Abstract
A solitary pelvic tumor after treating a primary endometriosis-related neoplasm is usually considered a recurrence but may actually be a primary endometriosis-related peritoneal neoplasm. A Japanese woman in her late 60s was referred to our hospital for a solitary pelvic tumor. The tumor was suspected as a recurrence of a previously treated stage IA ovarian clear-cell carcinoma, and resected. Pathological analysis revealed that the tumor was a peritoneal seromucinous carcinoma associated with pelvic endometriosis. Both tumors displayed distinct histopathologies, although both neoplasms were endometriosis related. An apparent recurrent tumor after treating of an endometriosis-related neoplasm might not be a true recurrent tumor but a second primary endometriosis-related neoplasm. Histological confirmation is indicated in such cases.
Keywords: Clear cell carcinoma, Endometriosis, Endometriosis-related neoplasm, Ovarian cancer, Seromucinous carcinoma
Highlights
-
•
Different histologic subtypes of endometriosis-related neoplasms can metachronically occur in a patient.
-
•
An apparent recurrent tumor after treating endometriosis-related ovarian neoplasms (ERONs) may be a primary tumor.
-
•
Histological confirmation of an apparent recurrent tumor after treating ERONs is indicated.
-
•
Peritoneal endometriosis resection may prevent the occurrence of endometriosis-related peritoneal neoplasms.
1. Introduction
Endometriosis has been established as a risk factor for epithelial ovarian cancer. A history of endometriosis is known to increase the risk of subsequent ovarian carcinoma by 2 to 3 fold, specifically that of clear-cell carcinoma (CCC), and endometrioid carcinoma (EC) (Pearce et al., 2012). For this reason, these tumors are termed as endometriosis-related ovarian neoplasms (ERONs) (Maeda and Shih Ie, 2013). Seromucinous tumors (seromucinous carcinoma or seromucinous borderline tumor), formerly known as endocervical-type mucinous and mixed epithelial carcinoma of the mullerian type, or endocervical-type mucinous borderline tumor (or as mullerian mucinous borderline tumor) (Kurman et al., 2014), are also categorized as ERONs (Maeda and Shih Ie, 2013).
Clinical management of endometriosis to prevent the occurrence of ERONs has not been standardized. The European Society of Human Reproduction and Embryology guideline for endometriosis management recommends no change in the current overall management protocol for endometriosis regarding development of cancer (Dunselman et al., 2014), probably due to lack of sufficient clinical data regarding methodologies to lower subsequent risks, although ovarian cancer risk is higher in women with endometriosis. Thus, accumulation of cases of ERONs is needed to understand the potential behavior of endometriosis-related neoplasms and establish the best clinical management of endometriosis with respect to subsequent malignancies.
Herein, we report a patient with a primary seromucinous carcinoma of the peritoneum, following the treatment of a primary CCC of the left ovary. We diagnosed these two tumors as independent cancers associated with endometriosis, since both tumors displayed distinct histopathological features.
2. Case
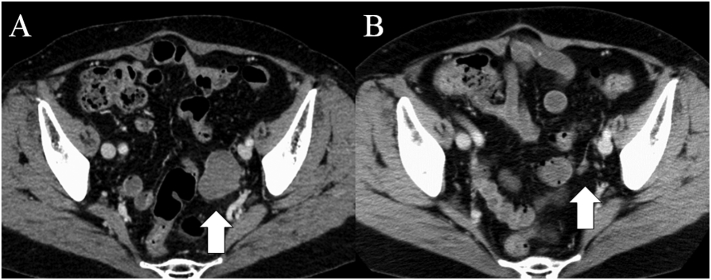
A Japanese woman in her late 60s was referred to our hospital for the treatment of recurrent ovarian CCC. She had a history of an endometrioma of the ovary in her late 30s, which was clinically diagnosed but not surgically treated. Three years prior to referral, she was treated for a Fédération Internationale de Gynécologie et d'Obstétrique stage IA CCC of the left ovary. Treatment included surgery (total abdominal hysterectomy, bilateral salpingo-oophorectomy, sub-total omentectomy) followed by 6 cycles of conventional paclitaxel/carboplatin adjuvant chemotherapy. She did not use hormone replace therapy. During a regular follow-up visit at 3 years and 3 months after adjuvant chemotherapy completion, a cystic and solid mass at the left pelvic wall was detected by a transvaginal ultrasound examination. Subsequent computed tomography (CT) (Fig. 1A) and 18F-fluorodeoxyglucose positron-emission tomography CT scans supported the diagnosis of a recurrent tumor. No other metastasis was revealed. A review of prior CT scans revealed that no tumor was detected at 1 year after adjuvant chemotherapy completion, but a 1-cm nodule was detected 10 months prior to the detection of the second tumor (Fig. 1B).
Fig. 1.
Computed tomography scans of the pelvis (A & B). A, A 4-cm tumor was detected in the left side of the pelvis (white arrow, 3 years and 3 months after adjuvant chemotherapy completion for the first tumor); B, A small 1-cm nodule was detected at the same location as the second tumor (white arrow, 10 months prior to the detection of the second tumor).
An elastic-firm mass fixed at the left pelvic wall was palpated by a pelvic examination. A transvaginal ultrasound examination revealed a 4-cm, cystic, solid tumor, devoid of blood flow in the solid component. A magnetic resonance image showed a 4.5-cm cystic and solid mass with hemorrhagic content. The serum cancer antigen 125 level was 14 units/mL. Although stage IA CCC of the ovary has been reported to have a favorable recurrence-free survival (Mizuno et al., 2012), we suspected the tumor to be a solitary recurrent tumor of CCC. A secondary debulking surgery was performed after informed consent was obtained.
A laparotomy revealed no ascites, and washing cytology revealed an absence of tumor cells. No metastatic lesion was suspected at the peritoneal surface. The tumor was encapsulated and adhered between the sigmoid colon and the left pelvic wall. The tumor was extirpated with a small portion of the adhered serosa of the mesocolon, without tumor rupture. A seromucinous carcinoma of the peritoneum was diagnosed. Six cycles of adjuvant chemotherapy with a dose-dense paclitaxel/carboplatin regimen were performed.
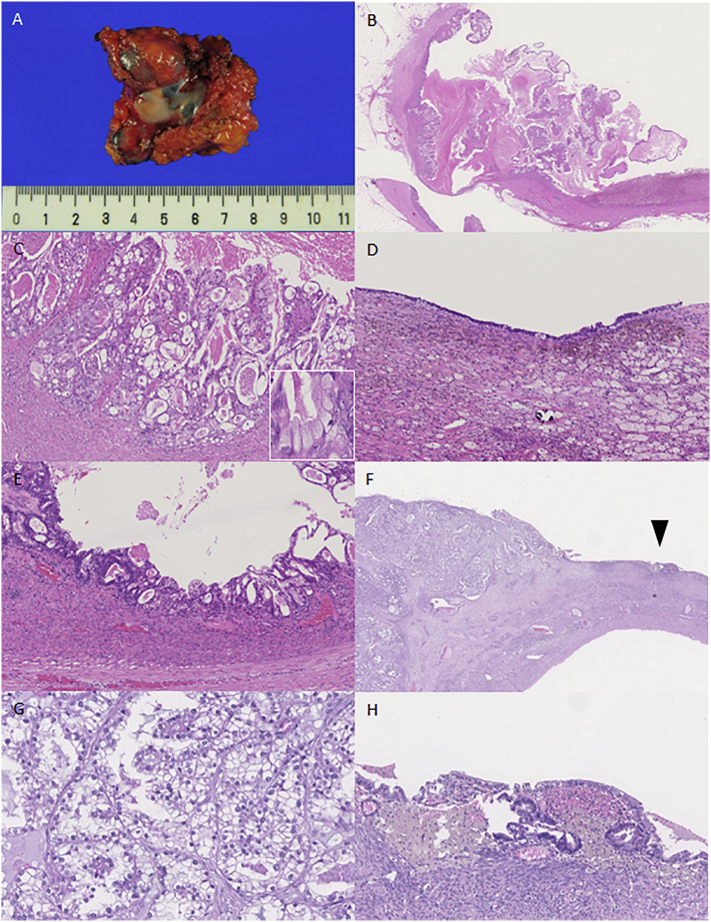
Macroscopically, the resected mass was ~6 cm in diameter (Fig. 2A), and the interior contained dark-green mucinous fluids. Dark-yellowish mossy protrusions and a 2-cm papillary protrusion were seen on the inner surface of the tumor. Microscopically, mucin- or eosinophilic vesicle-containing atypical cells formed dendritic or papillary structures, or proliferated in fused glands in the papillary protrusion (Fig. 2B and C). Hemosiderin-containing foamy cells aggregated in the dark-yellowish mossy protrusion and as a monolayer of mucin-containing normal cells and atypical cells, which indicated that a borderline tumor existed at the inner surface of the tumor (Fig. 2D and E). We diagnosed the tumor as an endometriosis-associated peritoneal seromucinous carcinoma. Pathological review of the primary ovarian tumor (which was distinct from the peritoneal seromucinous carcinoma) revealed that the CCC may have proliferated and invaded an endometrioma of the ovary (Fig. 2F–H).
Fig. 2.
Seromucinous carcinoma of the peritoneum (A–E) and clear-cell carcinoma of the ovary (F–H). A, Macroscopic view of an oligocystic lesion on the peritoneum; B, At lower magnification, papillary structures with edematous stroma are observed; C, At higher magnification, fused irregular glands show stromal invasion, while several tumor cells contain mucus (inset); D, Endometriosis observed on the background cyst wall; E, Components of a borderline seromucinous tumor present; F, Clear-cell carcinoma shows tubulo-cystic, papillary, and solid structure (left side) accompanied by endometriosis on the adjacent cystic wall (arrow head); G, Clear-cell carcinoma; H, At higher magnification, endometriosis and aggregation of hemosiderin-laden macrophages are observed. All microscopic slides (B–E and F–H) are hematoxylin and eosin-stained.
3. Discussion
We report a primary peritoneal seromucinous carcinoma following the treatment of a primary ovarian CCC. Both tumors were likely endometriosis associated.
The metachronous nature of the two endometriosis-related neoplasms is a clinically significant feature, so that a histological confirmation of a seemingly recurrent tumor after the treatment of ERON is thus necessary for treatment decisions. The second tumor was initially suspected to be a recurrent CCC, although it was subsequently diagnosed to be a primary peritoneal seromucinous carcinoma because of the co-existence of normal and atypical endometriosis in the second tumor. Although both tumors in the present case were endometriosis-related neoplasms, they were clearly distinct histologically.
ERONs (CCC, EC, and seromucinous carcinoma) are considered type I ovarian carcinomas, which develop from a benign precursor lesion, and the molecular genetic features of type I tumors have been elucidated (Kurman and Shih Ie, 2016). Endometriosis is the potential precursor of ERONs. ARID1A mutations are found in 46% of CCC and 30% of EC (Wiegand et al., 2010), and the mutation is considered an early molecular event of endometriosis in the development of CCC and EC (Wiegand et al., 2010; Ayhan et al., 2012). ARID1A is suggested to be an ERON associated gene (Maeda and Shih Ie, 2013). Several mutations have also been detected in each subtype of ERONs which include the following: PIK3CA mutation in CCC (Kuo et al., 2009), CTNNB1 and PTEN mutations in EC (Wu et al., 2007), and KRAS mutation in seromucinous carcinoma (Rambau et al., 2017). These results suggest that the histological subtype of ERONs is determined by mutations involved during the development of ERONs.
Carcinogenesis from ovarian endometriosis and peritoneal endometriosis may be different. Carcinogenesis from endometriomas of the ovary is possibly caused by chronic oxidative stress originating from hemorrhagic cystic contents (Yamaguchi et al., 2008). As mentioned above, ovarian CCC and EC are associated with somatic mutations of the ARID1A (Wiegand et al., 2010). Recently, normal-appearing deep-infiltrating endometriosis has been shown to harbor somatic mutations, including driver mutations of the ARID1A, PIK3CA, KRAS, or the PPP2R1A gene (Anglesio et al., 2017). Such mutations may lead to extra-ovarian endometriosis-related neoplasms, such as those from the vagina, fallopian tube or mesosalpinx, pelvic side wall, colon, or the parametrium (Leiserowitz et al., 2003). The second peritoneal seromucinous carcinoma of our case may already be harboring gene mutation different from that of the first ovarian CCC at the time of its potential precursor, peritoneal endometriosis.
Surgical resection and pathological confirmation of peritoneal endometriosis should be considered during surgery for ERONs. The European Society of Human Reproduction and Embryology guideline does not support surgical resection of asymptomatic lesions of peritoneal endometriosis, in general (Dunselman et al., 2014). In patients with ERONs, peritoneal endometriosis may be either a metastasis of the ERON or an abnormal peritoneal endometriosis harboring carcinogenic mutations, which may undergo a subsequent malignant change (Anglesio et al., 2017). If abnormal peritoneal endometriosis can be recognized and resected during surgical treatment for ERONs, subsequent tumors may be preventable.
In conclusion, we report the case of a patient with two different metachronous endometriosis-related neoplasms, a primary peritoneal seromucinous carcinoma, and a primary ovarian CCC. An apparent recurrent tumor after the treatment of ERONs might not be a true recurrence but a primary endometriosis-related neoplasm, so histological confirmation is necessary. Whether resection of suspicious lesions of peritoneal endometriosis during surgical treatment of an ERON can avoid a second primary cancer of the peritoneum should be elucidated further.
Consent
Written informed consent was obtained from the patient for the publication of this case report.
Conflict of interest
We have no conflict of interest to declare.
Author contribution
T.U. drafted and revised the manuscript and prepared the figures. H.Y. prepared the figures and revised the manuscript. K.T. and T.K. revised the manuscript. All the authors have read and approved the final manuscript.
Acknowledgments
No funding was obtained for this study.
We would like to thank Editage (www.editage.jp) for English language editing.
References
- Anglesio M.S., Papadopoulos N., Ayhan A., Nazeran T.M., Noe M., Horlings H.M., Lum A., Jones S., Senz J., Seckin T. Cancer-associated mutations in endometriosis without cancer. N. Engl. J. Med. 2017;376:1835–1848. doi: 10.1056/NEJMoa1614814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayhan A., Mao T.L., Seckin T., Wu C.H., Guan B., Ogawa H., Futagami M., Mizukami H., Yokoyama Y., Kurman R.J. Loss of ARID1A expression is an early molecular event in tumor progression from ovarian endometriotic cyst to clear cell and endometrioid carcinoma. Int. J. Gynecol. Cancer. 2012;22:1310–1315. doi: 10.1097/IGC.0b013e31826b5dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunselman G.A., Vermeulen N., Becker C., Calhaz-Jorge C., D'Hooghe T., De Bie B., Heikinheimo O., Horne A.W., Kiesel L., Nap A. ESHRE guideline: management of women with endometriosis. Hum. Reprod. 2014;29:400–412. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- Kuo K.T., Mao T.L., Jones S., Veras E., Ayhan A., Wang T.L., Glas R., Slamon D., Velculescu V.E., Kuman R.J. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am. J. Pathol. 2009;174:1597–1601. doi: 10.2353/ajpath.2009.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurman R.J., Shih Ie M. The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am. J. Pathol. 2016;186:733–747. doi: 10.1016/j.ajpath.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurman R.J., Carcangiu M.L., Herrington C.S., RH Young. International Agency for Research on Cancer; Lyon: 2014. WHO Classification of Tumours of Female Reproductive Organs. [Google Scholar]
- Leiserowitz G.S., Gumbs J.L., Oi R., Dalrymple J.L., Smith L.H., Ryu J., Scudder S., AH Russell. Endometriosis-related malignancies. Int. J. Gynecol. Cancer. 2003;(13):466–471. doi: 10.1046/j.1525-1438.2003.13329.x. [DOI] [PubMed] [Google Scholar]
- Maeda D., Shih Ie M. Pathogenesis and the role of ARID1A mutation in endometriosis-related ovarian neoplasms. Adv. Anat. Pathol. 2013;20:45–52. doi: 10.1097/PAP.0b013e31827bc24d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M., Kajiyama H., Shibata K., Mizuno K., Yamamuro O., Kawai M., Nakanishi T., Nagasaka T., Kikkawa F. Adjuvant chemotherapy for stage i ovarian clear cell carcinoma: is it necessary for stage IA? Int. J. Gynecol. Cancer. 2012;22:1143–1149. doi: 10.1097/IGC.0b013e31825c7cbe. [DOI] [PubMed] [Google Scholar]
- Pearce C.L., Templeman C., Rossing M.A., Lee A., Near A.M., Webb P.M., Nagle C.M., Doherty J.A., Cushing-Haugen K.L., Wicklund K.G. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;(13):385–394. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambau P.F., McIntyre J.B., Taylor J., Lee S., Ogilvie T., Sienko A., Morris D., Duggan M.A., McCluggage W.G., Kobel M. Morphologic reproducibility, genotyping, and immunohistochemical profiling do not support a category of seromucinous carcinoma of the ovary. Am. J. Surg. Pathol. 2017;41:685–695. doi: 10.1097/PAS.0000000000000812. [DOI] [PubMed] [Google Scholar]
- Wiegand K.C., Shah S.P., Al-Agha O.M., Zhao Y., Tse K., Zeng T., Senz J., McConechy M.K., Anglesio M.S., Kalloger S.E. ARID1A mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Hendrix-Lucas N., Kuick R., Zhai Y., Schwartz D.R., Akyol A., Hanash S., Misek D.E., Katabuchi H., Williams B.O. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell. 2007;(11):321–333. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Mandai M., Toyokuni S., Hamanishi J., Higuchi T., Takakura K., Fujii S. Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin. Cancer Res. 2008;14:32–40. doi: 10.1158/1078-0432.CCR-07-1614. [DOI] [PubMed] [Google Scholar]