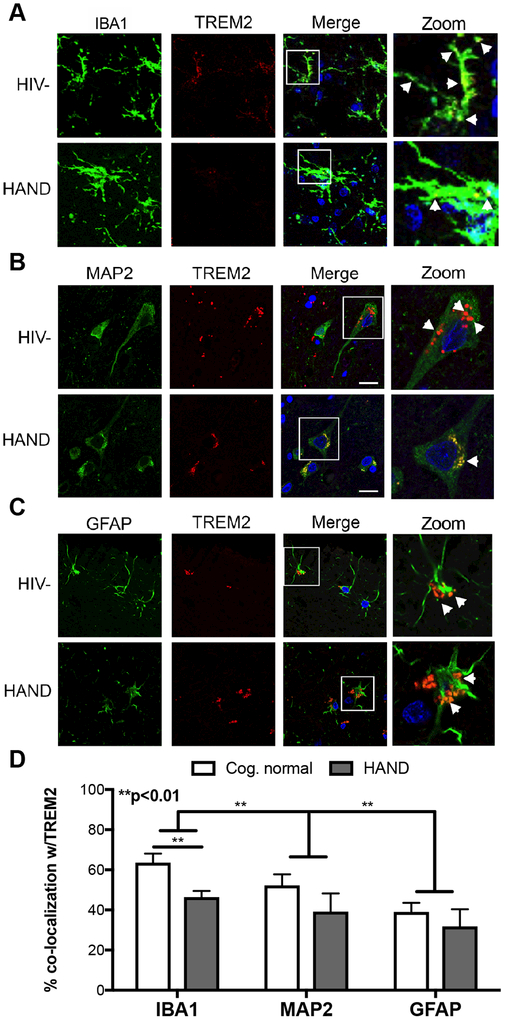
TREM2 was initially identified in myeloid-lineage cells, such as macrophages and microglia. However, recent studies in mouse and human brains have shown that TREM2 is also present in other brain cells, including neurons(Satoh et al. 2013). To determine the cellular localization of TREM2 in the human brain during HIV infection, we performed double immunolabeling of TREM2 with IBA1, MAP2, and GFAP for microglia, neurons, and astrocytes, respectively. Double labeling for IBA1, a microglial marker, revealed TREM2 is present throughout the cell body and on the surface of activated microglia of CN decedents; however, there is a noticeable reduction in TREM2 signal colocalizing with IBA1 signal in brains from HAND decedents (Fig. 4A). In neurons, MAP2 (green) signal emanated from throughout the neuronal body and apical dendrites, while TREM2 (red) showed a more punctate distribution mainly in the cell body in a perinuclear pattern, with some signal present in the dendrites (Fig. 4B). Interestingly, TREM2 signal colocalized with GFAP also in a perinuclear pattern, suggesting TREM2 is located on the surface and intracellular organelles of astrocytes. In astrocytes, GFAP (green) signal was present throughout the star-shaped cells, both in the cell body and in the processes and extensions (Fig. 4C). Quantification of colocalization of TREM2 with IBA1, MAP2, and GFAP revealed that most TREM2 in the brain colocalizes with the microglial marker, however, TREM2 signal is also colocalized with some neuronal and astroglial markers. (Fig. 4D). Importantly, TREM2 colocalizing with microglial marker, IBA1, was significantly (**p<0.01) reduced in brains of HAND decedents (Fig. 4D).
Figure 4. Microglial expression of TREM2 is variable among HAND donors.
Vibratome sections of frontal cortex were double immunolabeled for TREM2 (red) and cell-specific markers (green) for microglia (IBA1), neurons (MAP2), and astroglia (GFAP). (A) To identify TREM2 expression on microglia, vibratome sections of frontal cortex tissues were immunolabeled for TREM2 (red) and IBA1 (green), and then visualized using confocal microscopy. (B) To identify TREM2 expression on neurons, vibratome sections of frontal cortex tissues were immunolabeled for TREM2 (red) and MAP2 (green), and then visualized using confocal microscopy. (C) To identify TREM2 expression on astroglia, vibratome sections of frontal cortex tissues were immunolabeled for TREM2 (red) and GFAP (green), and then visualized using confocal microscopy. Bar = 10μm. Analyzed by two-way ANOVA; **p<0.01. (n=12, six CN and six HAND, where n= the number of human specimens analyzed).
Amyloid β 42 and 40 levels are increased in brains of Hispanic HAND donors and decreased in non-Hispanic HAND donors
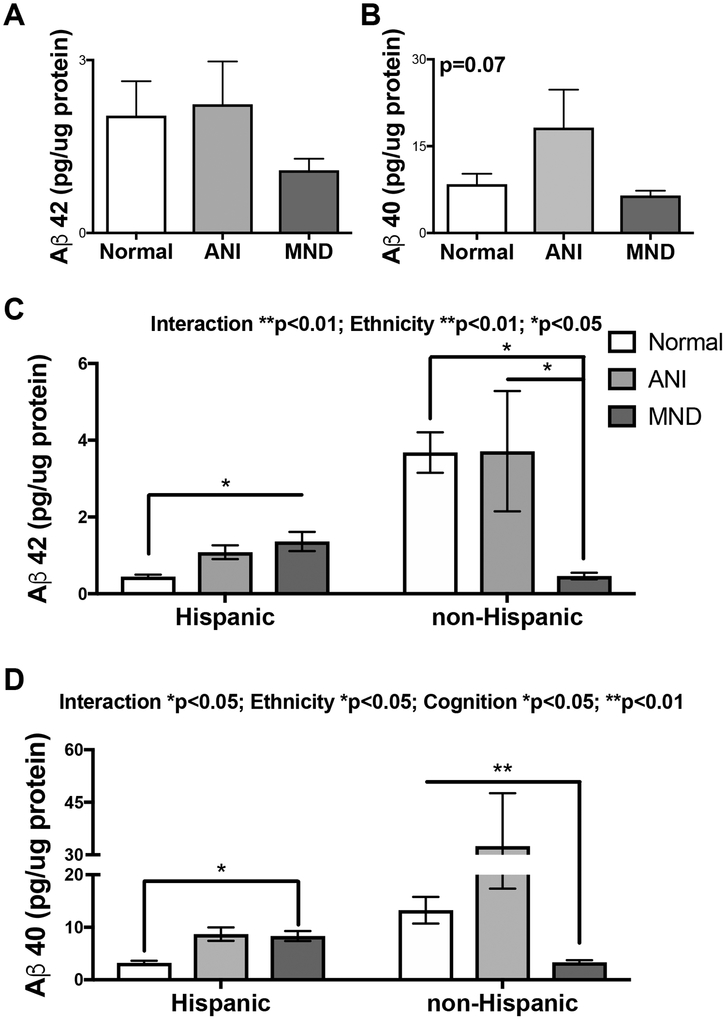
Increased levels of Aβ 1–42 and Aβ peptides 1–42 and 1–40 are associated with AD, aging, and inflammation (Sengupta et al. 2016). Moreover, decreased membrane-bound TREM2 is associated with reduced uptake of Aβ (Zhao et al. 2018). To determine levels of Aβ 1–42 and1–40 in the brains of our cohort, we analyzed brain lysates using the Aβ triplex Mesoscale Discovery kit. Analysis of brain lysates from all HIV+ samples for Aβ 1–42 revealed no significant differences among the HAND diagnosis groups; however, there is a trend for lower Aβ 1–42 levels in the MND group, as levels in this group are lower than CN or ANI groups (Fig. 5A). Aβ 1–40 mean levels appeared increased in ANI brains compared to CN, but these differences did not reach significance either (Fig. 5B). Importantly, when these data are further stratified by ethnicity, as Hispanic and non-Hispanic, distinct patterns emerge for both Aβ 1–42 and 1–40. In brain lysates from Hispanic donors, Aβ 1–42 levels are increased 2-fold in ANI and 3-fold in MND compared to CN samples (Fig. 5C). However, the opposite pattern is observed in non-Hispanic donors, the highest levels of Aβ 1–42 are observed in brains from CN cases, with a 50% reduction in ANI, and 90% reduction in MND brains (Fig. 5C). Similarly, in brain lysates from Hispanic donors, Aβ 1–40 levels are increased 2-fold in both ANI and MND cases when compared to controls (Fig. 5D). In the non-Hispanic samples, Aβ 1–40 levels are increased in ANI compared to CN cases, but the lowest levels of Aβ were detected in the MND group (Fig. 5D). The signal for Aβ 1–38 was not detectable in any specimens.
Figure 5. Aβ 42 and 40 levels are increased in brains of Hispanic HAND donors and decreased in non-Hispanic HAND donors.
Soluble-enriched fractions of brain lysates from HIV+ decedents were analyzed for Aβ 40 and 42 levels by sandwich ELISA (MSD). (A and B) Using soluble-enriched fractions from frontal cortices of the entire HIV+ cohort, levels of Aβ 42 and 40 were quantified, stratified by HAND diagnoses, and graphed as pg/μg of protein. (C and D) Using soluble-enriched fractions from frontal cortices of stratified by ethnicity (Hispanic and non-Hispanic), levels of Aβ 40 and 42 were quantified, also stratified by HAND diagnoses, and graphed as pg/μg of protein. Statistical significance was determined by one-way ANOVA or two-way ANOVA where appropriate (*p<0.05, **p<0.01) (n=42, where n= the number of human specimens analyzed).
Discussion
In this study, we report for the first time that TREM2 expression is altered in brain specimens from the frontal cortex of HAND decedents that were on ART when compared to HIV+ controls with no diagnoses of neurocognitive impairment. Specifically, increased levels of total TREM2 (mature + immature + CTF isoforms) in soluble-enriched fractions and decreased levels of total TREM2 in membrane-enriched fractions were observed in brains with HAND diagnoses. Interestingly, despite higher CD4+ cell counts and lower HIV viral load, the most robust changes in levels of total TREM2 was observed in brains from decedents that self-identified as Hispanics. Increased expression of the pro-inflammatory gene TNF-α was also increased in the brains from Hispanic decedents that were diagnosed with MND compared to CN controls. The levels of Aβ 1–42 and 1–40 were increased in Hispanics with HAND diagnoses compared to CN Hispanics; however, a different pattern of levels of Aβ 1–42 and 1–40 were observed in non-Hispanics, despite higher levels of Aβ 1–42 and 1–40 in CN non-Hispanics compared to CN Hispanics. These results show that alterations in the expression of TREM2 may be associated with increased inflammatory gene expression, Aβ deposition, and HAND progression in the ART era, although, these alterations in TREM2 and associated pathways may be more prominent in certain populations than others. Our findings are consistent with recent studies that show TREM2 is essential for Aβ clearance by microglia (Zhong et al. 2017, Yeh et al. 2016), modulates inflammatory gene expression (Ren et al. 2018), and protects neurons from inflammation (Raha et al. 2017).
HIV, Neuroinflammation, and TREM2
HIV-infection of the brain persists in the era of ART, and chronic neuroinflammation may contribute to the minor cognitive impairment that afflicts almost 50% of HIV+ individuals on ART (Heaton et al. 2015, Levine et al. 2016). Recently, TREM2 has emerged as an important immunomodulatory molecule that is crucial for clearance of extracellular protein aggregates such as Aβ (Wang et al. 2015, Rivest 2015, Yeh et al. 2016), as well as maintaining control of immune responses (Ren et al. 2018, Jiang et al. 2018). Accordingly, dysfunctional TREM2 is associated with multiple neurodegenerative diseases, such as AD and PD (Painter et al. 2015, Benitez et al. 2013), TREM2 functions as a membrane bound protein belonging to the immunoglobulin superfamily. When expressed by myeloid cells, TREM2 has anti-inflammatory and phagocytic functions (Zhong et al. 2017, Yeh et al. 2016), which may partly explain why mutated TREM2 predisposes individuals to AD, PD, FTD, and other neurodegenerative diseases. Indeed, a recent study reported soluble TREM2, derived from cleavage (Zhong et al. 2017) or from a specific mRNA for a soluble TREM2 isoform (Ma et al. 2016), is associated with increased neuroinflammation and age (Zhong et al. 2017, Byrne et al. 2018). These recent findings are consistent with our data showing reduced levels of total TREM2 protein detected in membrane-enriched fractions of MND brain lysates correspond with increased levels of total TREM2 protein detected in soluble-enriched fractions. Our findings also support a link between altered TREM2 protein expression, inflammation, and HAND. A recent study reported increased levels of TREM2 mRNA and total TREM2 protein in soluble enriched fractions of lysates from AD brains compared to age-matched controls (Ma et al. 2016), which is consistent with our findings, however, we did not observe an increase in the ratio of immature to mature TREM2 isoforms as was found in the AD brains. The question remains, however, as to how much of the changes in TREM2 mRNA and protein are due to increased microglial cell numbers entering the brains of some HIV+ individuals or if individual cells are increasing TREM2 expression. A larger study that includes more specimens amenable to double-immunolabeling would be useful to answer these questions.
Soluble TREM2 may exacerbate neuroinflammation (Zhong et al. 2017, Henjum et al. 2016), and based on a recent report by Ren et al., a lack of membrane-bound TREM2 could explain the increases in levels of mRNA for TNF-α detected in the HAND brains (Ren et al. 2018). Conversely, it is possible that inflammatory gene expression reduces levels of mRNA for TREM2, which could also explain reduced membrane-bound TREM2 in association with elevated levels TNF-〈 mRNA. Either of these scenarios would be consistent with our observations of reduced TREM2 colocalization with the microglial marker IBA1. Interpreting these data, however, are less straight forward as the reduced colocalization could reflect an overall increase in IBA1 expression or an infiltration of IBA1+ cells without a concomitant increase in TREM2 expression, an overall decrease in TREM2 expression, or an increase in TREM2 processing by cleavage or internalization. Larger neuropathological studies of need to be combined with genetic approaches as well as in vitro model systems to delineate the role of TREM2 expression and processing on microglia in neurodegenerative diseases.
Loss of function mutations or alterations in the cleavage of membrane-bound TREM2 could also contribute inflammation in the HIV+ brain; however, such mutations in TREM2 are extremely rare and the prevalence in Hispanic and other populations still needs to be investigated. Moreover, research is needed to determine the role of different proteases, such as ADAM10 (Colonna & Wang 2016), in modulating TREM2 cleavage in the brains of HAND cases. Identifying alternative, non-genetic, mechanisms of TREM2 expression and/or dysfunction may lead to novel therapies for HIV-induced neurotoxicity and other neurodegenerative diseases.
It is noteworthy that factors such as postmortem interval (PMI) can affect protein and mRNA degradation in brain specimens. Despite detecting no significant difference in PMI among the groups, the mean PMI for the ANI group was approximately twice that of the CN or MND groups. However, we found by statistical analysis that mean quantities of protein and RNA in these brain tissues were not significantly affected by the PMI of the specimens.
Aβ and TREM2 in HAND
Aβ plaques are protein aggregates associated with pathology of AD brains, but also, they have been associated with normal aging and HIV infection of the brain (An et al. 1997, Achim et al. 2009). Furthermore, Aβ may be deposited intraneuronal during HIV infection (Achim et al. 2009), or associated with vascular, axonal, and blood brain barrier damage (Andras & Toborek 2013). Interestingly, our findings that Aβ levels are increased in HAND brains from Hispanics may be partially explained by previous studies that have shown that HIV alters pathways that clear protein aggregates such as Aβ from the brain (Zhou et al. 2011, Fields et al. 2015a, Fields et al. 2013). Moreover, reduced TREM2 is associated with increased autophagy in a mouse model for AD (Ulland et al. 2017). Hence, the effects of reduced TREM2 on the autophagy pathway and Aβ accumulation in brains of HIV+ individuals warrant future investigation. Recently, multiple studies using cellular and transgenic animal models support a role for TREM2 to detect Aβ, facilitating clearance of damaged cells and protein aggregates, and moderating the immune response (Rivest 2015, Wang et al. 2015, Yeh et al. 2016). These findings are consistent with our observations of decreased cell-bound TREM2 and increased levels of Aβ in brains of Hispanic MND cases compared to Hispanic CN cases. However, this pattern was not evident in the specimens from non-Hispanics in which levels of Aβ were found to be reduced in MND compared to CN, but without corresponding significant changes in levels of total TREM2. We speculate that increases in total TREM2 detected in membrane-enriched fractions of non-Hispanic MND brains indicate more functional TREM2 in this group. On the other hand, the differences in the Aβ and TREM2 levels between Hispanics and non-Hispanics could be explained by differential TREM2 pathway regulation downstream of genetic or environmental factors, which requires more studies using larger cohorts and in vitro models. These data suggest that the role for TREM2 deficiency in Aβ accumulation in the brain during HIV infection is unclear and warrants further investigation.
Interestingly, while we did observe increased levels of Aβ in brains of Hispanics with MND compared to CN Hispanics, we observed no significant alterations in the levels of Aβ between CN and HAND (ANI or MND) when the cohort was analyzed in aggregate (Hispanics and non-Hispanics). Moreover, analysis by two-way ANOVA revealed that ethnicity significantly contributes to the variation in levels of both forms of Aβ, suggesting that Aβ production during HIV infection may vary by a group or even on an individual basis. The differences in levels of Aβ between Hispanics and non-Hispanics reported here may stem from differences in the initial responses to HIV infection. Studies have long suggested that Aβ shows antimicrobial properties that may be elicited upon viral infection (Gosztyla et al. 2018), and this could explain why HIV induces Aβ production. Future studies using brain samples from age-matched, healthy, HIV- controls would be helpful in determining differential responses to HIV in regard to Aβ expression in the brain. This differential relationship between HIV and Aβ expression among Hispanics and non-Hispanics may also indicate that Aβ may be a more promising therapeutic target in some, but not all HIV+ patients. Future studies using patient-derived induced pluripotent stem cells differentiated into brain cell types may help in determining the mechanisms underlying differential Aβ production during HIV infection.
Importantly, TREM2 dysfunction may be caused by mutations in the TREM2 gene that increase shedding from the cell surface or reduce TREM2 protein trafficking to the cell surface (Wunderlich et al. 2013, Kleinberger et al. 2014). Mutations in proteases that cause premature cleavage of TREM2 from the cell surface may also contribute to lack of Aβ clearance (Colonna & Wang 2016). In the case of deleterious TREM2 mutations, TREM2 levels or localization may yield no contribution to disease pathogenesis, as the mutation reduces TREM2 activity. Future studies using larger cohorts may be useful to determine if mutants of TREM2 may be associated with HAND.
TREM2 expression in neurons and astroglia
While TREM2 was originally identified in myeloid-lineage cells, several groups have reported expression by other cell types in human and mouse brains, including neurons and oligodendrocytes (Kiialainen et al. 2005, Sessa et al. 2004). Despite these findings, a definitive role for TREM2 in these cells has not been identified. Consistent with our studies, neurons in postmortem brain specimens from AD patients strongly react with antibodies for TREM2 (Satoh et al. 2013). Other studies found that TREM2 was expressed by microglia, oligodendrocytes, and neurons, but not astroglia in the mouse brain (Sessa et al. 2004, Kiialainen et al. 2005). While a role for TREM2 in neurons has yet to be identified, lipidic ligands for TREM2 are present on the surface of neurons (Wang et al. 2015); hence, soluble TREM2 may bind to these lipidic ligands on the surface of neurons. In vitro studies of TREM2 expression in different brain cell models combined with neuropathological analyses of TREM2 mRNA expression and localization to identify TREM2 expressing cells in the brain may shed light on neuronal TREM2 staining.
While astroglia have been shown to express ligands for TREM2 (Daws et al. 2003), to our knowledge, we are the first to report TREM2 immunoreactivity in cells expressing markers specific to astroglia. One explanation could be that TREM2 is localized to astroglia per binding to ligand and subsequent endocytosis. Further in vitro and neuropathological studies are needed to determine the role of astroglia in TREM2 expression and processing in the brain.
Ethnic-based disparities in HAND
Ethnic disparities in prevalence and severity of neurocognitive disorders have long been the subject of intense study and debate (Howell et al. 2017, Schoenberg et al. 1985, Prineas et al. 1995, Marquine et al. 2018, Mehta & Yeo 2017, Downer et al. 2016a, Chen et al. 2012). While many studies report differences in prevalence of dementia or other neurocognitive disorders between ethnicities, other large-scale studies observed no such differences. Regarding HAND, however, the data are clear that minorities, including Hispanics, are more likely to be diagnosed when compared to non-Hispanic whites (Marquine et al. 2018, Mehta & Yeo 2017, Hulgan et al. 2015). Despite the mounting evidence for ethnic-based differences in outcomes of neurodegenerative disorders, little is known about the molecular mechanisms underlying these differences.
Hispanics are disproportionately infected with HIV (Johnson et al. 2013) and may be more likely to suffer from HAND (Durvasula et al. 2001, Heaton et al. 2015, Marquine 2017, Rivera Mindt et al. 2008, Saez et al. 2014, Wojna et al. 2006). Specifically, Hispanics, who represent the largest ethnic/racial population in the USA (US Census Bureau), are three-times more likely to be infected with HIV compared to non-Hispanic whites (Centers for Disease Control and Prevention, 2015). In general, it takes longer for Hispanics to be diagnosed as HIV+, and hence, Hispanics receive delayed care engagement(Gardner et al. 2011, Chen et al. 2012). Hispanics often present with advanced HIV disease characteristics, including lower CD4 cell counts, more opportunistic infections, and higher rates of AIDS (Swindells et al. 2002). Moreover, minority populations, including Hispanics, in the USA are more likely to suffer from dementia compared to non-Hispanic Whites (Mehta & Yeo 2017). Our current data suggest that altered levels of TREM2, inflammation, and Aβ in the brain may be associated with neurocognitive impairment in HIV+ Hispanics.
Recent studies suggest that metabolic conditions related to diet may contribute to neuroinflammation and NCI (Mayeda et al. 2015, Downer et al. 2016b, Downer et al. 2016a). Other studies suggest that higher stress resulting from socioeconomic status may also contribute to conditions that lead to NCI and dementia symptoms (Russ et al. 2013, Yaffe et al. 2013). In this context, our results provide more evidence that Hispanics may be uniquely vulnerable to the progression of neurodegenerative diseases, such as HAND. Therefore, targeting TREM2 activity or related pathways may be a promising therapeutic strategy in HIV+ Hispanics, or other HIV+ individuals in which neuroinflammation and Aβ deposition is prominent.
Conclusions
Overall, our data show that TREM2 levels are altered concomitantly with Aβ and TNF-α levels in postmortem brains of HIV+ individuals suffering from HAND. Specifically, reduced TREM2 on the surface of microglia indicates a proinflammatory, M1, phenotype predominates in HAND brains in the ART era. Importantly and in agreement with previous studies, we show that TREM2 is localized to neurons, and we suggest that this phenomenon needs to be explored further. Remarkably, the expression patterns of TREM2 in Hispanics with HAND is distinct compared to TREM2 expression in non-Hispanics, suggesting that alterations in TREM2 function may contribute to neurodegeneration in Hispanics. Given that Hispanics are a heterogeneous population, it is likely that multiple factors, including genetic, cultural, socioeconomic, access to healthcare, or others, converge at the point of neuroinflammation and TREM2 function. To develop personalized therapeutics for patients suffering from HAND and other neurodegenerative diseases, future research should focus on how these factors can affect accumulation of protein aggregates, phagocytic function, and neuroinflammation in the brain.
Acknowledgements:
This work was supported by National Institutes of Health grants AG043384 from National Institute on Aging (to PD), MH115819 and MH062962 from the National Institute on Mental Health (to JAF and EM, respectively), and MH105319 from the National Institute on Mental Health (to CA). We would also like to thank the National NeuroAIDS Tissue Consortium for the human brain specimens and corresponding clinical data (tissue request numbers: R415, R434, R494).
Abbreviations:
- HAND
HIV-associated neurocognitive disorders
- ART
combined antiretroviral therapy
- TREM2
triggering receptor-expressed on myeloid cells
- Aβ
amyloid-β
- ANI
asymptomatic neurocognitive impairment
- MND
minor neurocognitive disorder
- AIDS
acquired immunodeficiency
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- NNTC
National NeuroAIDS Tissue Consortium
- NIH
National Institutes of Health
- CSF
Cerebrospinal fluid
- VL
viral load
- GDS
global deficit score
- LDS
learning deficit score
- MDS
motor deficit score
- ACTB
β-actin
- PBST
phosphate-buffered saline-tween20
- HRP
horseradish peroxidase
- MAP2
microtubule-associated protein 2
- GFAP
glial fibrillary acidic protein
- IBA1
ionized calcium-binding adaptor molecule 1
- cDNA
complementary DNA
- MSD
MesoScale Discovery
- TNF
tumor necrosis factor
- mRNA
messenger RNA
- PCR
polymerase chain reaction
- ANOVA
analysis of variance
- SEM
standard error of the mean
Footnotes
Scientific rigor and availability of the data and materials
Immunoblots were performed at least twice for reproducibility. Immunohistochemical analyses were performed on three tissue sections per case, and multiple images were taken of each section, as described in methods. Sample sizes are included in the figure legends. The datasets analyzed, or materials used in the current study are available from the corresponding author on a reasonable request. Data reported are less than n=52 if cases were determined to be outliers for all experiments except the double immunolabelling analyses, in which case 24 total cases were found to be amenable to analyses.
Ethics approval
The NNTC studies and tissue collections were performed in accordance with human subject protection protocols at participating institutions: Icahn School of Medicine at Mount Sinai, University of Texas Medical Branch, University of California, Los Angeles, and University of California, San Diego. The IRB’s (IRB#080323) have continuously reviewed NNTC protocols through the present.
Competing interests
The authors declare that they have no competing interests.
Open Science Badges
This article has received a badge for *Open Materials* because it provided all relevant information to reproduce the study in the manuscript. The complete Open Science Disclosure form for this article can be found at the end of the article. More information about the Open Practices badges can be found at https://cos.io/our-services/open-science-badges/.
References
- Achim CL, Adame A, Dumaop W, Everall IP and Masliah E (2009) Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol, 4, 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An SF, Giometto B, Groves M, Miller RF, Beckett AA, Gray F, Tavolato B and Scaravilli F (1997) Axonal damage revealed by accumulation of beta-APP in HIV-positive individuals without AIDS. J Neuropathol Exp Neurol, 56, 1262–1268. [DOI] [PubMed] [Google Scholar]
- Andras IE and Toborek M (2013) Amyloid beta accumulation in HIV-1-infected brain: The role of the blood brain barrier. IUBMB Life, 65, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT et al. (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology, 69, 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atagi Y, Liu CC, Painter MM et al. (2015) Apolipoprotein E Is a Ligand for Triggering Receptor Expressed on Myeloid Cells 2 (TREM2). J Biol Chem, 290, 26043–26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez BA, Cruchaga C and United States-Spain Parkinson’s Disease Research, G. (2013) TREM2 and neurodegenerative disease. N Engl J Med, 369, 1567–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedler JL, Roffler-Tarlov S, Schachner M and Freedman LS (1978) Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res, 38, 3751–3757. [PubMed] [Google Scholar]
- Bouchon A, Hernandez-Munain C, Cella M and Colonna M (2001) A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J Exp Med, 194, 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne LM, Rodrigues FB, Johnson EB, De Vita E, Blennow K, Scahill R, Zetterberg H, Heslegrave A and Wild EJ (2018) Cerebrospinal fluid neurogranin and TREM2 in Huntington’s disease. Sci Rep, 8, 4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelune GJ and Baer RA (1986) Developmental norms for the Wisconsin Card Sorting test. J Clin Exp Neuropsychol, 8, 219–228. [DOI] [PubMed] [Google Scholar]
- Chelune GJ, Heaton RK, & Lehman RA (1986) Neuropsychological and personality correlates of patients’ complaints of disability: Tarter RE (Ed.), Advances in clinical neuropsychology. [Google Scholar]
- Chen NE, Gallant JE and Page KR (2012) A systematic review of HIV/AIDS survival and delayed diagnosis among Hispanics in the United States. J Immigr Minor Health, 14, 65–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M and Wang Y (2016) TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat Rev Neurosci, 17, 201–207. [DOI] [PubMed] [Google Scholar]
- Daws MR, Sullam PM, Niemi EC, Chen TT, Tchao NK and Seaman WE (2003) Pattern recognition by TREM-2: binding of anionic ligands. J Immunol, 171, 594–599. [DOI] [PubMed] [Google Scholar]
- Downer B, Chen NW, Raji M and Markides KS (2016a) A longitudinal study of cognitive trajectories in Mexican Americans age 75 and older. Int J Geriatr Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer B, Rote S, Markides KS and Snih SA (2016b) The Comorbid Influence of High Depressive Symptoms and Diabetes on Mortality and Disability in Mexican Americans Aged 75 and Above. Gerontol Geriatr Med, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durvasula RS, Miller EN, Myers HF and Wyatt GE (2001) Predictors of neuropsychological performance in HIV positive women. J Clin Exp Neuropsychol, 23, 149–163. [DOI] [PubMed] [Google Scholar]
- Esiri MM, Biddolph SC and Morris CS (1998) Prevalence of Alzheimer plaques in AIDS. J Neurol Neurosurg Psychiatry, 65, 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields J, Dumaop W, Elueteri S et al. (2015a) HIV-1 Tat alters neuronal autophagy by modulating autophagosome fusion to the lysosome: implications for HIV-associated neurocognitive disorders. J Neurosci, 35, 1921–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields J, Dumaop W, Rockenstein E et al. (2013) Age-dependent molecular alterations in the autophagy pathway in HIVE patients and in a gp120 tg mouse model: reversal with beclin-1 gene transfer. J Neurovirol, 19, 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields JA, Serger E, Campos S et al. (2015b) HIV alters neuronal mitochondrial fission/fusion in the brain during HIV-associated neurocognitive disorders. Neurobiol Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Herbein G, Cossarizza A, Witkowski JM, Frost E, Dupuis G, Pawelec G and Larbi A (2017) Cellular Senescence, Immunosenescence and HIV. Interdiscip Top Gerontol Geriatr, 42, 28–46. [DOI] [PubMed] [Google Scholar]
- Gardner EM, McLees MP, Steiner JF, Del Rio C and Burman WJ (2011) The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis, 52, 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giometto B, An SF, Groves M, Scaravilli T, Geddes JF, Miller R, Tavolato B, Beckett AA and Scaravilli F (1997) Accumulation of beta-amyloid precursor protein in HIV encephalitis: relationship with neuropsychological abnormalities. Ann Neurol, 42, 34–40. [DOI] [PubMed] [Google Scholar]
- Gisslen M, Krut J, Andreasson U et al. (2009) Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol, 9, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosztyla ML, Brothers HM and Robinson SR (2018) Alzheimer’s Amyloid-beta is an Antimicrobial Peptide: A Review of the Evidence. J Alzheimers Dis, 62, 1495–1506. [DOI] [PubMed] [Google Scholar]
- Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ and Achim CL (2005) Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. Aids, 19, 407–411. [DOI] [PubMed] [Google Scholar]
- Gross AM, Jaeger PA, Kreisberg JF et al. (2016) Methylome-wide Analysis of Chronic HIV Infection Reveals Five-Year Increase in Biological Age and Epigenetic Targeting of HLA. Mol Cell, 62, 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G, Orlando G, Zona S et al. (2011) Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis, 53, 1120–1126. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr. et al. (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 75, 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ et al. (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol, 17, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR Jr., Deutsch R et al. (2015) Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis, 60, 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henjum K, Almdahl IS, Arskog V, Minthon L, Hansson O, Fladby T and Nilsson LN (2016) Cerebrospinal fluid soluble TREM2 in aging and Alzheimer’s disease. Alzheimers Res Ther, 8, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell JC, Watts KD, Parker MW, Wu J, Kollhoff A, Wingo TS, Dorbin CD, Qiu D and Hu WT (2017) Race modifies the relationship between cognition and Alzheimer’s disease cerebrospinal fluid biomarkers. Alzheimers Res Ther, 9, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulgan T, Samuels DC, Bush W et al. (2015) Mitochondrial DNA Haplogroups and Neurocognitive Impairment During HIV Infection. Clin Infect Dis, 61, 1476–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TR, von Saucken VE and Landreth GE (2017) TREM2 in Neurodegenerative Diseases. Mol Neurodegener, 12, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Zhang YD, Gao Q, Ou Z, Gong PY, Shi JQ, Wu L and Zhou JS (2018) TREM2 Ameliorates Neuronal Tau Pathology Through Suppression of Microglial Inflammatory Response. Inflammation. [DOI] [PubMed] [Google Scholar]
- Johnson AS, Beer L, Sionean C, Hu X, Furlow-Parmley C, Le B, Skarbinski J, Hall HI and Dean HD (2013) HIV infection - United States, 2008 and 2010. MMWR Surveill Summ, 62 Suppl 3, 112–119. [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S et al. (2013) Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med, 368, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiialainen A, Hovanes K, Paloneva J, Kopra O and Peltonen L (2005) Dap12 and Trem2, molecules involved in innate immunity and neurodegeneration, are co-expressed in the CNS. Neurobiol Dis, 18, 314–322. [DOI] [PubMed] [Google Scholar]
- Kleinberger G, Yamanishi Y, Suarez-Calvet M et al. (2014) TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med, 6, 243ra286. [DOI] [PubMed] [Google Scholar]
- Lawton MP and Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist, 9, 179–186. [PubMed] [Google Scholar]
- Levine AJ, Soontornniyomkij V, Achim CL, Masliah E, Gelman BB, Sinsheimer JS, Singer EJ and Moore DJ (2016) Multilevel analysis of neuropathogenesis of neurocognitive impairment in HIV. J Neurovirol, 22, 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Allen M, Sakae N, Ertekin-Taner N, Graff-Radford NR, Dickson DW, Younkin SG and Sevlever D (2016) Expression and processing analyses of wild type and p.R47H TREM2 variant in Alzheimer’s disease brains. Mol Neurodegener, 11, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquine JM, Heaton A, Johnson N, Rivera-Mindt M, Cherner M, Bloss C, Hulgan T, Umlauf A, Moore D, Fazeli P, Morgello S, Franklin D, Letendre S, Ellis R, Collier A, Marra C, Clifford D, Gelman B, Sacktor N, Simpson D, McCutchan J, Grant I, Heaton R (2017) Differences in Neurocognitive Impairment among HIV-infected Latinos in the United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquine MJ, Heaton A, Johnson N et al. (2018) Differences in Neurocognitive Impairment Among HIV-Infected Latinos in the United States. J Int Neuropsychol Soc, 24, 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeda ER, Haan MN, Yaffe K, Kanaya AM and Neuhaus J (2015) Does type 2 diabetes increase rate of cognitive decline in older Mexican Americans? Alzheimer Dis Assoc Disord, 29, 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta KM and Yeo GW (2017) Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement, 13, 72–83. [DOI] [PubMed] [Google Scholar]
- Mishra R and Singh SK (2014) HIV-1 Tat C phosphorylates VE-cadherin complex and increases human brain microvascular endothelial cell permeability. BMC Neurosci, 15, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihuela R, McPherson CA and Harry GJ (2016) Microglial M1/M2 polarization and metabolic states. Br J Pharmacol, 173, 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega M and Ances BM (2014) Role of HIV in amyloid metabolism. J Neuroimmune Pharmacol, 9, 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter MM, Atagi Y, Liu CC, Rademakers R, Xu H, Fryer JD and Bu G (2015) TREM2 in CNS homeostasis and neurodegenerative disease. Mol Neurodegener, 10, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathai S, Bajillan H, Landay AL and High KP (2014) Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci, 69, 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prineas RJ, Demirovic J, Bean JA, Duara R, Gomez-Marin O, Loewenstein D, Sevush S, Stitt F and Szapocznik J (1995) South Florida Program on Aging and Health. Assessing the prevalence of Alzheimer’s disease in three ethnic groups. J Fla Med Assoc, 82, 805–810. [PubMed] [Google Scholar]
- Raha AA, Henderson JW, Stott SR, Vuono R, Foscarin S, Friedland RP, Zaman SH and Raha-Chowdhury R (2017) Neuroprotective Effect of TREM-2 in Aging and Alzheimer’s Disease Model. J Alzheimers Dis, 55, 199–217. [DOI] [PubMed] [Google Scholar]
- Ren M, Guo Y, Wei X, Yan S, Qin Y, Zhang X, Jiang F and Lou H (2018) TREM2 overexpression attenuates neuroinflammation and protects dopaminergic neurons in experimental models of Parkinson’s disease. Exp Neurol, 302, 205–213. [DOI] [PubMed] [Google Scholar]
- Rivera Mindt M, Arentoft A, Kubo Germano K, D’Aquila E, Scheiner D, Pizzirusso M, Sandoval TC and Gollan TH (2008) Neuropsychological, cognitive, and theoretical considerations for evaluation of bilingual individuals. Neuropsychol Rev, 18, 255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S (2015) TREM2 enables amyloid beta clearance by microglia. Cell Res, 25, 535–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ TC, Stamatakis E, Hamer M, Starr JM, Kivimaki M and Batty GD (2013) Socioeconomic status as a risk factor for dementia death: individual participant meta-analysis of 86 508 men and women from the UK. Br J Psychiatry, 203, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez PA, Bender HA, Barr WB, Rivera Mindt M, Morrison CE, Hassenstab J, Rodriguez M and Vazquez B (2014) The impact of education and acculturation on nonverbal neuropsychological test performance among Latino/a patients with epilepsy. Appl Neuropsychol Adult, 21, 108–119. [DOI] [PubMed] [Google Scholar]
- Satoh J, Kawana N, Yamamoto Y, Ishida T, Saito Y and Arima K (2013) A survey of TREM2 antibodies reveals neuronal but not microglial staining in formalin-fixed paraffin-embedded postmortem Alzheimer’s brain tissues. Alzheimers Res Ther, 5, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N et al. (2016) HIV-associated neurocognitive disorder - pathogenesis and prospects for treatment. Nat Rev Neurol, 12, 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD and Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc, 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Schoenberg BS, Anderson DW and Haerer AF (1985) Severe dementia. Prevalence and clinical features in a biracial US population. Arch Neurol, 42, 740–743. [DOI] [PubMed] [Google Scholar]
- Sengupta U, Nilson AN and Kayed R (2016) The Role of Amyloid-beta Oligomers in Toxicity, Propagation, and Immunotherapy. EBioMedicine, 6, 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa G, Podini P, Mariani M, Meroni A, Spreafico R, Sinigaglia F, Colonna M, Panina P and Meldolesi J (2004) Distribution and signaling of TREM2/DAP12, the receptor system mutated in human polycystic lipomembraneous osteodysplasia with sclerosing leukoencephalopathy dementia. Eur J Neurosci, 20, 2617–2628. [DOI] [PubMed] [Google Scholar]
- Swindells S, Cobos DG, Lee N, Lien EA, Fitzgerald AP, Pauls JS and Anderson JR (2002) Racial/ethnic differences in CD4 T cell count and viral load at presentation for medical care and in follow-up after HIV-1 infection. AIDS, 16, 1832–1834. [DOI] [PubMed] [Google Scholar]
- Tang Y and Le W (2016) Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol Neurobiol, 53, 1181–1194. [DOI] [PubMed] [Google Scholar]
- Tavazzi E, Morrison D, Sullivan P, Morgello S and Fischer T (2014) Brain inflammation is a common feature of HIV-infected patients without HIV encephalitis or productive brain infection. Curr HIV Res, 12, 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulland TK, Song WM, Huang SC et al. (2017) TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell, 170, 649–663 e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich JD, Finn MB, Wang Y et al. (2014) Altered microglial response to Abeta plaques in APPPS1–21 mice heterozygous for TREM2. Mol Neurodegener, 9, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cella M, Mallinson K et al. (2015) TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell, 160, 1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojna V, Skolasky RL, Hechavarria R et al. (2006) Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J Neurovirol, 12, 356–364. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB et al. (2004) Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol, 26, 759–778. [DOI] [PubMed] [Google Scholar]
- Wunderlich P, Glebov K, Kemmerling N, Tien NT, Neumann H and Walter J (2013) Sequential proteolytic processing of the triggering receptor expressed on myeloid cells-2 (TREM2) protein by ectodomain shedding and gamma-secretase-dependent intramembranous cleavage. J Biol Chem, 288, 33027–33036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Falvey C, Harris TB, Newman A, Satterfield S, Koster A, Ayonayon H, Simonsick E and Health ABCS (2013) Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ, 347, f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh FL, Wang Y, Tom I, Gonzalez LC and Sheng M (2016) TREM2 Binds to Apolipoproteins, Including APOE and CLU/APOJ, and Thereby Facilitates Uptake of Amyloid-Beta by Microglia. Neuron, 91, 328–340. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Feng S, Nie K et al. (2018) TREM2 modulates microglia phenotypes in the neuroinflammation of Parkinson’s disease. Biochem Biophys Res Commun. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wu X, Li X et al. (2018) TREM2 Is a Receptor for beta-Amyloid that Mediates Microglial Function. Neuron, 97, 1023–1031 e1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Chen XF, Wang T et al. (2017) Soluble TREM2 induces inflammatory responses and enhances microglial survival. J Exp Med, 214, 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Masliah E and Spector SA (2011) Autophagy is increased in postmortem brains of persons with HIV-1-associated encephalitis. J Infect Dis, 203, 1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]