Abstract
We previously reported that AML transplants using KIR B haplotype Better or Best (≥2 B activating gene loci ± Cen B/B) unrelated donors (URD) yield less relapse and better survival. In this prospective trial we evaluated 535 AML searches from 14 participating centers with centralized donor KIR genotyping for donor selection. This represented 3–48% of all AML searches (median 20%) per center totaling 3 to 172 patients (median 22) per center. Donor KIR genotype was reported at a median of 14 days after request (≤ 26 days for 76% of searches). In 535 searches, 2,080 donors were requested for KIR genotyping (mean 4.3 per search); and a median of 1.8 (0 to 4.5) per search were KIR typed. Choosing more donors for confirmatory HLA and KIR haplotype identification enriched the likelihood of finding KIR Better or Best donors. The search process identified a mean of 30% KIR Better or Best donors; the success ranged from 24 to 38% in the 11 centers enrolling ≥ 8 patients. More donors requested for KIR genotyping increased the likelihood of identifying KIR Better or Best haplotype donors. Of the 247 transplants, 9.3% used KIR Best, 19% used KIR Better, 48% used KIR neutral donors while 24% used a non-KIR tested donor. KIR genotyping did not delay transplantation. The time from search to transplant was identical for transplants using a KIR genotyped versus a non-KIR genotyped donor. Prospective evaluation can rapidly identify KIR favorable genotype donors, but choosing more donors per search would substantially increase the likelihood of having a KIR Best or Better donor available for transplantation. Transplant centers and donor registries must both commit extra effort to incorporate new characteristics (beyond HLA, age and parity) into improved donor selection. Deliberate efforts to present additional genetic factors for donor selection will require novel procedures.
Keywords: Allogeneic hematopoietic cell transplant, Unrelated Donor selection, KIR, HLA
INTRODUCTION
The preferred unrelated donor is HLA allele-matched [1, 2], young, not previously alloimmunized (through pregnancy or transfusion) [3] and in some, but not all reports, for certain recipients, CMV seronegative [4]. However, other factors have been proposed to identify better donors yielding prompt graft recovery and improved post-transplant outcomes. We previously reported on KIR B haplotype donors and their association with reduced relapse risks and improved disease free-survival (DFS) after unrelated donor (URD) transplants for AML [5,6,7]. We postulated that more robust expression or more activating receptors on donor NK cells was, at least in part, the mechanism for this protection against leukemia recurrence.
We initiated a prospective trial to rapidly provide KIR haplotype information to transplant centers on their potential donors and thus potentially enrich the use of KIR Best or Better donors in selection for transplantation. We report the outcome of this KIR donor selection (KIR DS) trial along with insights into the feasibility of rapid and efficient distribution of relevant genetic information to improve donor selection and transplantation outcomes.
METHODS
Between 2012 and 2016, 535 patients were enrolled from 14 participating transplant centers and eventually 49 participating donor centers. After recipient consent to allow donor KIR information to be considered in selection of the best URD for transplantation, transplant center-selected potential donors were identified through the National Marrow Donor Program® (NMDP) Be the Match® registry and contacted by the Center for International Blood and Marrow Transplant Research (CIBMTR) for KIR genotyping. Samples were self-collected by the donor using buccal swabs and sent to the KIR typing lab initially at Children’s Hospital of Oakland HLA laboratory and later at Stanford University. Gene content typing for these prospective clinical samples were typed for informative KIR loci and ligands using the Olerup KIR Genotyping Kits. The Olerup KIR Genotyping kits (and each lot thereafter) were validated against the UCLA International KIR Exchange and previous samples genotyped by MALDI-TOF, used in typing the retrospective study samples [5,8,9], then transmitted to the coordinating center and reported to the requesting transplant center as: KIR Best (displaying the homozygous CenB/B and 2 or more KIR B haplotype genes; KIR Better expressing two or more B haplotype KIR gene loci but not Cen B/B; or KIR neutral expressing zero or one KIR haplotype defining genes). Other details of each transplant centers’ search outcomes and reasons for selecting a donor other than the best available KIR genotyped donor were recorded.
Not all donors requested had KIR genotyping performed. Some donor centers were not participating; for some donors (n=741), we could not confirm if they were recontacted to submit confirmatory HLA typing and thus the KIR typing kit; some donors (n=217) declined to submit the typing swab kit; some had already submitted confirmatory HLA typing samples and therefore were not again contacted by the donor centers. Thus, of 2080 requested for KIR typing, only 916 had samples submitted and were typed.
Statistical analysis compared the study efficiency of sample collection, typing and reporting donor KIR haplotype data to the centers, numbers of donors requested, participation of each center in recruiting potential transplant recipients for study, choosing potential donors for genotyping, efficiency in proceeding to transplantation and overall search efficiency in enriching populations for KIR Better or Best donors being available to consider and being chosen for transplantation. Descriptive and comparative statistics are reported. The study was approved by the IRB at the University of Minnesota, the NMDP and the participating transplant and donor centers. Recipients and potential donors consented to collecting these data to inform the search process.
RESULTS
From study initiation in June 2011 through the end of study in June 2016 at 14 participating centers, 7,359 searches were initiated for 2,839 patients with AML. (Table 1) Throughout the period of study, 535 (19%) of AML study searches were enrolled on this KIR DS study. In the participating centers enrollment ranged from 3–48% of AML searches (median 20%) per center totaling a range of 3 to 172 patients (median 22 per center). Four centers closed participation early because of institutional priorities while all others continued enrollment and participation through the end of the study. The KIR haplotype evaluation for each donor included: the time from donor request to confirmed KIR genotyping; from sample collection kit delivery to the donor, sample delivered to the typing lab and genotyping report sent to the transplant center. From the date of donor request it took a median of 14 days until the KIR genotype grouping was reported to the transplant center. Seventy-five percent of KIR genotype requests were reported within 26 days.
Table 1.
KIR DS Trial Demographics, Timing and Success
| Centers participating: Transplant/Donor | 14/49 |
| Searches N (% of AML searches/center) | Total = 535 median 20% of AML searches per center (range 3–48%) median 22 searches per center (range 3–172) |
| Donors Selected for KIR typing | 2080 total Mean 4.3 per search by center (range 0–8.5) |
| Donors KIR typed Total N N per search, median (range) |
916 Median 2 (range 0–4.5 per search) |
| Time from Donor sample at typing lab to Results reported to Transplant Center (days) |
Median 14 (IQR 8–26) |
| KIR Preferred (Best + Better) donors identified. N (%) |
Overall 278 (30%) Range 24–38% per center |
| KIR Preferred donors chosen for 247 Transplants N (%) |
KIR Best 23(9.3%)/ Better 48(19.4%) |
| Time from Search to Transplant (days) Median (range; IQR) KIR DS trial donor chosen Other donor chosen |
Median 84 (range 24–846; IQR 70–107) Median 92 (range 33–870; IQR 69–132) |
KIR DS, KIR donor selection trial; IQR, interquartile range
From our and other previously reported studies [5,6 10–12], in the general and NMDP donor population, 11, 20 and 69 % of donors selected for transplantation will have the KIR Best, Better and Neutral phenotypes, respectively [12, 13]. Thus, random KIR typing should approximate this frequency. In this current, prospective KIR typing study of 916 donors, 9%, 21% and 70% of donors had KIR Best, Better and Neutral haplotypes identified; no enrichment of favorable donors was noted. Choosing more donors per patient search did allow additional chances to identify a KIR Better or Best donor to select for transplantation. In the overall study, 2080 donors were requested for KIR genotyping for 535 searches (median donors requested: 1.8 per center, range 1–3; donors KIR genotyped: median 1.61, range 0–5 per individual patient search). Choosing more donors for confirmatory HLA typing and KIR haplotype identification enriched the likelihood of finding KIR Better or Best donors. While overall the search process identified a mean of 30% KIR better and best donors for all searches, the success of enriching the donor pool for KIR Better or Best donors ranged from 24.4% to 37.9% in the 11 centers that enrolled more than eight patients. More donors requested per search and especially more donors KIR genotyped per search led to a higher likelihood of identifying a KIR Best or Better haplotype donor for transplantation. (Figure 1)
Fig 1.
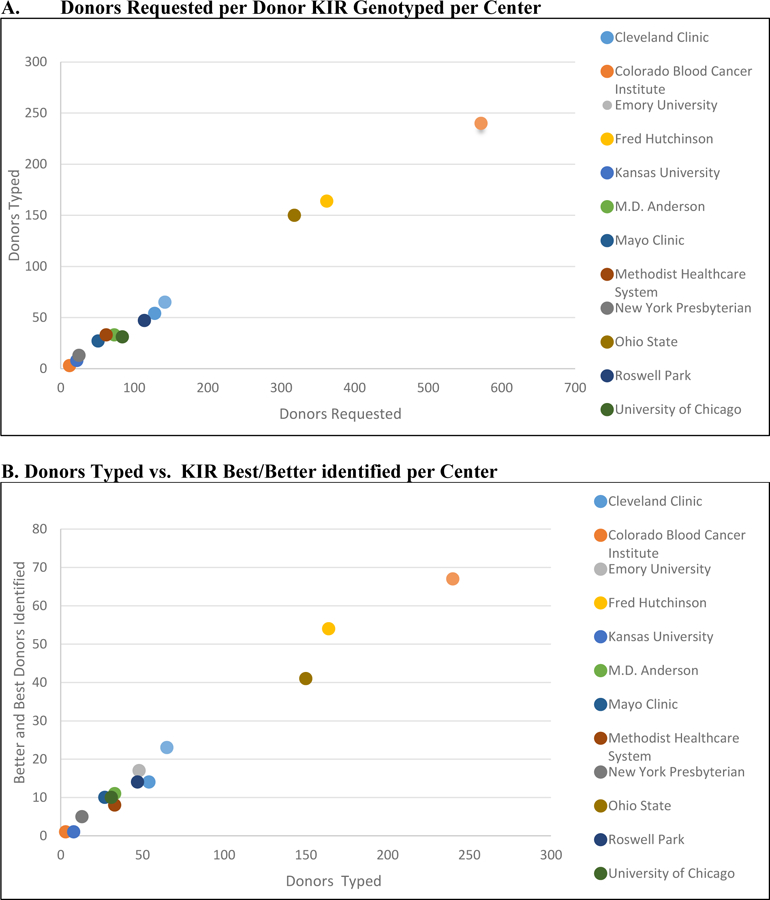
(A) Donors Requested vs. KIR genotyped and (B) Donors Typed vs. KIR Best or Better Identified per Center
Of the 535 patients enrolled in this search trial, 247 (46%) proceeded to URD transplant, and 189 (76%) used a KIR DS trial typed donor while 58 (24%) transplants were performed with donors who were not KIR genotyped during the study. Of the transplants performed, 23 (9.3%) had a KIR Best donor selected while 48 (19.4%) had a KIR Better donor and 118 (47.8%) had a KIR Neutral donor selected for transplantation. Of the 247 total transplants, 46 were performed with a donor other than a KIR Better or Best haplotype donor identified during the search process. Prior to selecting the donor for HCT, each Transplant Center had potential access to the Search success score predicted by HapLogic. The search model (14) (targeting a 10/10 allele matched donor) predicted that donors would be Good in 201/247 (81%) (>2 10/10 matched donors available); Fair in 45/247 (19%) (1–2 10/10 or no 10/10 and >2 9/10); and only Poor in 1/247 (<1%) (no 10/10 and <3 9/10). Therefore, the search prediction model identified that the vast majority (>81%) of the patients that went to transplant on the trial had a good search prognosis and likely had multiple HLA matched donors available for potential KIR genotype enrichment. Amongst the 46 transplants performed bypassing an identified KIR Better or Best donor, the transplant centers indicated: ABO status (n=4), donor availability and logistics (n=26), CMV serostatus (n=4), HLA match (n=6) and HLA antibodies or donor size (n=4) and unknown (n=2) as the stated reasons for choosing other than the preferred KIR haplotype donor.
Donor KIR genotyping did not delay the transplants. The time from trial enrollment and donor search to transplant ranged from 24 to 846 days (median 84; interquartile range 70–107) and was similar for transplants using donors who were not KIR genotyped in this trial with search to transplant interval ranging from 33 to 870 days (median 92; interquartile range 69–132).
DISCUSSION
This prospective trial tackled the challenging aim of providing prompt sample collection and KIR genotyping of the HLA-preferred donors selected by the transplant center and thus to better inform the URD search and selection process. Donor enrollment, DNA sample collection and KIR haplotype genetic information was collected and reported to the transplant center within 2 weeks. This allowed the majority of transplants performed for these patients (189 of 247, 76%) to use a KIR typed donor. In addition, the majority of transplants from the trial selected the best available KIR donor for transplantation.
Somewhat disappointingly, however, despite ongoing participating center guidance and encouragement to enroll, only a minority of searches were included in the trial. In addition, even for the 535 searches enrolled, this KIR DS trial process did not enrich the donor file with potential donors having the KIR Best or Better haplotype. Current transplant center procedural habits (even for those committed to participation in this KIR DS trial) in donor searching are likely the reasons for this failure. The average search selected only a median of 1.8 (range 1–5) donors for confirmatory HLA typing and simultaneous KIR genotyping. With an expected KIR Best frequency of 11, KIR Better 20% and the remainder being KIR Neutral, selection of fewer than 4 donors per search would hamstring the likelihood of identifying a KIR Best or Better donor beyond those available in the random population. The pre-study sample size projection was based upon estimates that 70% of searches would KIR genotype 5 or more donors and 30% would type 3 donors. These genotyping goals were not met during the study despite repeated search method education sessions and encouragement throughout the trial period. Since the KIR haplotype defining genes on chromosome 19 are inherited independently of the major histocompatibility complex (MHC) genes on chromosome 6, restricting KIR genotyping to only these minimal numbers of possible donors limits the potential for identifying preferred KIR haplotype donors required to test the previously identified favorable protection against relapse and improved DFS reported with KIR Best and Better donors or that have been reported with other KIR gene models (15–22). Currently, the available NMDP/Be the Match tools of HapLogic (which uses patient ethnicity and HLA type to predict and thus select donor HLA haplotypes) along with center concerns about delay in identifying the HLA preferred (8/8 or 10/10 HLA allele matched) URD for transplantation may have induced pressure to limit the number of donors selected for additional or confirmatory typing. Additionally, some payers will only cover typing for a limited number of donors or will cover only the donor chosen for transplantation-further constraining the number of donors selected for additional testing.
It will be difficult, if not impossible, to adopt additional donor selection procedures to enrich a favorable KIR haplotype donor pool with only a minimal cohort of donors selected in each search for additional genotyping. As a consequence, if KIR donor haplotypes or other donor pool features are to be incorporated into donor search and identification protocols, donor pool KIR haplotype testing along with age, CMV serostatus, gender, alloimmunization or other, still to be identified, genetic factors will need to be predetermined for chosen donors. Alternatively, transplant centers must be willing to pursue more donors for each search and thus potentially enrich the number available to choose the best donor for transplantation, beyond allele level HLA matching. Restricting choices to only the minimum necessary to identify a preferred HLA match limits the donor choice process and potentially compromises patients’ chances of having the best outcomes.
If Donor Centers or Registries were assured that added value in pre-search KIR genotyping of their donor files would accelerate the search process and provide useful data to provide the best donors quickly, these cost and time restrictions perceived by the Transplant Centers could be overcome. Yet the costs and added value of large scale donor KIR genotyping would need to be acknowledged and shared amongst the Networks of Donor Centers and their Transplant Center customers. Donor Registries and Transplant Centers must both commit to more intensive efforts to incorporate new factors into the search process for any meaningful advances in donor selection.
Highlights.
Favorable KIR unrelated donors for transplantation may improve outcome.
Prospective testing did not enrich the donor options with favorable donors: too few were genotyped.
Donor search relying on additional factors beyond HLA may require extra effort and more donors considered.
Acknowledgements:
This work reflects valuable efforts from other participating centers who are listed as additional members of the writing committee including: Joseph McGuirk, DO, James Slack, MD, Mark Brunvand, Carlos Bachier, Betul Oran, Sherif Farag, Tsiporah Shore, MD, Koen Van Besien MD.
Supported in part by a grant from the National Cancer Institute PO1 CA 111412
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lee S, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood 2007;110:4576–4583. [DOI] [PubMed] [Google Scholar]
- 2.Morishima Y, Kashiwase K, Matsuo K, et al. Biological significance of HLA locus matching in unrelated donor bone marrow transplantation. Blood 2015;125:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kollman C, Spellman SR, Zhang M, et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood 2016;127:260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw BE, Mayor NP, Szydlo RM, et al. Recipient/Donor HLA and CMV matching in recipients of T cell depleted unrelated donor haematopoietic cell transplants. Bone Marrow Transplantation 2017;52:717–725. [DOI] [PubMed] [Google Scholar]
- 5.Cooley S, Trachtenberg E, Bergemann TL, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood 2009;113(3):726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood 2010;116(14):2411–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor killer cell Ig-like receptor B haplotypes, recipient HLA-C1, and HLA-C mismatch enhance the clinical benefit of unrelated transplantation for acute myelogenous leukemia. J Immunol 2014;192(10):4592–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houtchens KA, Nichols RJ, Ladner MB, et al. High-throughput killer cell immunoglobulin-like receptor genotyping by MALDI-TOF mass spectrometry with discovery of novel alleles. Immunogenetics 2007;59(7):525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollenbach JA, Ladner MB, Saeteurn K, et al. Susceptibility to Crohn’s disease is mediated by KIR2DL2/KIR2DL3 heterozygosity and the HLA-C ligand. Immunogenetics 2009;61:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu KC, Keever-Taylor CA, Wilton A, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood 2005;105(12):4878–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook MA, Milligan DW, Fegan CD, et al. The impact of donor KIR and patient HLA-C genotypes on outcome following HLA-identical sibling hematopoietic stem cell transplantation for myeloid leukemia. Blood 2004;103(4):1521–1526. [DOI] [PubMed] [Google Scholar]
- 12.Beelen DW, Ottinger HD, Ferencik S, et al. Genotypic inhibitory killer immunoglobulin-like receptor ligand incompatibility enhances the long-term antileukemic effect of unmodified allogeneic hematopoietic stem cell transplantation in patients with myeloid leukemias. Blood 2005;105(6):2594–2600. [DOI] [PubMed] [Google Scholar]
- 13.Bornhauser M, Schwerdtfeger R, Martin H, et al. Role of KIR ligand incompatibility in hematopoietic stem cell transplantation using unrelated donors. Blood 2004;103(7):2860–2861. [DOI] [PubMed] [Google Scholar]
- 14.Wadsworth K, Albrecht M, Fonstad R, Spellman S, Maiers M, Dehn J. Unrelated donor search prognostic score to support early HLA consultation and clinical decisions. Bone Marrow Transplant 2016;51(11):1476–1481. [DOI] [PubMed] [Google Scholar]
- 15.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002;295:2097–2100. [DOI] [PubMed] [Google Scholar]
- 16.Davies SM, Ruggieri L, DeFor T, et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Blood 2002;100(10):3825–3827. [DOI] [PubMed] [Google Scholar]
- 17.Venstrom JM, Gooley TA, Spellman S, et al. Donor activating KIR3DS1 is associated with decreased acute GVHD in unrelated allogeneic hematopoietic stem cell transplantation. Blood 2010;115(15)3162–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venstrom JM, Pittari G, Gooley TA, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med 2012;367(9):805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu KC, Gooley T, Malkki M, et al. KIR ligands and prediction of relapse after unrelated donor hematopoietic cell transplantation for hematologic malignancy. Biol Blood Marrow Transplant 2006;12(8):828–836. [DOI] [PubMed] [Google Scholar]
- 20.Chen DF, Prasad VK, Broadwater G, et al. Differential impact of inhibitory and activating Killer Ig-Like Receptors (KIR) on high-risk patients with myeloid and lymphoid malignancies undergoing reduced intensity transplantation from haploidentical related donors. Bone Marrow Transplant 2012;47(6):817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verheyden S, Schots R, Duquet W, Demanet C. A defined donor activating natural killer cell receptor genotype protects against leukemic relapse after related HLA-identical hematopoietic stem cell transplantation. Leukemia 2005;19(8):1446–1451. [DOI] [PubMed] [Google Scholar]
- 22.Gagne K, Busson M, Bignon JD, et al. Donor KIR3DL1/3DS1 gene and recipient Bw4 KIR ligand as prognostic markers for outcome in unrelated hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2009;15(11):1366–1375. [DOI] [PubMed] [Google Scholar]


