Abstract
Stroke is the leading cause of long-term disability and individuals post-stroke often experience impaired walking ability. The plantarflexor (PF) muscles are critical to walking through their contributions to the ground reaction forces and body segment energetics. Previous studies have shown muscle activity during walking can be grouped into co-excited muscle sets, or modules. Improper co-activation, or merging of modules, is a common impairment in individuals post-stroke. The purpose of this study was to determine the influence of merged PF modules on walking performance in individuals post stroke by examining balance control, body support and propulsion, and walking symmetry. Muscle modules were identified using nonnegative matrix factorization to classify subjects as having an independent or merged PF module. The merged group had decreased balance control with a significantly higher frontal plane wholebody angular momentum than both the indepedent and control groups, while the independent and control groups were not significantly different. The merged group also had higher paretic braking and nonparetic propulsion than both the indepdendent and control groups. These results remained when comparisons were limited to subjects who had the same number of modules, indicating this was not a general effect due to subjects with merged PF having fewer modules. It is likely that a merged PF module is indicative of general PF dysfunction even when some activation occurs at the appropriate time. These results suggest an independent PF module is critical to walking performance, and thus obtaining an independent PF module should be a crucial aim of stroke rehabilitation.
Keywords: Muscle modules, synergies, gait, rehabilitation, biomechanics
1. Introduction
Stroke is the leading cause of long-term disability in the United States with approximately 795,000 people experiencing a stroke each year (Benjamin et al., 2017). Individuals post-stroke often experience reduced mobility and impaired balance control, with over 70% falling at least once within six months of leaving the hospital (Forster and Young, 1995). Falls can lead to long-term injuries, limited community involvement and reduce quality of life. Thus, there is a need for more targeted and efficient rehabilitation to maximize walking performance while reducing time spent in therapy.
The ankle plantarflexors (PFs), including the soleus (SOL) and gastrocnemius (GAS), are critical to walking performance as they are primary contributors to important biomechanical functions such as body support, forward propulsion and leg swing initiation (Anderson and Pandy, 2003; McGowan et al., 2008; Neptune et al., 2001). However, PF output is often impaired post-stroke. For example, simulation analyses have shown PF activation and function is impaired in individuals post-stroke (Higginson et al., 2006; Knarr et al., 2013), while others have shown those with limited community ambulation can have reduced paretic PF contributions to propulsion (Peterson et al., 2010) and paretic propulsion has been shown to correlate with hemiparetic severity (Bowden et al., 2006).
The PFs also contribute to balance control through mediolateral acceleration of the body’s center of mass (Pandy et al., 2010) and regulation of frontal plane angular momentum (H) (Neptune and McGowan, 2016). The range of frontal plane angular momentum (HR) has been shown to correlate with common clinical balance measures and can reveal the underlying mechanisms affecting balance in hemiparetic walking, with post-stroke subjects exhibiting a higher HR due to poor regulation of H in early stance (Nott et al., 2014).
Appropriate muscle activity is critical to the execution of these biomechanical functions and previous studies have shown that muscle activity during walking can be grouped into sets of co-excited muscles or modules (Cappellini et al., 2006; Clark et al., 2010; Ivanenko et al., 2004). These modules may originate from neuroplasticity caused by repeated activities and may reduce the computational expense involved in choosing muscle coordination strategies (Ting et al., 2015). Simulation studies have shown that well-coordinated walking in healthy subjects can be produced by five co-activation modules: 1) hip and knee extensors in early stance, 2) ankle plantarflexors (PFs) in late stance, 3) tibialis anterior and rectus femoris in swing, 4) hamstrings in late swing and early stance, and 5) hip flexors in pre- and early swing (Allen and Neptune, 2012; Neptune et al., 2009). Most experimental studies do not include hip flexor activity measurements (e.g., iliopsoas), and thus only identify modules 1–4 (e.g., Clark et al., 2010).
Research has found that in post-stroke hemiparetic walking, modules in the paretic leg can merge, or become co-activated, resulting in reduced modular complexity. Merged modules prevent the independent activation of specific muscles, which causes suboptimal execution of biomechanical functions (Clark et al., 2010). Improper co-activation of muscles is a common impairment in individuals post-stroke, with the PFs often co-activating prematurely with the hip and knee extensors in early stance.(Den Otter et al., 2007; Higginson et al., 2006; Knutsson and Richards, 1979). One mechanism identified for increased co-activation of the PFs and knee extensors is an increase in intersegmental facilitative pathways between the paretic leg knee extensors and soleus, suggesting that the co-activation is related to changes in neural pathways post-stroke (Dyer et al., 2014). However, it is unclear how a PF module merging with any other module would influence walking performance. Based on the critical functional roles of the PFs in unimpaired walking, we hypothesize that a merged PF module would result in 1) increased paretic leg braking, 2) reduced paretic leg body support, 3) increased stepping asymmetry, and 4) higher frontal plane HR compared to both healthy controls and individuals post-stroke with an independent PF module.
2. Methods
2.1. Experimental data
Kinematic, kinetic and electromyography (EMG) data were collected from 56 hemiparetic post stroke individuals (22 left hemiparesis, 29 female; age: 57 ± 13 years) and 17 healthy controls (7 female, age 55 ± 8 years). Subjects gave informed consent to participate in this IRB-approved study; see Tables A1–4 in the Appendix for demographics. The subjects walked on a split-belt instrumented treadmill (Bertec, Columbus, Ohio) at their self-selected speed. Prior to data collection, the subjects practiced treadmill walking to get comfortable with the experimental setup. Subjects walked for at least 10 seconds to reach a steady-state walking pattern before each 30-second trial. Kinematic data were collected at 120 Hz using a twelvecamera motion capture system (PhaseSpace, Inc.) and a modified Helen Hayes marker set. Kinematic and kinetic data were processed with a low-pass fourth-order Butterworth filter at 9 Hz and 20 Hz, respectively. EMG data were collected (Motion Labs) at 1000 Hz from bilateral electrodes placed on the GAS, SOL, tibialis anterior, rectus femoris, gluteus medius, vastus medialis, lateral hamstrings, and medial hamstrings. EMG data were high-pass filtered with a zero-lag fourth-order Butterworth filter at 40Hz, demeaned, rectified and low-pass filtered with a zero-lag fourth-order Butterworth filter at 4 Hz. For each muscle, the filtered signal was normalized to its peak value during each trial. Each step was normalized to 100 percent of the gait cycle and then averaged across steps.
2.2. Data analysis
The processed EMG signals were analyzed using nonnegative matrix factorization (NNMF) as previously described (Clark et al., 2010). NNMF determined the minimum number of muscle modules required to account for >90% of the EMG variability and the weighted contribution of each muscle to the module. Each subject’s module compositions were compared to the four average control modules using Pearson’s correlations. If a subject had a PF module with a Pearson’s correlation coefficient of 0.8 or greater compared to the average control PF module, they were classified as having an independent PF module. If that criterion was not met, the subject was classified as having a PF module that was merged with another muscle group.
Walking performance was assessed by examining balance control, body support and propulsion, and walking symmetry. Balance control was assessed using the range of frontal plane whole-body angular momentum (HR). Whole-body angular momentum (H) was determined using a 13-segment inverse dynamics model created in Visual3D (C-Motion, Germantown, MD) and summing the angular momentum of each body segment about the whole-body center of mass in the frontal plane. Whole-body angular momentum was normalized by subject mass, walking speed and leg length. HR was defined as the difference between the highest positive and lowest negative peaks of H and averaged over all strides. Contributions from each leg to body support were calculated from the time integral of the vertical GRF, averaged across all steps and normalized by body weight. Contributions from each leg to braking and propulsion were calculated from the time integral of the negative and positive regions of the anterior and posterior GRF, respectively. For each subject, braking and propulsion were averaged across all steps and normalized by body weight and walking speed. Walking symmetry between the paretic and nonparetic legs was assessed using both stance and stepping measures. Stance symmetry measures were defined as the paretic propulsion ratio (PP), paretic braking ratio (PB) and paretic body support ratio, which were defined as the paretic value divided by the sum of the paretic and non-paretic values, with 0.5 being perfectly symmetric. Stepping symmetry measures were defined as the percentage of total stance on the paretic and nonparetic legs and paretic step ratio, or paretic step length divided by the sum of paretic and nonparetic step lengths (Balasubramanian et al., 2009).
2.3. Statistical tests
For each dependent measure (HR, body support, braking and propulsion, and stance and stepping symmetries), ANOVAs and two-sample t-tests were used to test for significant differences between each group (independent PF module, merged PF module and control groups). A Bonferroni-Holm correction for multiple comparisons was used for the t-tests (uncorrected p<0.05).
3. Results
3.1. Module analysis
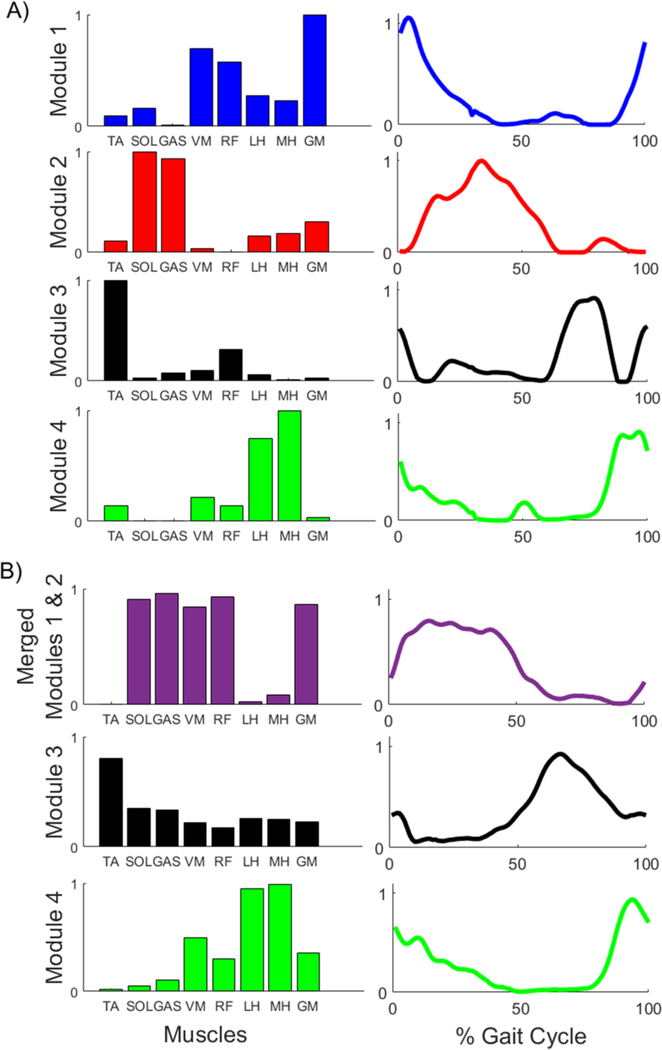
Twenty-nine of the hemiparetic subjects had a merged PF module and sixteen hemiparetic subjects had an independent PF module (e.g., Fig. 1). Twelve subjects (one control and eleven post-stroke) were excluded from analysis due to poor EMG signals and/or missing kinematic markers. The average self-selected speeds of the merged, independent and control groups were 0.27 ± 0.12, 0.64 ± 0.12, and 0.75 ± 0.22 m/s, respectively. The merged group’s self-selected speeds were significantly slower than the independent and control groups’ (p < 0.001). The 0.12 m/s difference between the independent and control group’s self-selected walking speeds did not reach significance (p = 0.09). See Tables A1–4 in the Appendix for individual subject demographics, Pearson’s correlations and walking performance assessments.
Fig. 1.
Representative NNMF results, where each row represents one module and shows the weighted contribution of each muscle to that module (left column) and the activation of that module throughout a gait cycle (right column). Results are shown for: A) control subject with four independent modules, and B) subject with a PF module merged with module 1 (purple trace).
3.2. Balance control
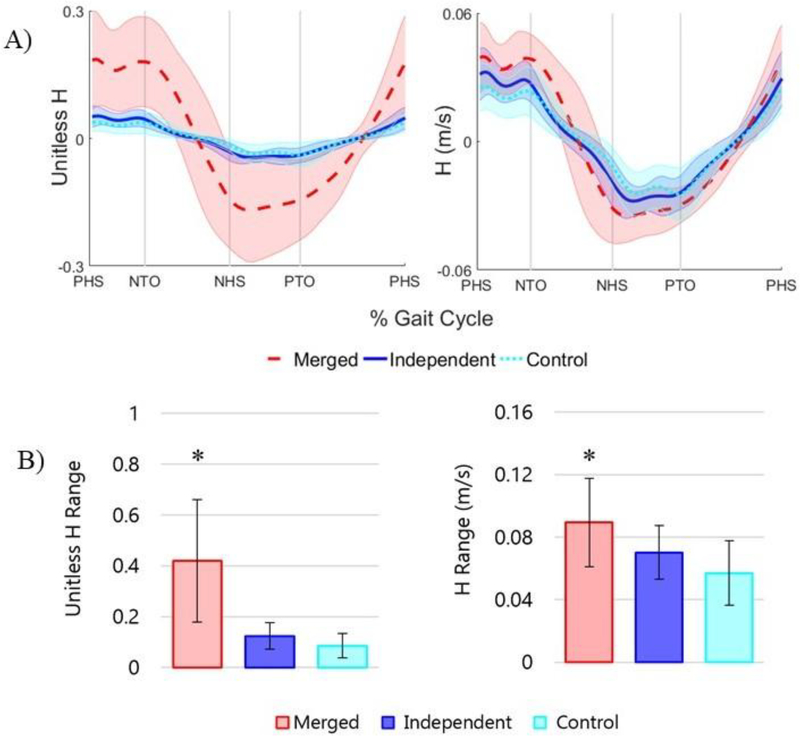
The merged group had a significantly higher HR than both the independent and control groups (p < 0.0001) (Fig. 2). There was no difference between independent and control groups (p = 0.12). The increased HR was not dependent on the differences in speed between the groups as the merged group had significantly higher HR than the independent and control groups (p = 0.003 and p < 0.001, respectively) even without normalizing H by speed (Fig. 2).
Fig. 2.
Group averaged frontal plane A) H and B) HR ± one standard deviation for the merged, independent and control groups. Results are shown for unitless H (normalized by body mass, leg length, and walking speed) and H not normalized by walking speed (units = m/s, normalized by body mass and leg length). Vertical lines show average paretic and nonparetic heel strikes (PHS, NHS) and toe-offs (PTO, NTO) as indicated on the horizontal axis. ‘*” indicates a significant difference between the HR of the merged group and the independent and control groups.
3.3. Body support and propulsion and stance symmetry
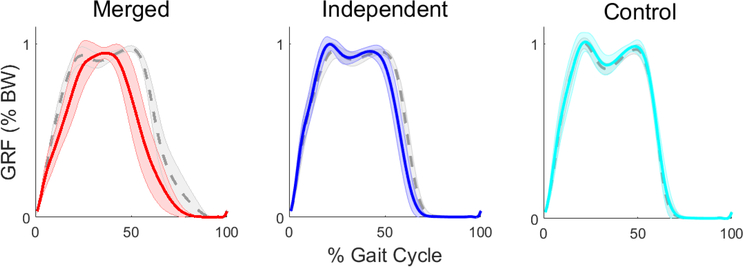
On average, the merged group produced less body support with the paretic leg compared to the nonparetic leg (Fig. 3, Table 1). The paretic body support ratio for the merged group was significantly lower than the independent and control groups (p<0.001 for both). The independent and control groups did not have significantly different paretic body support ratios (p=0.070).
Fig. 3.
Group averaged vertical ground reaction forces ± one standard deviation for the paretic leg (solid line) and non-paretic leg (dashed line) for the merged and independent groups (left and right sides of the control group, respectively).
Table 1.
Paretic body support, braking and propulsion ratios ± one standard deviation for the merged, independent and control groups. A bolded value with ‘*’ indicates the result was significantly different from both the other groups.
| Group | Paretic Body Support (%) | Paretic Propulsion (%) | Paretic Braking (%) |
|---|---|---|---|
| Merged | 43 ± 4* | 33 ± 23 | 63 ± 18 |
| Independent | 49 ± 2 | 39 ± 10 | 58 ± 9 |
| Control | 50 ± 0 | 55 ± 5* | 46 ± 6* |
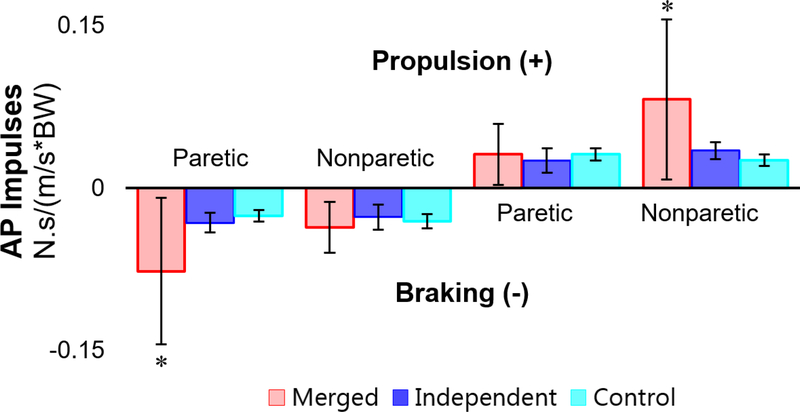
The control group had significantly lower paretic (left) braking ratio and significantly higher paretic (left) propulsion ratio than the merged and independent groups (p<0.001 for both groups and ratios, Table 1). The merged group had significantly higher paretic braking and nonparetic propulsion compared to both the independent (p=0.002, p=0.002) and control groups (p<0.001, p<0.001, Fig. 4). For paretic propulsion and nonparetic braking, differences were not significant between any groups.
Fig. 4.
Group averaged braking and propulsion ± one standard deviation (normalized by subject weight and walking speed) for the paretic and non-paretic legs of the merged and independent groups (left and right legs for the control group, respectively). ‘*’ indicates significance between the merged group and both other groups.
The merged group spent less time in stance on their paretic leg than on their nonparetic leg as a percentage of the total gait cycle (p < 0.001). There were no significant differences between paretic and nonparetic stance percentage in the independent and control groups.
3.4. Stepping symmetry
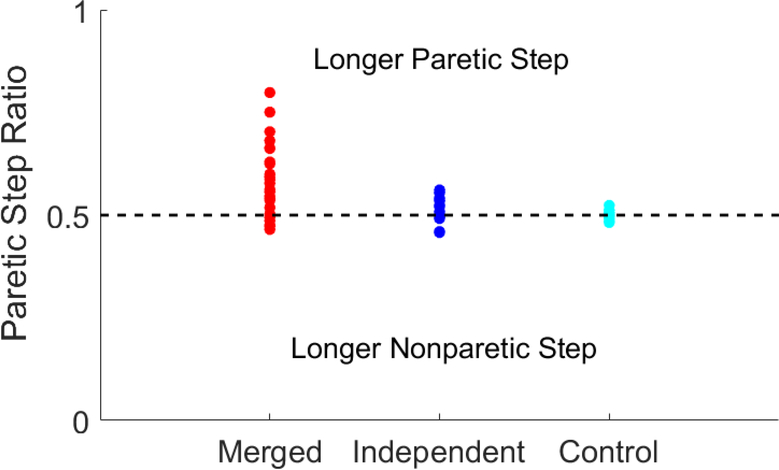
The merged group had highly variable step length asymmetries with a higher paretic step length ratio on average (0.58 ± 0.9) compared to the merged and independent groups (p=0.002, p<0.001, Fig. 5). There was no significant step length asymmetry in the independent and control groups.
Fig. 5.
Paretic step length ratio for each subject in the merged, independent and control groups. The horizontal dashed line indicates perfect symmetry.
3.5. Controlling for the effect of fewer modules
The merged group had an average of 2.8 ± 0.7 total modules, the independent group had an average of 3.7 ± 0.6 total modules and the control group had an average of 3.75 ± 0.56 modules. Previous research has shown that subjects with fewer modules have slower selfselected walking speeds and greater stepping and propulsive asymmetry (Clark et al., 2010). Because the merged group had fewer modules on average than the other groups, an additional analysis was performed on the merged and independent subjects with three (n=7 and n=5, respectively) and four (n= 4 and n=10, respectively) modules to isolate the effects of a merged PF module. Despite having the same number of modules, the merged group still had significantly lower self-selected walking speeds and higher HR, and higher paretic braking, nonparetic propulsion and propulsive asymmetries (Table 2).
Table 2.
Post-hoc comparison of merged and independent group subjects with the same total number of modules (either 3 or 4). A bolded p-value with ‘*’ indicates a significant difference between the merged and independent groups.
| Modules | Group | Speed (m/s) | Unitless HR | Paretic Braking (N.s/BW) | Nonparetic Propulsion (N.s/BW) | Paretic Ratio (%) | |
|---|---|---|---|---|---|---|---|
| Braking | Propulsion | ||||||
| 3 | Merged | 0.30±0.13 | 0.35±0.17 | −0.08±0.06 | 0.09±0.07 | 67±20 | 27±24 |
| Independent | 0.58±0.08 | 0.12±0.04 | −0.03±0.01 | 0.04±0.00 | 59±8 | 35±10 | |
| p-value | 0.002* | 0.015* | 0.119 | 0.118 | 0.442 | 0.485 | |
| 4 | Merged | 0.30±0.09 | 0.38±0.14 | −0.06±0.03 | 0.07±0.04 | 65±17 | 29±26 |
| Independent | 0.67±0.12 | 0.10±0.03 | −0.03±0.01 | 0.03±0.01 | 49±2 | 51±3 | |
| p-value | <0.001* | 0.044* | 0.150 | 0.181 | 0.513 | 0.462 | |
4. Discussion
The goal of this study was to examine the influence of a merged PF module on walking performance post-stroke. Walking performance was assessed using the dependent measures of balance control, body support, braking and propulsion, and stance and stepping symmetries, which are directly related to the functional roles of the ankle plantarflexors in healthy walking (Higginson et al., 2006; Neptune et al., 2001; Neptune and McGowan, 2016). We found that subjects with a merged PF module had decreased performance in these measures, while participants with an independent PF module walked more similarly to the control group than the merged group.
4.1. Balance control
Previous research has shown individuals post stroke have a higher HR than control subjects, indicating poor balance control (Nott et al., 2014). Our results support the expectation that a merged PF module would lead to poor balance control, with the merged group having a higher HR than both the independent and control groups. Whole-body angular momentum is regulated through foot placement and GRF generation, with the plantarflexors being primary contributors to both the vertical and mediolateral GRFs (Neptune and McGowan, 2016). Thus, subjects who do not have independent control of their plantarflexor module are likely unable to modulate the timing and magnitude of GRFs and adequately regulate frontal plane H and control balance.
4.2. Body support and propulsion and stance symmetry
As expected, the merged PF group produced less paretic body support than the independent and control groups. While prolonged activity of modules 1 and 2 during paretic stance would seem to increase body support, quicker offloading of the paretic leg (i.e., lower percentage of paretic stance) resulted in a lower overall vertical impulse. A number of subjects in the merged group lacked the second peak in the vertical GRF associated with the PF push-off (Fig. 3), suggesting that the paretic leg functioned as a passive strut for body support rather than actively generating needed forces. While slower speeds are associated with less of a trough between the first and second peaks of the vertical GRF (Cook et al., 1997; Nilsson and Thorstensson, 1989) and the merged group did walk more slowly, the nonparetic leg did not exhibit single peak behavior to the extent of the paretic leg.
We expected that the merged group would have increased paretic leg braking due to the premature PF co-activation with the knee extensor group in early stance (Den Otter et al., 2007; Higginson et al., 2006). To maintain a constant walking speed (i.e., produce net zero anterior/posterior impulse), subjects who produced higher paretic braking would then have to produce higher nonparetic propulsion. Consistent with our expectations, the merged PF group had higher mean paretic braking and nonparetic propulsion than both the independent and control groups relative to walking speed.
Hemiparetic individuals generally spend a lower percentage of the gait cycle in paretic stance than in nonparetic stance (Patterson et al., 2010; von Schroeder et al., 1995). We observed this trend in the merged PF group but it was not significant in the independent group. A lower percentage of gait spent in paretic stance versus nonparetic stance is consistent with the observed reduced paretic body support in the merged group.
4.3. Stepping symmetry
Because GAS contributes to initiating leg swing (Neptune et al., 2001), we hypothesized that the merged PF group would have altered spatiotemporal stepping characteristics following stroke. Generally, the merged group had greater step length asymmetry than the other groups. However, there was high variability between merged subjects (Fig. 5). Previous work found that a high paretic step length ratio was related to lower paretic propulsion and poor PF function (Allen et al., 2011). However, post-hoc analysis of these individuals showed no relationship between the percentage of paretic step length and propulsive measures.
4.4. Modules
It is unlikely that the merging of modules is the only cause of post-stroke biomechanical abnormalities during gait, as the merging identified through matrix factorization may also be associated with poorly activated PFs such that even when the combined module is activated in late stance when healthy PFs should be activated, the PFs are not producing adequate force. Thus, the merged module is likely indicative of general dysfunction of the PFs even during the period of the gait cycle when the merged activation overlaps with the usual PF activation. Impaired coordination of module 1 (hip and knee extensors) when it is merged with the PF module could also affect results (e.g., prolonged knee extensor activation may prevent the knee from flexing during the swing phase (Yelnik et al., 1999). Post-stroke individuals may be able to improve walking without improving muscle coordination (Den Otter et al., 2006). However, these results further support that PF coordination is a strong predictor of gait performance.
4.5. Limitations and future work
One potential limitation of this study is that by having subjects walk at their self-selected speed, we did not control for possible speed-dependent differences in our dependent measures (Lelas et al., 2003; Zeni and Higginson, 2009). Since the merged group was also the slowest group, reduced speed may have added to the observed differences.
This study also raised some questions about the protocol for normalizing frontal plane whole-body angular momentum. Normalizing by mass, leg length, and walking speed is a common protocol for H calculations (e.g. Herr and Popovic, 2008; Silverman and Neptune, 2011). However, due to the much slower walking speed of the merged group, we also examined HR without normalizing by speed to better understand the behavior of absolute HR. Frontal plane HR was still higher in the merged group than in the independent and control groups. Future work is needed to determine whether absolute or relative HR is more important for assessing fall risk and balance control. It is possible that frontal plane H does not scale linearly with walking speed, and other normalization techniques should be explored to compare subjects with a high range of body weight, height and walking speed.
5. Conclusions
In summary, we found that having an independent PF module is essential to walking performance. Post-stroke individuals whose PFs were co-activated with other muscle groups had slower walking speeds, decreased balance control and decreased walking symmetry. Thus, strategies should be developed to improve PF output during walking (Clark et al., 2016; Hsu et al., 2017). Previous research has shown that individuals can gain independent modules through locomotor training, even years after a stroke occurs (Routson et al., 2013). Thus, obtaining an independent PF module should be a priority in post-stroke rehabilitation.
Supplementary Material
Acknowledgements
This work was supported in part by NIH P20HD109040, VA RR& D 1I01RX001935 and the Rehabilitation Research & Development service of the VA.
Appendix
Table A-1:
Control subject demographics and results
| Pearson’s Correlation | Paretic (Left) Ratio (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Age (years) | Mass (kg) | Speed (m/s) | # Modules | Mod 1 | Mod 2 | Mod 3 | Mod 4 | Unitless HR | Braking | Propulsion | Body Support |
Step Length |
| 8 | 50 | 93 | 0.75 | 4 | 0.95 | 0.99 | 0.81 | 0.98 | 0.046 | 40 | 57 | 49 | 50 |
| 10 | 48 | 96 | 0.7 | 3 | 0.35 | 0.87 | 0.86 | 0.82 | 0.060 | 41 | 58 | 49 | 50 |
| 14 | 53 | 79 | 1.1 | 5 | 0.54 | 0.86 | 0.79 | 0.96 | 0.020 | 43 | 61 | 49 | 49 |
| 17 | 46 | 115 | 0.5 | 4 | 0.96 | 0.96 | 0.95 | 0.99 | 0.172 | 47 | 54 | 50 | 50 |
| 18 | 51 | 84 | 0.7 | 4 | 0.91 | 0.92 | 0.98 | 0.87 | 0.136 | 46 | 50 | 50 | 48 |
| 23 | 52 | 106 | 1 | 4 | 0.79 | 0.99 | 0.77 | 0.99 | 0.052 | 47 | 56 | 49 | 50 |
| 28 | 65 | 87 | 0.8 | 4 | 0.82 | 0.94 | 0.71 | 0.98 | 0.034 | 58 | 48 | 50 | 52 |
| 34 | 58 | 65 | 0.8 | 3 | 0.97 | 0.98 | 0.36 | 0.90 | 0.059 | 49 | 53 | 49 | 50 |
| 35 | 64 | 78 | 0.5 | 4 | 0.48 | 0.87 | 0.85 | 0.67 | 0.068 | 45 | 55 | 49 | 51 |
| 36 | 48 | 81 | 0.4 | 4 | 0.84 | 0.55 | 0.72 | 0.54 | 0.191 | 34 | 60 | 49 | 48 |
| 37 | 59 | 80 | 0.5 | 4 | 0.95 | 0.97 | 0.88 | 0.96 | 0.109 | 36 | 63 | 49 | 48 |
| 38 | 59 | 81 | 0.55 | 3 | 0.80 | 0.90 | 0.80 | 0.49 | 0.134 | 44 | 52 | 49 | 51 |
| 39 | 56 | 99 | 1.1 | 3 | 0.80 | 0.99 | 0.52 | 0.99 | 0.067 | 53 | 46 | 49 | 50 |
| 41 | 47 | 95 | 0.75 | 3 | 0.94 | 0.98 | 0.66 | 0.72 | 0.087 | 51 | 52 | 50 | 50 |
| 43 | 51 | 94 | 0.8 | 4 | 0.96 | 0.97 | 0.88 | 0.99 | 0.077 | 44 | 58 | 50 | 48 |
| 44 | 40 | 80 | 1.1 | 4 | 0.87 | 0.95 | 0.91 | 0.89 | 0.060 | 53 | 50 | 50 | 50 |
| Mean | 53±7 | 88±12 | 0.75±0.22 | 3.75±0.56 | 0.81±0.18 | 0.92± 0.10 | 0.78±0.15 | 0.86±0.16 | 0.086±0.048 | 46±6 | 55±5 | 50±0 | 50±1 |
Table A-2:
Independent group subject demographics and results
| Pearson’s Correlation | Paretic Ratio (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Age (years) | Mass (kg) | Speed (m/s) | # Modules | Mod 1 | Mod 2 | Mod 3 | Mod 4 | Unitless HR | Braking | Propulsion | Body Support |
Step Length |
| 3 | 72 | 138 | 0.7 | 4 | 0.71 | 0.98 | 0.57 | 0.95 | 0.100 | 52 | 46 | 50 | 51 |
| 4 | 67 | 76 | 0.55 | 3 | 0.60 | 0.93 | 0.90 | 0.38 | 0.095 | 64 | 35 | 51 | 49 |
| 5 | 54 | 65 | 0.9 | 4 | 0.85 | 0.97 | 0.77 | 0.97 | 0.057 | 65 | 34 | 51 | 46 |
| 6 | 58 | 77 | 0.55 | 3 | 0.76 | 0.95 | 0.82 | 0.31 | 0.100 | 50 | 42 | 47 | 50 |
| 9 | 48 | 115 | 0.75 | 3 | 0.12 | 0.89 | 0.90 | 0.86 | 0.107 | 68 | 23 | 49 | 55 |
| 12 | 60 | 107 | 0.6 | 5 | 0.73 | 0.83 | 0.83 | 0.75 | 0.115 | 54 | 37 | 47 | 52 |
| 13 | 68 | 94 | 0.75 | 4 | 0.72 | 0.97 | 0.87 | 0.69 | 0.115 | 69 | 19 | 47 | 54 |
| 22 | 51 | 86 | 0.55 | 3 | 0.46 | 0.89 | 0.81 | 0.53 | 0.102 | 48 | 53 | 49 | 52 |
| 27 | 28 | 79 | 0.5 | 3 | 0.01 | 0.92 | 0.95 | 0.68 | 0.216 | 68 | 24 | 45 | 56 |
| 31 | 68 | 120 | 0.55 | 4 | 0.67 | 0.97 | 0.80 | 0.71 | 0.140 | 32 | 52 | 45 | 46 |
| 45 | 42 | 88 | 0.8 | 4 | 0.84 | 0.97 | 0.69 | 0.93 | 0.064 | 63 | 40 | 49 | 56 |
| 46 | 62 | 88 | 0.65 | 4 | 0.74 | 0.94 | 0.70 | 0.57 | 0.098 | 56 | 35 | 46 | 54 |
| 49 | 50 | 134 | 0.5 | 4 | 0.65 | 0.95 | 0.85 | 0.87 | 0.172 | 58 | 43 | 48 | 52 |
| 51 | 58 | 106 | 0.65 | 4 | 0.75 | 0.95 | 0.71 | 0.93 | 0.105 | 64 | 52 | 50 | 49 |
| 61 | 35 | 64 | 0.5 | 4 | 0.93 | 0.87 | 0.76 | 0.80 | 0.086 | 57 | 51 | 52 | 54 |
| 63 | 59 | 111 | 0.7 | 4 | 0.87 | 0.94 | 0.94 | 0.97 | 0.104 | 59 | 44 | 50 | 49 |
| Mean | 54±12 | 97±22 | 0.64±0.12 | 3.7±0.6 | 0.65±0.25 | 0.93±0.04 | 0.80±0.10 | 0.74±0.20 | 0.111±0.037 | 58±9 | 39±10 | 48±2 | 52±3 |
Table A-3:
Merged group subject demographics and results
| Pearson’s Correlation | Paretic Ratio (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Age (years) | Mass (kg) | Speed (m/s) | # Modules | Mod 1 | Mod 2 | Mod 3 | Mod 4 | Unitless HR | Braking | Propulsion | Body Support |
Step Length |
| 2 | 75 | 67 | 0.44 | 3 | 0.50 | 0.54 | 0.92 | 0.78 | 0.174 | 55 | 28 | 46 | 58 |
| 11 | 59 | 74 | 0.3 | 3 | 0.84 | 0.22 | 0.80 | 0.88 | 0.308 | 35 | 66 | 39 | 63 |
| 15 | 43 | 85 | 0.2 | 3 | 0.55 | 0.65 | 0.70 | 0.43 | 0.481 | 35 | 67 | 37 | 56 |
| 16 | 71 | 102 | 0.15 | 4 | 0.91 | 0.67 | 0.83 | 0.83 | 0.549 | 58 | 45 | 40 | 80 |
| 19 | 71 | 70 | 0.1 | 2 | 0.47 | 0.25 | 0.74 | 0.08 | 0.588 | 55 | 37 | 46 | 66 |
| 20 | 49 | 114 | 0.15 | 3 | 0.60 | 0.27 | 0.61 | 0.89 | 0.720 | 47 | 33 | 38 | 56 |
| 21 | 69 | 74 | 0.2 | 3 | 0.77 | 0.21 | 0.39 | 0.61 | 0.330 | 57 | 74 | 46 | 49 |
| 24 | 40 | 96 | 0.1 | 2 | 0.67 | 0.52 | 0.55 | 0.20 | 1.180 | 88 | 0 | 35 | 68 |
| 25 | 61 | 92 | 0.15 | 2 | 0.61 | 0.18 | 0.66 | 0.03 | 0.413 | 90 | 7 | 46 | 66 |
| 29 | 70 | 104 | 0.15 | 3 | 0.83 | 0.65 | 0.88 | 0.20 | 0.494 | 70 | 14 | 46 | 60 |
| 33 | 58 | 115 | 0.15 | 3 | 0.39 | 0.76 | 0.38 | 0.85 | 0.923 | 88 | 9 | 38 | 75 |
| 40 | 53 | 113 | 0.35 | 4 | 0.42 | 0.77 | 0.65 | 0.91 | 0.163 | 40 | 63 | 50 | 48 |
| 47 | 41 | 75 | 0.2 | 3 | 0.47 | 0.55 | 0.95 | 0.68 | 0.343 | 95 | 1 | 48 | 50 |
| 48 | 66 | 99 | 0.4 | 4 | 0.67 | 0.77 | 0.75 | 0.88 | 0.450 | 84 | 4 | 39 | 70 |
| 50 | 63 | 115 | 0.4 | 3 | 0.58 | 0.52 | 0.78 | 0.89 | 0.220 | 56 | 28 | 45 | 55 |
| 55 | 62 | 85 | 0.25 | 2 | 0.53 | 0.17 | 0.39 | −0.03 | 0.284 | 66 | 44 | 44 | 50 |
| 56 | 50 | 116 | 0.25 | 2 | −0.04 | 0.52 | 0.34 | 0.75 | 0.495 | 51 | 47 | 45 | 52 |
| 57 | 58 | 74 | 0.1 | 2 | 0.11 | 0.09 | 0.59 | 0.51 | 0.700 | 50 | 58 | 40 | 47 |
| 58 | 26 | 78 | 0.3 | 4 | 0.29 | 0.64 | 0.84 | 0.55 | 0.368 | 78 | 2 | 40 | 59 |
| 60 | 49 | 93 | 0.4 | 2 | 0.21 | 0.47 | 0.93 | 0.15 | 0.273 | 48 | 49 | 42 | 59 |
| 62 | 76 | 92 | 0.45 | 2 | 0.47 | 0.62 | 0.02 | 0.67 | 0.108 | 53 | 43 | 49 | 49 |
| 65 | 25 | 79 | 0.15 | 3 | 0.49 | 0.49 | 0.93 | 0.53 | 0.578 | 90 | 1 | 35 | 54 |
| 66 | 33 | 69 | 0.2 | 3 | 0.18 | 0.80 | 0.97 | 0.73 | 0.606 | 84 | 3 | 34 | 60 |
| 68 | 70 | 85 | 0.3 | 3 | 0.18 | 0.49 | 0.61 | 0.96 | 0.310 | 50 | 57 | 43 | 56 |
| 69 | 70 | 86 | 0.3 | 3 | 0.42 | 0.67 | 0.90 | 0.91 | 0.295 | 43 | 55 | 46 | 47 |
| 70 | 56 | 88 | 0.55 | 3 | 0.80 | 0.79 | 0.67 | 0.51 | 0.122 | 51 | 46 | 49 | 50 |
| 71 | 55 | 54 | 0.3 | 2 | 0.57 | 0.00 | 0.51 | 0.13 | 0.234 | 92 | 7 | 43 | 63 |
| 72 | 60 | 76 | 0.45 | 2 | 0.46 | 0.30 | 0.07 | 0.72 | 0.229 | 59 | 29 | 43 | 54 |
| 73 | 64 | 107 | 0.3 | 2 | 0.66 | 0.62 | 0.70 | −0.03 | 0.214 | 55 | 46 | 49 | 54 |
| Mean | 57±14 | 89±17 | 0.27±0.12 | 2.8±0.7 | 0.50±0.23 | 0.49±0.23 | 0.66±0.24 | 0.56±0.32 | 0.124±0.052 | 63±18 | 33±23 | 43±4 | 58±8 |
Table A-4:
Subjects excluded from further analysis and reasons for exclusion.
| Subject | Age (years) | Mass (kg) | Speed (m/s) | Type | Reason for Exclusion |
|---|---|---|---|---|---|
| 1 | 74 | 80.3 | 0.3 | Hemiparetic | Missing heel markers |
| 7 | 74 | 82.9 | 0.7 | Control | Missing heel markers |
| 26 | 72 | 98.2 | 0.15 | Hemiparetic | Poor quality EMG |
| 30 | 53 | 82.0 | 0.1 | Hemiparetic | Poor quality EMG |
| 32 | 47 | 83.5 | 0.4 | Hemiparetic | Poor quality EMG |
| 42 | 66 | 78.3 | 0.5 | Hemiparetic | Poor quality EMG |
| 52 | 42 | 95.6 | 0.25 | Hemiparetic | Poor quality EMG |
| 53 | 63 | 106.6 | 0.45 | Hemiparetic | Poor quality EMG |
| 54 | 64 | 51.6 | 0.3 | Hemiparetic | Poor quality EMG |
| 59 | 82 | 70.9 | 0.3 | Hemiparetic | Poor quality EMG |
| 64 | 61 | 73.9 | 0.85 | Hemiparetic | Unable to calculate H due to missing markers |
| 67 | 49 | 94.3 | 0.45 | Hemiparetic | Poor quality EMG |
| Mean | 54±12 | 97±22 | 0.64±0.12 |
Footnotes
Conflict of Interest Statement
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JL, Kautz SA, Neptune RR, 2011. Step Length Asymmetry is Representative of Compensatory Mechanisms Used in Post-Stroke Hemiparetic Walking. Gait Posture 33, 538–543. 10.1016/j.gaitpost.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JL, Neptune RR, 2012. Three-Dimensional Modular Control of Human Walking. J. Biomech 45, 2157–2163. 10.1016/j.jbiomech.2012.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson FC, Pandy MG, 2003. Individual muscle contributions to support in normal walking. Gait Posture 17, 159–169. 10.1016/S0966-6362(02)00073-5 [DOI] [PubMed] [Google Scholar]
- Balasubramanian CK, Neptune RR, Kautz SA, 2009. Variability in spatiotemporal step characteristics and its relationship to walking performance post-stroke. Gait Posture 29, 408–414. 10.1016/j.gaitpost.2008.10.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics Committee and Stroke Statistics Subcommittee, 2017. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 135, e146–e603. 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA, 2006. Anterior-Posterior Ground Reaction Forces as a Measure of Paretic Leg Contribution in Hemiparetic Walking. Stroke 37, 872–876. 10.1161/01.STR.0000204063.75779.8d [DOI] [PubMed] [Google Scholar]
- Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F, 2006. Motor Patterns in Human Walking and Running. J. Neurophysiol 95, 3426–3437. 10.1152/jn.00081.2006 [DOI] [PubMed] [Google Scholar]
- Clark DJ, Neptune RR, Behrman AL, Kautz SA, 2016. Locomotor Adaptability Task Promotes Intense and Task-Appropriate Output From the Paretic Leg During Walking. Arch. Phys. Med. Rehabil 97, 493–496. 10.1016/j.apmr.2015.10.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA, 2010. Merging of Healthy Motor Modules Predicts Reduced Locomotor Performance and Muscle Coordination Complexity Post-Stroke. J. Neurophysiol 103, 844–857. 10.1152/jn.00825.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook TM, Farrell KP, Carey IA, Gibbs JM, Wiger GE, 1997. Effects of Restricted Knee Flexion and Walking Speed on the Vertical Ground Reaction Force During Gait. J. Orthop. Sports Phys. Ther 25, 236–244. 10.2519/jospt.1997.25.4.236 [DOI] [PubMed] [Google Scholar]
- Den Otter AR, Geurts ACH, Mulder T, Duysens J, 2007. Abnormalities in the temporal patterning of lower extremity muscle activity in hemiparetic gait. Gait Posture 25, 342–352. 10.1016/j.gaitpost.2006.04.007 [DOI] [PubMed] [Google Scholar]
- Den Otter AR, Geurts ACH, Mulder T, Duysens J, 2006. Gait recovery is not associated with changes in the temporal patterning of muscle activity during treadmill walking in patients with post-stroke hemiparesis. Clin. Neurophysiol 117, 4–15. 10.1016/j.clinph.2005.08.014 [DOI] [PubMed] [Google Scholar]
- Dyer J-O, Maupas E, de Andrade Melo S, Bourbonnais D, Nadeau S, Forget R, 2014. Changes in activation timing of knee and ankle extensors during gait are related to changes in heteronymous spinal pathways after stroke. J. NeuroEngineering Rehabil 11, 148 10.1186/1743-0003-11-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A, Young J, 1995. Incidence and consequences offalls due to stroke: a systematic inquiry. BMJ 311, 83–86. 10.1136/bmj.311.6997.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr H, Popovic M, 2008. Angular momentum in human walking. J. Exp. Biol 211, 467–481. 10.1242/jeb.008573 [DOI] [PubMed] [Google Scholar]
- Higginson JS, Zajac FE, Neptune RR, Kautz SA, Delp SL, 2006. Muscle contributions to support during gait in an individual with post-stroke hemiparesis. J. Biomech 39, 1769–1777. 10.1016/j.jbiomech.2005.05.032 [DOI] [PubMed] [Google Scholar]
- Hsu C-J, Kim J, Roth EJ, Rymer WZ, Wu M, 2017. Forced Use of the Paretic Leg Induced by a Constraint Force Applied to the Nonparetic Leg in Individuals Poststroke During Walking. Neurorehabil. Neural Repair 31, 1042–1052. 10.1177/1545968317740972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Poppele RE, Lacquaniti F, 2004. Five basic muscle activation patterns account for muscle activity during human locomotion. J. Physiol 556, 267–282. 10.1113/jphysiol.2003.057174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knarr BA, Kesar TM, Reisman DS, Binder-Macleod SA, Higginson JS, 2013. Changes in the activation and function of the ankle plantar flexor muscles due to gait retraining in chronic stroke survivors. J. NeuroEngineering Rehabil 10, 12 10.1186/1743-0003-10-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsson E, Richards C, 1979. Different types of disturbed motor control in gait of hemiparetic patients. Brain J. Neurol 102, 405–430. [DOI] [PubMed] [Google Scholar]
- Lelas JL, Merriman GJ, Riley PO, Kerrigan DC, 2003. Predicting peak kinematic and kinetic parameters from gait speed. Gait Posture 17, 106–112. 10.1016/S0966-6362(02)00060-7 [DOI] [PubMed] [Google Scholar]
- McGowan CP, Neptune RR, Kram R, 2008. Independent effects of weight and mass on plantar flexor activity during walking: implications for their contributions to body support and forward propulsion. J. Appl. Physiol Bethesda Md 1985 105, 486–494. 10.1152/japplphysiol.90448.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune RR, Clark DJ, Kautz SA, 2009. Modular Control of Human Walking: A Simulation Study. J. Biomech 42, 1282–1287. 10.1016/j.jbiomech.2009.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE, 2001. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J. Biomech 34, 1387–1398. 10.1016/S0021-9290(01)00105-1 [DOI] [PubMed] [Google Scholar]
- Neptune RR, McGowan CP, 2016. Muscle contributions to frontal plane angular momentum during walking. J. Biomech 49, 2975–2981. http://dx.doi.org.ezproxy.lib.utexas.edu/10.1016/j.jbiomech.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, Thorstensson A, 1989. Ground reaction forces at different speeds of human walking and running. Acta Physiol. Scand 136, 217–227. 10.1111/j.17481716.1989.tb08655.x [DOI] [PubMed] [Google Scholar]
- Nott CR, Neptune RR, Kautz SA, 2014. Relationships between frontal-plane angular momentum and clinical balance measures during post-stroke hemiparetic walking. Gait Posture 39, 129–134. 10.1016/j.gaitpost.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandy MG, Lin Y-C, Kim HJ, 2010. Muscle coordination of mediolateral balance in normal walking. J. Biomech 43, 2055–2064. 10.1016/j.jbiomech.2010.04.010 [DOI] [PubMed] [Google Scholar]
- Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE, 2010. Evaluation of gait symmetry after stroke: A comparison of current methods and recommendations for standardization. Gait Posture 31, 241–246. 10.1016/j.gaitpost.2009.10.014 [DOI] [PubMed] [Google Scholar]
- Peterson CL, Hall AL, Kautz SA, Neptune RR, 2010. Pre-swing deficits in forward propulsion, swing initiation and power generation by individual muscles during hemiparetic walking. J. Biomech 43, 2348–2355. 10.1016/j.jbiomech.2010.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routson RL, Clark DJ, Bowden MG, Kautz SA, Neptune RR, 2013. The influence of locomotor rehabilitation on module quality and post-stroke hemiparetic walking performance. Gait Posture 38, 511–517. 10.1016/j.gaitpost.2013.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman AK, Neptune RR, 2011. Differences in whole-body angular momentum between below-knee amputees and non-amputees across walking speeds. J. Biomech 44, 379–385. 10.1016/j.jbiomech.2010.10.027 [DOI] [PubMed] [Google Scholar]
- Ting LH, Chiel HJ, Trumbower RD, Allen JL, McKay JL, Hackney ME, Kesar TM, 2015. Neuromechanical principles underlying movement modularity and their implications for rehabilitation. Neuron 86, 38–54. 10.1016/j.neuron.2015.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schroeder HP, Coutts RD, Lyden PD, Billings E, Nickel VL, 1995. Gait parameters following stroke: a practical assessment. J. Rehabil. Res. Dev 32, 25–31. [PubMed] [Google Scholar]
- Yelnik A, Albert T, Bonan I, Laffont I, 1999. A Clinical Guide to Assess the Role of Lower Limb Extensor Overactivity in Hemiplegic Gait Disorders. Stroke 30, 580–585. 10.1161/01.STR.30.3.580 [DOI] [PubMed] [Google Scholar]
- Zeni JA, Higginson JS, 2009. Differences in gait parameters between healthy subjects and persons with moderate and severe knee osteoarthritis: A result of altered walking speed? Clin. Biomech. Bristol Avon 24, 372–378. 10.1016/j.clinbiomech.2009.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.