Abstract
Introduction
There is limited data regarding in hospital determinants of clinical deterioration and outcome in sub massive pulmonary embolism (PE). We aimed to evaluate these determinants by comparing biomarkers, CT pulmonary angiogram echocardiography, electrocardiography variables.
Methods
57 patients of sub massive PE diagnosed on CT pulmonary angiogram were included. All patients received UFH on admission and were divided into two groups based on their clinical course. Group 1 comprised of patients who remained stable, group 2 of patients who showed signs of clinical deterioration.
Results
There were 34(59.6%) patients in group 1 and 23(40.4%) patients in group 2. No significant difference in age, gender, BMI. 59.37% had sub massive PE, 5.26% had mortality and 40.4% had clinical deterioration. Intravenous UFH infusion given to 59.6%, systemic thrombolysis 22.8%, catheter directed mechanical breakdown 14%, surgical embolectomy in 3.5% patients. S1Q3T3, new onset RBBB, T wave inversion > 1.63 mm, Basal RV size > 40 mm, RV: LV ratio > 1.2, Global RV longitudinal strain <−10.75% and RVSP > 39 mmHg profiled high risk group. Serum BNP and CT pulmonary angiogram derived scores didn’t differ significantly although CT findings helped to exclude low risk patients (specificity 88%, sensitivity 95%).
Conclusions
Physicians should be aware that patients who have ECG and Echocardiography changes suggestive of right ventricular strain and dysfunction above the cut off values and have documented thrombus in Proximal branches (RPA/LPA) or in distal portion of main pulmonary artery may require aggressive management with systemic/catheter based thrombolysis besides routine anticoagulation with heparin to prevent clinical deterioration.
Keywords: BNP, CT pulmonary angiography, Echocardiography, Sub Massive pulmonary embolism
1. Introduction
Sub-massive pulmonary embolism (PE) is defined as an acute PE event without hemodynamic instability, systolic blood pressure (SBP) >90 mmHg. It constitutes approximately 40% of all acute PE cases with a mortality rate ranging from 3% to 15%.1 European Society of Cardiology further divides sub-massive PE into two groups: intermediate-low risk and intermediate-high risk. The intermediate-high risk group has both signs of right ventricular dysfunction on imaging and abnormal biomarkers of myocardial injury while intermediate low risk has either of both.
Diagnosis of sub-massive PE requires evidence of any of the following: right ventricular (RV) dysfunction, RV dilatation (RV:LV [left ventricular] diameter >0.9), elevated serum biomarkers (BNP > 500 pg/ml), Troponin-I >0.4 ng/ml or Troponin-T >0.1 ng/ml or new ECG Changes (Complete or incomplete RBBB [right bundle branch block], antero septal ST-segment elevation/depression or T-wave inversion).2
Conventionally, these patients are treated using intravenous heparin anticoagulation only, a practice which requires serious reconsideration, as a significant number of these patients tend to deteriorate soon after hospitalization. These patients may therefore require expedited aggressive measures for clot resolution using fibrin specific thrombolytic therapy (2016 Chest guidelines grade 1B), catheter directed mechanical breakdown of thrombus or rarely surgical embolectomy.3, 4, 5
Assessing and identifying which of these patients may clinical deteriorate after admission still remains a challenge. Current literature provides abundant risk stratification algorithms for assessing patients presenting with massive PE; however, the same clinical models are not currently validated for patients with sub-massive PE.
In this study we evaluated predictive factors influencing in-hospital course and outcome in patients with sub-massive PE.
2. Materials and methods
A prospective analysis was performed at a tertiary level referral cardiac care centre in North India for a period of six months.
2.1. Inclusion criteria
Hemodynamically stable patients (normotensive at admission) presenting with acute PE (diagnosed on computed tomographic [CT] pulmonary angiogram) with evidence of RV dysfunction on echocardiography were included for the study.
2.2. Exclusion criteria
Patients with previous history of PE, severe sepsis, valvular heart disease, chronic obstructive pulmonary disease, chronic kidney disease, prior myocardial infarction, congenital heart disease and patients with sub optimal window were excluded.
After an informed consent, following variables were recorded – Patient’s pertinent history, vitals, on admission serum B-type natriuretic peptide (BNP) levels (using Alere Triage® BNP Test kit in ethylene diamine tetra acetic acid anti-coagulated whole blood specimen), 12 lead ECG along with right side V4R lead (using GE Mac 2000) and two-dimensional trans-thoracic bedside echocardiography (using Philips Affinity 50 C S5 probe).
All ECG patterns were grouped into three and assigned a point score:
-
•
S1Q3T3 pattern, RV strain pattern in V1–V3 and RBBB : 10 points
-
•
Anterior leads T wave inversion simulating ACS: 5 points
-
•
Normal ECG: 2.5 points.
{T wave inversion >1 mm in lead III and >0.5 mm in lead V4R was taken as sign of right ventricular enlargement and strain}
Specific Echocardiography variables recorded were - (1) RV size at base 1 cm below tricuspid annulus in diastole (in apical four chamber view), (2) RV:LV ratio (3) tricuspid annular planar systolic excursion (TAPSE), (4) right ventricular fractional area change (RVFAC) calculated as [RV area diastole –RV area systole]/RV area diastole, (5) right ventricular systolic pressure (RVSP) (6) tissue Doppler based tricuspid annular S’ (7) two-dimensional global longitudinal strain of RV (GLSRV) including longitudinal strain of inter-ventricular septum, RV free wall and the apex (8) tissue Doppler based right ventricular myocardial performance index (RV MPI).
CT pulmonary angiography findings were numerically scored based on the thrombus location into the following:
1 - Thrombus in main pulmonary artery
2 - Thrombus in the Main Branches right or left pulmonary artery,
3 - Segmental branch occlusion,
0 - None
Patients were subsequently divided into two groups. Patients who maintained hemodynamic stability on admission and during the course of hospitalization were labeled as group 1 whereas those who had either hemodynamic or respiratory decompensation (shock index [heart rate/SBP]>1, SpO2 <95% on room air with symptoms of severe dyspnea), and lack of any alternative plausible explanation for their deterioration such as hypovolemia or sepsis during their hospital were labeled as group 2.
Treatment of these patients was based on physician judgement, besides anticoagulation systemic thrombolysis or catheter-based therapy was chosen accordingly.
2.3. Statistical analysis
Data was described in terms of range; mean ± standard deviation (± SD), median, frequencies (number of cases) and relative frequencies (percentages) as appropriate. Comparison of quantitative variables between the study groups was done using Student t-test and Mann Whitney U test for independent samples for parametric and non-parametric data, respectively. For comparing categorical data, Chi square (χ2) test was performed and exact test was used when the expected frequency was less than 5. A probability value (p value) less than 0.05 was considered statistically significant. For multivariate analysis we used receiver operating characteristics (ROC) curve to derive parameters predicting high risk patients. All statistical calculations were done using SPSS 21 version.
3. Results
Out of 96 patients with sub-massive PE enrolled for the study, 57 patients met the inclusion criteria and were included in the study. Group 1 comprised of 34 patients (60%) and group 2 comprised of 23 patients (40%). Commonest presenting symptoms were acute onset dyspnea (68%), palpitations (12%), pleuritic chest pain (3%), hemoptysis (4%), cough (7%) and pre-syncope or postural symptoms like light-headedness (6%). Associated lower limb deep vein thrombosis (DVT) was noted in 44% patients in group 1 and 56% patients in group 2.
There were 23 males and 11 females in group 1 and 14 males and 9 females in group 2 (p value = 0.6). Mean age in two groups was comparable (48.26 years ± 13.61 in group 1 vs. 51.35 years ± 13.58 in group 2).
Patient’s age, body mass index (BMI) and presenting complaints including history of bed rest >3 days and presence of associated leg DVT were comparable between the two groups with no statistical significance (Table 1).
Table 1.
Baseline Patient Characteristics.
| Group 1(Low Risk) |
Group 2 (High Risk) |
p-value | |||
|---|---|---|---|---|---|
| MEAN | SD | MEAN | SD | ||
| Age (in years) | 48.3 | 13.6 | 51.35 | 13.58 | 0.405 |
| BMI | 29.7 | 3.7 | 30.25 | 2.31 | 0.537 |
| Heart Rate (bpm) | 102.0 | 13.1 | 103.6 | 20.5 | 0.719 |
| Systolic BP (mm Hg) | 108.5 | 10.8 | 105.7 | 9.4 | 0.309 |
| Hospital stay (no of days) | 9.1 | 3.10 | 8.9 | 5.2 | 0.821 |
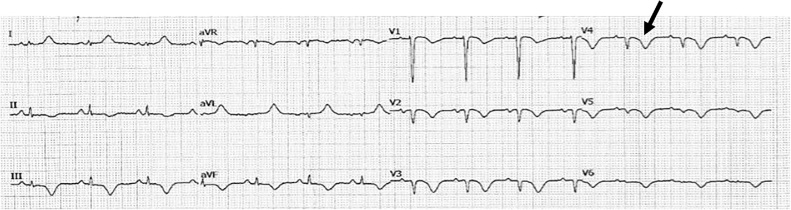
Utilizing our ECG scoring system, group 1 patients had an average ECG score of 1.34 ± 0.84 and patients in group 2, 7.33 ± 2.36 (p value < 0.05). T wave inversion depth in the right sided leads (Fig. 1) was compared between the two groups, with lead V4R at a cut off of 1.63 mm showing better sensitivity and specificity than lead III, to distinguish patients into low and high risk. (95% sensitivity, 97% specificity, AUC [area under curve] 0.98, p value < 0.001 in lead V4R versus 60% sensitivity,100% specificity, AUC 0.75, p value = 0.001 in lead III).
Fig. 1.
Right side V4R lead black arrow showing significantly inverted T wave.
All echocardiography variables showed significant statistical difference between two groups on univariate analysis except for RV myocardial performance index.
On multivariate analysis only echocardiography parameters, RV size, RV:LV ratio and strain derived parameter GLSRV achieved statistical significance.
RVSP showed borderline significance whereas TAPSE, S’ of RV free wall and RVFAC showed no significance.
When RV size was used a cut off value of 40.5 mm was successfully able to segregate sub-massive PE patients into low risk (RV size <40.5 mm) and high risk (>40.5 mm) with a sensitivity of 87% and specificity of 85% (AUC 0.927, p value < 0.001)
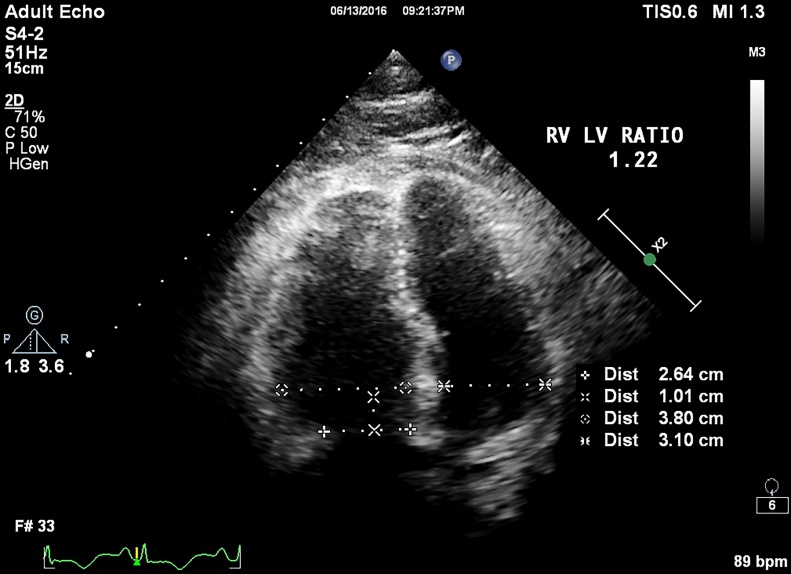
Similarly an RV:LV ratio of ≤1.2 and >1.2 successfully divided patients into group 1 and group 2 with a 95% sensitivity and 97% specificity (AUC 0.99, p value < 0.001) (Fig. 2).
Fig. 2.
RV size and RV LV ratio is taken at end diastole using ECG gating in apical four chamber view 1 cm from tricuspid annular plane; in this case it was 1.22.
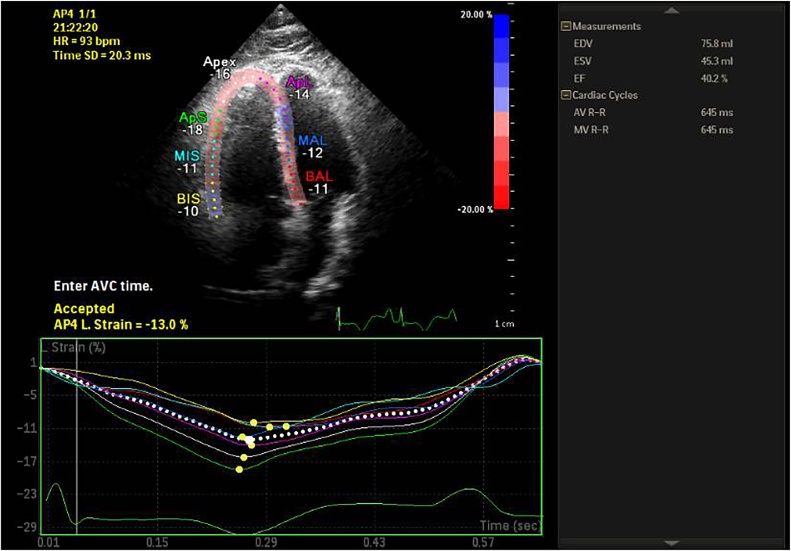
Strain derived parameter GLSRV at a cut off of −10.75% effectively divided patients into group 1 with GLSRV < −10.75% and group 2 with GLSRV ≥ −10.75% (95% sensitivity, 97% specificity, AUC 0.972, p value < 0.001) (Fig. 3).
Fig. 3.
Global right ventricular strain in apical four chamber view showing significantly reduced value −13%.
Right ventricular systolic pressure gave borderline results at a cut off of 39 mmHg with 87% sensitivity but only 53% specificity (AUC 0.818) (Table 2)
Table 2.
Cut-off values for discriminative variables on ECG and Echocardiogram.
| Predictive Parameters | Low Risk (Group 1) | High Risk (Group 1) | Sensitivity (%) |
Specificity (%) |
|---|---|---|---|---|
| TWI Lead v4r (in mm) | <1.63 | >1.63 | 95 | 97 |
| RV size (in mm) | <40.5 | >40.5 | 87 | 85 |
| RV: LV ratio | ≤1.2 | ≥1.2 | 95 | 97 |
| GLSRV (in %) | ≥−10.75 | ≤−10.75 | 95 | 97 |
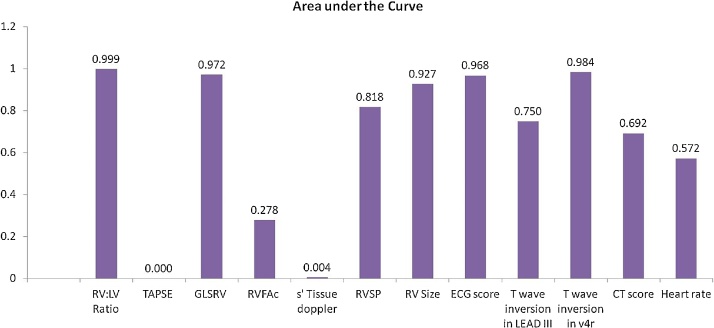
When comparing CT scores between the two groups on univariate analysis a statistically significant difference was seen (p value = 0.01) but on further plotting the AUC a value of 0.69 was seen thus diminishing its statistical significance (Fig. 4).
Fig. 4.
Accuracy of various parameters for predicting clinical deterioration. Areas under the curve derived from receiver operator characteristics curve is depicted here.
BNP values between the two groups did not show any significant difference (p value = 0.4) with mean levels of 426 pg/ml in group 1 and 530 pg/ml in group 2, respectively.
3.1. Patient outcome and mortality
There was no statistical significant difference in number of days of hospital stay between two groups. (9.1 ± 3.1 vs 8.9 ± 5.2 days, p = 0.821)
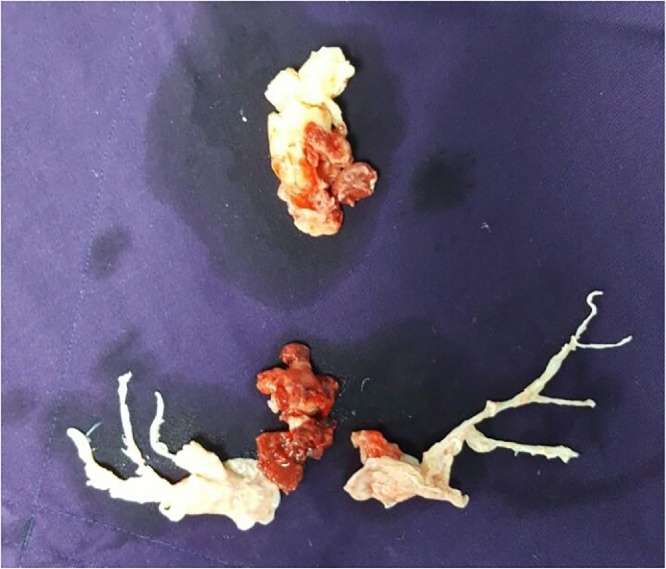
Physician’s judgement was used to decide the initial management approach in these patients which involved unfractionated heparin infusion in 59.6% of these patients, systemic thrombolysis in 22.8% patients, catheter directed mechanical breakdown combined with intra-lesional thrombolysis in 14% patients and surgical embolectomy in 3.5% patients (Fig. 5).
Fig. 5.
Surgically excised thrombus from main pulmonary artery and its branches.
In group 1, there were three mortalities massive hemoptysis in one patient and severe hypoxia failing ventilator support in the other two. All three of these patients were on currently chemotherapy.
In group 2, one patient deteriorated post thrombolysis requiring emergency surgical embolectomy. Another patient was managed by surgical embolectomy due to two large mobile RV thrombi on presentation.
4. Discussion
This study demonstrates a clinically important application of simple non-invasive bedside measurements derived from electrocardiography and echocardiography in distinguishing patients sub-massive PE into low and high risk.
RV ECG leads and RV dilatation and dysfunction using echocardiography are well recognized to identify acute right ventricular strain.6
Patients with sub-massive PE although hemodynamically stable, have a high risk of decompensating during their in-hospital course.
Echocardiographic and ECG parameters derived from our study helped us identify these patients with a relatively high sensitivity and specificity.
In our study, ECG findings with its relevant scoring was successful in categorizing patients with sub-massive PE into low risk and high risk groups with sensitivity of 91%, and a specificity of 97%. Patients with T wave inversion simulating ACS in anterior leads and normal ECG favoured a low risk clinical profile (group 1) whereas patients with S1Q3T3 pattern, Right ventricular strain, new onset RBBB or T wave inversion >1.63 mm in V4R favoured high risk profile (group 2). Qaddoura et al7 in their meta-analysis of 39 studies including 9198 patients, analyzed ECG features to predict a negative outcome in patients with acute PE and found that specific features most predictive of in-hospital death and clinical deterioration included S1Q3T3, complete RBBB, TWI, ST-segment depression V4–V6, ST segment elevation in V1, III, aVr Qr-V1, right axis deviation, atrial fibrillation, and RV trans mural ischemic pattern. Zhan et al in a study including only hemodynamically stable acute PE patients found that S1Q3T3 pattern, ST segment elevation (in leads V1, V2, III, aVr), ST-segment depression (in leads I, V4–V6) to be significantly associated with hemodynamic instability.8,9 In normal subjects, right-sided leads may show minimal (<0.5 mm) T wave inversion whereas in PE patients it can reach >2 mm. We also found that right side V4R may be useful adjunct to depict RV dilatation and strain at a T wave inversion cut off value >1.63 mm.
Echocardiography variables deserve special attention in our study as almost all variables measured (except the RV myocardial performance index), showed statistically significant difference on univariate analysis.
Based on echocardiographic variables, patients having basal Right ventricular diameter >40.5 mm, RV:LV ratio >1.2, GLSRV < −10.75% and RVSP > 39 mmHg (TR velocity >3.1 m/s) should be termed as high risk.
These findings are of immense clinical importance as nowadays echocardiography is one of the cardinal diagnostic modality which is used both at admission and during hospital stay to risk stratify patients and gauging the treatment success.
Meta-analysis of studies on sub-massive PE have concluded that In normotensive patients, 30 day mortality with RV dysfunction is two-fold compared to normotensive patients without RV dysfunction, probably due to poor pulmonary clot resolution after the initial event and higher recurrence rate.10 reported that 37% patients have evidence of RV dysfunction on echo with increased short-term mortality (OR 2.29; 13.7% with RV dysfunction vs 6.5% without RV dysfunction).10, 11, 12, 13
Our study also emphasizes quantifying RV functions using GLSRV is helpful in distinguishing high and low risk patients with sub-massive PE.
Although elevated BNP levels can help identify patients with acute PE at high risk of short-term death and adverse outcome events, albeit it has low specificity as seen in our study, which showed a grossly variable standard deviation in maximum and minimum ranges in both groups. The positive predictive value of elevated BNP levels alone remains low and its high negative predictive value is more useful to identify individuals with a likely favourable outcome.14, 15, 16 In our study, although significant elevations in serum BNP levels (>400 pg/ml) were observed in both groups but it showed no statistically significance amongst them on univariate analysis. This may be explained due to an obligatory delay in BNP mRNA up regulation and subsequent protein release in the serum as it takes several hours for BNP levels to increase after the onset of acute myocardial stretch.
CT pulmonary angiogram has a definite diagnostic role in acute PE but the prognostic value based on clot location remains questionable.
Meta-analysis of clinically significant CT pulmonary angiogram findings showed that the localization of emboli in the central branches of pulmonary arteries was associated with an increased risk of 30- day mortality but no association was observed between the overall involvement of pulmonary arterial branches (obstruction index) and mortality, assessed at either 1 month or 3 months.17 In our study, CT pulmonary angiography based scores did not differ much in two groups (p value = 0.010, AUC 0.692).
4.1. Limitations
Although the results of our study strongly suggest risk stratification using ECG and echocardiogram, but it is limited by being a single centre experience with a small group of patients. More studies in future are required comprising of a multicenter analysis with a large patient cohort to add to the current knowledge and formulate an effective risk stratification model for sub-massive PE patients. Such a model shall be helpful both regarding improving patient outcomes and early decision making need for aggressive management pathways.
5. Conclusion
A significant proportion of patient with acute PE present with features consistent with sub-massive PE. Current literature provides exhaustive risk stratification models for massive PE while patients with sub-massive PE are treated according to variable institutional policies leading to high rates of in-hospital decompensation and mortality. Physicians should be aware that patients who have ECG and Echocardiography changes suggestive of RV strain and dysfunction above the cut off values described above may require aggressive management with systemic/catheter based thrombolysis besides routine anticoagulation with heparin to prevent clinical deterioration during their hospital stay.
Conflict ofinterest
We all have no conflict of interest.
References
- 1.Jaff M.R., McMurtry M.S., Archer S.L. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 2.Wood K.E. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121:877–905. doi: 10.1378/chest.121.3.877. [DOI] [PubMed] [Google Scholar]
- 3.Sekhri V., Mehta N., Rawat N., Lehrman S.G., Aronow W.S. Management of massive and nonmassive pulmonary embolism. Arch Med Sci. 2012;8:957–969. doi: 10.5114/aoms.2012.32402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer G., Vicaut E., Danays T. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402–1411. doi: 10.1056/NEJMoa1302097. for the PEITHO Investigators. [DOI] [PubMed] [Google Scholar]
- 5.Kline J.A., Nordenholz K.E., Courtney D.M. Treatment of submassive pulmonary embolism with tenecteplase or placebo:cardiopulmonary outcomes at three months (TOPCOAT): multicenter double-blind placebo-controlled randomized trial. J Thromb Haemost. 2014;12:459–468. doi: 10.1111/jth.12521. [DOI] [PubMed] [Google Scholar]
- 6.Akula R., Hasan S.P., Alhassen M., Mujahid H., Amegashie E. Right sided EKG with pulmonary embolism. J Natl Med Assoc. 2003;95:714–717. [PMC free article] [PubMed] [Google Scholar]
- 7.Qaddoura A., Digby G.C., Kabali C., Kukla P., Zhan Z.Q., Baranchuk A.M. The value of electrocardiography in prognosticating clinical deterioration and mortality in acute pulmonary embolism: a systematic review and meta-analysis. Clin Cardiol. 2017;40:814–824. doi: 10.1002/clc.22742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniel K.R., Courtney D.M., Kline J.A. Assessment of cardiac stress from massive pulmonary embolism with 12-lead ECG. Chest. 2001;120:474–481. doi: 10.1378/chest.120.2.474. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari E., Imbert A., Chevalier T., Mihoubi A., Morand P., Baudouy M. The ECG in pulmonary embolism. Predictive value of negative T waves in precordial leads – 80 case reports. Chest. 1997;111:537–543. doi: 10.1378/chest.111.3.537. [DOI] [PubMed] [Google Scholar]
- 10.Cho J.H., Kutti Sridharan G., Kim S.H., Kaw R., Abburi T., Irfan A. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. BMC Cardiovasc Disord. 2014;14(6):64. doi: 10.1186/1471-2261-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frémont B., Pacouret G., Jacobi D. Prognostic value of echocardiographic Right/Left ventricular End-diastolic diameter ratio in patients with acute pulmonary embolism results from a monocenter registry of 1416 patients. Chest. 2008;133:358–362. doi: 10.1378/chest.07-1231. [DOI] [PubMed] [Google Scholar]
- 12.Kline J.A., Steuerwald M.T., Marchick M.R., Hernandez-Nino J., Rose G.A. Prospective evaluation of right ventricular function and functional Status 6 months after acute submassive pulmonary embolism. Chest. 2009;136:1202–1210. doi: 10.1378/chest.08-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grifoni S., Olivotto I., Cecchini P. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation. 2000;101:2817–2822. doi: 10.1161/01.cir.101.24.2817. [DOI] [PubMed] [Google Scholar]
- 14.Coutance G., Le Page O., Lo T., Hamon M. Prognostic value of brain natriuretic peptide in acute pulmonary embolism. Crit Care. 2008;12:R109. doi: 10.1186/cc6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ten Wolde M., Tulevski I.I., Mulder J.W. Brain natriuretic peptide as a predictor of adverse outcome in patients with pulmonary embolism. Circulation. 2003;107:2082–2084. doi: 10.1161/01.CIR.0000070020.79932.DB. [DOI] [PubMed] [Google Scholar]
- 16.Kucher N., Printzen G., Goldhaber S.Z. Prognostic role of brain natriuretic peptide in acute pulmonary embolism. Circulation. 2003;107:2545–2547. doi: 10.1161/01.CIR.0000074039.45523.BE. [DOI] [PubMed] [Google Scholar]
- 17.Vedovati M.C., Germini F., Agnelli G., Becattini C. Prognostic role of embolic burden assessed at computed tomography angiography in patients with acute pulmonary embolism: systematic review and meta-analysis. J Thromb Haemost. 2013;11:2092–2102. doi: 10.1111/jth.12429. [DOI] [PubMed] [Google Scholar]