Abstract
Objective
Neutrophil-to-lymphocyte ratio (NLR) has prognostic value in acute coronary syndromes. We investigated its utility for predicting heart failure (HF) admissions and major adverse cardiac outcomes in patients undergoing transcatheter aortic valve replacement (TAVR).
Methods
Data on clinical, laboratory, procedural, HF admissions, and major adverse cardiac events (MACEs) (all-cause mortality, recurrence of myocardial infarction requiring intervention, stroke) for 298 consecutive patients who underwent TAVR between 2012 and 2016 in our tertiary center were collected.
Results
Analysis included 298 patients. The mean age was 83 ± 8 years, 51% were males, and 95% were Caucasians. The median Society of Thoracic Surgeons risk score was 9 (interquartile range: 6.3–11.8). Receiver-operating curve analysis identified a cutoff value of NLR of 4.0 for MACE after TAVR and sensitivity of 68% and specificity of 68% {area under the curve [AUC] = 0.65 [95% confidence interval (CI): 0.51–0.79], p = 0.03}. An NLR of 4.0 for HF hospitalizations after TAVR and sensitivity of 60% and specificity of 57% [AUC = 0.61 (95% CI: 0.53–0.69), p = 0.01]. NLR ≥4.0 before TAVR significantly predicted MACE after TAVR (68.4% vs. 31.6%, p = 0.02) and HF hospitalizations (58.3% vs. 41.7%, p = 0.03). NLR with TAVR risk score increased the predictive value for MACE after TAVR from AUC = 0.61 (95% CI: 0.50–0.72, p = 0.06) to AUC = 0.69 (95% CI: 0.57–0.80, p = 0.007).
Conclusion
NLR predicts all-cause mortality, MACE, and HF hospitalization 1 year after TAVR. NLR with TAVR risk score improved predictability for MACE. Further studies for prognostication using NLR are warranted.
Keywords: Heart failure, MACE, Mortality, NLR, Readmissions, TAVR
1. Introduction
Inflammation plays an integral role in the pathogenesis of atherosclerotic cardiovascular disease and calcific valvular heart disease. Inflammation causes activation of valvular endothelial cells with subendothelial lipid deposition and infiltration with monocytes transforming into macrophages. Ultimately, the combination of oxidized lipids, angiotensin II, and macrophages promote activation of the inflammatory cascade responsible for matrix remodeling, valvular fibrosis, and eventually calcification.1, 2
Transcatheter aortic valve replacement (TAVR) is a well-established procedure for the treatment of severe aortic stenosis (AS) in intermediate- to high-risk patients who are poor candidates for surgical valve replacement.3, 4 Owing to the global uptrend in the utilization of TAVR, there is an increasing need for improved risk stratification of patients undergoing the procedure. Neutrophil-to-lymphocyte ratio (NLR) is a noninvasive, inexpensive, and widely available hematologic marker of inflammation.1 It has been studied as a prognostic marker in patients with severe calcific stenosis5, 6 and heart failure (HF).7, 8
Post-TAVR hospitalizations are associated with increased rates of mortality and constitute a significant cost burden to the health-care system. There is a growing body of research to identify causes and predictors of HF admissions after TAVR.9, 10, 11 To date, the role of NLR as a prognostic factor in predicting long-term outcomes after TAVR, including HF readmissions, remains to be determined. The goal of our study is four-fold: (1) to determine if NLR is associated with increased risk of major adverse cardiac events (MACE); (2) to determine if NLR is associated with increased risk of HF readmissions; (3) to determine if NLR is an independent predictor for short-term (30 days) and long-term (1 year) mortality in patients undergoing TAVR; and (4) to determine if NLR can be utilized for risk stratification of patients undergoing TAVR.
2. Methods
2.1. Study population
Our study is a retrospective review of a prospectively collected database. The study population comprised patients (n = 610) who were enrolled in the prospective TAVR registry of patients undergoing TAVR from January 2012 to July 2016 at our tertiary care center (Gates Vascular Institute, Buffalo, NY). Out of those, 141 patients did not have baseline echocardiographic variables, and 109 patients did not have baseline laboratory data pertaining to our study available for review. In addition, 42 patients were who had active malignancy or chronic inflammatory diseases were excluded. Finally, 20 patients who were lost to follow-up were excluded from the study. The remainder (n = 298) formed the study group and were included in the final analysis. All data variables were collected according to the standardized definitions adherent with the standards of the Society of Thoracic Surgeons (STS) and American College of Cardiology's National Transcatheter Valve Therapy registry.
2.2. Clinical characteristics
Patient demographics and baseline characteristics including relevant clinical variables, such as medical comorbidities, were collected. In addition, use of home medications, specifically, diuretics, beta-blockers, angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARBs), and potassium-sparing diuretics was reviewed and recorded. Pre-TAVR noninvasive testing data including routine electrocardiogram and echocardiogram variables were collected. Pre-TAVR laboratory data including complete blood count (CBC), complete metabolic panel, and brain natriuretic peptide (BNP) were collected. In addition, data on MACE (all-cause mortality, recurrence of myocardial infarction [MI] requiring percutaneous coronary intervention [PCI] or stroke) and HF admissions 1 year after TAVR were collected.
2.3. Neutrophil-lymphocyte ratio
All study participants underwent pre-TAVR laboratory data testing including CBC. Blood sampling was performed in the TAVR clinic 1–3 weeks before the procedure. Patients whose CBC included differential counts were analyzed specifically. A CBC with five-part differential was measured using Coulter LH 750 Hematology Analyzer. Neutrophil and lymphocyte values were reported in standard international units and cell count ×109/L. Receiver-operating curve (ROC) analysis of NLR identified the NLR cutoff value of the highest accuracy for our primary end points.
2.4. TAVR risk score
TAVR risk score was calculated after incorporating the following variables: age, sex, race, serum creatinine (mg/dl) on the same blood sample from which NLR was calculated, procedural access site, dialysis dependence, presence of the New York Heart Association class IV symptoms within 2 weeks of TAVR, and presence of severe chronic lung disease. The following variables were also included to assess the acuity of TAVR: procedural status (elective vs. urgent vs. emergency vs. salvage), prior cardiac arrest or cardiogenic shock, preprocedure inotrope use, and use of any mechanical assist device.
2.5. Outcomes assessment
The primary end points of this study were in-hospital mortality and MACEs (defined as death from all cause, recurrence of MI requiring PCI, or stroke) at 1 year. The U.S. Social Security Death Index and New York State Death Index records and patient electronic medical records were reviewed to obtain mortality data. The secondary outcome of our study was HF readmissions 1 year after TAVR. HF readmission was defined using the standard definitions of the Multi-Ethnic Study of Atherosclerosis study.15, 12 The University at Buffalo Institutional Review Board approved all procedures with a waiver of individual informed consent. The study conforms to widely accepted ethical principles guiding human research.
2.6. Statistical analysis
The categorical variables were described as frequency (%) and were compared using either chi-square tests of independence or Fishers exact test as appropriate. Continuous variables were summarized as mean ± standard deviation for normally distributed data and medians with interquartile range (IQR) if the data were skewed. Continuous variables were compared across groups using a two-sample/paired t-test or Mann–Whitney U test, as appropriate. ROCs were used to determine the area under the curve (AUC), and Kaplan–Meier survival analysis was used to evaluate freedom from MACE and HF readmissions. Statistical analysis was performed using STATA v13.0 (StataCorp, College Station, Texas). A p-value of <0.05 was considered significant.
3. Results
3.1. Study population
The baseline demographic and clinical characteristics of all patients (n = 298) are outlined in Table 1. The mean age among all patients was 83 ± 8 years, 51% were males, and 95% were Caucasians. The median STS risk score was 9.0 (IQR: 6.3–11.8). Ninety-four percent had hypertension; 43%, atrial fibrillation; 41%, peripheral arterial disease, and 36%, diabetes. Among all patients, diuretic use was 65%; beta-blocker, 68%; ACEi, 24%; ARB, 17%; and potassium-sparing diuretic, 7%.
Table 1.
Baseline characteristics.
| Variables | NLR <4.0 (n = 168 | NLR ≥ 4.0 (n = 130) | p value |
|---|---|---|---|
| Demographics | |||
| Sex (male) | 81 (55%) | 67 (45%) | 0.57 |
| Age at procedure | 82 ± 8 | 83 ± 8 | 0.34 |
| Race (white) | 158 (94%) | 123 (95%) | 0.83 |
| Risk factors | |||
| Smoker | 6 (4%) | 1 (0.8%) | 0.14 |
| Diabetes | 52 (31%) | 53 (41%) | 0.08 |
| Hypertension | 159 (95%) | 121 (93%) | 0.57 |
| Systolic heart failure | 44 (27%) | 38 (30%) | 0.59 |
| Peripheral vascular disease | 67 (49%) | 53 (41%) | 0.88 |
| STS risk score | 8.6 (6.0–11.2) | 9,5 (6.5–12.8) | 0.08 |
| Prior myocardial infarction | 25 (15%) | 26 (20%) | 0.25 |
| Atrial fibrillation/flutter | 72 (43%) | 50 (39%) | 0.44 |
| NYHA class (III or higher) | 53 (33%) | 42 (35%) | 0.81 |
| Stroke history | 19 (11%) | 10 (8%) | 0.30 |
| Baseline BNP | 405 (174–838) | 527 (241–1160) | 0.05 |
| Echocardiographic variables | |||
| Baseline LVEF (%) | 53 ± 13 | 55 ± 14 | 0.29 |
| PAP (mmHg) | 31 ± 15 | 32 ± 114 | 0.6 |
| Home medications | |||
| Diuretics | 104 (62%) | 91 (70%) | 0.15 |
| Beta-blockers | 115 (69%) | 87 (67%) | 0.78 |
| Calcium channel blockers | 36 (21%) | 29 (22%) | 0.86 |
| Angiotensin-converting enzyme inhibitor | 45 (27%) | 31 (24%) | 0.56 |
| Aldosterone receptor blockers | 25 (15%) | 25 (19%) | 0.32 |
| Potassium-sparing diuretics | 10 (6%) | 13 (10%) | 0.19 |
Values are expressed as mean ± standard deviation, median (interquartile range), N (%).
BNP, brain natriuretic peptide; LVEF, left ventricular ejection fraction; NLR, neutrophil-to-lymphocyte ratio; NYHA, New York Heart Association; PAP, pulmonary artery pressure; STS, Society of Thoracic Surgeons.
3.2. NLR and MACE
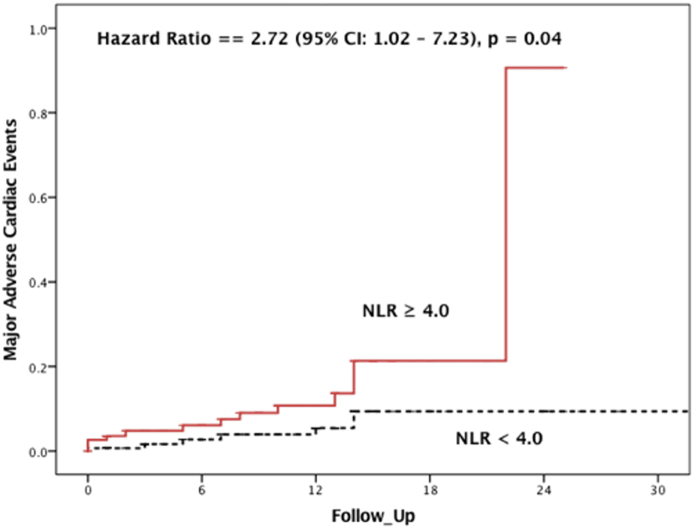
Among all patients, there were 19 (7.2%) patients who experienced a MACE at 1 year after TAVR. As shown in Fig. 1, ROC analysis identified a cutoff value of NLR = 4.0 for MACE after TAVR with a sensitivity of 68% and specificity of 68% [AUC = 0.65 (95% CI: 0.51–0.79), p = 0.03]. In an unadjusted analysis, patients with NLR ≥4.0 before TAVR were significantly more likely to experience MACE after TAVR (68.4% vs. 31.6%, p = 0.02). Next, we adjusted for STS risk score in a Cox proportional model after confirming that the assumptions of Cox model were met. As shown in Fig. 2, survival from MACE was significantly worse among patients with NLR ≥4.0 before TAVR than those with NLR <4.0 [hazard ratio (HR) = 2.72 (95% CI: 1.02–7.23), p = 0.04].
Fig. 1.
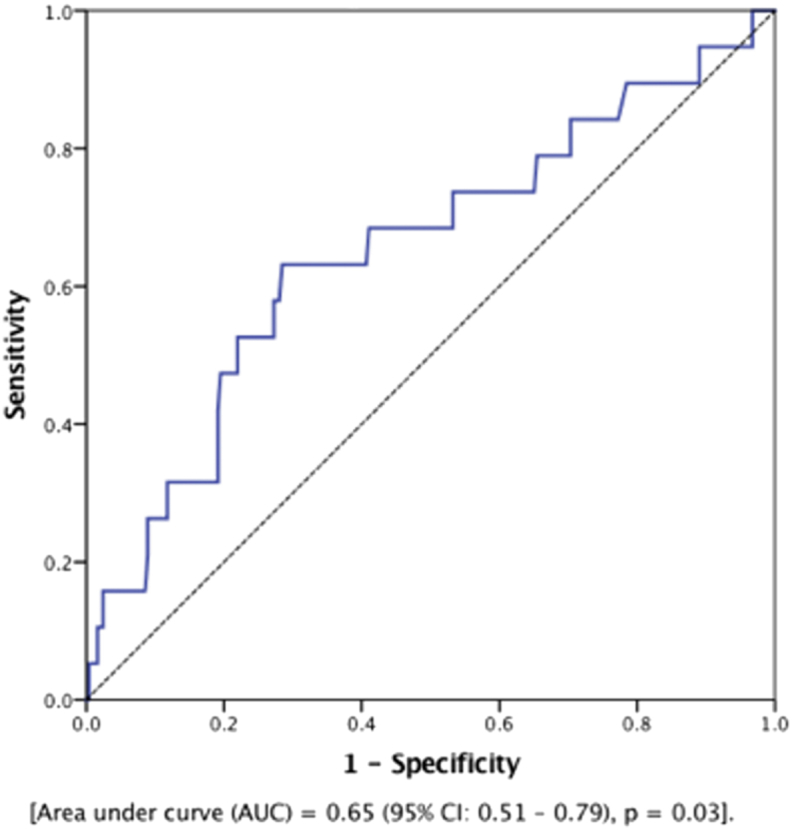
ROC curve showing an NLR cutoff value of 4 for MACE after TAVR. A sensitivity of 68% and specificity of 68% is identified at this value. MACE, major adverse cardiac events; NLR, neutrophil-to-lymphocyte ratio; TAVR, transcatheter aortic valve replacement.
Fig. 2.
Cox proportional hazard model demonstrating survival from MACE was worse among patients with NLR ≥4.0 before TAVR. MACE, major adverse cardiac events; NLR, neutrophil-to-lymphocyte ratio; TAVR, transcatheter aortic valve replacement.
3.3. NLR and HF readmissions
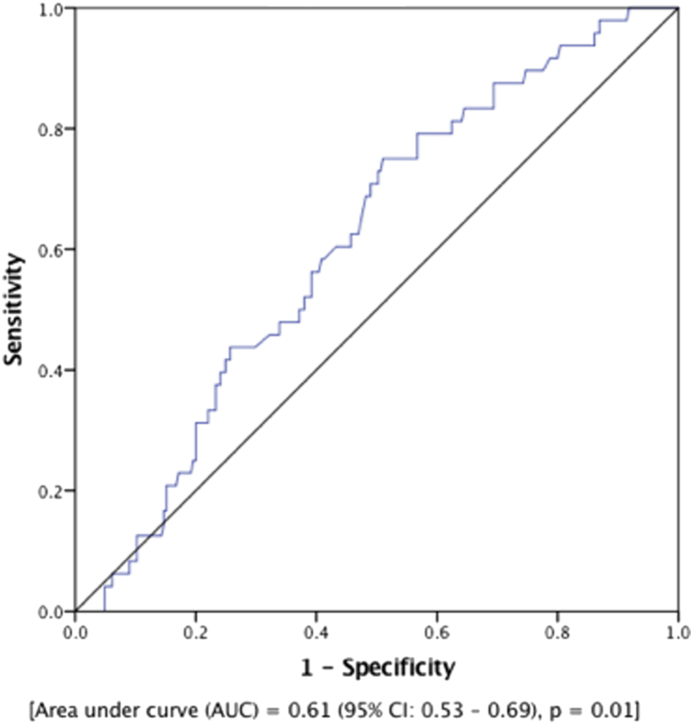
Among all patients who underwent TAVR, there were 48 (16.4%) patients who required hospitalization for HF. Patients with NLR ≥4.0 before TAVR had higher BNP levels during follow-up without significant difference in left ventricular ejection fraction compared to those with NLR <4.0, as shown in Table 2. As shown in Fig. 3, ROC analysis identified a cutoff value of NLR = 4.0 for HF hospitalizations after TAVR with a sensitivity of 60% and specificity of 57% [AUC = 0.61 (95% CI: 0.53–0.69), p = 0.01]. In an unadjusted analysis, patients with NLR ≥4.0 before TAVR had a significantly higher risk of HF hospitalizations compared to those with NLR <4.0 (58.3% VS. 41.7%, p = 0.03). Next, we performed an age-adjusted analysis for preprocedural BNP levels using a Cox proportional model after confirming the assumptions of Cox model were met. As shown in Fig. 4, patients with NLR ≥4.0 before TAVR had significantly worse survival from HF readmissions [HR = 1.9 (95% CI: 1.02–3.39), p = 0.04].
Table 2.
Post-transcatheter aortic valve replacement outcomes.
| Variables | NLR <4.0 (n = 168) | NLR ≥ 4.0 (n = 130) | p value |
|---|---|---|---|
| Post-TAVR aortic valve insufficiency | 48 (31.5%) | 43 (35%) | 0.60 |
| Follow-up BNP | 585 ± 658 | 984 ± 1088 | 0.03 |
| LVEF (%) | 59 ± 10.5 | 58 ± 12.6 | 0.46 |
Values are expressed as mean ± standard deviation, N (%).
BNP, brain natriuretic peptide; LVEF, left ventricular ejection fraction; NLR, neutrophil-to-lymphocyte ratio; TAVR, transcatheter aortic valve replacement.
Fig. 3.
ROC curve showing an NLR cutoff value of 4 for HF admissions after TAVR. A sensitivity of 60% and specificity of 57% are identified at this value. HF, heart failure; NLR, neutrophil-to-lymphocyte ratio; ROC, receiver-operating curve; TAVR, transcatheter aortic valve replacement.
Fig. 4.
Cox proportional hazard model demonstrating NLR ≥4.0 before TAVR had significantly worse survival from HF admissions. HF, heart failure; NLR, neutrophil-to-lymphocyte ratio; TAVR, transcatheter aortic valve replacement.
3.4. Incremental value of NLR
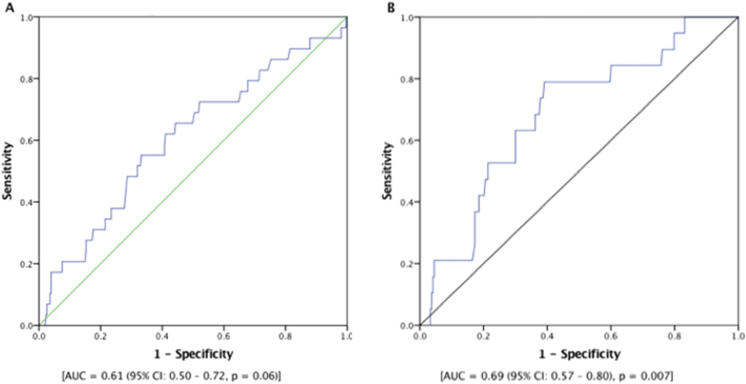
Finally, we calculated the risk of mortality using the TAVR mortality risk calculator. As shown in Fig. 5A, ROC analysis demonstrated that TAVR risk score predicted MACE with AUC = 0.61 (95% CI: 0.50–0.72, p = 0.06). Next, we combined the TAVR mortality risk with NLR in which we assigned one point for NLR ≥4.0 before TAVR. After repeating ROC analysis, combining NLR with TAVR risk score increased the predictive value for occurrence of MACE after TAVR [AUC = 0.69 (95% CI: 0.57–0.80), p = 0.007].
Fig. 5.
(A-B) ROC curves showing that combining NLR with TAVR risk score increased the predictive value for occurrence of MACE after TAVR. MACE, major adverse cardiac events; NLR, neutrophil-to-lymphocyte ratio; ROC, receiver-operating curve; TAVR, transcatheter aortic valve replacement.
4. Discussion
Our study showed that NLR has a prognostic value in the risk stratification of patients undergoing TAVR. The key findings were (1) NLR cutoff of 4 has the highest discriminatory capability to differentiate incidence of MACE or HF hospitalizations after TAVR. (2) Patients with pre-TAVR NLR >4 had a statistically significant higher incidence of MACEs within 1 year after TAVR (3) Patients with pre-TAVR NLR >4 had a significantly higher incidence of HF readmissions within 1 year after TAVR. (4) Combining NLR >4 (as one point) to TAVR risk score increased the predictive value for MACE after TAVR.
In the PARTNER trials, approximately 25% of patients who had undergone TAVR were deceased at 1 year. Among those who survived, a number reported significant HF symptoms, poor quality of life, and functional limitation.4, 13, 14 As such, consensus exists that TAVR should be offered to patients who may derive morbidity and mortality benefit. Identifying such patients remains a current challenge.15 NLR is a useful assessment tool to predict cardiovascular outcomes in several studies.16, 17, 18, 19 A representative sample of the general population of the United States showed that the average value of NLR is 2.15; values were significantly higher in individuals with chronic inflammatory states, such as diabetes, heart disease, obesity, and smoking.20
4.1. Role of NLR in predicting MACE
In stable coronary artery disease and acute coronary syndrome, NLR is independently associated with mortality.16, 17 A recent study showed that patients with left main disease or triple-vessel disease at NLR ≥ 3.39 have significantly higher incidence of 2-year MACEs, including stroke.21 Furthermore, a meta-analysis of eight cohort studies demonstrated a higher NLR as a predictor of MACE in patients undergoing coronary angiography or revascularization.22 A large variation in NLR levels from baseline to time of hospital discharge correlated with worse 1-year outcomes after TAVR; specifically, hospital admissions and mortality.23 Interestingly, high NLR was independently associated with stroke incidence in a retrospective cohort study of over 24,000 adults.24 A greater than 2.5 times risk for in-hospital mortality was identified in nonatrial fibrillation ischemic stroke.25, 26 Furthermore, a high NLR at approximately 1 year after stroke correlated with increased risk of recurrent ischemic stroke.27
4.2. Role of NLR as a prognostic marker in TAVR
In patients with AS, high NLR has been associated with AS severity and MACEs.5, 6 These studies suggest the potential utility of NLR in identifying patients with AS who may require intervention. Our study demonstrates that preoperative NLR independently predicts MACE at 1 year after TAVR. In patients with AS, NLR predicted a twofold higher risk for MACE after TAVR. In addition, subgroup analysis of patients who suffered from MACEs after TAVR also revealed that high NLR is associated with a higher risk of death. NLR maybe a highly valuable, inexpensively obtained, and quickly identifiable tool in aiding with risk stratification and an informed decision. It is one of a number of parameters that should be evaluated independently and in conjunction with other variables to offer the most predictive rate of morbidity and mortality for those undergoing TAVR.
4.3. Role of NLR in predicting HF hospitalizations
The value of NLR in prognostication of patients with HF has been previously studied. NLR was found to be associated with adverse outcomes, regardless of whether it is calculated at the time of hospitalization or during follow-up after index hospitalization.28, 29 Admission NLR was found to serve as a prognostic indicator of mortality in patients with HF, predict poor functional capacity, and the need for transplant in patients with HF.7, 29, 30, 31, 32 The proposed mechanism for NLR as a prognostic marker in patients with HF remains consistent across studies. It is theorized to be two-pronged: firstly, inflammatory reactions are known to contribute to the development of HF33, 34; and secondly, inflammatory stimuli lead to the release of cytokines and proteolytic enzymes causing destruction of the myocardium and decreased left ventricular function.35, 36 In HF, the hypothalamic–pituitary axis is activated leading to increased cortisol production resulting in decrease in the relative concentration of lymphocytes.37, 38 Lymphopenia has also been associated with a poor prognosis in HF.30, 39
Currently, information is scarce in the literature regarding the association between NLR and post-TAVR hospitalization. Our study demonstrates that a higher NLR carries a significantly higher risk of HF hospitalizations after TAVR. In addition to assisting in informed decision-making, NLR can also serve to reduce national and global health-care expenditure costs by decreasing hospital readmissions.
4.4. Incremental value of NLR when combined with TAVR risk score
The TAVR risk score represents a validated tool for the prediction of in-hospital mortality for the unique population undergoing TAVR. Currently, it stands as a reliable predictor of in-hospital mortality and is used in conjunction with a history, physical examination, laboratory data, and clinical judgment to stratify patients. It also provides objective insight into center- and patient-specific outcomes. Of note, limitations include acute mortality prognostication, and currently, the STS surgical aortic valve replacement (SAVR) model remains the most widely model used for 30-day mortality.40 Our study demonstrates that NLR can improve the predictability of MACE when combined with the TAVR risk score. This can help further risk stratify patients once they have been selected for a TAVR procedure even after considering the STS-patient-reported outcome measure (PROM) score. Patients who are less likely to benefit and more likely to have MACE may opt to forego TAVR.
4.5. Study limitations
Our study is a single-center experience, and the results may not be representative of the general population. However, our patient population had equal representation of both sexes, which is uncommon in cardiovascular research. NLR is a nonspecific inflammatory marker that could be affected by various inflammatory conditions. Hence, we excluded patients with chronic inflammatory conditions, history of malignancy, and those on steroid therapy from our study population to minimize possible confounding factors. Although we have adjusted for the majority of factors associated with increased risk of 1-year mortality, MACE, and HF hospitalizations, additional variables that are not routinely measured could potentially confound the results. Patients lost to follow-up and excluded may potentially represent a different study population with better or worse clinical characteristics. In our study, NLR was obtained at one timepoint preoperatively. Postoperative NLR values at various time intervals might augment its prognostic value. Also, cutoff value of NLR with the highest discriminatory capability identified in our study is different than what was reported in prior studies. This points out the need to identify standardized cutoff levels for consistent application.
5. Conclusion
Our study demonstrates NLR as a potentially valuable tool in the prognostication of patients who undergo TAVR. NLR is independently associated with mortality and HF readmissions. The addition of NLR to TAVR risk score improved the predictability of MACE after TAVR. Future larger prospective studies to evaluate the role of NLR at 1 year after TAVR and its utility as a cost-effective screening tool in providing clinically significant data for risk stratification of patients who undergo TAVR patients when combined with other variables are warranted.
Funding
This research did not receive any specific grant from any funding agencies in the public, commercial or not-for-profit sectors.
Conflicts of interest
All authors have none to declare.
References
- 1.Mahler G.J., Butcher J.T. Inflammatory regulation of valvular remodeling: the good(?), the bad, and the ugly. Int J Inflamm. 2011;2011:721419. doi: 10.4061/2011/721419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dweck M.R., Boon N.A., Newby D.E. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol. 2012;60:1854–1863. doi: 10.1016/j.jacc.2012.02.093. [DOI] [PubMed] [Google Scholar]
- 3.Leon M.B., Smith C.R., Mack M.J. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 4.Leon M.B., Smith C.R., Mack M. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 5.Cho K.I., Cho S.H., Her A.Y., Singh G.B., Shin E.S. Prognostic utility of neutrophil-to-lymphocyte ratio on adverse clinical outcomes in patients with severe calcific aortic stenosis. PLoS One. 2016;11:e0161530. doi: 10.1371/journal.pone.0161530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avci A., Elnur A., Goksel A. The relationship between neutrophil/lymphocyte ratio and calcific aortic stenosis. Echocardiography. 2014;31:1031–1035. doi: 10.1111/echo.12534. [DOI] [PubMed] [Google Scholar]
- 7.Benites-Zapata V.A., Hernandez A.V., Nagarajan V., Cauthen C.A., Starling R.C., Tang W.H. Usefulness of neutrophil-to-lymphocyte ratio in risk stratification of patients with advanced heart failure. Am J Cardiol. 2015;115:57–61. doi: 10.1016/j.amjcard.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uthamalingam S., Patvardhan E.A., Subramanian S. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am J Cardiol. 2011;107:433–438. doi: 10.1016/j.amjcard.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 9.Durand E., Doutriaux M., Bettinger N. Incidence, prognostic impact, and predictive factors of readmission for heart failure after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2017;10:2426–2436. doi: 10.1016/j.jcin.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Nombela-Franco L., del Trigo M., Morrison-Polo G. Incidence, causes, and predictors of early (</=30 Days) and late unplanned hospital readmissions after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2015;8:1748–1757. doi: 10.1016/j.jcin.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Kolte D., Khera S., Sardar M.R. Thirty-day readmissions after transcatheter aortic valve replacement in the United States: insights from the nationwide readmissions database. Circ Cardiovasc Interv. 2017;10(1) doi: 10.1161/CIRCINTERVENTIONS.116.004472. e004472. [DOI] [PubMed] [Google Scholar]
- 12.Bhardwaj A., Ramanan T., Khalil C. Valvuloarterial impedance predicts heart failure readmissions in patients undergoing transcatheter aortic valve replacement. Structural Heart. 2017;1:277–284. [Google Scholar]
- 13.Reynolds M.R., Magnuson E.A., Lei Y. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124:1964–1972. doi: 10.1161/CIRCULATIONAHA.111.040022. [DOI] [PubMed] [Google Scholar]
- 14.Smith C.R., Leon M.B., Mack M.J. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 15.Mack M.J., Holper E.M. TAVR risk assessment: does the eyeball test have 20/20 vision, or can we do better? J Am Coll Cardiol. 2016;68:353–355. doi: 10.1016/j.jacc.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 16.Papa A., Emdin M., Passino C., Michelassi C., Battaglia D., Cocci F. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta. 2008;395:27–31. doi: 10.1016/j.cca.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Azab B., Zaher M., Weiserbs K.F. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. 2010;106:470–476. doi: 10.1016/j.amjcard.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 18.Akpek M., Kaya M.G., Lam Y.Y. Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2012;110:621–627. doi: 10.1016/j.amjcard.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 19.Tokgoz S., Kayrak M., Akpinar Z., Seyithanoglu A., Guney F., Yuruten B. Neutrophil lymphocyte ratio as a predictor of stroke. J Stroke Cerebrovasc Dis. 2013;22:1169–1174. doi: 10.1016/j.jstrokecerebrovasdis.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Azab B., Camacho-Rivera M., Taioli E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects. PLoS One. 2014;9:e112361. doi: 10.1371/journal.pone.0112361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu N., Tang X.F., Yao Y. Predictive value of neutrophil to lymphocyte ratio in long-term outcomes of left main and/or three-vessel disease in patients with acute myocardial infarction. Cathet Cardiovasc Interv. 2018;91(S1):551–557. doi: 10.1002/ccd.27495. [DOI] [PubMed] [Google Scholar]
- 22.Wang X., Zhang G., Jiang X., Zhu H., Lu Z., Xu L. Neutrophil to lymphocyte ratio in relation to risk of all-cause mortality and cardiovascular events among patients undergoing angiography or cardiac revascularization: a meta-analysis of observational studies. Atherosclerosis. 2014;234:206–213. doi: 10.1016/j.atherosclerosis.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Condado J.F., Junpaparp P., Binongo J.N. Neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) can risk stratify patients in transcatheter aortic-valve replacement (TAVR) Int J Cardiol. 2016;223:444–449. doi: 10.1016/j.ijcard.2016.08.260. [DOI] [PubMed] [Google Scholar]
- 24.Suh B., Shin D.W., Kwon H.M. Elevated neutrophil to lymphocyte ratio and ischemic stroke risk in generally healthy adults. PLoS One. 2017;12:e0183706. doi: 10.1371/journal.pone.0183706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ertas G., Sonmez O., Turfan M. Neutrophil/lymphocyte ratio is associated with thromboembolic stroke in patients with non-valvular atrial fibrillation. J Neurol Sci. 2013;324:49–52. doi: 10.1016/j.jns.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 26.Fang Y.N., Tong M.S., Sung P.H. Higher neutrophil counts and neutrophil-to-lymphocyte ratio predict prognostic outcomes in patients after non-atrial fibrillation-caused ischemic stroke. Biomed J. 2017;40:154–162. doi: 10.1016/j.bj.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue J., Huang W., Chen X. Neutrophil-to-Lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26:650–657. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Yan W., Li R.J., Jia Q., Mu Y., Liu C.L., He K.L. Neutrophil-to-lymphocyte ratio compared to N-terminal pro-brain natriuretic peptide as a prognostic marker of adverse events in elderly patients with chronic heart failure. J Geriatr Cardiol. 2017;14:127–134. doi: 10.11909/j.issn.1671-5411.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu C., Chen M., Chen Z., Lu G. Predictive and prognostic value of admission neutrophil-to-lymphocyte ratio in patients with CHD. Herz. 2016;41:605–613. doi: 10.1007/s00059-015-4399-8. [DOI] [PubMed] [Google Scholar]
- 30.Durmus E., Kivrak T., Gerin F., Sunbul M., Sari I., Erdogan O. Neutrophil-to-Lymphocyte ratio and platelet-to-lymphocyte ratio are predictors of heart failure. Arq Bras Cardiol. 2015;105:606–613. doi: 10.5935/abc.20150126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turfan M., Erdogan E., Tasal A. Neutrophil-to-lymphocyte ratio and in-hospital mortality in patients with acute heart failure. Clinics (Sao Paulo). 2014;69:190–193. doi: 10.6061/clinics/2014(03)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cakici M., Cetin M., Dogan A. Neutrophil to lymphocyte ratio predicts poor functional capacity in patients with heart failure. Turk Kardiyol Dernegi Arsivi. 2014;42:612–620. doi: 10.5543/tkda.2014.16363. [DOI] [PubMed] [Google Scholar]
- 33.Yndestad A., Damas J.K., Oie E., Ueland T., Gullestad L., Aukrust P. Systemic inflammation in heart failure--the whys and wherefores. Heart Fail Rev. 2006;11:83–92. doi: 10.1007/s10741-006-9196-2. [DOI] [PubMed] [Google Scholar]
- 34.Anker S.D., von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart. 2004;90:464–470. doi: 10.1136/hrt.2002.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mann D.L., Young J.B. Basic mechanisms in congestive heart failure. Recognizing the role of proinflammatory cytokines. Chest. 1994;105:897–904. doi: 10.1378/chest.105.3.897. [DOI] [PubMed] [Google Scholar]
- 36.Torre-Amione G., Kapadia S., Benedict C., Oral H., Young J.B., Mann D.L. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 37.Maisel A.S., Knowlton K.U., Fowler P. Adrenergic control of circulating lymphocyte subpopulations. Effects of congestive heart failure, dynamic exercise, and terbutaline treatment. J Clin Invest. 1990;85:462–467. doi: 10.1172/JCI114460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ommen S.R., Hodge D.O., Rodeheffer R.J., McGregor C.G., Thomson S.P., Gibbons R.J. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation. 1998;97:19–22. doi: 10.1161/01.cir.97.1.19. [DOI] [PubMed] [Google Scholar]
- 39.Rudiger A., Burckhardt O.A., Harpes P., Muller S.A., Follath F. The relative lymphocyte count on hospital admission is a risk factor for long-term mortality in patients with acute heart failure. Am J Emerg Med. 2006;24:451–454. doi: 10.1016/j.ajem.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Edwards F.H., Cohen D.J., O'Brien S.M. Development and validation of a risk prediction model for in-hospital mortality after transcatheter aortic valve replacement. JAMA Cardiol. 2016;1:46–52. doi: 10.1001/jamacardio.2015.0326. [DOI] [PubMed] [Google Scholar]