Abstract
Objective
Deterioration in ventricular function is often observed in patients treated with anthracyclines for cancer. There is a paucity of evidence on interventions that might provide cardio-protection. We investigated whether prophylactic use of carvedilol can prevent doxorubicin-induced cardiotoxicity and whether any observed effect is dose related.
Methods
A prospective, randomized, double-blind study in patients treated with doxorubicin, comparing placebo (n = 38) with different doses of carvedilol [6.25 mg/day (n = 41), 12.5 mg/day (n = 38) or 25 mg/day (n = 37)]. The primary endpoint was the measured change in left ventricular ejection fraction (LVEF) from baseline to 6 months.
Results
LVEF decreased from 62 ± 5% at baseline to 58 ± 7% at 6-months (p = 0.002) in patients assigned to placebo but no statistically significant changes were observed in any of the 3 carvedilol groups. At 6 months, only one of 116 patients (1%) assigned to carvedilol had an LVEF < 50% compared to four of the 38 assigned to placebo (11%), (p = 0.013). No significant differences were noted between carvedilol and placebo in terms of the development of diastolic dysfunction, clinically overt heart failure or death.
Conclusions
Carvedilol might prevent deterioration in LVEF in cancer patients treated with doxorubicin. This effect may not be dose related within the studied range.
Keywords: Doxorubicin, Carvedilol, Ejection fraction, Cardiomyopathy
1. Introduction
Anthracyclines are used to treat a wide spectrum of human malignancies but their use is associated with an increased risk of developing ventricular dysfunction that may be irreversible. Post-chemotherapy left ventricular ejection fraction (LVEF) and cumulative anthracycline dose are independent correlates of its occurrence. Early detection of cardiotoxicity and its prompt treatment may be crucial for the preservation of cardiac function.1 Although the risk of cardiac dysfunction is proportional to the cumulative anthracycline exposure,2 a substantial number of patients still develop severe cardiotoxicity at doses well below 550 mg/m2.3 In a study on the early detection and prediction of cardiotoxicity, 27.6% of patients developed chemotherapy-related cardiotoxicity.4 Another study found that doxorubicin in doses of less than 300 mg/m2 could induce cardiotoxicity.5 Strategies that might prevent chemotherapy-induced cardiomyopathy are receiving increased attention from oncologists and cardiologists. However, there is a dearth of randomized controlled trials reported to date. These include carvedilol which blocks β1, β2 and α1-adrenergic receptors with antioxidant properties,6 metoprolol, which only blocks β1 receptors, perindopril (an angiotensin converting enzyme inhibitor)7 and dexrazoxane, a chelating agent that is a derivative of ethylene diamine tetra acetic acid, which reduces the number of metal ions that can complex with anthracyclines and therefore the formation of superoxide free-radicals.8 Two randomized trials, have reported that once-daily, low-dose carvedilol reduces the development of ventricular dysfunction in patients treated for cancer with doxorubicin or epirubicin.9,10
Accordingly, we conducted a prospective, randomized, double-blind, placebo-controlled dose-ranging study to assess the prophylactic use of carvedilol for preventing left ventricular dysfunction in cancer patients treated with doxorubicin.
2. Patients and methods
This double-blind, randomized, placebo-controlled study was conducted at the King Faisal Cardiac Centre in King Abdulaziz Medical City-Jeddah, Saudi Arabia. The study was approved by a local institutional review board. Patients were randomly assigned to placebo or to one of three doses of carvedilol: 6.25 mg OD, 12.5 mg OD and 25 mg OD. Randomization was performed by an independent statistician using computer generated random number numbers and allocation concealment was achieved with opaque sealed envelopes. Cancer patients aged >16 years who were treated with doxorubicin and met the inclusion and exclusion criterion were recruited for the study and were followed up for six months. We conducted the study to observe the early anthracycline cardiac toxicity (like all other similar trials), but late anthracycline toxicity needs many years of follow-up, which could be done, but with different protocol. Written informed consent was obtained from each participant.
Exclusion Criteria: Patients with left ventricular ejection fraction (LVEF) <50% before enrolment to the study, known cardiomyopathy or on therapy for heart failure, bronchial asthma that required regular daily beta-2 stimulant therapy, severe peripheral arterial disease, second or third degree heart block, severe valvular heart disease, earlier therapy with anthracycline derivatives, coronary artery disease, thyroid function disorder and patients who were on beta-blockers or angiotensin converting-enzyme inhibitors were excluded.
Data were collected including a history, physical examinations, electrocardiograms and echocardiograms at baseline and after 2, 4 and 6 months after initiation of doxorubicin. Mainly two-dimensional (2D) echocardiography was used and only in few cases, three-dimensional (3D) echo was done because 3D echo was not routinely available at our center. LVEF, and left ventricular diastolic (LVDd), and systolic diameters (LVSd) were measured from 2D echocardiograms by two consultant cardiologists, independently. An LVEF > 50% was considered normal. Doppler ultrasound measures of LV diastolic dysfunction included the ratio of E (early mitral inflow velocity) to A (late mitral inflow velocity), mitral flow deceleration time (DT) and lateral E` on tissue Doppler imaging. Diastolic dysfunction was graded using the American Society of Echocardiography guidelines.11
3. Statistical analysis
The sample size calculation was based on the incidence of doxorubicin-induced subclinical cardiomyopathy at 6 months of 27.6% in the control and a 4% in the carvedilol group patients, reported in previous studies.4,9 The sample size was carried out based on alpha value, statistical power, and effect size. The effect size to detect reduction in the prevalence of doxorubicin-induced subclinical cardiomyopathy was estimated from previous studies to be 23.6%. Using a 2-sided α of 0.05, a sample size of 37 patients per group in the study would provide 80% power to detect a 23.6% difference in the prevalence of cardiomyopathy between control and carvedilol groups. Statistical analyses were done by intention-to-treat. Continuous variables were reported as mean ± standard deviations (SD) and categorical variables as frequencies and percentages. Chi-square or Fisher’s exact tests were used to compare categorical variables. Baseline measurements of the four treatment groups were analyzed using one-way analyses of variance (ANOVA) and paired Student’s t-test was used to compare baseline and last visit (month 6) values for each of the treatment groups with p < 0.05 considered statistically significant. The primary analyses of interest were the differences in the change in LVEF from baseline to six months for patients assigned to placebo compared to carvedilol, regardless of dose and then analysis for a carvedilol dose-response. Missing data were imputed using the last observation carried forward (LOCF) method.
The reproducibility of LVEF measurements was tested using intra-class correlation coefficients (ICC). A total of 20 subjects were randomly selected. Two independent observers were involved in reading the echocardiography images. To determine intra-observer reproducibility, the first observer performed two readings at two different time points. To determine inter-observer reproducibility, a second observer who was blinded to the results of the first observer, provided independent readings on the same subjects.
4. Results
4.1. Baseline characteristics
One hundred and fifty-four patients were randomized to 4 groups (38 to placebo, 41 to carvedilol 6.25 mg OD, 38 to carvedilol 12.5 mg OD and 37 to carvedilol 25 mg OD); their baseline characteristics are summarized in Table 1. Patient characteristics, cardiac function, cancer type and cumulative doxorubicin dose was similar amongst treatment groups.
Table 1.
Baseline Clinical Characteristics of Patients.
| Carvedilol |
p-value* | ||||
|---|---|---|---|---|---|
| Placebo (n = 38) | 6.25 mg (n = 41) | 12.5 mg (n = 38) | 25 mg (n = 37) | ||
| Age (yrs) | 40.4 ± 14.0 | 46.1 ± 13.0 | 41.3 ± 18.2 | 42.0 ± 15.0 | 0.345 |
| Women (%) | 76 | 80 | 58 | 77 | 0.119 |
| BMI (kg/m2) | 27.4 ± 6.5 | 29.1 ± 6.3 | 27.1 ± 6.4 | 28.3 ± 7.2 | 0.511 |
| Baseline EF (%) | 62.0 ± 4.6 | 61.4 ± 3.9 | 60.0 ± 4.2 | 60.5 ± 4.2 | 0.177 |
| LVDd (mm) | 45.3 ± 5.3 | 46.0 ± 5.1 | 44.8 ± 4.3 | 44.6 ± 6.3 | 0.634 |
| LVSd (mm) | 28.0 ± 4.4 | 29.3 ± 4.4 | 29.2 ± 4.2 | 30.2 ± 5.7 | 0.243 |
| E/A (%) | 1.2 ± 0.4 | 1.2 ± 0.5 | 1.3 ± 0.5 | 1.2 ± 0.3 | 0.836 |
| E' | 11.3 ± 3.0 | 11.9 ± 3.2 | 13.2 ± 5.1 | 11.8 ± 3.4 | 0.150 |
| DT | 190.8 ± 33.8 | 200.6 ± 28.2 | 203.6 ± 38.7 | 199.5 ± 38.8 | 0.421 |
| E/E' | 7.2 ± 2.6 | 7.4 ± 3.0 | 7.0 ± 3.3 | 6.9 ± 2.6 | 0.836 |
| Cancer Type (%) | |||||
| Breast | 20 (53) | 23 (56) | 13 (34) | 16 (43) | 0.208 |
| NHL | 5 (13) | 8 (20) | 11 (29) | 12 (33) | 0.177 |
| Other | 13 (34) | 10 (24) | 14 (37) | 9 (24) | 0.502 |
| Cum Doxo dose (mg/m2) | 265.6 ± 98.5 | 252 ± 65.0 | 282 ± 78.5 | 261.0 ± 101.8 | 0.473 |
| Hypertension | 4 | 4 | 3 | 7 | 0.524 |
| Diabetes Mellitus | 6 | 8 | 5 | 8 | 0.712 |
| Dyslipidemia | 2 | 0 | 3 | 3 | 0.267 |
Data are mean ± standard deviation or percentage. Cum Doxo dose and NHL denote cumulative doxorubicin dose and Non-Hodgkin Lymphoma respectively.
P-value by ANOVA for continuous variables and Chi Square or Fisher’s test for categorical variables.
4.2. Reproducibility assessment
Based on baseline data, the intra-observer variability, ICC, was high: 0.975 (95% CI: 0.926–0.991) and the inter-observer variability was 0.809 (95% CI: 0.509–0.925) for LVEF. Similarly, for follow-up data, the intra and inter ICC were 0.955 (95% CI: 0.887–0.982) and 0.982 (95% CI: 0.995–0.993), respectively.
4.3. Treatment outcomes at end of six months
At 6 months, four (11%) patients assigned to placebo but only one (1%) assigned to carvedilol 12.5 mg had an LVEF < 50% (p = 0.013).
The cumulative doxorubicin dose in the five patients who developed an LVEF < 50% were: 160 mg/m2 (one patient), 300 mg/m2 (two patients) and 360 mg/m2 (one patient) in the placebo group and 240 mg/m2 in one patient who received carvedilol 12.5 mg.
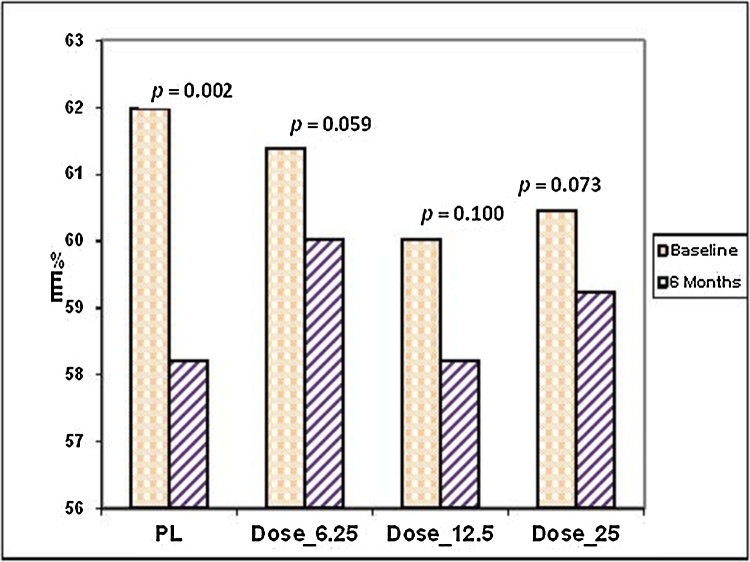
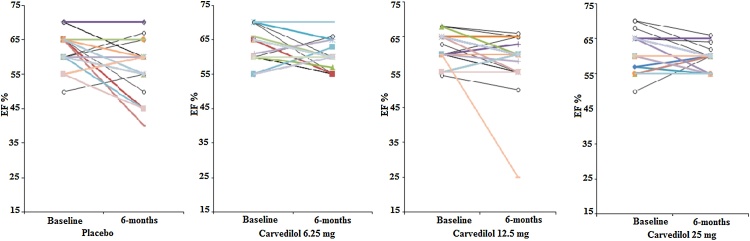
By six months, for patients assigned to placebo, LVEF had fallen by 3.8 ± 7.1% (p = 0.002) with 45% having some reduction in LVEF. In contrast, there was no significant decline in LVEF for any group assigned to carvedilol [6.25 mg (p = 0.059), 12.5 mg (p = 0.100), or 25 mg (p = 0.073)] and depicted as bar and line graphs in Fig. 1, Fig. 2. On inter-group comparison, no significant change was observed in LVEF between placebo and all carvedilol groups (p = 0.185). Similarly, no difference noted in the inter group comparison with any carvedilol dose: 6.25 mg (p = 0.080); 12.5 mg (p = 0.219); or 25 mg (p = 0.060).
Fig. 1.
LVEF at baseline and 6 months among different doses.
Fig. 2.
Individual left ventricle ejection fraction at baseline and 6-months.
4.4. Effect of doxorubicin on LV size
At 6-months there was an increase in mean LVSd in patients assigned to placebo from baseline of 28 ± 4 mm to 31 ± 6 mm (p = 0.002) but not amongst patients assigned to carvedilol. The differences between placebo and carvedilol, either in individual dose groups or overall were not significant [6.25 mg (29 ± 4 vs. 30 ± 3, p = 0.059); 12.5 mg (29 ± 4 vs. 30 ± 4, p = 0.178); 25 mg (30 ± 5 vs. 31 ± 4, p = 0.459)]. The mean changes in LVDd for patients assigned to placebo or carvedilol were similar (Table 2).
Table 2.
Comparison of echocardiography and tissue Doppler variables at baseline and 6 month of follow-up.
| Placebo | p value | Car (6.25 mg) | p value | Car (12.5 mg) | p value | Car (25 mg) | p value | ||
|---|---|---|---|---|---|---|---|---|---|
| LVSd | Baseline | 28.0 ± 4.4 | 0.002 | 29.3 ± 4.4 | 0.059 | 29.2 ± 4.2 | 0.178 | 30.2 ± 5.7 | 0.458 |
| 6 months | 30.7 ± 5.7 | 30.4 ± 3.0 | 30.3 ± 4.3 | 30.9 ± 4.1 | |||||
| LVDd | Baseline | 45.3 ± 5.3 | 0.566 | 46.0 ± 5.1 | 0.166 | 44.8 ± 4.3 | 0.011 | 44.6 ± 6.3 | 0.368 |
| 6 months | 45.9 ± 7.5 | 46.8 ± 4.0 | 46.0 ± 3.7 | 45.5 ± 5.3 | |||||
| EF | Baseline | 62.0 ± 4.6 | 0.002 | 61.4 ± 3.9 | 0.059 | 60.0 ± 4.1 | 0.100 | 60.4 ± 4.2 | 0.073 |
| 6 months | 58.2 ± 6.6 | 60.0 ± 2.9 | 58.2 ± 6.6 | 59.2 ± 2.8 | |||||
| E' | Baseline | 11.3 ± 3.0 | 0.365 | 11.9 ± 3.2 | 0.067 | 13.2 ± 5.0 | 0.001 | 11.8 ± 3.4 | 015 |
| 6 months | 10.9 ± 3.8 | 10.9 ± 3.4 | 11.8 ± 4.9 | 10.8 ± 3.0 | |||||
| E/A | Baseline | 1.22 ± 0.4 | 0.533 | 1.20 ± 0.5 | 0.359 | 1.26 ± 0.5 | 0.949 | 1.17 ± 0.3 | 0.251 |
| 6 months | 1.20 ± 0.4 | 1.14 ± 0.4 | 1.27 ± 0.6 | 1.13 ± 0.3 | |||||
| DT | Baseline | 190.7 ± 33.9 | 0.188 | 200.8 ± 28.2 | 0.637 | 203.6 ± 38.7 | 0.228 | 199.5 ± 38.8 | 0.139 |
| 6 months | 199.7 ± 39.8 | 197.1 ± 30.0 | 213.3 ± 45.1 | 209.3 ± 40.9 | |||||
| E/E' | Baseline | 7.2 ± 2.6 | 0.413 | 7.4 ± 3.0 | 0.730 | 7.0 ± 3.3 | 0.033 | 6.9 ± 2.6 | 0.788 |
| 6 months | 7.6 ± 3.3 | 7.6 ± 3.6 | 8.0 ± 4.0 | 7.0 ± 2.2 |
Car* stands for carvedilol.
4.4.1. Effect of doxorubicin on diastolic function
E' decreased only in patients treated with carvedilol 12.5 mg or carvedilol 25 mg (Table 2). However, mean E' remained above 10 and mean E/E' ratio below 13 in all groups (8.0 ± 4.0). Furthermore, no significant differences in Doppler echocardiography variables were observed for patients assigned to placebo or carvedilol.
4.5. Mortality
Overall 11 patients died. Two deaths (5.3%) occurred on placebo, one (2.4%) on carvedilol 6.25 mg, four (10.5%) on carvedilol 12.5 mg, and four (10.8%) on carvedilol 25 mg. Of these, 8 died in hospital which was attributed to end stage cancer with metastasis in addition to respiratory failure and massive pleural effusion in some cases; and sepsis in other patients. We did not find any significant difference in the hemodynamic response (blood pressure and heart rate) between all carvedilol doses (see Table 4).
Table 4.
Blood pressure and heart rate at baseline and 6 months for the 4 groups.
| Group Dose | Heart Rate |
Systolic BP |
Diastolic BP |
||||
|---|---|---|---|---|---|---|---|
| Baseline | 6-month | Baseline | 6-month | Baseline | 6-month | ||
| Placebo | Mean | 89.33 | 86.88 | 120.49 | 112.76 | 72.84 | 73.92 |
| Carvedilol 6.25 mg | Mean | 87.08 | 84.89 | 129.47 | 119.64 | 74.44 | 73.21 |
| Carvedilol 12.5 mg | Mean | 85.26 | 77.56 | 121.71 | 112.59 | 72.97 | 70.12 |
| Carvedilol 25 mg | Mean | 85.45 | 81.79 | 120.53 | 116.78 | 72.41 | 74.11 |
5. Discussion
This study provides further evidence that carvedilol may provide protection against anthracycline-induced cardiomyopathy. Moreover, this effect may be observed with a broad range of carvedilol doses. This latter observation is important because the side effects of beta-blockers appear strongly dose-related12 and cancer patients may experience symptoms that might be ascribed to higher doses of beta-blocker. Lower doses of carvedilol may offer myocardial protection but with fewer side effects.
Earlier, smaller randomized studies by Kalay et al. and Salehi et al. suggested that carvedilol offered myocardial protection to cancer patients treated with anthracyclines.9,10 Kalay et al. investigated the protective effect of prescribing carvedilol 12.5 mg once daily to 50 patients treated with doxorubicin or epirubicin.9 There was no attempt at blinding. Measures of both systolic and diastolic function deteriorated less amongst patients assigned to carvedilol. Salehi et al. conducted a study in 66 patients treated with doxorubicin or epirubicin who were randomized to receive placebo, carvedilol 12.5 mg once daily or carvedilol 25 mg once daily. The paper does not explicitly state whether or not the study was blinded. Overall, there was no significant benefit with carvedilol but the authors claimed that the lower dose was associated with improved diastolic function and the higher dose with both improved diastolic and systolic function. The prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA) trial results were recently reported. They studied women with breast cancer in a double-blind, placebo-controlled study with approximately 30 patients per group assigned either to placebo, metoprolol succinate titrated to 100 mg/day or candesartan titrated to 32 mg/day. Compared to those assigned to placebo, patients assigned to candesartan had a smaller decline in LVEF after chemotherapy (p = 0.03) but did not observe a similar effect with metoprolol and questioned if other beta-blockers, such as carvedilol, might have led to different results.13
Our study differs from previous trials of carvedilol in several important ways. This is the first unequivocally, double-blind, randomized, placebo-controlled study to investigate the effects of carvedilol in patients treated with anthracyclines. It is the only study to evaluate three doses of carvedilol and is also substantially larger than the previous two studies (Table 3).9,10 In previous randomized trials, the mean cumulative doxorubicin dose was ≥500 mg/m2 but only four patients received a cumulative doxorubicin dose of >400 mg/m2 in our study, which may explain the lower incidence of LV dysfunction in the control group in our study. Doxorubicin cardiotoxicity is cumulative and typically occurs at an average total dose of >500 mg/m2 but can occur at cumulative doses as low as 300 mg/m2.
Table 3.
Comparison of studies on the prophylactic use of carvedilol in adult patients receiving anthracycline.
| Studies |
||||
|---|---|---|---|---|
| Present | Kalay et al. | Salehi et al. | Total | |
| Study Design | ||||
| Single center | Yes | Yes | Yes | |
| Placebo controlled | Yes | Yes | Yes | |
| Blind | Double | Single | NA | |
| Treatment Groups | 4 | 2 | 3 | |
| Sample Size | ||||
| Placebo | 38 | 25 | 22 | 85 |
| Carvedilol 6.25 mg od | 41 | – | – | 41 |
| Carvedilol 12.50 mg od | 38 | 25 | 22 | 75 |
| Carvedilol 25.00 mg od | 37 | – | 22 | 59 |
| Chemotherapy Treatment | Doxorubicin | Doxorubicin or epirubicin | Doxorubicin or epirubicin | |
| Cumulative Doxorubicin dose | <400 mg/m2 | ∼520 mg/m2 | ∼530 mg/m2 | |
| Follow up period | 6 months | 6 months | 4 months | |
| Echo studies | Baseline, then at 2, 4 & 6 months. | Baseline then after chemotherapy. | Baseline, then only at 4 months. | |
| Beneficial effect of carvedilol on LV function | Yes | Yes | Yes | |
| Number of patients who developed cardiomyopathy | Placebo: 4 (11%) Carvedilol: 1 (1%) (p = 0.013). |
Placebo: 5 (20%) Carvedilol: 1 (4%) |
Placebo: 5 (23%). Carvedilol: 6 (14%) |
14/85 (16%) 8/145 (6%) P < 0.01 |
During six months of chemotherapy, four (11%) patients in the placebo group but only one on carvedilol (3%) developed LV systolic dysfunction (LVSD). Kalay et al. observed the development of LVSD in one patient (4%) treated with carvedilol and 5 patients (23%) assigned to placebo and Salehi et al. in five patients (22.7%) treated with carvedilol 12.5 mg/day but in only one (5%) treated with carvedilol 25 mg/day and 5 (23%) patients in control group. Cumulatively, including the current study, 14 of 85 patients (16%) assigned to placebo developed LVSD compared to 8 of 145 patients (6%) assigned to carvedilol (p < 0.01).
We found no evidence to support a beneficial effect of carvedilol on diastolic dysfunction; the incidence of different grades of diastolic dysfunction were similar across treatment groups. This finding differs from those reported in earlier studies.9,10 At 6 months, there was an increase in the E/E', which was statistically significant in the carvedilol 12.5 mg group when compared to baseline. In the PRADA study, the increase in E/E' on metoprolol succinate was highly significant. Sympathetic activation increases the velocity of myocardial relaxation and so it is not surprising that beta-blockers may slow the rate of ventricular relaxation.
In clinical trials of heart failure, carvedilol was usually given twice daily and in total daily doses of up to 100 mg/day. Whether other doses or dosing regimens would carry greater benefit than we observed should be considered in other trials. Whether, heart rate rather than dose achieved is the more important target for beta-blocker therapy is also uncertain.12, 13, 14 It is also unclear whether similar effects would be observed with other beta-blockers. Neither the study size nor the mechanistic character of the primary outcome (change in LVEF) is sufficient to provide conclusive evidence of clinically relevant benefits with carvedilol for this population. However, this study does give hope for patients with few alternatives, adds to the existing evidence-base and provides valuable data to inform the design of definitive multi-centre trials.
5.1. Study limitations
While this is the largest trial on the topic, including men and women with different doses of carvedilol, it was still underpowered to be able to detect a difference in outcomes between treatment groups. Data was missed for troponin and creatinine from a good percentage of patients. Long-term outcome was not assessed.
5.2. Conclusion
These data provide further evidence suggesting that prophylactic use of carvedilol might attenuate or prevent the decline in LVEF associated with the use of anthracyclines for the treatment of cancer.
Conflicts of interest
None.
Acknowledgements
Carvedilol®.
Saudi Pharmaceutical Industries & Medical Appliances Corporation (SPIMACO ADDWAEIH). They supplied us with the different carvedilol doses and the placebo.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ihj.2018.06.011.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Cardinale Daniela, Colombo Alessandro, Bacchiani Giulia. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer B., Tziros C., Katz Br R. J Cardiol. 2009;16:85–89. [Google Scholar]
- 3.Pfeffer B., Tziros C., Katz R. Current concepts of anthracycline cardiotoxicity: pathogenesis, diagnosis and prevention. Br J Cardiol. 2009;16:85–89. [Google Scholar]
- 4.Hequet O., Le Q.H., Moullet I. Subclinical late cardiomyopathy after doxorubicin therapy for lymphoma in adults. J Clin Oncol. 2004;22:1864–1871. doi: 10.1200/JCO.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 5.Sawaya H., Sebag I.A., Plana J.C. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107:1375–1380. doi: 10.1016/j.amjcard.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carreira R.S., Monteiro P., Gon Alves L.M., Providencia L.A. Carvedilol: just another beta-blocker or a powerful cardioprotector? Cardiovasc Hematol Disord Drug Targets. 2006;6:57–66. doi: 10.2174/187152906779010746. [DOI] [PubMed] [Google Scholar]
- 7.Jaylo M.S.N., Narraval C., Chiew B., Lapuz F. The cardioprotective effects of angiotensin converting enzyme inhibitor (perindopril) as prophylaxis of doxorubicin-induced cardiomyopathy—a pilot study. Intern Med Phil J. 2006;44:75–82. [Google Scholar]
- 8.Hellmann K. Preventing the cardiotoxicity of anthracyclines by dexrazoxane. BMJ. 1999;319:1085–1086. doi: 10.1136/bmj.319.7217.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalay N., Basar E., Ozdogru I. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006;48:2258–2262. doi: 10.1016/j.jacc.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 10.Salehi R., Zamani B., Esfehani A., Ghafari S., Abasnezhad M., Goldust M. Protective effect of carvedilol in cardiomyopathy caused by anthracyclines in patients suffering from breast cancer and lymphoma. Am Heart Hosp J. 2011;9:95–98. doi: 10.15420/ahhj.2011.9.2.95. [DOI] [PubMed] [Google Scholar]
- 11.Nagueh S.F., Appleton C.P., Gillebert T.C. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. JASE. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Cleland J.G., Coletta A.P., Freemantle N., Ahmed D., Rubis P., Clark A.L. Clinical trials update from the American Heart Association Meeting 2009: HEAAL, FAIR-HF, J-CHF, HeartMate II, PACE and a meta-analysis of dose-ranging studies of beta-blockers in Heart failure. Eur J Heart Fail. 2010;12:197–201. doi: 10.1093/eurjhf/hfp199. [DOI] [PubMed] [Google Scholar]
- 13.Gulati G., Heck S.L., Ree A.H. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671–1680. doi: 10.1093/eurheartj/ehw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cullington D., Goode K.M., Clark A.L., Cleland J.G. Heart rate or beta-bloker dose in patients with chronic heart failure: which is the better target? Eur J Heart Fail. 2012;14:737–747. doi: 10.1093/eurjhf/hfs060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.