Abstract
Metastasis from primary cancer to the thyroid is uncommon in breast cancer. Here we present a case of lobular breast carcinoma that metastasized to the thyroid. A 54-year-old woman without symptoms was admitted to our institution for staging of the lymph node above the left clavicle. An 18F-fluoro-deoxy-D-glucose positron emission tomography scan was performed for staging, and low uptakes were observed in the left supraclavicular and cervical lymph nodes. High uptake was seen in the posterior and lower left lobe of the thyroid. Histologic findings indicated lobular breast carcinoma (positive GATA3, loss of E-cadherin expression) metastatic to the thyroid with a luminal profile. Immunohistochemical analysis was negative for primary thyroid or parathyroid carcinoma. To our knowledge, this is the first report of a patient presenting a metastatic invasive lobular carcinoma in the thyroid and lymph nodes without a prior diagnosis of breast cancer.
Keywords: Breast neoplasms, Immunohistochemistry, Lobular carcinoma, Neoplasm metastasis, Thyroid gland
INTRODUCTION
Approximately 80% of invasive breast cancers are typically classified into invasive ductal carcinoma; the remaining 20% are invasive lobular carcinoma. The metastatic patterns of invasive lobular carcinoma and invasive ductal carcinoma are different [1]. In invasive lobular carcinoma, metastases are found more frequently in the bone and/or other sites such as the central nervous system, body cavities, and visceral organs [2], and the frequency of metastasis is higher in invasive lobular carcinoma than in invasive ductal carcinoma [2].
Although the thyroid gland is richly vascularized, it is a relatively uncommon site for metastatic disease. Thyroid metastases from breast cancer, particularly invasive lobular carcinoma, are rare. To the best of our knowledge, only one case of invasive lobular carcinoma with metastasis has been reported in the literature [3].
Here we report a case of a metastatic lesion in the thyroid from invasive lobular carcinoma in a 54-year-old woman along with a short review of the literature.
CASE REPORT
A 54-year-old woman was admitted to the Limoges University Hospital on July 2016 for exploration of the lymph node above the left clavicle without other symptoms. Her medical history included hypertension and diabetes mellitus, and she was overweight (body mass index, 45 kg/m2). She stopped using tobacco in 2000. Her family history included breast cancer in her sister and a daughter with the p53 mutation. This patient did not have the p53 mutation.
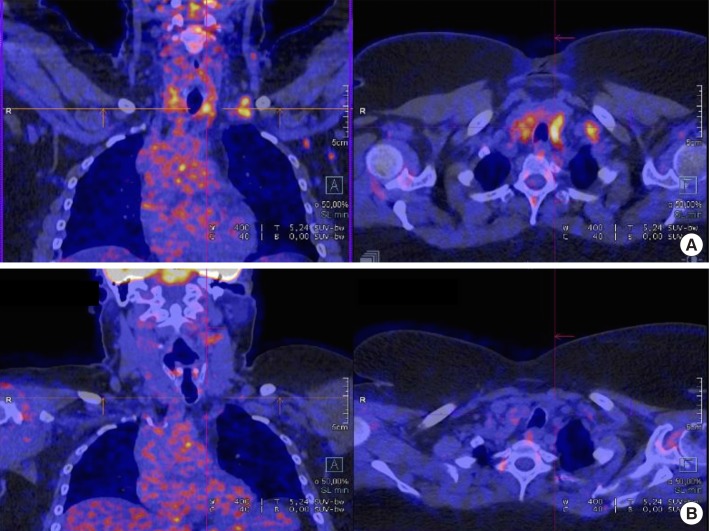
An 18F-fluoro-deoxy-D-glucose positron emission tomography (18FDG PET) scan was performed for staging. Low uptake values were observed in the left supraclavicular and cervical lymph nodes. A significantly high uptake value was seen in the posterior and lower part of the left lobe of the thyroid (Figure 1). Ultrasound sonography and fine-needle aspiration cytology were carried out and were inconclusive in the left supraclavicular lymph node and positive in the thyroid with a malignancy classified as Bethesda VI. The patient was euthyroid (serum thyroid-stimulating hormone, T4, and T3 levels in the normal range). The lymph node exploration by airway panendoscopy was negative. Technetium-99m bone scintigraphy, computed tomography scan, and breast MRI were all unremarkable, and tumor marker CA15-3 was negative.
Figure 1. 18F-fluoro-deoxy-D-glucose positron emission tomography staging scan for patient with lobular breast carcinoma metastasis to the thyroid gland. (A) April 2016. (B) July 2016.
A multidisciplinary board decided to perform a total thyroidectomy and cervical lymph node dissection. The morphological aspect was strongly suggestive of invasive lobular carcinoma with positive lymph node invasion (17 positive lymph nodes) after pathological analysis. A carcinomatous infiltration was found at the upper right lobe and the lower and middle left lobe, made up of monomorphic cells without any glandular organization. These elements do not exhibit the nuclear characteristics of a papillary or vesicular carcinoma.
Immunohistochemical staining was negative for thyroglobulin (Agilent Dako, Santa Clara, USA), thyroid transcription factor 1 (TTF1) (clone 8G7G3/1; Roche, Meylan, France), mammaglobin (clone 31A5; Roche), E-cadherin (clone EP700Y; Roche), and human epidermal growth factor receptor 2 (HER2) (DA45; Ventana, Roche Diagnostic, Meylan, France).
Immunohistochemical staining was positive for AE1/AE3 (clone AE1-AE3; Agilent Dako), estrogen receptors (clone Sp1; Ventana, Roche Diagnostic), and progesterone receptors (clone 1E2; Ventana, Roche Diagnostic). The Ki-67 cutoff level (1:80 dilution; Agilent Dako) was defined at 15%.
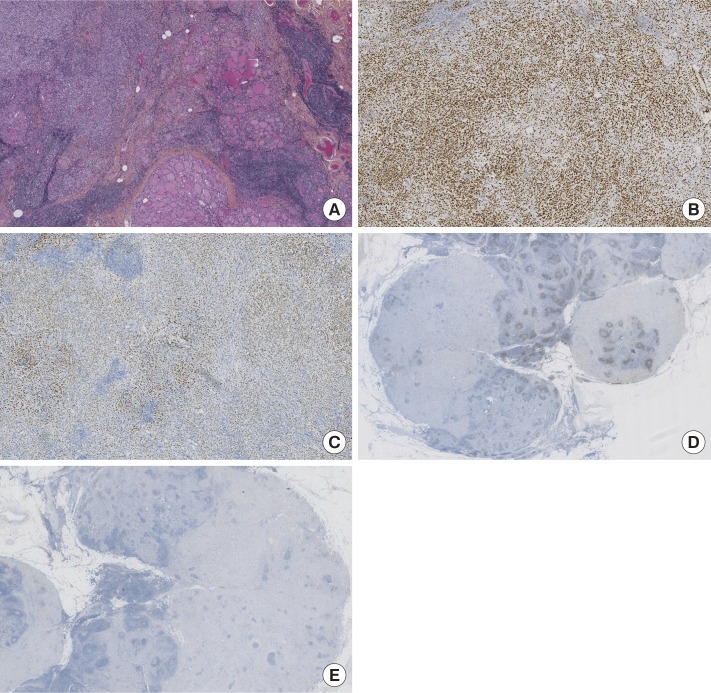
Given these results, a complementary immunohistochemical study was carried out which found negative calcitonin (polyclonal; Agilent Dako), CDX2 (clone Sp65; Agilent Dako), and PAX8 (clone MRP-50; Roche), and strongly positive GATA3 (L50823; Diagomics, Blagnac, France) (Figure 2).
Figure 2. Histologic section from thyroid. Immunohistochemical features were presented. Infiltrating carcinoma which was made up of monomorphic cells without any glandular organization. (A) H&E-stained section of tumor. (B) Immunohistochemical positive staining for GATA3. (C) Immunohistochemical positive staining for estrogen receptor. (D) Immunohistochemical negative staining for PAX8. (E) Immunohistochemical negative staining for thyroid transcription factor 1 (A–E, ×50).
Considering the comorbidities, the pathological examination (lobular carcinoma and luminal profile), and the absence of an imaging target, the decision was made to treat the patient by endocrine therapy with an aromatase inhibitor.
Assessment on July 13, 2016 by 18FDG PET did not reveal any disease recurrence; therefore, we decided to continue endocrine therapy (Figure 1).
DISCUSSION
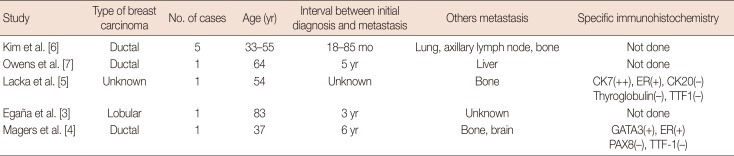
To our knowledge, this is the first report of a case of a patient presenting a metastatic invasive lobular carcinoma in the thyroid and lymph nodes without a prior diagnosis of breast cancer. Metastases from breast to the thyroid are uncommon, although cases of metastatic disease involving the thyroid have been reported in the literature (Table 1) [3,4,5,6,7]. In a large series from the Mayo Clinic (97 patients), the most frequent primary tumor sites for thyroid metastasis were the kidney (22%), lung (22%), and head and neck (12%) [8]. Egaña et al. [3] reported the first case of a patient with thyroid metastasis from a lobular breast carcinoma with a prior history of breast cancer (operated on 3 years previously).
Table 1. Reports of metastatic breast carcinoma involving the thyroid gland.
CK=cytokeratin; ER=estrogen receptor; TTF1=thyroid transcription factor 1.
In our case, the patient presented initial metastasis of invasive lobular carcinoma without a prior diagnosis of breast cancer. All secondary thyroid tumors in the literature had been diagnosed after a primary breast cancer (Table 1). The time to detection of thyroid metastasis after a primary breast cancer was more than 1 year.
Metastasis to the thyroid generally results in poor prognosis, with many patients surviving less than 3 years, and is treated differently than primary thyroid neoplasms. If we take into account the neoadjuvant setting, the chemotherapy effect is less important in lobular carcinoma than in ductal carcinoma [9]. Therefore, our patient was treated with surgery and hormone therapy, because of comorbidities and the pathological examination (lobular carcinoma, strong expression of estrogen and progesterone receptors).
For the pathologist, it is important to distinguish metastatic disease from the more common primary thyroid malignancy. Differential diagnosis between thyroid carcinoma and a secondary tumor is often difficult. Fine needle aspiration cytology remains a sensitive and specific method to detect metastases to the thyroid [8]. For the thyroidectomy, we utilized an immunohistochemical panel to discriminate primary thyroid carcinoma from metastatic invasive lobular carcinoma.
The diagnosis of invasive lobular carcinoma was based on a set of immunohistochemical markers, specifically positive expression of hormone receptors related to breast cancer. The loss of E-cadherin staining distinguishes lobular from ductal carcinoma [10]. The expression of GATA3 is a sensitive and specific labeling of breast cancer and is more sensitive than mammaglobin, which was negative in our case [11]. Thus, combined expression of GATA3, estrogen and progesterone receptors, and the loss of E-cadherin expression was compatible with the invasive lobular carcinoma diagnosis.
Diagnosis of primary thyroid and parathyroid malignancy was excluded by the negative expression of panel markers (thyroglobulin, TTF1, calcitonin, CDX2, and PAX8). CDX2 is a sensitive and specific marker for intestinal differentiation, and the columnar cells variant of papillary thyroid carcinomas has recently been shown to be CDX2 positive [12]. PAX8 is a highly sensitive marker for cells of thyroid and thymic tumor origin [13]. It is expressed in 90% of thyroid cancer cases and is negative in 100% of breast cancer cases [14]. Primary thyroid origin was also ruled out by the TTF1, thyroglobulin, and calcitonin negativity [15].
It seems to be necessary to use a panel of immunohistochemistry markers, including GATA3, hormonal receptors, TTF1, and PAX8, and to take the clinical history into account [4]. Another study came to the same conclusion using another panel of immunohistochemistry markers, including hormonal receptors, TTF1, mammaglobin, and carcinoembryonic antigen [5].
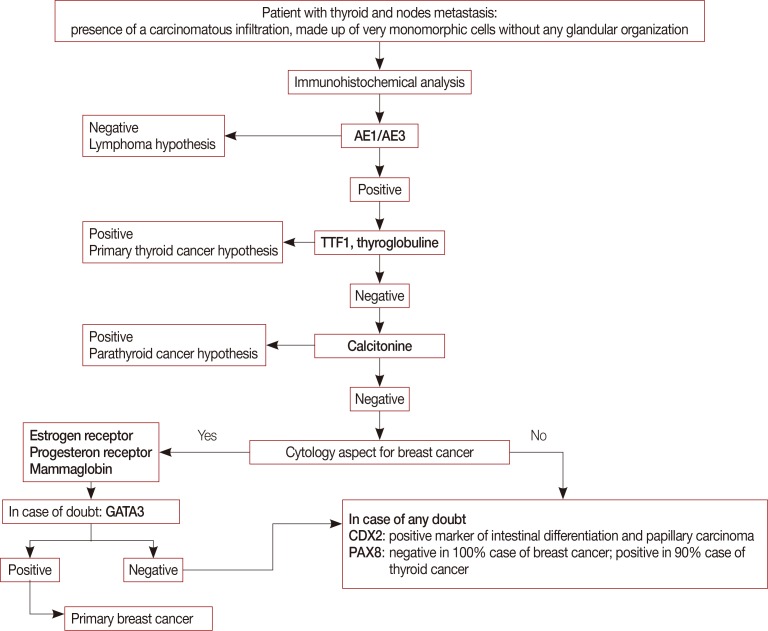
In conclusion, we suggest that immunohistochemical panels are helpful in differentiating between primary and secondary tumors (Figure 3). Thyroid metastases from cancer, specifically breast cancer, are rare. Few cases of metastasis to the thyroid have been described in the literature; to the best of our knowledge, this is the first case of initial thyroid metastasis of lobular breast carcinoma. Despite the rare presentation, this case reminds the medical community of the importance of the diagnosis of metastasis using an immunohistochemistry panel and appropriate imaging.
Figure 3. A decision tree to diagnose lobular breast carcinoma metastasis to the thyroid gland.
TTF1=thyroid transcription factor 1.
Footnotes
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Fernández B, Paish EC, Green AR, Lee AH, Macmillan RD, Ellis IO, et al. Lymph-node metastases in invasive lobular carcinoma are different from those in ductal carcinoma of the breast. J Clin Pathol. 2011;64:995–1000. doi: 10.1136/jclinpath-2011-200151. [DOI] [PubMed] [Google Scholar]
- 2.Ferlicot S, Vincent-Salomon A, Médioni J, Genin P, Rosty C, Sigal-Zafrani B, et al. Wide metastatic spreading in infiltrating lobular carcinoma of the breast. Eur J Cancer. 2004;40:336–341. doi: 10.1016/j.ejca.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Egaña N, Socias C, Matteucci T, Bilbao I, Alvarez-Coca M. Thyroid metastasis of lobular breast carcinoma. Endocrinol Nutr. 2012;59:219–220. doi: 10.1016/j.endonu.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Magers MJ, Dueber JC, Lew M, Pang JC, Davenport RD. Metastatic ductal carcinoma of the breast to the thyroid gland diagnosed with fine needle aspiration: a case report with emphasis on morphologic and immunophenotypic features. Diagn Cytopathol. 2016;44:530–534. doi: 10.1002/dc.23462. [DOI] [PubMed] [Google Scholar]
- 5.Lacka K, Breborowicz D, Uliasz A, Teresiak M. Thyroid metastases from a breast cancer diagnosed by fine-needle aspiration biopsy: case report and overview of the literature. Exp Oncol. 2012;34:129–133. [PubMed] [Google Scholar]
- 6.Kim TY, Kim WB, Gong G, Hong SJ, Shong YK. Metastasis to the thyroid diagnosed by fine-needle aspiration biopsy. Clin Endocrinol (Oxf) 2005;62:236–241. doi: 10.1111/j.1365-2265.2005.02206.x. [DOI] [PubMed] [Google Scholar]
- 7.Owens CL, Basaria S, Nicol TL. Metastatic breast carcinoma involving the thyroid gland diagnosed by fine-needle aspiration: a case report. Diagn Cytopathol. 2005;33:110–115. doi: 10.1002/dc.20311. [DOI] [PubMed] [Google Scholar]
- 8.Hegerova L, Griebeler ML, Reynolds JP, Henry MR, Gharib H. Metastasis to the thyroid gland: report of a large series from the Mayo Clinic. Am J Clin Oncol. 2015;38:338–342. doi: 10.1097/COC.0b013e31829d1d09. [DOI] [PubMed] [Google Scholar]
- 9.Cristofanilli M, Gonzalez-Angulo A, Sneige N, Kau SW, Broglio K, Theriault RL, et al. Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. J Clin Oncol. 2005;23:41–48. doi: 10.1200/JCO.2005.03.111. [DOI] [PubMed] [Google Scholar]
- 10.Choi YJ, Pinto MM, Hao L, Riba AK. Interobserver variability and aberrant E-cadherin immunostaining of lobular neoplasia and infiltrating lobular carcinoma. Mod Pathol. 2008;21:1224–1237. doi: 10.1038/modpathol.2008.106. [DOI] [PubMed] [Google Scholar]
- 11.Cimino-Mathews A, Subhawong AP, Illei PB, Sharma R, Halushka MK, Vang R, et al. GATA3 expression in breast carcinoma: utility in triple-negative, sarcomatoid, and metastatic carcinomas. Hum Pathol. 2013;44:1341–1349. doi: 10.1016/j.humpath.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sujoy V, Pinto A, Nosé V. Columnar cell variant of papillary thyroid carcinoma: a study of 10 cases with emphasis on CDX2 expression. Thyroid. 2013;23:714–719. doi: 10.1089/thy.2012.0455. [DOI] [PubMed] [Google Scholar]
- 13.Laury AR, Perets R, Piao H, Krane JF, Barletta JA, French C, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011;35:816–826. doi: 10.1097/PAS.0b013e318216c112. [DOI] [PubMed] [Google Scholar]
- 14.Tacha D, Zhou D, Cheng L. Expression of PAX8 in normal and neoplastic tissues: a comprehensive immunohistochemical study. Appl Immunohistochem Mol Morphol. 2011;19:293–299. doi: 10.1097/PAI.0b013e3182025f66. [DOI] [PubMed] [Google Scholar]
- 15.Hsiao SJ, Nikiforov YE. Molecular approaches to thyroid cancer diagnosis. Endocr Relat Cancer. 2014;21:T301–T313. doi: 10.1530/ERC-14-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]