Abstract
Background
Hepatitis C virus (HCV) infection and alcohol use disorder (AUD) both adversely affect the immune system resulting in alterations in immune cell signaling and inflammatory processes. The aim of this study was to investigate how co-morbid AUD contributes to abnormalities in inflammatory mediators and psychiatric impairments in adults with HCV.
Methods
Alcohol use, mood, and inflammatory factors were evaluated at three time points (baseline, week 4, and week 12) in Veterans with HCV, with (n = 42) and without (n = 13) co-morbid AUD. Peripheral indices of immune activation, blood brain barrier (BBB) damage (S100B), liver function, and viral load were measured using immunoassays and polymerase chain reaction assays.
Results
Co-morbid AUD was associated with increased symptoms of depression and anxiety, elevated levels of liver enzymes, and altered expression of inflammatory factors. Alcohol consumption was positively correlated with the severity of psychiatric symptoms. Univariate analysis identified significant group differences for interleukin (IL)-8 (p = 0.006), IL-10 (p = 0.03), and S100B (p = 0.048), with increased levels in participants with AUD, which persisted over time despite reductions in alcohol use and no significant change in HCV viral load. Statistically significant effects of study group or time were not found for the other immune factors assessed. Exploratory receiver operating characteristics (ROC) curve analysis evaluated the ability of IL-8, IL-10, and S100B to differentiate between levels of alcohol consumption and generated biomarker cut-off values used to identify low risk and unhealthy alcohol use groups.
Conclusions
These results demonstrate that HCV and co-morbid AUD is associated with greater psychiatric impairments, potentially resulting from increased inflammation, dysregulated cytokine expression, and compromised BBB function. Alcohol-induced BBB damage may increase the risk of neuropathological consequences within the context of chronic HCV infection.
Keywords: Blood Brain Barrier, Cytokines, Depression, Alcohol Use Disorders, Hepatitis C
INTRODUCTION
Alcohol use disorders (AUDs) are almost three times more prevalent in Veterans with chronic hepatitis C virus (HCV) infection compared to those without HCV (Butt et al., 2007), and the prevalence of chronic HCV infection among individuals with AUD ranges from 2.1% to over 50% (in non-Veteran and Veteran populations) (Novo-Veleiro et al., 2013). Infection with HCV and the presence of an AUD are both associated with neuropathogenic effects as well as impairments in mood and cognition, but the mechanisms by which co-morbid HCV infection and AUD exert adverse effects on brain function remain unknown. Depression and cognitive dysfunction are common in patients with chronic HCV infection and co-morbid substance use disorders, including those who have mild liver disease (Huckans et al., 2009, Kramer et al., 2002, Loftis et al., 2008, Monaco et al., 2015, Nelligan et al., 2008, Thames et al., 2015). In HCV infection, elevated circulating inflammatory cytokines may influence brain function [reviewed in (Mathew et al., 2016)], and dysregulated expression of peripheral immune factors has been suggested to play a key role in the adverse neuropsychiatric sequelae associated with HCV (Huckans et al., 2014, Pawlowski et al., 2014). Reports of HCV infection of the central nervous system (CNS) (Fletcher et al., 2012, Forton et al., 2004, Letendre et al., 2007, Radkowski et al., 2002, Vargas et al., 2002, Wilkinson et al., 2009) speculate that HCV neuroinvasion may also contribute to CNS inflammation and neuropsychiatric symptoms [e.g., (Forton et al., 2001, Forton et al., 2002, Heeren et al., 2011)]. However, to date, the scientific literature does not provide a clear indication that HCV replicates in brain cells (Liu et al., 2014), and it is important to differentiate between the effects of common co-morbidities, such as AUDs from the effects of HCV (Silverstein et al., 2014).
Like HCV infection, chronic alcohol use induces inflammatory responses that contribute to its adverse CNS and neuropsychiatric effects. A growing body of research suggests that neuroinflammation is evident in the brains of adults with a history of excessive alcohol use (as compared to controls without a history of heavy alcohol consumption), and is associated with increased activation of microglia and elevated expression of central and peripheral inflammatory factors (Achur et al., 2010, He and Crews, 2008). Animal studies also show that alcohol induces gliosis, and specifically, activation of immune receptors that stimulate microglia and the induction of pro-inflammatory factors [e.g., interleukin (IL)-1β and IL-18 signaling pathways] that putatively contribute to alcohol-induced blood brain barrier (BBB) permeability (Alfonso-Loeches et al., 2016). Although the introduction of direct-acting antiviral medications has revolutionized the treatment of HCV, with current regimens showing 90-95% sustained viral response (SVR) rates (Kohli et al., 2014, Fitz, 2016), a large retrospective study conducted in the VA healthcare system found that individuals with unhealthy levels of alcohol use (defined by an AUDIT-C score of 4-12 for men and 3-12 for women) may be somewhat less likely to achieve SVR following antiviral therapy (Tsui et al., 2016). The aim of this study was to investigate how co-morbid AUD contributes to abnormalities in inflammatory mediators and psychiatric impairments in Veterans with HCV. Answering questions about the effects of a co-morbid AUD is especially relevant to the VA, not only because of their leadership role in HCV treatment but also because of the high prevalence of AUD and associated mental health disorders in Veterans. A recent, large sample-sized, retrospective study analyzed data from the National Health and Resilience in Veterans Study (3,157 U.S. Veterans aged 21 years and older) and found that the prevalence of lifetime AUD was 42.2%. Further, compared with Veterans without AUD, those with lifetime AUD had higher rates of lifetime and current mood and anxiety disorders, drug use disorder, lifetime suicide attempt, and current suicidal ideation (Fuehrlein et al., 2016).
MATERIAL AND METHODS
Participants and Procedures
Veterans with HCV (n = 55) were recruited from VA Portland, Long Beach, San Diego, and Minneapolis Health Care Systems. Participants with and without active AUDs were evaluated at three time points to assess changes in alcohol use and inflammatory factors over a 12-week period. The group with AUD included a subset of participants (placebo only) from a larger clinical trial, described previously (Hauser et al., 2017). In addition to the placebo treatment, participants were provided brief behavioral compliance enhancement treatment (BBCET), which is a standardized 15-min psychosocial intervention that emphasizes medication adherence as a crucial element to change alcohol use behavior. General exclusion criteria for participants with and without AUD included: i) dependence on cocaine, methamphetamine, or opioids within the past 6 months, ii) any known pre-existing medical conditions that could interfere with participation in the protocol, such as CNS trauma, known cognitive impairment, and acute psychiatric instability, and iii) use of the following medications: ondansetron, disulfiram, topirmamate, naltrexone, acamprosate, buprenorphine, or methadone. Further, participants without AUD also had no history of AUD or heavy drinking in the past 10+ years (as defined by > 7 drinks in a week, or one heavy drinking day in a week, consisting of 5 > drinks for men, 4 > for women). A standard drink was defined as 14 g of pure alcohol, equivalent to 12 ounces of beer, 5 ounces of wine or 1.5 ounces of 80 proof alcohol. The research was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and approved by the Institutional Review Boards at the VA Portland, Long Beach, San Diego, and Minneapolis Health Care Systems. Research participants gave informed consent after the procedures of the study were explained in full.
Following the informed consent process, participants completed a breathalyzer test and initial assessment instruments, which included the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (SCID), AUD Identification Test-C (AUDIT-C), Timeline Followback (TLFB), Beck Depression Inventory, Second Edition (BDI-II), and the Posttraumatic Stress Disorder (PTSD)-Civilian Version (PCL-C). Participants also provided blood samples for the biomarker assessments. Follow-up visits occurred at weeks 4 and 12, which involved administration of a breathalyzer test, completion of the TLFB, BDI-II, and PCL-C instruments, and collection of blood samples.
Alcohol and Psychiatric Symptom Assessments
Structured Clinical Interview for DSM-IV (SCID)
The SCID is a semi-structured psychiatric interview based on DSM-IV criteria (First, 2005) and was administered during the initial, baseline assessment. The SCID assesses both current and lifetime diagnoses and was used to identify substance dependence and abuse disorders and to determine study eligibility.
AUD Identification Test-C (AUDIT-C)
The AUDIT-C is a 3-item alcohol screening tool that is scored on a scale of 0-12. In men, a score of 4 or more is considered positive for hazardous drinking or active AUD. In women, a score of 3 or more is considered positive. Participants were administered the AUDIT-C as an initial screen for study entry. This is a modification of a standard clinical tool used to assess quantity and frequency of alcohol use (Bush et al., 1998).
Alcohol Timeline Followback (TLFB)
Alcohol use was assessed using the TLFB. The TLFB is a self-report measure of daily alcohol use collected in a calendar format and asks that participants retrospectively estimate their alcohol use (Sobell et al., 1996, Sobell, 1992). The TLFB was administered at baseline (30-day follow-back) and during follow-up visits at week 4 (2-week follow-back) and week 12 (2-week follow-back). (Note: for TLFB data analysis, 2-week segments were used for all time points).
Beck Depression Inventory, Second Edition (BDI-II)
The BDI-II is a brief 21-item self-report instrument that was used to assess depressive symptomatology at baseline, week 4, and week 12. A total score of 0-13 is considered within the minimal range, 14-19 is mild, 20-28 is moderate, and 29-63 is severe (Beck, 1996).
PTSD Checklist-Civilian Version (PCL-C)
The PCL-C is a 17-item self-report instrument that was used to assess PTSD symptoms. The PCL-C was administered at baseline and follow-up visits (weeks 4 and 12). A total score of 0-49 is considered not indicative of severe symptoms of PTSD, 50-85 is considered indicative of symptoms of PTSD. The PCL-C has good validity and reliability in various medical and psychiatric populations (Norris, 2004). The PCL‐C is used in VA medical centers to measure the level of symptom severity for Veterans and covers any stressful experience from the past.
Biomarker Assessments
Human plasma samples were analyzed in duplicate using a multiplex bead-based immunoassay (AssayGate, Inc., Ijamsville, MD, USA) to measure peripheral immune biomarkers associated with inflammation, mood disorders, and infection control [i.e., C-reactive protein (CRP), IL-1β, IL-1 receptor antagonist (IL-1RA), IL-6, IL-8, IL-10, monocyte chemoattractant protein-1 (MCP-1), and tumor necrosis factor-α (TNF-α)]. Immune factors that were below assay sensitivity limits were assigned the lowest detectable concentration in order to conduct statistical analysis. Values were assigned as follows: IL-1β = 0.11 pg/mL [3 participants at baseline (3 AUD, 0 controls) and 7 participants at week 4 (7 AUD, 0 controls)]; IL-1RA = 5.5 pg/mL [4 participants at baseline (3 AUD, 1 control), 3 participants at week 4 (1 AUD, 2 controls), and 6 participants at week 12 (3 AUD, 3 controls)]; IL-6 = 0.10 pg/mL [2 participants at baseline (1 AUD, 1 control) and 3 participants at week 4 (3 AUD, 0 controls)]; IL-10 = 0.9 pg/mL [15 participants at baseline (10 AUD, 5 controls), 10 participants at week 4 (4 AUD, 6 controls), and 13 participants at week 12 (6 AUD, 7 controls)]. To investigate the effects of AUD on BBB integrity, we measured S100 calcium-binding protein B (S100B) in plasma samples using enzyme-linked immunosorbent assays following a protocol adapted from Leite and colleagues (Leite et al., 2008), similar to published methods (Loftis et al., 2013). Monoclonal anti-S100B (1:1000; Sigma-Aldridge, St. Louis, MO, USA) and polyclonal anti-S100B (1:5000; Agilent, Santa Clara, CA, USA) were used for the capture and detection antibodies, respectively. Microplates were read at 450 nm on a Benchmark Plus Spectrophotometer System (Bio Rad, Hercules, CA, USA). Liver enzyme and viral load assessments were conducted by VA Pathology and Laboratory Services per standard operating procedures.
Statistical Analysis
Data were analyzed using Prism 6.05 (GraphPad Software, Inc., La Jolla, CA, USA) and IBM SPSS Statistics (IBM Corporation, Armonk, NY, USA). A Student’s t-test was used to compare baseline AUDIT-C scores. Repeated measures analyses of variance (ANOVA) were used to compare psychiatric measures, inflammatory factors, and viremia between groups and across time, and Holm-Sidak’s multiple comparisons tests were used for post hoc comparisons, when appropriate. Spearman’s rank correlations were used to evaluate the strength of the relationships between TLFB scores and psychiatric symptom severities, liver enzymes, and viral load. Receiver operating characteristic (ROC) analysis explored immune factor cut-off values and their sensitivities and specificities to discriminate between low risk and unhealthy levels of alcohol consumption. Post hoc t-tests were conducted as follow-up to ROC analyses to compare groups with high and low levels of IL-8 and S100B. Within the AUD group, t-tests were also used to evaluate the effects of abstinence on immune factors. See table footnotes and figure legends for additional details regarding the statistical tests used for the specific comparisons of interest. Statistical significance was maintained at p < 0.05.
RESULTS
Physical and Mental Health Characteristics in Veterans with HCV
Demographically-matched groups of Veterans with HCV and co-morbid AUDs [n = 42; mean age (SD) = 59 (5.55)] and those with HCV and no AUDs or history of heavy alcohol drinking in the past 10+ years [(n = 13); mean age (SD) = 60 (4.62)] were compared on physical and mental health factors relevant to HCV and addiction recovery outcomes. Co-morbid AUD was associated with elevated liver enzymes and increased symptoms of depression and PTSD (Table 1). Levels of ALT and AST were higher in the group of participants with AUD, as compared to the group without AUD (p = 0.03 and p = 0.01, respectively); however, statistically significant between-group differences in HCV viral load were not found. Further, liver enzymes and HCV viremia did not show any significant effects of time. Within the total sample, correlations between TLFB scores and liver enzymes detected a positive association with AST (r = 0.30, p = 0.04) at baseline and a trending association at week 12 (r = 0.31, p = 0.056). There were no significant correlations between TLFB scores and ALT levels or TLFB and HCV viremia (Supplementary Table 1). Among those participants with an AUD, self-reported alcohol consumption (as measured using the TLFB) decreased during the 12-week period (p < 0.0001, Table 1), and nine participants in the AUD reported obtaining abstinence from alcohol by week 4. Alcohol use was positively correlated with the severity of depressive and PTSD symptoms (Fig. 1).
Table 1.
Physical and mental health characteristics in Veterans with HCV
| AUD+ (n = 42-34)a | AUD- (n = 13-12)a | p-valueb | |
|---|---|---|---|
|
| |||
| Liver enzymes and HCV viremia | |||
|
| |||
| ALT (Unit/L) | 0.03c, 0.69, 0.98 | ||
| Baseline | 99.09 (80.16) | 62.17 (45.62) | |
| Week 12 | 93.39 (79.39) | 55.08 (26.47) | |
|
| |||
| AST(Unit/L) | 0.01c, 0.78, 0.87 | ||
| Baseline | 95.11 (63.87) | 57.33 (35.54) | |
| Week 12 | 89.80 (61.09) | 55.83 (27.69) | |
|
| |||
| HCV (IntUnit/mL) | 0.42, 0.83, 0.59 | ||
| Baseline | 6,418,684 (11,505,130) | 3,377,121 (3,874,565) | |
| Week 12 | 4,605,181 (6,227,033) | 4,257,697 (5,007,696) | |
|
| |||
| Alcohol use and psychiatric symptoms | |||
|
| |||
| AUDIT-C | < 0.0001 (group) | ||
| Baseline | 7.78 (2.51) | 0.17 (0.39) | |
|
| |||
| TLFB | < 0.0001c (time) | ||
| Baseline | 55.11 (42.82) | 0 (0) | |
| Week 4 | 29.54 (32.90) | 0 (0) | |
| Week 12 | 18.73 (28.02) | 0 (0) | |
|
| |||
| BDI-II | 0.003c, 0.39, 0.52 | ||
| Baseline | 13.76 (10.40) | 4.92 (5.11) | |
| Week 4 | 9.32 (11.47) | 4.67 (5.77) | |
| Week 12 | 8.27 (10.35) | 4.33 (6.71) | |
|
| |||
| PCL-C | 0.008c, 0.29, 0.90 | ||
| Baseline | 32.94 (14.66) | 25.58 (10.25) | |
| Week 4 | 30.00 (16.33) | 23.17 (10.43) | |
| Week 12 | 26.81 (11.75) | 21.92 (7.22) | |
Variations in sample size were due to unavailable data points; participants without baseline blood samples were excluded from liver enzyme and viremia analyses.
p-values reported in Table 1 were obtained from a t-test (AUDIT-C), one-way ANOVA (TLFB; within the AUD+ group only), or two-way ANOVAs (ALT, AST, and HCV viremia: 2 groups × 2 time points; BDI-II and PCL-C: 2 groups × 3 time points). Unless otherwise indicated, p-values are listed in the following order: group, time, interaction; statistically significant p-values are bolded.
Holm-Sidak’s multiple comparison tests were used to follow-up on ANOVAs that detected significant differences. Adjusted p-values from statistically significant post hoc tests were as follows: i) TLFB, baseline vs. week 4, p < 0.0001; baseline vs. week 12, p < 0.0001; week 4 vs. week 12, p = 0.009. ii) BDI-II, AUD+ vs. AUD- at baseline, p = 0.02. Post hoc comparisons for ALT, AST, and PCL-C were not statistically significant. For the physical and mental health variables, mean values (SD) are shown. Abbreviations: AUD, alcohol use disorder; AUD+, study group with AUD; AUD-, study group without AUD; AUDIT-C, AUD Identification Test; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BDI-II, Beck Depression Inventory-II; PCL-C, PTSD Check List-Civilian Version; TLFB, Timeline Followback
Figure 1. Level of alcohol consumption, as measured using the TLFB, positively correlates with psychiatric symptom severity.
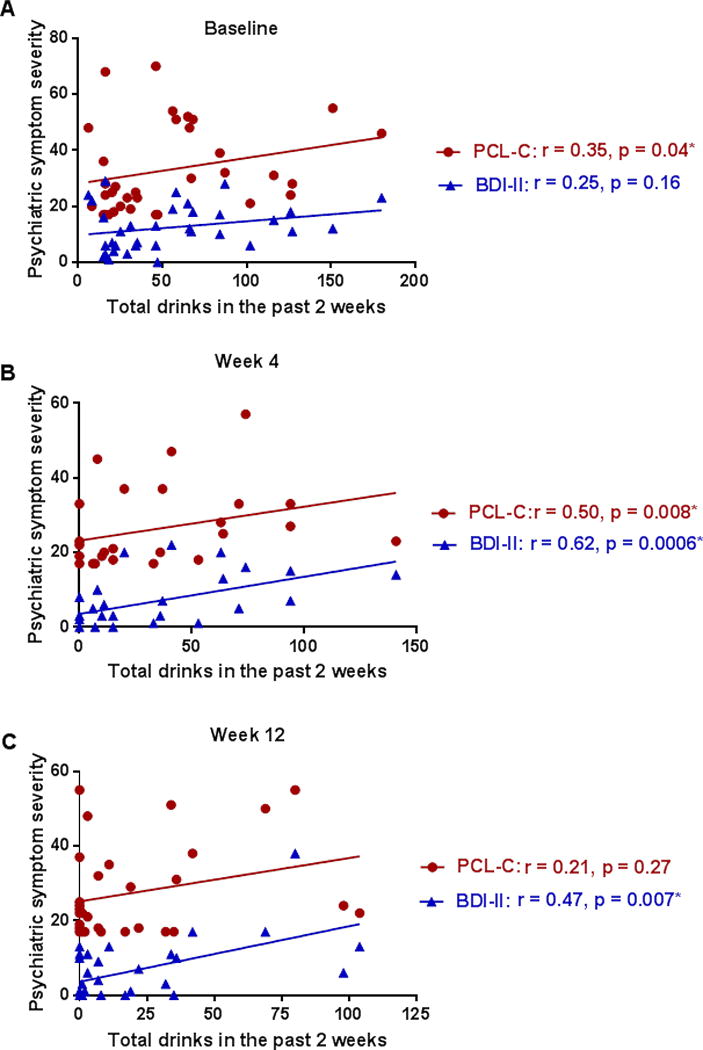
Correlations were used to evaluate the relationships between alcohol use and depressive symptoms (BDI-II; blue triangles) and alcohol use and PTSD symptoms (PCL-C; red circles) in patients with HCV and co-morbid AUD at baseline (A), week 4 (B), and week 12 (C). P-values and correlation coefficients are shown in the figure (* = statistically significant correlations). Nine participants in the AUD group obtained abstinence from alcohol by week 4. Excluding these participants from the analyses at week 4 and week 12 yielded the following correlations: week 4 BDI-II r = 0.60, p = 0.005, PCL-C r = 0.65, p = 0.002 and week 12 BDI-II r = 0.44, p = 0.03, PCL-C r = 0.35, p = 0.11. When the participants without AUD (not shown on graph) were included in the analyses with the AUD group, the following correlations were obtained: baseline BDI-II r = 0.53, p = 0.0001, PCL-C r = 0.41, p = 0.003; week 4 BDI-II r = 0.49, p = 0.002, PCL-C r = 0.42, p = 0.009; week 12 BDI-II r = 0.40, p = 0.008; PCL-C r = 0.23, p = 0.14.
Circulating Cytokines, Chemokines, and Other Immune Factors in Veterans with HCV
Co-morbid AUD was associated with altered expression of inflammatory factors in plasma. Two-way ANOVA (group × time) identified significant group differences for IL-8 (F (1, 47) = 7.96; p = 0.006), IL-10 (F (1, 47) = 4.33, p = 0.03), and S100B (F (1, 134) = 3.97, p = 0.048) (Table 2). Participants with HCV and co-morbid AUD had increased expression of all three factors, which persisted over time despite reductions in self-reported alcohol consumption and no significant change in HCV viral load (Table 1). When participants who obtained abstinence by week 4 were compared to those who reported ongoing alcohol use, no significant differences in IL-8, IL-10, or S100B were found (Supplementary Table 2). Statistically significant effects of group or time were not found for the other immune factors assessed; however, the mean levels of CRP, IL-1RA, and IL-6 were higher in the group with co-morbid AUD, as compared with the group without AUD, across all time points (Table 2).
Table 2.
Circulating cytokines, chemokines, and other immune factors in Veterans with HCV
| AUD+ (n = 42-37)a | AUD- (n = 13-12)a | p-valueb | |
|---|---|---|---|
|
| |||
| Immune factors | |||
|
| |||
| CRP (ng/mL) | 0.19, 0.29, 0.91 | ||
| Baseline | 1,731.29 (2,580.07) | 588.18 (745.23) | |
| Week 4 | 2,418.95 (4,389.74) | 888.68 (1,032.74) | |
| Week 12 | 2,514.33 (4,683.86) | 1,317.91 (1,894.31) | |
|
| |||
| IL-1RA (pg/mL) | 0.26, 0.67, 0.61 | ||
| Baseline | 36.63 (24.56) | 29.50 (13.99) | |
| Week 4 | 31.69 (15.02) | 29.70 (16.99) | |
| Week 12 | 39.76 (31.73) | 29.25 (27.74) | |
|
| |||
| IL-1β (pg/mL) | 0.64, 0.67, 0.65 | ||
| Baseline | 0.40 (0.21) | 0.36 (0.050) | |
| Week 4 | 0.24 (0.18) | 0.36 (0.07) | |
| Week 12 | 0.76 (2.56) | 0.35 (0.07) | |
|
| |||
| IL-6 (pg/mL) | 0.16, 0.29, 0.53 | ||
| Baseline | 3.75 (7.34) | 1.40 (3.15) | |
| Week 4 | 3.79 (4.13) | 2.56 (5.82) | |
| Week 12 | 8.82 (18.57) | 2.85 (6.05) | |
|
| |||
| IL-8 (pg/mL) | 0.006c, 0.80, 0.74 | ||
| Baseline | 27.81 (21.75) | 11.61 (6.97) | |
| Week 4 | 31.73 (39.81) | 12.39 (7.50) | |
| Week 12 | 24.92 (14.63) | 13.03 (9.37) | |
|
| |||
| IL-10 (pg/mL) | 0.03c, 0.71, 0.82 | ||
| Baseline | 3.42 (5.70) | 1.26 (0.59) | |
| Week 4 | 3.45 (3.45) | 1.24 (0.59) | |
| Week 12 | 2.70 (1.93) | 1.155 (0.41) | |
|
| |||
| MCP-1 (pg/mL) | 0.72, 0.56, 0.43 | ||
| Baseline | 96.19 (66.97) | 100.51 (32.06) | |
| Week 4 | 92.73 (53.77) | 110.99 (36.84) | |
| Week 12 | 108.66 (85.44) | 105.79 (56.51) | |
|
| |||
| S100B (pg/mL) | 0.048c, 0.64, 0.77 | ||
| Baseline | 23.86 (40.15) | 7.36 (10.02) | |
| Week 4 | 16.14 (31.31) | 5.99 (7.61) | |
| Week 12 | 14.24 (38.49) | 5.55 (7.18) | |
|
| |||
| TNF-α (pg/mL) | 0.77, 0.72, 0.25 | ||
| Baseline | 18.34 (20.80) | 12.03 (5.52) | |
| Week 4 | 16.03 (11.66) | 17.61 (22.01) | |
| Week 12 | 17.78 (21.85) | 18.14 (15.64) | |
Variations in sample size were due to unavailable data points; participants without baseline blood samples were excluded from immune factor analyses.
p-values reported in Table 2 were obtained from two-way ANOVAs (2 groups × 3 time points) and are listed in the following order: group, time, interaction. Statistically significant ANOVA p-values are bolded.
Holm-Sidak’s multiple comparison tests were used to follow-up on ANOVAs that detected significant differences. The adjusted p-value for the significant post hoc test was as follows: IL-8, AUD+ vs. AUD- at week 4, p = 0.04. Post hoc comparisons for IL-10 and S100B were not statistically significant. For the immune factors, mean values (SD) are shown. Abbreviations: AUD, alcohol use disorder; AUD+, study group with AUD; AUD-, study group without AUD; CRP, C-reactive protein; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; S100B, S100 calcium-binding protein B; TNF-α, tumor necrosis factor-alpha
Alcohol Consumption and Immune Factor Levels: Post hoc exploratory analyses
To begin to investigate the potential clinical relevance of increased IL-8, IL-10, and S100B in patients with HCV and AUD, we evaluated the ability of these biomarkers to differentiate between levels of alcohol consumption [i.e., low risk vs. unhealthy alcohol drinking (“at-risk” and “heavy”)], as determined by the reference standard TLFB. We used the NIAAA definition of “at-risk” drinking for men (NIAAA, 2007), but placed it in the context of a two-week period: > 14 drinks per week on average. We defined “heavy” drinking as ≥ five drinks per day in past 14 days—amounts at which physiologic and/or organ damage become more likely (Conigrave et al., 2002, White et al., 2002). Baseline levels of IL-8, IL-10, and S100B were selected for ROC analysis to identify the immune factors’ cut-off values and their sensitivities and specificities to discriminate unhealthy amounts of alcohol consumption. Values with the highest sum of the sensitivity and specificity were reported as the best cut-off values. The sensitivities and specificities of the biomarker cut-off values to identify low risk and unhealthy alcohol use groups appear in Table 3. An overall indication of the diagnostic accuracy of a ROC curve is the area under the curve (AUC). The ROC curves with calculated AUC (± 95% CI, with p-values) for IL-8 and S100B are shown in Figure 2A. The AUC for the anti-inflammatory cytokine, IL-10 was 0.54 (0.38 - 0.69; p = 0.66) and is not shown on the graph. Follow-up analyses indicated that participants with circulating S100B concentrations greater than 12.0 pg/mL reported consuming more alcohol (measured by TLFB scores), as compared to participants with S100B concentrations less than 12.0 pg/mL (Fig. 2B). Alcohol consumption was not significantly different between participants with “low” and “high” IL-8 concentrations (i.e., less than or greater than 14.96 pg/mL, respectively) (Fig. 2C).
Table 3.
Best cut-off values of plasma immune factor levels to differentiate alcohol groups according to receiver operating characteristic analysis
| Biomarker | Plasma concentration | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|
| IL-8 (pg/mL) | 14.96 | 81 (60.7 - 93.5) | 59 (38.9 - 76.5) |
| IL-10 (pg/mL) | 1.21 | 69 (48.2 - 85.7) | 55 (35.7 - 73.6) |
| S100B (pg/mL) | 12.0 | 50 (27.2 - 72.8) | 83 (58.6 - 94.4) |
Figure 2. Exploratory receiver operating characteristic (ROC) curve analysis of immune factors for differentiating unhealthy alcohol use in the context of HCV.
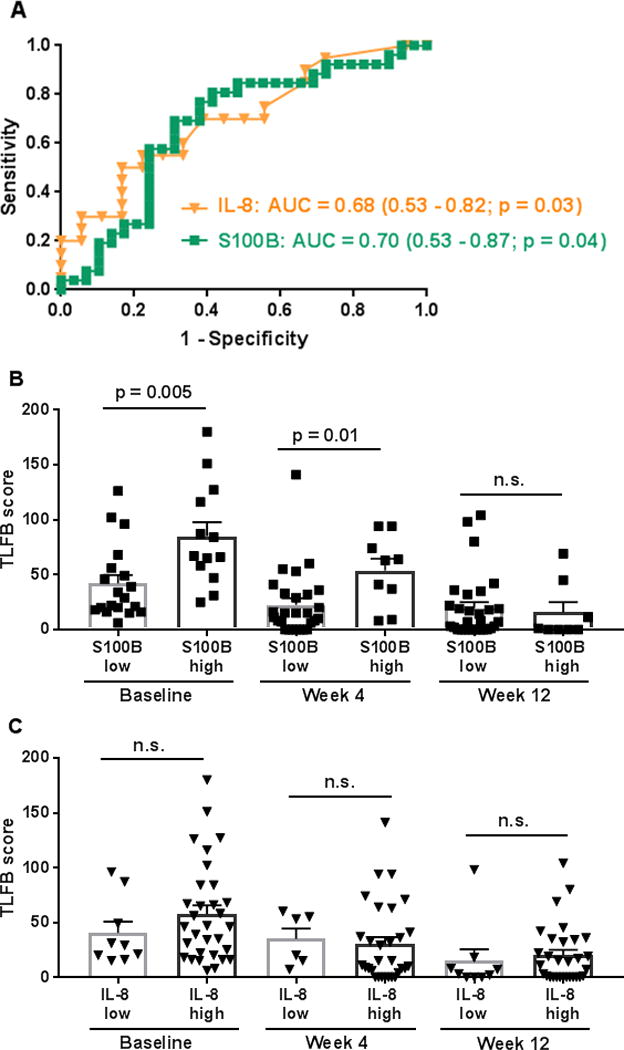
ROC curves of plasma IL-8 and S100B levels to differentiate low risk from unhealthy amounts of alcohol consumption (based on the reference standard TLFB and NIAAA guidelines) are shown (A). Follow-up t-tests indicated that participants with S100B concentrations greater than 12.0 pg/mL (Table 3) (S100B high group) had significantly higher TLFB scores at baseline and week 4, as compared to participants with S100B concentrations less than 12.0 pg/mL (S100B low group) (B). There were no significant between-group differences in alcohol consumption at week 12 (p = 0.74) (B). TLFB scores were not statistically different between participants with IL-8 concentrations less than (IL-8 low group) or greater than (IL-8 high group) 14.96 pg/mL (Table 3). P-values were greater than 0.05 for all between-group comparisons (baseline, p = 0.29; week 4, p = 0.75; week 12, p = 0.63) (C). Abbreviations: AUC, area under curve; IL, interleukin; TLFB, Timeline Followback; NIAAA, National Institute of Alcohol Abuse and Alcoholism.
DISCUSSION
In this study, we investigated the impact of co-morbid AUD on inflammatory mediators and psychiatric symptom severities in adults with HCV. Our results expand on previous reports regarding the psychiatric effects of HCV and AUD (Carta et al., 2012, Huckans et al., 2014, Petrakis, 2002) and demonstrate increased symptoms of depression and anxiety in adults with HCV and co-morbid AUD, relative to those with HCV and no history of alcohol abuse. Further, we found that the amount of alcohol consumption was positively associated with AST levels [as has been shown previously (Gough et al., 2015)], as well as with the severity of psychiatric symptoms (Fig. 1). The pathological interactions between alcohol and HCV, which contribute, in part, to these adverse mental health effects involve shared immunological consequences—most notably the modulation of cytokine production [reviewed in (Montesinos et al., 2016, Szabo et al., 2010)].
Research is converging on specific inflammatory signaling pathways and the role of peripheral and central immune factors in CNS impairment accompanying HCV and AUD, such as interferon-alpha (IFN-α) (Ganesan et al., 2016, Huckans et al., 2015), IL-8 (French et al., 2017, Gonzalez-Quintela et al., 1999, Huckans et al., 2014, Leclercq et al., 2014, Maes et al., 1998, Vivithanaporn et al., 2010, Wilkinson et al., 2010), and IL-10 (Armah et al., 2013, Gonzalez-Quintela et al., 1999, Huckans et al., 2014, Song et al., 1998). Consistent with this body of evidence, in the current study we identified significant group differences for IL-8 and IL-10, with increased plasma concentrations in participants with AUD. Research in adults with HCV shows IL-8 and IL-10 levels comparable to those observed in our participants with HCV and no AUD (e.g., Rahman et al., 2011), but studies that compare groups of participants with and without HCV and co-morbid AUD are needed to determine the individual and combined effects of the illnesses on cytokine levels. IL-8 [a.k.a. C-X-C motif ligand 8 (CXCL8)] is a chemokine produced by macrophages and other cell types, and although neutrophils are the primary target cells of IL-8, there are a wide range of cells (including endothelial cells) that respond to this chemokine. Inducible IL-8 functions as a chemoattractant which attracts and activates immune cells in inflammatory regions. Secretion of IL-8 is increased under conditions of oxidant stress [e.g., TNF-α-induced reactive oxygen species (Vlahopoulos et al., 1999)], which can occur with alcohol abuse and HCV infection (Gonzalez-Reimers et al., 2014, Medvedev et al., 2017). In addition to inflammatory signals, IL-8 production is also regulated by IL-10 (Méndez-Samperio et al., 2002). IL-10 [a.k.a. human cytokine synthesis inhibitory factor (CSIF)] is produced by many different cell types and is considered one of the most important cytokines with anti-inflammatory properties (Couper et al., 2008, Mingomataj and Bakiri, 2016).
In adults with HCV and co-morbid AUD, increased concentrations of IL-8 were accompanied by elevated IL-10 plasma levels (Table 1). The anti-inflammatory and modulatory actions of IL-10 (e.g., inhibiting the production of pro-inflammatory cytokines) appear to target the transcription factor nuclear factor-κB (NF-κB) pathway (Bhattacharyya et al., 2004), an inflammatory signaling pathway induced by alcohol and associated with addiction-related behavior (Nennig and Schank, 2017). In contrast to some reports (Heberlein et al., 2014), we did not detect statistically significant group differences in the expression of other inflammatory cytokines produced via NF-κB activation, such as IL-6; however, we did find that mean levels of IL-6, as well as IL-1RA and CRP, were higher in the group with co-morbid AUD, as compared with the group without AUD. Thus, cytokine balance alterations likely contribute to the systemic and neurological manifestations of alcohol abuse.
Alcohol, HCV, and inflammatory cytokines are involved in BBB disruption (Rubio-Araiz et al., 2017, Silverstein et al., 2014, Miner and Diamond, 2016). We found that participants with HCV and co-morbid AUD had increased levels of S100B, relative to those without AUD. Elevated S100B levels in biological fluids (i.e., cerebrospinal fluid, blood, urine, saliva, amniotic fluid) have been associated with a range of neuropathological conditions, such as acute brain injury, neurodegenerative disorders, and psychiatric disorders [reviewed in (Michetti et al., 2012)]. In the CNS, S100B is primarily concentrated in glial cells, although it has also been detected in other cell types. Preclinical studies indicate that S100B protein levels are increased in the cerebrospinal fluid of rats exposed to alcohol, which are accompanied by alterations in astrocyte expression [as measured using glial fibrillary acid protein (GFAP) immunodetection] (Brolese et al., 2014). Extracellular S100B signals through interaction with the Receptor for Advanced Glycation End Product (RAGE) [(Hofmann et al., 1999); reviewed in (Michetti et al., 2012)]. Although not the only receptor for S100B [e.g., see (Riuzzi et al., 2011)], S100B induced activation of RAGE triggers the generation of pro-inflammatory factors and contributes to neuroinflammation (Han et al., 2011, Michetti et al., 2012). Like S100B (Brin et al., 2011), RAGE is increased following a history of alcohol abuse. Upregulation of RAGE is evident in post-mortem frontal cortices from humans with alcohol dependence, relative to controls with moderate alcohol consumption (Vetreno et al., 2013). It is yet to be determined whether increased levels of S100B are the result of leakage from injured cells and/or increased production as a result of pathological conditions.
In clinical studies of AUD and recovery, peripheral S100B levels are influenced by the amount of alcohol consumption and duration of remission. For instance, in a study of 20 adults with a history of alcohol abuse/dependence receiving inpatient treatment for alcohol detoxification, S100B levels were decreased in 10 patients with moderate alcohol-consumption, but increased in 10 patients with high alcohol consumption. The levels of S100B were also positively correlated with AST levels upon admission as well as with alcohol consumption over the last year (Liappas et al., 2006). More recently, in a study of 115 adults with alcohol dependence admitted to a psychiatric hospital for alcohol detoxification, inflammatory factors (e.g., S100B, TNF-α, IL-1β, IL-8, IL-6, MCP-1) were assessed during the first 48 hours of withdrawal and at one, two, four, and six months after. The levels of TNF-α, IL-1β, IL-8, IL-6, and MCP-1 decreased after withdrawal and remained low at six months post-detoxification; however, serum S100B concentrations were significantly increased at one month, and then decreased, regardless of abstinence status, at six months (Girard et al., 2017). Our findings in participants with HCV and co-morbid AUD indicate that inflammatory factors, such as IL-8 and S100B may take longer to recover following reductions in alcohol use. When we compared cytokine and S100B concentrations from adults who obtained abstinence by week 4 to those with ongoing alcohol use, there were no statistically significant differences between the groups (Supplementary Table 2), suggesting that the presence of a chronic viral infection may contribute to perpetuating inflammatory processes.
A few studies have also investigated the effects of HCV infection on S100B levels. In patients with viral hepatitis (HCV and HBV), S100B levels were significantly reduced following two weeks of IFN-α based antiviral therapy, as compared to baseline. Further, after two weeks of IFN-α therapy, S100B concentrations were higher in the individuals with increased symptoms of depression, but the difference was not statistically significant (Cicek et al., 2014). One recent study found decreased S100B mRNA in liver specimens in patients with HCV, relative to controls (Baik et al., 2017), suggesting that the liver is not likely the source of increased circulating S100B levels in individuals with HCV. In vitro, S100B has been shown to facilitate viral neuroinvasion, and similarly, inactivated viral particles (i.e., West Nile virus, a single-stranded RNA virus in the Flaviviridae family of viruses, as is HCV) can induce S100B expression (Kuwar et al., 2015). To our knowledge, our study is the first to examine the role of S100B in HCV and co-morbid AUD.
A number of biomarkers are affected by unhealthy alcohol consumption (e.g., liver enzymes, lipids, and cytokines) and have been evaluated for their sensitivity and specificity as screening and diagnostic indices. For example, IL-8 has been included as a factor (pro-fibrogenic marker) to predict the development of liver fibrosis in HIV-HCV co-infection (Moqueet et al., 2017). Another study found that IL-8 (as well as IL-1β) was positively correlated with alcohol consumption and alcohol-craving scores (Leclercq et al., 2014). We conducted exploratory ROC curve analyses to assess the ability of IL-8, IL-10, and S100B (i.e., factors that were significantly increased in participants with AUD, as compared to those without AUD) to differentiate between levels of alcohol consumption and to identify biomarker cut-off values that could potentially be used to identify unhealthy alcohol use. Participants with higher circulating S100B concentrations (> 12.0 pg/mL) reported consuming more alcohol, as compared to participants with S100B concentrations less than 12.0 pg/mL. Alcohol consumption was not significantly different between participants with “low” and “high” IL-8 concentrations. Given the sensitivity (81%) and specificity (59%) of IL-8 to differentiate between the alcohol groups (Table 3), it may be that IL-8 would be better utilized in a composite measure that includes additional, concurrently elevated inflammatory factors [e.g., (Armah et al., 2013)].
Strengths and Limitations
This longitudinal study was strengthened by the use of stringent inclusionary criteria for the group without AUD (AUD- group), excluding participants with a history of heavy drinking in the past 10+ years (> 7 drinks in a week, or one heavy drinking day consisting of 5 > drinks for men, 4 > for women). A limitation of this study is that the results included only male Veterans, as AUD (a heterogeneous disorder) has different effects on inflammatory processes in women, including liver enzyme expression, fatty acid levels, and other factors (Wilhelm et al., 2017, Vatsalya et al., 2016). However, there are not thought to be sex differences in the expression of S100B, in healthy control participants or in patients under the influence of alcohol (Brin et al., 2011). An additional limitation is that this study did not include comparative groups of participants (i.e., with AUD and no HCV or without either illness).
Conclusions
In summary, these cross-sectional data show that HCV and co-morbid AUD is associated with altered expression of plasma immune factors and proteins suggestive of BBB damage. Higher levels of S100B may be indicative of CNS injury in individuals with HCV and AUD. Our initial findings suggest a predictive value of S100B and its inflammatory signaling effects in the neuropathological consequences of co-morbid HCV and AUD; however, follow-up mechanistic studies are necessary to corroborate these observations. The recent introduction of direct-acting antiviral therapies has revolutionized the treatment of HCV, with viral clearance rates of 90% and higher. Although a history of alcohol abuse may not significantly impact peripheral viral clearance, as indicated by SVR (Belperio et al., 2017, Tsui et al., 2016), it is unknown whether heavy alcohol consumption contributes to HCV-related brain pathology following direct-acting antiviral therapy. The potential for pathology to persist following antiviral therapy is suggested by the inflammatory profile (Radkowski et al., 2014) and psychiatric and neurological symptoms reported in patients attaining an SVR following IFN-α based therapies (Huckans et al., 2015, Kuhn et al., 2017); however, research is needed to assess the impact of HCV and co-morbid AUD on CNS function following direct-acting antiviral (Mathew et al., 2016).
Supplementary Material
Acknowledgments
The authors would like to thank the study participants, staff at the recruitment sites (particularly, Ms. Alissa Phelps for study coordination), and the VA Pathology and Laboratory Services.
Sources of support: This work was supported by the U.S. Department of Veterans Affairs Biomedical Laboratory Research and Development Merit Review grant (grant number: I01 BX002061) (JML), VA Merit project # NURA-014-09S (PH) [ClinicalTrials.gov Info: # NCT01008280 Baclofen to Reduce Alcohol Use in Veterans With HCV (BRAC) https://clinicaltrials.gov/ct2/show/NCT01008280?term=NCT01008280&rank=1] and the National Institute on Drug Abuse (grant number: DA P50DA018165) (JML). The work was conducted using facilities at Veterans Affairs Health Care Systems (Portland, OR; Long Beach, CA; San Diego, CA; and Minneapolis, MN) and Oregon Health & Science University, Portland, OR, USA.
Role of Funding Source
This work was supported by the U.S. Department of Veterans Affairs Biomedical Laboratory Research and Development Merit Review grant # I01 BX002061 (JML) and Clinical Sciences Research and Development Merit Review project # NURA-014-09S (PH). The work was also supported by the National Institute on Drug Abuse grant #: DA P50DA018165 (JML). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
DR. JENNIFER LOFTIS (Orcid ID : 0000-0003-1773-9365)
Conflict of Interest
No conflicts declared.
References
- ACHUR RN, FREEMAN WM, VRANA KE. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J Neuroimmune Pharmacol. 2010;5:83–91. doi: 10.1007/s11481-009-9185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALFONSO-LOECHES S, URENA-PERALTA J, MORILLO-BARGUES MJ, GOMEZ-PINEDO U, GUERRI C. Ethanol-Induced TLR4/NLRP3 Neuroinflammatory Response in Microglial Cells Promotes Leukocyte Infiltration Across the BBB. Neurochem Res. 2016;41:193–209. doi: 10.1007/s11064-015-1760-5. [DOI] [PubMed] [Google Scholar]
- ARMAH KA, QUINN EK, CHENG DM, TRACY RP, BAKER JV, SAMET JH, FREIBERG MS. Human immunodeficiency virus, hepatitis C, and inflammatory biomarkers in individuals with alcohol problems: a cross-sectional study. BMC Infect Dis. 2013;13:399. doi: 10.1186/1471-2334-13-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAIK SJ, KIM TH, YOO K, MOON IH, CHOI JY, CHUNG KW, SONG DE. Decreased S100B expression in chronic liver diseases. Korean J Intern Med. 2017;32:269–276. doi: 10.3904/kjim.2015.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECK AT, STEER RA, BROWN GK. Beck Depression Inventory–2nd edition (BDI-II) San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- BELPERIO PS, CHARTIER M, ROSS DB, ALAIGH P, SHULKIN D. Curing Hepatitis C Virus Infection: Best Practices From the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167:499–504. doi: 10.7326/M17-1073. [DOI] [PubMed] [Google Scholar]
- BHATTACHARYYA S, SEN P, WALLET M, LONG B, BALDWIN AS, JR, TISCH R. Immunoregulation of dendritic cells by IL-10 is mediated through suppression of the PI3K/Akt pathway and of IkappaB kinase activity. Blood. 2004;104:1100–9. doi: 10.1182/blood-2003-12-4302. [DOI] [PubMed] [Google Scholar]
- BRIN T, BORUCKI K, AMBROSCH A. The influence of experimental alcohol load and alcohol intoxication on S100B concentrations. Shock. 2011;36:356–60. doi: 10.1097/SHK.0b013e31822bd07d. [DOI] [PubMed] [Google Scholar]
- BROLESE G, LUNARDI P, BROETTO N, ENGELKE DS, LIRIO F, BATASSINI C, TRAMONTINA AC, GONCALVES CA. Moderate prenatal alcohol exposure alters behavior and neuroglial parameters in adolescent rats. Behav Brain Res. 2014;269:175–84. doi: 10.1016/j.bbr.2014.04.023. [DOI] [PubMed] [Google Scholar]
- BUSH K, KIVLAHAN DR, MCDONELL MB, FIHN SD, BRADLEY KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–95. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- BUTT AA, KHAN UA, MCGINNIS KA, SKANDERSON M, KENT KWOH C. Co-morbid medical and psychiatric illness and substance abuse in HCV-infected and uninfected veterans. J Viral Hepat. 2007;14:890–6. doi: 10.1111/j.1365-2893.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- CARTA MG, ANGST J, MORO MF, MURA G, HARDOY MC, BALESTRIERI C, CHESSA L, SERRA G, LAI ME, FARCI P. Association of chronic hepatitis C with recurrent brief depression. J Affect Disord. 2012;141:361–6. doi: 10.1016/j.jad.2012.03.020. [DOI] [PubMed] [Google Scholar]
- CICEK IE, CICEK E, KAYHAN F, UGUZ F, ERAYMAN I, KURBAN S, YERLIKAYA FH, KAYA N. The roles of BDNF, S100B, and oxidative stress in interferon-induced depression and the effect of antidepressant treatment in patients with chronic viral hepatitis: a prospective study. J Psychosom Res. 2014;76:227–32. doi: 10.1016/j.jpsychores.2014.01.003. [DOI] [PubMed] [Google Scholar]
- CONIGRAVE KM, DEGENHARDT LJ, WHITFIELD JB, SAUNDERS JB, HELANDER A, TABAKOFF B, GROUP, W. I. S. CDT, GGT, and AST as markers of alcohol use: the WHO/ISBRA collaborative project. Alcohol Clin Exp Res. 2002;26:332–9. [PubMed] [Google Scholar]
- COUPER KN, BLOUNT DG, RILEY EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–7. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- FIRST MB. Research Version of the Structured Clinical Interview for DSM-IV-TR AXIS I Disorders: (SCID-1) New York: New York State Psychiatric Institute; 2005. [Google Scholar]
- FITZ JG. Hepatology after Hepatitis C. Dig Dis. 2016;34:603–6. doi: 10.1159/000445276. [DOI] [PubMed] [Google Scholar]
- FLETCHER NF, WILSON GK, MURRAY J, HU K, LEWIS A, REYNOLDS GM, STAMATAKI Z, MEREDITH LW, ROWE IA, LUO G, LOPEZ-RAMIREZ MA, BAUMERT TF, WEKSLER B, COURAUD PO, KIM KS, ROMERO IA, JOPLING C, MORGELLO S, BALFE P, MCKEATING JA. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142:634–643 e6. doi: 10.1053/j.gastro.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORTON DM, ALLSOP JM, MAIN J, FOSTER GR, THOMAS HC, TAYLOR-ROBINSON SD. Evidence for a cerebral effect of the hepatitis C virus. Lancet. 2001;358:38–9. doi: 10.1016/S0140-6736(00)05270-3. [DOI] [PubMed] [Google Scholar]
- FORTON DM, KARAYIANNIS P, MAHMUD N, TAYLOR-ROBINSON SD, THOMAS HC. Identification of unique hepatitis C virus quasispecies in the central nervous system and comparative analysis of internal translational efficiency of brain, liver, and serum variants. J Virol. 2004;78:5170–83. doi: 10.1128/JVI.78.10.5170-5183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORTON DM, THOMAS HC, MURPHY CA, ALLSOP JM, FOSTER GR, MAIN J, WESNES KA, TAYLOR-ROBINSON SD. Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatology. 2002;35:433–9. doi: 10.1053/jhep.2002.30688. [DOI] [PubMed] [Google Scholar]
- FRENCH SW, MENDOZA AS, AFIFIYAN N, TILLMAN B, VITOCRUZ E, FRENCH BA. The role of the IL-8 signaling pathway in the infiltration of granulocytes into the livers of patients with alcoholic hepatitis. Exp Mol Pathol. 2017;103:137–140. doi: 10.1016/j.yexmp.2017.08.005. [DOI] [PubMed] [Google Scholar]
- FUEHRLEIN BS, MOTA N, ARIAS AJ, TREVISAN LA, KACHADOURIAN LK, KRYSTAL JH, SOUTHWICK SM, PIETRZAK RH. The burden of alcohol use disorders in US military veterans: results from the National Health and Resilience in Veterans Study. Addiction. 2016;111:1786–94. doi: 10.1111/add.13423. [DOI] [PubMed] [Google Scholar]
- GANESAN M, POLUEKTOVA LY, TUMA DJ, KHARBANDA KK, OSNA NA. Acetaldehyde Disrupts Interferon Alpha Signaling in Hepatitis C Virus-Infected Liver Cells by Up-Regulating USP18. Alcohol Clin Exp Res. 2016;40:2329–2338. doi: 10.1111/acer.13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRARD M, MALAUZAT D, NUBUKPO P. Serum inflammatory molecules and markers of neuronal damage in alcohol-dependent subjects after withdrawal. World J Biol Psychiatry. 2017:1–15. doi: 10.1080/15622975.2017.1349338. [DOI] [PubMed] [Google Scholar]
- GONZALEZ-QUINTELA A, VIDAL C, LOJO S, PEREZ LF, OTERO-ANTON E, GUDE F, BARRIO E. Serum cytokines and increased total serum IgE in alcoholics. Ann Allergy Asthma Immunol. 1999;83:61–7. doi: 10.1016/S1081-1206(10)63514-4. [DOI] [PubMed] [Google Scholar]
- GONZALEZ-REIMERS E, SANTOLARIA-FERNANDEZ F, MARTIN-GONZALEZ MC, FERNANDEZ-RODRIGUEZ CM, QUINTERO-PLATT G. Alcoholism: a systemic proinflammatory condition. World J Gastroenterol. 2014;20:14660–71. doi: 10.3748/wjg.v20.i40.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOUGH G, HEATHERS L, PUCKETT D, WESTERHOLD C, REN X, YU Z, CRABB DW, LIANGPUNSAKUL S. The Utility of Commonly Used Laboratory Tests to Screen for Excessive Alcohol Use in Clinical Practice. Alcohol Clin Exp Res. 2015;39:1493–500. doi: 10.1111/acer.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAN SH, KIM YH, MOOK-JUNG I. RAGE: the beneficial and deleterious effects by diverse mechanisms of actions. Mol Cells. 2011;31:91–7. doi: 10.1007/s10059-011-0030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUSER P, FULLER B, HO SB, THURAS P, KERN S, DIEPERINK E. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112:1173–1183. doi: 10.1111/add.13787. [DOI] [PubMed] [Google Scholar]
- HE J, CREWS FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–58. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEBERLEIN A, KASER M, LICHTINGHAGEN R, RHEIN M, LENZ B, KORNHUBER J, BLEICH S, HILLEMACHER T. TNF-alpha and IL-6 serum levels: neurobiological markers of alcohol consumption in alcohol-dependent patients? Alcohol. 2014;48:671–6. doi: 10.1016/j.alcohol.2014.08.003. [DOI] [PubMed] [Google Scholar]
- HEEREN M, WEISSENBORN K, ARVANITIS D, BOKEMEYER M, GOLDBECKER A, TOUNTOPOULOU A, PESCHEL T, GROSSKREUTZ J, HECKER H, BUCHERT R, BERDING G. Cerebral glucose utilisation in hepatitis C virus infection-associated encephalopathy. J Cereb Blood Flow Metab. 2011;31:2199–208. doi: 10.1038/jcbfm.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFMANN MA, DRURY S, FU C, QU W, TAGUCHI A, LU Y, AVILA C, KAMBHAM N, BIERHAUS A, NAWROTH P, NEURATH MF, SLATTERY T, BEACH D, MCCLARY J, NAGASHIMA M, MORSER J, STERN D, SCHMIDT AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- HUCKANS M, FULLER B, WHEATON V, JAEHNERT S, ELLIS C, KOLESSAR M, KRIZ D, ANDERSON JR, BERGGREN K, OLAVARRIA H, SASAKI AW, CHANG M, FLORA KD, LOFTIS JM. A longitudinal study evaluating the effects of interferon-alpha therapy on cognitive and psychiatric function in adults with chronic hepatitis C. J Psychosom Res. 2015;78:184–92. doi: 10.1016/j.jpsychores.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUCKANS M, FULLER BE, OLAVARRIA H, SASAKI AW, CHANG M, FLORA KD, KOLESSAR M, KRIZ D, ANDERSON JR, VANDENBARK AA, LOFTIS JM. Multi-analyte profile analysis of plasma immune proteins: altered expression of peripheral immune factors is associated with neuropsychiatric symptom severity in adults with and without chronic hepatitis C virus infection. Brain Behav. 2014;4:123–42. doi: 10.1002/brb3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUCKANS M, SEELYE A, PARCEL T, MULL L, WOODHOUSE J, BJORNSON D, FULLER BE, LOFTIS JM, MORASCO BJ, SASAKI AW, STORZBACH D, HAUSER P. The cognitive effects of hepatitis C in the presence and absence of a history of substance use disorder. J Int Neuropsychol Soc. 2009;15:69–82. doi: 10.1017/S1355617708090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON BA, DICLEMENTE CC, AIT-DAOUD N, STOKS SM. Brief Behavioral Compliance Enhancement Treatment (BBCET) manual. In: Johnson BA, Ruiz P, Galanter M, editors. Handbook of clinical alcoholism treatment. Lippincott Williams & Wilkins; Baltimore, MD: 2003. pp. 282–301. [Google Scholar]
- KOHLI A, SHAFFER A, SHERMAN A, KOTTILIL S. Treatment of hepatitis C: a systematic review. JAMA. 2014;312:631–40. doi: 10.1001/jama.2014.7085. [DOI] [PubMed] [Google Scholar]
- KRAMER L, BAUER E, FUNK G, HOFER H, JESSNER W, STEINDL-MUNDA P, WRBA F, MADL C, GANGL A, FERENCI P. Subclinical impairment of brain function in chronic hepatitis C infection. J Hepatol. 2002;37:349–54. doi: 10.1016/s0168-8278(02)00172-1. [DOI] [PubMed] [Google Scholar]
- KUHN T, SAYEGH P, JONES JD, SMITH J, SARMA MK, RAGIN A, SINGER EJ, ALBERT THOMAS M, THAMES AD, CASTELLON SA, HINKIN CH. Improvements in brain and behavior following eradication of hepatitis C. J Neurovirol. 2017;23:593–602. doi: 10.1007/s13365-017-0533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUWAR RB, STOKIC DS, LEIS AA, BAI F, PAUL AM, FRATKIN JD, VIG PJ. Does astroglial protein S100B contribute to West Nile neuro-invasive syndrome? J Neurol Sci. 2015;358:243–52. doi: 10.1016/j.jns.2015.09.003. [DOI] [PubMed] [Google Scholar]
- LECLERCQ S, DE SAEGER C, DELZENNE N, DE TIMARY P, STARKEL P. Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biol Psychiatry. 2014;76:725–33. doi: 10.1016/j.biopsych.2014.02.003. [DOI] [PubMed] [Google Scholar]
- LEITE MC, GALLAND F, BROLESE G, GUERRA MC, BORTOLOTTO JW, FREITAS R, ALMEIDA LM, GOTTFRIED C, GONCALVES CA. A simple, sensitive and widely applicable ELISA for S100B: Methodological features of the measurement of this glial protein. J Neurosci Methods. 2008;169:93–9. doi: 10.1016/j.jneumeth.2007.11.021. [DOI] [PubMed] [Google Scholar]
- LETENDRE S, PAULINO AD, ROCKENSTEIN E, ADAME A, CREWS L, CHERNER M, HEATON R, ELLIS R, EVERALL IP, GRANT I, MASLIAH E, GROUP, H. I. V. N. R. C. Pathogenesis of hepatitis C virus coinfection in the brains of patients infected with HIV. J Infect Dis. 2007;196:361–70. doi: 10.1086/519285. [DOI] [PubMed] [Google Scholar]
- LIAPPAS I, TZAVELLAS EO, KARIYANNIS C, PIPERI C, SCHULPIS C, PAPASSOTIRIOU I, SOLDATOS CR. Effect of alcohol detoxification on serum S-100B levels of alcohol-dependent individuals. In Vivo. 2006;20:675–80. [PubMed] [Google Scholar]
- LIU Z, ZHAO F, HE JJ. Hepatitis C virus (HCV) interaction with astrocytes: nonproductive infection and induction of IL-18. J Neurovirol. 2014;20:278–93. doi: 10.1007/s13365-014-0245-7. [DOI] [PubMed] [Google Scholar]
- LOFTIS JM, HUCKANS M, RUIMY S, HINRICHS DJ, HAUSER P. Depressive symptoms in patients with chronic hepatitis C are correlated with elevated plasma levels of interleukin-1beta and tumor necrosis factor-alpha. Neurosci Lett. 2008;430:264–8. doi: 10.1016/j.neulet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOFTIS JM, PATTERSON AL, WILHELM CJ, MCNETT H, MORASCO BJ, HUCKANS M, MORGAN T, SAPERSTEIN S, ASGHAR A, HAUSER P. Vulnerability to somatic symptoms of depression during interferon-alpha therapy for hepatitis C: a 16-week prospective study. J Psychosom Res. 2013;74:57–63. doi: 10.1016/j.jpsychores.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAES M, LIN A, BOSMANS E, VANDOOLAEGHE E, BONACCORSO S, KENIS G, DE JONGH R, VERKERK R, SONG C, SCHARPE S, NEELS H. Serotonin-immune interactions in detoxified chronic alcoholic patients without apparent liver disease: activation of the inflammatory response system and lower plasma total tryptophan. Psychiatry Res. 1998;78:151–61. doi: 10.1016/s0165-1781(98)00010-9. [DOI] [PubMed] [Google Scholar]
- MATHEW S, FAHEEM M, IBRAHIM SM, IQBAL W, RAUFF B, FATIMA K, QADRI I. Hepatitis C virus and neurological damage. World J Hepatol. 2016;8:545–56. doi: 10.4254/wjh.v8.i12.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEDVEDEV R, HILDT E, PLOEN D. Look who’s talking-the crosstalk between oxidative stress and autophagy supports exosomal-dependent release of HCV particles. Cell Biol Toxicol. 2017;33:211–231. doi: 10.1007/s10565-016-9376-3. [DOI] [PubMed] [Google Scholar]
- MICHETTI F, CORVINO V, GELOSO MC, LATTANZI W, BERNARDINI C, SERPERO L, GAZZOLO D. The S100B protein in biological fluids: more than a lifelong biomarker of brain distress. J Neurochem. 2012;120:644–59. doi: 10.1111/j.1471-4159.2011.07612.x. [DOI] [PubMed] [Google Scholar]
- MINER JJ, DIAMOND MS. Mechanisms of restriction of viral neuroinvasion at the blood-brain barrier. Curr Opin Immunol. 2016;38:18–23. doi: 10.1016/j.coi.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINGOMATAJ EC, BAKIRI AH. Regulator Versus Effector Paradigm: Interleukin-10 as Indicator of the Switching Response. Clin Rev Allergy Immunol. 2016;50:97–113. doi: 10.1007/s12016-015-8514-7. [DOI] [PubMed] [Google Scholar]
- MONACO S, MARIOTTO S, FERRARI S, CALABRESE M, ZANUSSO G, GAJOFATTO A, SANSONNO D, DAMMACCO F. Hepatitis C virus-associated neurocognitive and neuropsychiatric disorders: Advances in 2015. World J Gastroenterol. 2015;21:11974–83. doi: 10.3748/wjg.v21.i42.11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTESINOS J, ALFONSO-LOECHES S, GUERRI C. Impact of the Innate Immune Response in the Actions of Ethanol on the Central Nervous System. Alcohol Clin Exp Res. 2016;40:2260–2270. doi: 10.1111/acer.13208. [DOI] [PubMed] [Google Scholar]
- MOQUEET N, KANAGARATHAM C, GILL MJ, HULL M, WALMSLEY S, RADZIOCH D, SAEED S, PLATT RW, KLEIN MB, CANADIAN CO-INFECTION COHORT, S A prognostic model for development of significant liver fibrosis in HIV-hepatitis C co-infection. PLoS One. 2017;12:e0176282. doi: 10.1371/journal.pone.0176282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELLIGAN JA, LOFTIS JM, MATTHEWS AM, ZUCKER BL, LINKE AM, HAUSER P. Depression comorbidity and antidepressant use in veterans with chronic hepatitis C: results from a retrospective chart review. J Clin Psychiatry. 2008;69:810–6. doi: 10.4088/jcp.v69n0514. [DOI] [PubMed] [Google Scholar]
- NENNIG SE, SCHANK JR. The Role of NFkB in Drug Addiction: Beyond Inflammation. Alcohol Alcohol. 2017;52:172–179. doi: 10.1093/alcalc/agw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA. What’s “Low-Risk” Drinking? 2007 [Online]. Available: http://rethinkingdrinking.niaaa.nih.gov/IsYourDrinkingPatternRisky/WhatsLowRiskDrinking.asp [Accessed October 6 2017]
- NORRIS FH, HAMBLEN JL. Standardized self-report measures of civilian trauma and PTSD. In: WILSON JP, KEANE TM, editors. Assessing Psychological Trauma and PTSD. 2nd. New York: Guilford Press; 2004. [Google Scholar]
- NOVO-VELEIRO I, CALLE CDE L, DOMINGUEZ-QUIBEN S, PASTOR I, MARCOS M, LASO FJ. Prevalence of hepatitis C virus infection in alcoholic patients: cohort study and systematic review. Alcohol Alcohol. 2013;48:564–9. doi: 10.1093/alcalc/agt044. [DOI] [PubMed] [Google Scholar]
- PAWLOWSKI T, RADKOWSKI M, MALYSZCZAK K, INGLOT M, ZALEWSKA M, JABLONSKA J, LASKUS T. Depression and neuroticism in patients with chronic hepatitis C: correlation with peripheral blood mononuclear cells activation. J Clin Virol. 2014;60:105–11. doi: 10.1016/j.jcv.2014.03.004. [DOI] [PubMed] [Google Scholar]
- PETRAKIS IL, GONZALEZ G, ROSENHECK R, KRYSTAL JH. Comorbidity of Alcoholism and Psychiatric Disorders: An Overview [Online] National Institute on Alcohol Abuse and Alcoholism; 2002. Available: https://pubs.niaaa.nih.gov/publications/arh26-2/81-89.htm [Accessed September 29 2017] [Google Scholar]
- RADKOWSKI M, OPOKA-KEGLER J, CORTES KC, BUKOWSKA-OSKO I, PERLEJEWSKI K, PAWELCZYK A, LASKUS T. Evidence for immune activation in patients with residual hepatitis C virus RNA long after successful treatment with IFN and ribavirin. J Gen Virol. 2014;95:2004–9. doi: 10.1099/vir.0.064709-0. [DOI] [PubMed] [Google Scholar]
- RADKOWSKI M, WILKINSON J, NOWICKI M, ADAIR D, VARGAS H, INGUI C, RAKELA J, LASKUS T. Search for hepatitis C virus negative-strand RNA sequences and analysis of viral sequences in the central nervous system: evidence of replication. J Virol. 2002;76:600–8. doi: 10.1128/JVI.76.2.600-608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAHMAN S, CONNOLLY JE, MANUELM SL, CHEHIMI J, MONTANER LJ, JAIN P. Unique Cytokine/Chemokine Signatures for HIV-1 and HCV Mono-infection versus Co-infection as Determined by the Luminex® Analyses. J Clin Cell Immunol. 2011;2 doi: 10.4172/2155-9899.1000104. pii: 1000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIUZZI F, SORCI G, DONATO R. S100B protein regulates myoblast proliferation and differentiation by activating FGFR1 in a bFGF-dependent manner. J Cell Sci. 2011;124:2389–400. doi: 10.1242/jcs.084491. [DOI] [PubMed] [Google Scholar]
- RUBIO-ARAIZ A, PORCU F, PEREZ-HERNANDEZ M, GARCIA-GUTIERREZ MS, ARACIL-FERNANDEZ MA, GUTIERREZ-LOPEZ MD, GUERRI C, MANZANARES J, O’SHEA E, COLADO MI. Disruption of blood-brain barrier integrity in postmortem alcoholic brain: preclinical evidence of TLR4 involvement from a binge-like drinking model. Addict Biol. 2017;22:1103–1116. doi: 10.1111/adb.12376. [DOI] [PubMed] [Google Scholar]
- SILVERSTEIN PS, KUMAR S, KUMAR A. HIV-1, HCV and alcohol in the CNS: potential interactions and effects on neuroinflammation. Curr HIV Res. 2014;12:282–92. doi: 10.2174/1570162x12666140721122956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOBELL LC, BROWN J, LEO GI, SOBELL MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 1996;42:49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- SOBELL LC, SOBELL MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: LITTEN RZ, ALLEN J, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Totowa, NJ: Humana Press; 1992. [Google Scholar]
- SONG C, LIN A, BONACCORSO S, HEIDE C, VERKERK R, KENIS G, BOSMANS E, SCHARPE S, WHELAN A, COSYNS P, DE JONGH R, MAES M. The inflammatory response system and the availability of plasma tryptophan in patients with primary sleep disorders and major depression. J Affect Disord. 1998;49:211–9. doi: 10.1016/s0165-0327(98)00025-1. [DOI] [PubMed] [Google Scholar]
- SZABO G, WANDS JR, EKEN A, OSNA NA, WEINMAN SA, MACHIDA K, JOE WANG H. Alcohol and hepatitis C virus–interactions in immune dysfunctions and liver damage. Alcohol Clin Exp Res. 2010;34:1675–86. doi: 10.1111/j.1530-0277.2010.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THAMES AD, CASTELLON SA, SINGER EJ, NAGARAJAN R, SARMA MK, SMITH J, THALER NS, TRUONG JH, SCHONFELD D, THOMAS MA, HINKIN CH. Neuroimaging abnormalities, neurocognitive function, and fatigue in patients with hepatitis C. Neurol Neuroimmunol Neuroinflamm. 2015;2:e59. doi: 10.1212/NXI.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUI JI, WILLIAMS EC, GREEN PK, BERRY K, SU F, IOANNOU GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend. 2016;169:101–109. doi: 10.1016/j.drugalcdep.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARGAS HE, LASKUS T, RADKOWSKI M, WILKINSON J, BALAN V, DOUGLAS DD, HARRISON ME, MULLIGAN DC, OLDEN K, ADAIR D, RAKELA J. Detection of hepatitis C virus sequences in brain tissue obtained in recurrent hepatitis C after liver transplantation. Liver Transpl. 2002;8:1014–9. doi: 10.1053/jlts.2002.36393. [DOI] [PubMed] [Google Scholar]
- VATSALYA V, SONG M, SCHWANDT ML, CAVE MC, BARVE SS, GEORGE DT, RAMCHANDANI VA, MCCLAIN CJ. Effects of Sex, Drinking History, and Omega-3 and Omega-6 Fatty Acids Dysregulation on the Onset of Liver Injury in Very Heavy Drinking Alcohol-Dependent Patients. Alcohol Clin Exp Res. 2016;40:2085–2093. doi: 10.1111/acer.13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VETRENO RP, QIN L, CREWS FT. Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Neurobiol Dis. 2013;59:52–62. doi: 10.1016/j.nbd.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIVITHANAPORN P, MAINGAT F, LIN LT, NA H, RICHARDSON CD, AGRAWAL B, COHEN EA, JHAMANDAS JH, POWER C. Hepatitis C virus core protein induces neuroimmune activation and potentiates Human Immunodeficiency Virus-1 neurotoxicity. PLoS One. 2010;5:e12856. doi: 10.1371/journal.pone.0012856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VLAHOPOULOS S, BOLDOGH I, CASOLA A, BRASIER AR. Nuclear factor-kappaB-dependent induction of interleukin-8 gene expression by tumor necrosis factor alpha: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood. 1999;94:1878–89. [PubMed] [Google Scholar]
- WHITE IR, ALTMANN DR, NANCHAHAL K. Alcohol consumption and mortality: modelling risks for men and women at different ages. BMJ. 2002;325:191. doi: 10.1136/bmj.325.7357.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILHELM CJ, FULLER BE, HUCKANS M, LOFTIS JM. Peripheral immune factors are elevated in women with current or recent alcohol dependence and associated with altered mood and memory. Drug Alcohol Depend. 2017;176:71–78. doi: 10.1016/j.drugalcdep.2017.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON J, RADKOWSKI M, ESCHBACHER JM, LASKUS T. Activation of brain macrophages/microglia cells in hepatitis C infection. Gut. 2010;59:1394–400. doi: 10.1136/gut.2009.199356. [DOI] [PubMed] [Google Scholar]
- WILKINSON J, RADKOWSKI M, LASKUS T. Hepatitis C virus neuroinvasion: identification of infected cells. J Virol. 2009;83:1312–9. doi: 10.1128/JVI.01890-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


