INTRODUCTION
Pain remains a significant problem for individuals with cancer.1 The management of acute and chronic pain in cancer patients and survivors requires the use of opioid therapy for individuals who do not respond to conservative management (e.g., nonopioid and adjuvant analgesics).2,3 If pain is moderate to severe and persistent, cancer patients are prescribed a long-acting opioid formulation with an around-the-clock (ATC) dosing schedule. This strategy can reduce pill burden, simplify the administration schedule, and provide consistent pain relief.4,5 The key to effectiveness of ATC dosing is adherence to the fixed schedule.
Unfortunately, cancer pain relief is sometimes inadequate because of poor adherence to pain medication. Medication nonadherence to therapeutic regimens has been studied extensively in chronic conditions such as diabetes, cardiac disease, and HIV. Studies frequently find Black patients less adherent than White patients even after adjusting for differences in demographic characteristics6,7 or access to medications.8 However, research on opioid adherence among cancer patients is limited and shows mixed results. Adherence rates across all types of pain medications vary from 41% to 90.8%.9–13 Adherence to ATC opioids in a primarily White oncology outpatient sample ranged from 84.5% to 90.8%.12 This contrasts with a comparable sample in terms of education of Black patients with cancer, who had considerably lower opioid rates (44% to 53%).11,14 Of note, several of these studies used self-report as the only measure for adherence,11–14 and some combined ATC and as needed (PRN) medications in the calculation of adherence.9,10
Limited research has explored factors that may influence opioid adherence. In some studies, women and individuals with limitied financial resources are less adherent to analgesic medications.9,14 Findings about the relationship between pain levels and opioid adherence are mixed, varying from no relationship10 to a significant correlation.11 Negative attitudes regarding opioids have been shown to adversely influence adherence.13–16 The relationship of opioid adherence and other factors have not yet been studied, but research on general medication adherence provides clues. Depressed individuals are three times more likely to be non-adherent to treatment guidelines than non-depressed individuals.17 Research with Black patients has reached mixed conclusions about the impact of education on adherence11,18 Social support has been shown to help overcome barriers to medication adherence in chronic diseases6 but has not been examined in relation to opioid adherence.
More information is needed to understand adherence to opioids, especially among minorities. Therefore, the purpose of this study was to identify factors associated with adherence to ATC opioids among Black individuals being treated for cancer pain.
Methods
This was a prospective, observational study with data collected at two time points: baseline and one-month follow-up. Patients were recruited from March 2014 through June 2016, from medical oncology, radiation oncology, and palliative care clinics of an urban safety-net hospital and from a tertiary cancer center. To be included in the study, all potential participants needed to have an extended release (ATC) opioid prescription; have a cancer diagnosis; self-identify as Black; be 21 years of age or older; be mentally competent according to their medical provider’s assessment; have lived in the United States for >10 years; currently living at home (rather than in an assisted living facility); and have lived at their current residence for >6 months. Those who had surgery in the previous month were excluded because the pain these patients were experiencing was expected to improve over time. In addition, individuals using a pillbox for their opioid prescription were unable to participate because the method used to measure adherence required that participants take medication directly from the bottle. Approval for research with human participants was obtained from the institutional review board. After discussing the purpose and requirements of the study, individuals who agreed to participate signed an informed consent form.
Interviews took place at the participant’s home or at a private location in the clinic. The research staff verbally administered the instruments, with the baseline interview taking about 60 minutes. The baseline visit included the completion of a demographic questionnaire, and measures of pain, depression, social support, and barriers to adherence. All data were entered and managed using REDCap.19 At the baseline visit, a Medication Event Monitoring System (MEMS) cap, which can record every time a bottle is opened, was placed on one ATC opioid drug vial. Written directions on how to use the MEMS cap were reviewed and reinforced one week after the baseline visit by phone. The one month visit lasted about 15 minutes and included collection of pain data and the MEMS cap.
The main outcome of this study was adherence to the ATC opioid, which was measured using the MEMS cap. MEMS is considered the gold standard for the measurement of medication adherence and correlates with pill counts, pharmacy refills, and self-report adherence measures.20 The MEMS cap uses a pressure-activated microprocessor to record the date and time when the bottle is opened, which most likely corresponds with the time of pill ingestion. Data were retrieved from the cap using PowerView software (MVW Switzerland Ltd). Participants were instructed to take their MEMS-monitored medication as they usually do. Participants were given a form to record any behavior that would interfere with the accuracy of the MEMS data, such as opening the bottle for any reason other than removing a dose or pocket dosing (removing >1 dose at a time for later use). If the participant received a refill of the prescription, the person was instructed to add the new medicine to the old bottle or place the MEMS cap on the new bottle at the same time they were taking a dose out. The primary measures of ATC analgesic adherence obtained from the MEMS data were dose adherence, the percentage of the total number of prescribed doses taken over the course of the study period, and 2) schedule adherence, the percentage of all prescribed doses taken on schedule over the course of the study period. This measure incorporates the schedule window defined as an hour before or after the appointed dosing time.
The Brief Pain Inventory (BPI) was used to measure cancer pain within the past 24 hours at both baseline and follow-up. There are two subscales: pain severity and pain interference. Pain severity is assessed at its “worst,” “least,” “on average,” and “current” on a 0-to-10 scale.21 An average of the four scores is calculated to determine the pain severity, and higher averages are interpreted as more severe pain. The pain interference score, an average of 7 items, measures the degree to which pain hinders mood, general activities, and relationships. In addition, change in pain was examined and measured as the difference between average pain severity between baseline and 30-day follow-up. The BPI has been validated for assessing pain in cancer patients22 and tested in minority groups.23 High internal consistency of the BPI subscales was demonstrated by Cronbach’s alpha between 0.85 and 0.87.24
Depressive symptoms were measured using the Patient Health Questionnaire 8 (PHQ-8).25 Items are scored from 0 (not at all) to 3 (nearly every day). Depressive symptoms scores can range from 0 to 24 and were calculated by summing the individual responses. The validity and reliability of the PHQ-8 (Cronbach α = 0.82) has been established across diverse populations.26–28
The Edmonton Symptom Assessment Scale (ESAS) measures the intensity of symptoms experienced during cancer treatment. The scale assesses the intensity of pain, tiredness, drowsiness, nausea, appetite, shortness of breath, depression, anxiety, and well-being at the time of the assessment.29 For the current study, an additional item, constipation, was added because it is a frequent side effect of opioid drugs.30 Respondents rate the intensity of their symptoms on a scale from 0 (absence of the symptom) to 10 (worst possible severity). Moderate intensity is generally determined to be a 4 or greater on this 0 to 10 scale.31,32 The ESAS has been evaluated in many studies33 and has a demonstrated reliability with a Cronbach’s alpha of 0.79.29
Social support was measured with the Multidimensional Scale of Perceived Social Support (MSPSS). This scale measures the individual’s perception that family, friends, and a partner would provide support if needed.34 Patients rated each item, with scores ranging from very strongly disagree (0) to very strongly agree (6). Scores for the 12 items were summed for a total score, ranging from 0 to 72; with higher scores indicating more support.35 The MSPSS had a Cronbach’s alpha of 0.91.36
The Barriers Questionnaire-13 (BQ-13) measures beliefs regarding pain and pain control.37,38 Participants taking the BQ-13 indicate their level of agreement with 13 statements; the responses range from 0 (do not agree) to 5 (agree very much). Higher scores reflect that the responder possesses more beliefs that act as barriers to pain relief. The BQ-13 scale has been validated and has demonstrated reliability with a Cronbach’s alpha of 0.86.38
Self-reported demographic data were gathered at the baseline interview. Variables such as cancer type, presence of metastasis, and co-morbidities (measured with Charlson Comorbidity Index39) were gathered by review of the medical record.
Data Analysis
Data were analyzed using both descriptive and inferential test statistics. Differences in pain scores at baseline and follow-up were examined with a paired t-test. Continuous analysis (Pearson correlation, t-test, analysis of variance) and categorical analyses (chi-square) were implemented to explore the relationships between study variables. Based on these exploratory analyses and previous literature, multiple linear regression models were built to examine the relationships between the independent variables (demographic factors [sex, education, marital status, income]), recent treatment, symptoms, depression, social support, barriers to pain management (total score and items of BQ-13), pain [interference and severity] and changes of pain severity between baseline and 30-day follow-up and the dependent variables (overall adherence to prescribed dose and schedule). We employed stepwise variable selection methods (probability of F of .05 for entry and .10 for removal) to build the multiple linear regression models. We examined both main effects and all first-order interactions between all the independent variables. Using the initial covariance matrix, we evaluated multicollinearity among the independent variables. Diagnostics (e.g., condition index, variance inflation factors, and tolerance levels) were calculated to determine if multicollinearity issues were present. Using this approach, we examined the univariate F tests for each variable to interpret their respective effect. Statistical analysis was conducted using IBM SPSS 23 and significance level was set at p<0.05.
RESULTS
A flowchart of participation is presented in Figure 1. Of the 350 patients who were initially screened for eligibility, 122 completed the informed consent process and baseline interview. Of those 122 participants, 118 completed the follow-up visit, and 105 had usable MEMS cap data. We report the findings from the 105 participants with complete data.
Figure 1.
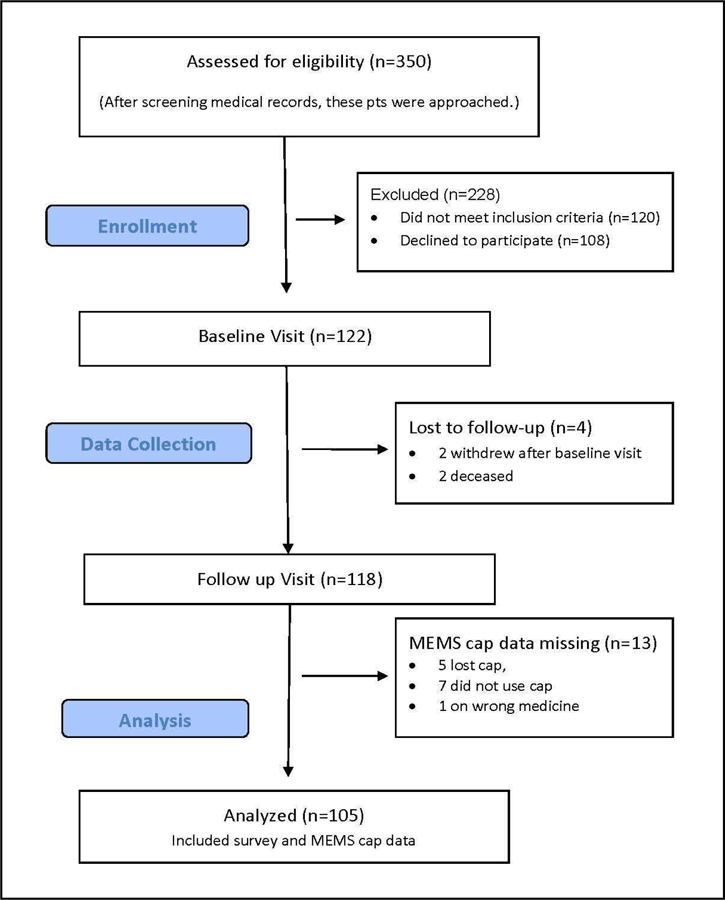
Participant and Recruitment Flowchart
Demographic and clinical characteristics are summarized in Table 1. The majority of the sample was female, middle aged, and low to middle income. Fifty-five percent had metastatic disease, and the majority (61%) were currently receiving chemotherapy. Symptom scores which were generally mild, except for tiredness and loss of appetite which fell in the moderate range. The mean score of the PHQ- 8 was 8.4, falling under the cutoff score of 10, which has been validated as indicative of depression.26 The majority of participants were prescribed extended-release (ER) morphine (64%), followed by ER oxycodone (32%) and methadone (4%).
Table 1.
Patient Characteristics at Baseline (N=105 except when noted)
| N (%) or Mean(SD) |
|
|---|---|
| Mean Age, years | 56 (10.1) |
| Sex | |
| Female | 66 (62.9) |
| Individual Income (n=87) | |
| ≤$10,000 | 20 (20.2) |
| $10,000–50,000 | 62 (62.6) |
| ≥$ 50,000 | 17 (17.2) |
| Education | |
| Less than High School | 19 (18.1) |
| High School | 27 (25.7) |
| Some College | 23 (21.9) |
| College | 24 (22.9) |
| Graduate School | 12 (11.4) |
| Marital Status | |
| Single | 28 (26.7) |
| Married | 35 (33.3) |
| Divorced/Separated | 29 (27.6) |
| Widowed | 13 (12.4) |
| History of Treatment for Alcohol Abuse | 8 (7.6) |
| History of Treatment for Drug Abuse (other than alcohol) | 3 (2.9) |
| Years of Regular Drug/Alcohol Use, (n=104) | 41.2 (44.6) |
| Years of Regular Use of Alcohol Intoxication, (n=102) | 3.1 (8.5) |
| Number of Days of Drug/Alcohol Use in past month | 6.7 (15.7) |
| Cancer Diagnosis | |
| Myeloma | 29 (27.6) |
| Gastro-Intestinal | 26 (24.8) |
| Breast | 18 (17.1) |
| Lung | 11 (10.5) |
| Other | 21 (20.0) |
| Metastatic Disease | 53 (55.8) |
| Treatment in Last Month | |
| Chemotherapy (n=101) | 62 (61.4) |
| Radiation (n=100) | 21 (21.0) |
| Charlson Comorbidity Index, mean (SD) | 5.7 (6.0) |
| Depression | |
| PHQ Score (0–23), mean (SD) | 8.4 (5.4) |
| Mean Symptom Severity per ESAS (0–10) | |
| Pain | 4.0 (3.2) |
| Tiredness | 4.7 (3.3) |
| Lack of Appetite | 4.0 (3.6) |
| Lack of Well-being | 3.6 (2.9) |
| Drowsiness | 3.3 (3.3) |
| Constipation | 3.0 (3.4) |
| Depression Symptoms | 2.1 (3.1) |
| Anxiety | 2.0 (2.8) |
| Nausea | 1.3 (2.7) |
| Shortness of breath | 1.5 (2.4) |
| Mean Social Support Score, (0–72)< (n=90) | 63.3 (12.1) |
| ATC Opioid | |
| ER Morphine | 67 (63.8) |
| ER Oxycodone | 34 (32.4) |
| Methadone | 4 (3.8) |
Pain and Adherence
Despite being treated with ATC opioids, participants reported moderate pain (Table 2). Pain severity at baseline was 4.6 and pain interference was 5.1. No significant differences between baseline and follow-up pain scores were noted per paired t-test. Participants reported 73% (baseline) and 70% (follow-up) pain relief from using medications plus nonmedical treatments. Participants’ adherence was monitored for an average of 32.4 days. Mean MEMS dose adherence equaled 60% and mean MEMS schedule adherence equaled 33% over the study period.
Table 2.
Pain (0–10) and ATC Adherence per MEMS
| Baseline | Follow-Up | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Pain Severity (0–10) | 4.6 | 2.3 | 4.7 | 2.6 |
| Pain Interference (0–10) | 5.1 | 2.6 | 4.9 | 3.0 |
| Least Pain | 3.0 | 2.7 | 3.3 | 2.7 |
| Worst Pain | 6.5 | 2.7 | 6.3 | 3.0 |
| Current Pain | 3.5 | 3.2 | 4.0 | 3.2 |
| Average Pain | 5.0 | 2.5 | 5.1 | 2.8 |
| Percent Pain Relief | 73.1 | 24.9 | 70.1 | 28.3 |
| % Dose Adherence | 60 | 28.5 | ||
| % Schedule Adherence | 33 | 31.0 | ||
Patient-Related Barriers to Pain Management
The responses to the BQ-13 (Table 3) showed that patients were concerned about addiction to pills (3.5), disliked taking pills (3.4), and were worried about constipation (3.4) using a 0 (do not agree) to 5 (agree very much) scale. Greater than 60% of the sample also agreed that the pain medication might have diminished effectiveness over time, and that the doctor should focus on a cure instead of managing pain.
Table 3:
Perceived Barrier Items about Pain Management (n=105)
| Barrier item | % agreeing with statement |
Mean ± SD (0 to 5 scale) |
|---|---|---|
| People get addicted to pain medication easily | 85.6 | 3.5 ± 1.8 |
| I do not like taking pills | 81.0 | 3.4 ± 2.0 |
| Constipation from pain medicine is really upsetting | 76.0 | 3.4 ± 2.1 |
| If you take pain medicine when you have some pain, then it might not work as well if the pain becomes worse |
63.5 | 2.4 ± 2.1 |
| It is more important for the doctor to focus on curing illness than to put time into controlling pain |
60.2 | 2.4 ± 2.2 |
| Drowsiness from pain medication is really a bother | 59.0 | 2.2 ± 2.1 |
| Nausea from pain medicine is really distressing | 53.8 | 2.0 ± 2.1 |
| Having pain means the disease is getting worse | 53.3 | 1.8 ± 1.9 |
| Confusion from pain medication is really a bother | 48.6 | 1.7 ± 2.0 |
| It is important to be strong by not talking about pain | 43.8 | 1.4 ± 2.0 |
| Pain medication cannot really control pain | 42.9 | 1.5 ± 1.9 |
| It is easier to put up with pain than with the side effects that come from pain medicine |
39.0 | 1.3 ± 1.9 |
| Pain medicine often makes you say or do embarrassing things. |
10.5 | 0.7 ± 1.5 |
Factors Associated with Adherence
The best-fitting multiple regression models are shown in Tables 4 and 5. The model in Table 4 shows 26% of the variance in dose adherence was explained by recent history of receiving chemotherapy, change in average perceived pain, perceptions about doctor’s treatment focus and concerns about nausea. The model in Table 5 estimates the total percentage of prescribed doses taken on schedule. Approximately 27% of the total variance was explained by recent history of receiving chemotherapy, change in average perceived pain, perceptions about doctor’s treatment focus and the total symptom score. The total score of BQ-13 was significantly contributed to total percentages of dose and schedule adherence. To compare with current literature, items of BQ-13 significantly associated with dose and schedule adherence were reported in Tables 4 and 5. Social support was not significantly entered into both regression models.
Table 4.
Factors Associated with Opioid Dose Adherence
| Variable | Standardized β |
SD | p | 95% CI | ∆R2 | p for ∆R2 |
|---|---|---|---|---|---|---|
| Change in average pain |
0.30 | 2.72 | 0.003 | 1.02 to 4.94 | 0.09 | 0.005 |
| Chemo received in last month |
−0.28 | 0.49 | 0.005 | −26.54 to − 4.84 |
0.085 | 0.005 |
| Fear that if doctors deal with the pain that will not focus on curing the disease |
−0.20 | 2.19 | 0.046 | −4.95 to −0.51 | 0.045 | 0.034 |
| Concern that nausea can be distressing |
−0.20 | 2.14 | 0.048 | −5.02 to −0.02 | 0.037 | 0.048 |
Note: Model: R2=0.26, F (1, 80) = 6.90, p< 0.0001
CI, confidence interval; SD, standardized deviation.
Table 5.
Factors Associated with Opioid Schedule Adherence
| Variable | Standardized β |
SD | p | 95% CI | ∆R2 | p for ∆R2 |
|---|---|---|---|---|---|---|
| Chemo received in last month |
−0.35 | 0.493 | 0.001 | −35.04 to −9.99 | 0.083 | 0.008 |
| Fear that if doctors deal with the pain that will not focus on curing the disease |
−0.29 | 2.186 | 0.004 | −7.07 to −1.42 | 0.093 | 0.003 |
| Change in average pain |
0.25 | 2.722 | 0.012 | 0.655 to 5.219 | 0.046 | 0.032 |
| Total Symptom Score | −0.23 | 17.95 | 0.020 | −0.759 to −0.066 | 0.051 | 0.020 |
Note: Model: R2=0.27, F (1, 80) = 7.5, p< 0.0001
CI, confidence interval; SD, standard deviation.
DISCUSSION
These findings support and extend previous research on factors that influence adherence to pain medication, only the second study to focus exclusively on Black cancer patients using electronic monitoring. We found that adherence was poor; mean dose adherence was 60% and mean schedule adherence was 33%. The one previous study that compared Black and White outpatients taking ATC long-acting opioids for cancer pain using MEMS (n=207; 42% Black patients) reported a comparable dose adherence of 62.9% for their subsample of Black patients.14 It is important to consider that 60% dose adherence could capture several different dosing patterns. It could represent a person who took their ER morphine medication perfectly on some days and skipped other days, or it could mean that the person took every evening dose but skipped the morning ones. Although we often think of medication-taking behavior as stable, adherence behavior can vary among patients and within the same patient over time40 and across different treatments.14 In one of the few studies exploring longitudinal ATC analgesic adherence patterns among cancer patients using MEMS, Meghani and Knafl 40 identified unique analgesic adherence patterns. The interaction of inconsistent adherence and strong opioids was a significant risk factor for acute healthcare use; of note, identifying as a Black cancer patient was a significant correlate of inconsistent ATC analgesic adherence.40 Future work should consider these unique patterns and target interventions to address the variability.
Limited information exists about schedule adherence to ATC opioids which is particularly important since this extended-release formulation of opioids requires a specific schedule of administration to provide a slow release of drug resulting in maintenance of a therapeutic dose. In this study, our measure of schedule adherence incorporates the schedule window defined as an hour before or after the appointed dosing time. Oldenmenger and colleagues reported “timing” adherence (n = 54) for a sample in which 61% were taking ER opioids.41 “Timing adherence” defined as the percentage of days on which all doses were taken within 25% of the correct dosing interval (a more lenient definition compared to the current study) and timing adherence was 64–78% for their sample. In comparison, we reported only a 33% schedule adherence for those taking ATC opioids, which demonstrates patients may not be getting adequate therapeutic coverage and raises concern about safe administration of these drugs. Future work should include examination of adherence with different scheduling windows related to the metabolism of the medication.
Receiving chemotherapy in the past month was associated with decreased adherence for both dose and schedule adherence. Perhaps the complexity of care and resulting self-care activities required when patients are actively receiving treatments, including travel to clinics, doctors’ appointments, and symptom management, result in decreased adherence. In this sample, chemotherapy treatment and symptoms were not correlated, but symptoms are still important to consider. Meghani’s et al. study14 showed the side effects attributed to opioids were negatively associated with adherence among Black patients. The current study, in contrast, did not specifically measure side effects attributed to opioids but rather measured general side effects that could be related to multiple factors such as opioids, cancer itself, treatments, or other chronic conditions. Overall the participants reported low symptom severity but nonetheless symptom burden was associated with worse schedule adherence though not dose adherence. Interestingly although nausea levels were mild in this sample, patients concerns about the distressing nature of nausea were associated with poor adherence. These concerns about nausea may have originated from early experiences since many patients initially experience nausea when starting opioids but become tolerant of effects within days.4 Interestingly, in a study that examined the relative impact of pain relief and opioid side effects on patients’ preferences for medication, nausea was the side effect that opioid-treated patients most wanted to avoid and would accept a higher level of pain to do so.42
This study confirms that a patient’s perceived barriers influence the decision they make about taking opioids. Consistent with other studies,11,14 Black cancer patients concerns about the doctors’ focus (cure versus pain relief) was associated with adherence. Patients’ concerns must be better understood when designing patient education programs and patients need to be assured that pain management and a cancer treatment focus can occur concurrently.
This study and others14,43 show that cancer patients who are prescribed ATC opioids are not taking their pain medication as prescribed despite reporting moderate pain. A change in pain between the baseline and follow-up assessment was associated with better adherence which likely means patients adjust medication when pain changes. Patients may be looking for a balance between an acceptable amount of pain and the minimum amount of pain medication needed. The act of taking a stigmatized drug around the clock on a set schedule might be difficult for patients when their pain level is decreased, stable, or just bearable resulting in poor adherence. Patients might be working to achieve a balance; they often require opioids to acquire pain relief, but they look for opportunities to take less pain medication.
Data collection for the current study occurred from 2014 through 2016 as the epidemic of opioid overuse was being publicly uncovered. The discussion of how to determine the appropriate use of opioids for the treatment of cancer pain was and still is an active topic of discussion among clinicians, researchers, and policy makers.3,44 Palliative care professionals, pain specialists, and oncologists have long been advocating for the aggressive management of pain for patients with advanced cancer.45 Progress has been made but barriers remain. It is a tricky time to be taking opioids. Despite or because of concerns about abuse, our study and others found that individuals being treated for pain with opioids underuse or take less than prescribed rather than overuse them or take more than prescribed.46
Our results should be interpreted in the context of a number of limitations. Although using MEMS provides an objective way to measure drug use, this method can only determine when the drug container is opened and does not guarantee that medication is ingested. In addition, we only measured adherence to one pain medication and additional detailed information on PRN pain medication use would have given a more complete picture of how patients were managing their pain. In addition, because only 26 to 27 percent of the variance in adherence was explained in this study, research is needed to determine what additional factors influence Black cancer patients’ adherence to ATC opioids. Finally, recruitment for our study took place in one urban setting and the extent to which these findings generalize to other geographic regions is unclear.
There are several clinical implications of our findings, including enhancing methods of assessment. In our study, the majority of patients were not adherent to ATC opioids posing possible risk of opioid toxicity. Patients potentially may have reported ineffective pain control to their provider rather than poor adherence that may have led to inappropriate dose escalation. Clinicians should partner with cancer patients, especially Black cancer patients who might have concerns about taking opioids, to provide a personalized pain treatment plan, addressing goals of care, and patient safety.47 Findings reported here indicate the need for enhanced assessment of symptoms and adherence patterns when patients are receiving chemotherapy. A thorough discussion of adherence patterns can help tailor their treatment recommendations and the level of patient education needed. Providers also need to communicate clearly that pain management can be addressed along with cancer treatment goals and that focus on pain does not sacrifice attention to treatment or cure. Additionally, clinicians will need to explore patients attitudes and previous experience with opioids and recognize that some patients desire to avoid opioid use may require revised goals about pain relief and additional strategies for relief pain.
Pain continues to be a prevalent symptom in patients with cancer. Continued research is needed to understand barriers toward effective pain treatment, especially for the Black community. The problem of underuse or poor adherence of prescribed opioids for cancer patients reporting moderate pain needs to be balanced with the emerging concerns about potential abuse and diversion of opioids for recreational use.47 In this complex context, development and testing of interventions for Black patients with cancer pain are needed to optimally manage pain. Challenges to cancer pain management include the need to find the right balance between benefits and harms of opioids from the patient perspective. Prescribing an opioid is only the first step toward treating cancer pain.
Acknowledgements:
Funding: This study was supported by the National Institutes of Health, National Institute of Nursing Research, 1K01NR014673. REDCap is supported in part by the National Institutes of Health (NIH/NCATS UL1 TR000445)
We are indebted to the individuals who participated in the study. We also want to thank the research staff that managed the recruitment of participants and data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
K. Yeager reports no potential conflicts of interest.
B. Williams reports no potential conflicts of interest.
J. Bai reports no potential conflicts of interest
H.L.F. Cooper reports no potential conflicts of interest
T. Quest reports no potential conflicts of interest.
S. Meghani reports no potential conflicts of interest
D. Bruner no potential conflicts of interest.
References
- 1.van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: Systematic review and meta-analysis. J. Pain Symptom Manage 2016;51(6):1070–1090 10.1016/j.jpainsymman.2015.12.340 [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Adult Cancer Pain, Volume 2 2016. www.nccn.org [DOI] [PubMed] [Google Scholar]
- 3.Paice JA, Portenoy R, Lacchetti C, et al. Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol 2016;34(27):3325. 10.1200/Jco.2016.68.5206 [DOI] [PubMed] [Google Scholar]
- 4.Bruera E, Paice JA. Cancer pain management: safe and effective use of opioids. American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Meeting 2015:e593–599. 10.14694/EdBook_AM.2015.35.e593 [DOI] [PubMed]
- 5.Portenoy RK, Ahmed E. Principles of opioid use in cancer pain. J. Clin. Oncol 2014;32(16):1662–1670. 10.1200/jco.2013.52.5188 [DOI] [PubMed] [Google Scholar]
- 6.Gerber BS, Cho YI, Arozullah AM, Lee SYD. Racial differences in medication adherence: a cross-sectional study of medicare enrollees. Am. J. Geriatr. Pharmacother 2010;8(2):136–145. 10.1200/jco.2013.52.5188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qato DM, Lindau ST, Conti RM, Schumm LP, Alexander GC. Racial and ethnic disparities in cardiovascular medication use among older adults in the United States. Pharmacoepidemiol. Drug Saf 2010;19(8):834–842. 10.1002/pds.1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trinacty CM, Adams AS, Soumerai SB, et al. Racial differences in long-term adherence to oral antidiabetic drug therapy: a longitudinal cohort study. BMC Health Serv. Res 2009;9 10.1186/1472-6963-9-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valeberg BT, Miaskowski C, Hanestad BR, Bjordal K, Moum T, Rustoen T. Prevalence rates for and predictors of self-reported adherence of oncology outpatients with analgesic medications. Clin. J. Pain 2008;24(7):627–636. 10.1097/AJP.0b013e31816fe020 [DOI] [PubMed] [Google Scholar]
- 10.Tzeng JI, Chang CC, Chang HJ, Lin CC. Assessing analgesic regimen adherence with the Morisky Medication Adherence Measure for Taiwanese patients with cancer pain. J. Pain Symptom Manage 2008;36(2):157–166. 10.1016/j.jpainsymman.2007.10.015 [DOI] [PubMed] [Google Scholar]
- 11.Rhee YO, Kim E, Kim B. Assessment of pain and analgesic use in African American cancer patients: factors related to adherence to analgesics. Journal of immigrant and minority health / Center for Minority Public Health 2012;14(6):1045–1051. 10.1007/s10903-012-9582-x [DOI] [PubMed] [Google Scholar]
- 12.Miaskowski C, Dodd MJ, West C, et al. Lack of adherence with the analgesic regimen: a significant barrier to effective cancer pain management. J. Clin. Oncol 2001;19(23):4275–4279. 10.1200/JCO.2001.19.23.4275 [DOI] [PubMed] [Google Scholar]
- 13.Liang SY, Chen KP, Tsay SL, et al. Relationship between belief about analgesics, analgesic adherence and pain experience in taiwanese cancer outpatients. Asian Pac. J. Cancer Prev 2013;14(2):713–716. 10.7314/Apjcp.2013.14.2.713 [DOI] [PubMed] [Google Scholar]
- 14.Meghani SH, Thompson AML, Chittams J, Bruner DW, Riegel B. Adherence to analgesics for cancer pain: a comparative study of African Americans and Whites using an electronic monitoring device. J. Pain 2015;16(9):825–835. 10.1016/j.jpain.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang S, Yates P, Edwards H, Tsay S. Factors influencing opioid-taking self-efficacy and analgesic adherence in Taiwanese outpatients with cancer. Psychooncology 2008;17(11):1100–1107. 10.1002/pon.1326 [DOI] [PubMed] [Google Scholar]
- 16.Nguyen LM, Rhondali W, De la Cruz M, et al. Frequency and predictors of patient deviation from prescribed opioids and barriers to opioid pain management in patients with advanced cancer. J. Pain Symptom Manage 2013;45(3):506–516. 10.1016/j.jpainsymman.2012.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment - Meta-analysis of the effects of anxiety and depression on patient adherence. Arch. Intern. Med 2000;160(14):2101–2107. 10.1001/archinte.160.14.2101 [DOI] [PubMed] [Google Scholar]
- 18.Braverman J, Dedier J. Predictors of medication adherence for African American patients diagnosed with hypertension. Ethn. Dis 2009;19(4):396–400. [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform 2009; 42(2):377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams AB, Amico KR, Bova C, Womack JA. A Proposal for quality standards for measuring medication adherence in research. AIDS Behav 2013;17(1):284–297 10.1007/s10461-012-0172-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N. Engl. J. Med 1994;330(9):592–596. 10.1056/NEJM199403033300902 [DOI] [PubMed] [Google Scholar]
- 22.Kumar SP. Utilization of brief pain inventory as an assessment tool for pain in patients with cancer: a focused review. Indian J. Palliat. Care 2011;17(2):108–115 10.4103/0973-1075.84531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson KO, Richman SP, Hurley J, et al. Cancer pain management among underserved minority outpatients - Perceived needs and barriers to optimal control. Cancer 2002;94(8):2295–2304. 10.1002/Cncr.10414 [DOI] [PubMed] [Google Scholar]
- 24.Wu JS, Beaton D, Smith PM, Hagen NA. Patterns of pain and interference in patients with painful bone metastases: a brief pain inventory validation study. J. Pain Symptom Manage 2010;39(2):230–240. 10.1016/j.jpainsymman.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 25.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord 2009;114(1–3):163–173. 10.1016/j.genhosppsych.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 26.Razykov I, Ziegelstein RC, Whooley MA, Thombs BD. The PHQ-9 versus the PHQ-8-Is item 9 useful for assessing suicide risk in coronary artery disease patients? Data from the Heart and Soul Study. J. Psychosom. Res 2012;73(3):163–168. 10.1016/j.jpsychores.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 27.Pressler SJ, Subramanian U, Perkins SM, et al. Measuring depressive symptoms in heart failure: validity and reliability of the patient health questionnaire-8. Am. J. Crit. Care 2011;20(2):146–152. 10.4037/ajcc2010931 [DOI] [PubMed] [Google Scholar]
- 28.Kroenke K, Spitzer RL, Williams JBW, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen. Hosp. Psychiatry 2010;32(4):345–359. 10.1016/j.genhosppsych.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 29.Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer 2000;88(9):2164–2171. [DOI] [PubMed] [Google Scholar]
- 30.Panchal SJ, Muller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int. J. Clin. Pract 2007;61(7):1181–1187. 10.1111/j.1742-1241.2007.01415.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oldenmenger WH, de Raaf PJ, de Klerk C, van der Rijt CC. Cut points on 0–10 numeric rating scales for symptoms included in the Edmonton Symptom Assessment Scale in cancer patients: a systematic review. J. Pain Symptom Manage 2013;45(6):1083–1093. 10.1016/j.jpainsymman.2012.06.007 [DOI] [PubMed] [Google Scholar]
- 32.Given B, Given CW, Sikorskii A, et al. Establishing mild, moderate, and severe scores for cancer-related symptoms: how consistent and clinically meaningful are interference-based severity cut-points? J. Pain Symptom Manage 2008;35(2):126–135. doi: S0885-3924(07)00669-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nekolaichuk C, Watanabe S, Beaumont C. The Edmonton Symptom Assessment System: a 15-year retrospective review of validation studies (1991−−2006). Palliat. Med 2008;22(2):111–122. 10.1177/0269216307087659 [DOI] [PubMed] [Google Scholar]
- 34.Sarason IG, Sarason BR, Shearin EN, Pierce GR. A brief measure of social support - practical and theoretical implications. J Soc Pers Relat 1987;4(4):497–510 10.1177/0265407587044007 [DOI] [Google Scholar]
- 35.Shumaker SC, Frazier SK, Moser DK, Chung ML. Psychometric properties of the multidimensional scale of perceived social support in patients with heart failure. J. Nurs. Meas 2017;25(1):90–102. 10.1891/1061-3749.25.1.90 [DOI] [PubMed] [Google Scholar]
- 36.Dahlem NW, Zimet GD, Walker RR. The Multidimensional Scale of Perceived Social Support: a confirmation study. J. Clin. Psychol 1991;47(6):756–761. [DOI] [PubMed] [Google Scholar]
- 37.Boyd-Seale D, Wilkie DJ, Kim YO, et al. Pain barriers: psychometrics of a 13-item questionnaire. Nurs. Res 2010;59(2):93–101. 10.1097/NNR.0b013e3181d1a6de [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkie DJ, Ezenwa MO, Yao Y, et al. Pain intensity and misconceptions among hospice patients with cancer and their caregivers: status after 2 decades. American Journal of Hospice & Palliative Medicine 2017;34(4):318–324. 10.1177/1049909116639612 [DOI] [PubMed] [Google Scholar]
- 39.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J. Clin. Epidemiol 1994;47(11):1245–1251. [DOI] [PubMed] [Google Scholar]
- 40.Meghani SH, Knafl GJ. Patterns of analgesic adherence predict health care utilization among outpatients with cancer pain. Patient Prefer Adher 2016;10:81–98 10.2147/Ppa.S93726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oldenmenger WH, Sillevis Smitt PAE, de Raaf PJ, van der Rijt CCD. Adherence to Analgesics in Oncology Outpatients: Focus on Taking Analgesics on Time. Pain Pract 2017;17(5):616–624. 10.1111/papr.12490 [DOI] [PubMed] [Google Scholar]
- 42.Gregorian RS Jr, Gasik A, Kwong WJ, Voeller S, Kavanagh S. Importance of side effects in opioid treatment: a trade-off analysis with patients and physicians. J Pain 2010. November;11(11):1095–108. 10.1016/j.jpain.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Yeager KA, Sterk CE, Quest TE, DiIorio C, Vena C, Bauer-Wu S. Managing one’s symptoms: a qualitative study of low-income African Americans with advanced cancer. Cancer Nurs 2016;39(4):303–312. 10.1097/NCC.0000000000000284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm. Rep 2016;65(1):1–49. 10.15585/mmwr.rr6501e1 [DOI] [PubMed] [Google Scholar]
- 45.Paice JA, Von Roenn JH. Under- or overtreatment of pain in the patient with cancer: how to achieve proper balance. J. Clin. Oncol 2014;32(16):1721–1726. 10.1200/jco.2013.52.5196 [DOI] [PubMed] [Google Scholar]
- 46.Lewis ET, Combs A, Trafton JA. Reasons for under-use of prescribed opioid medications by patients in pain. Pain Med 2010;11(6):861–871. 10.1111/j.1526-4637.2010.00868.x [DOI] [PubMed] [Google Scholar]
- 47.Hui D, Bruera E. A personalized approach to assessing and managing pain in patients with cancer. J. Clin. Oncol 2014;32(16):1640–1646. 10.1200/jco.2013.52.2508 [DOI] [PMC free article] [PubMed] [Google Scholar]


