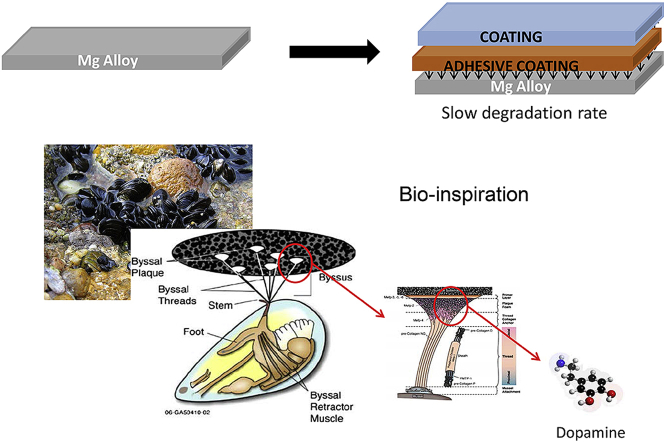
Abstract
Magnesium alloys are candidates to be used as biodegradable biomaterials for producing medical device. Their use is restricted due to the high degradation rate in physiological media. To contribute to solving this problem, a polydopamine (PDOPA) layer could be used to increase adhesion between the metallic substrate and external organic coating. In this paper, the corrosion behaviour of samples was investigated to determine their performance during the long-term exposure in simulated body fluid. Electrochemical methods including Open Circuit Potential (OCP) and Electrochemical Impedance Spectroscopy (EIS) were used to investigate the corrosion resistance of samples. The results demonstrated a decreasing of the substrate degradation rate when PDOPA was used as interlayer supposing a synergistic effect when it was used together with the organic coating.
Keywords: Magnesium alloy, Polydopamine coating, Corrosion resistance, Biodegradability
Graphical abstract
Highlights
-
•
The corrosion resistance improvement of a AZ31 magnesium alloy by using polydopamine (PDOPA) film.
-
•
Electrochemical response revealed a good corrosion resistance of the metallic substrate coated with PDOPA and organic film.
-
•
PDOPA film, used as intermediate layer, has a great potential for improving the degradation rate of magnesium alloy.
1. Introduction
Magnesium and its alloys can be used as biodegradable biomaterials [[1], [2], [3]] to produce medical devices [4,5] due to their biocompatibility. Conversely, the main limitations of the medical use of magnesium alloys are their high susceptibility to corrosion in physiological environments and the release of hydrogen gas during degradation. Nowadays, researches focused on improving the corrosion resistance of magnesium alloys have received increasing attention [[6], [7], [8]].
As for other metals that are used to produce medical devices, i.e. titanium and its alloys [[9], [10], [11], [12]], it is necessary to consider surface treatments to improve their corrosion resistance, antibacterial activity and cellular adhesion. A way to enhance devices surface properties is to use organic coatings. Several authors [[13], [14], [15], [16]] tested different biodegradable polymers, like polycaprolactone (PCL) and polylactic acid (PLA), to slow the degradation rate of the substrate, but it is well known that the Mg degradation rate accelerated when the pH increase as happening in the corrosion processes of Mg. In fact, Chen et al. [16] proved that the alkaline environment degraded the coating of PCL and PLA that covered the surface of high purity magnesium. A possible procedure to slow down the metal dissolution rate could be the usage of a biodegradable and biocompatible intermediate layer between the metal substrate and the external coating, in order to increase the bonding at the interface between them.
Particular curiosity was inspired by the adhesion mechanism used by mussels to bond to various surfaces, like natural inorganic and organic materials, synthetic materials, in wet and dry environments [[17], [18], [19]]. This feature is due to fibres containing polydopamine (PDOPA) that as well as promoting cell adhesion, is biocompatible and biodegradable [20,21]. To the best of the author's knowledge, few papers [6,[22], [23], [24], [25]] dealt with the use of PDOPA as an insulating layer to slow down the substrate degradation rate, therefore, it is necessary to acquire new knowledge on this field. The present work aims to study the corrosion behaviour of AZ31 magnesium alloy, when coated using a PDOPA intermediate layer between substrate metallic and external organic coating, during exposure to Hank's solution.
2. Materials and methods
AZ31 magnesium alloy plates with dimensions of 130 mm × 70 mm × 3 mm were used as the substrate, its composition is reported in Table 1.
Table 1.
Nominal composition of AZ31 magnesium alloy in weight %.
| Element | Al | Zn | Mn | Si | Cu | Fe | Ni | Others | Mg |
|---|---|---|---|---|---|---|---|---|---|
| 2.5–3.5 | 0.7–1.3 | 0.2–1 | 0.05 | 0.01 | >0.05 | >0.05 | 0.4 | Bal. |
All the samples used in this work were ground using SiC papers ranging from 240 to 1200 grits using high purity ethanol to remove contamination layers and native oxide, as suggested by Geels [26]. After mechanical lapping process, a wet chemical etching was performed in 0.15 M hydrochloride acid (HCl) aqueous solution for 10 s and stirred at 300 rpm. Then, each sample was rinsed in the deionized water and dried in air.
Some samples were coated by using a water-based epoxy resin and cured at 150 °C for 10 min. The choice of using an epoxy resin, instead of polymer coatings made of PLC or PLA, was due to that the epoxy resins are stable in a wide range of pH and degraded much slower than the biodegradable materials. In addition, the epoxy resin chosen did not contain anticorrosive pigments, but it contains 63% wt. of solid, mainly titanium oxide additionally barium sulphate and calcium carbonate so that its protective properties are scarce. More details were discussed in a previous paper and not reported herein for brevity [27]. The thickness measurement of the epoxy coating was evaluated and the value was of the 40 μm ± 2 μm.
Other samples were coated using the PDOPA or the PDOPA and the epoxy coating. The polydopamine layer was obtained by immersion the samples in the aqueous solution containing 2 mg/ml of dopamine hydrochloride and 10 mM of Trizma-base, stirred at 500 rpm at pH of 8.5 for 24 h. At the end of the dipping, the samples were rinsed in deionized water and cured at 150 °C for 10 min in the oven [28,29]. The measurement of the thickness of PDOPA coating was also assessed and the value was of 4.5 μm±1 μm.
Moreover the coating/substrate adhesion was evaluated in the previous paper and we do not repeat the test here [27].
The acronyms used in order to identify the samples are reported in Table 2.
Table 2.
Acronyms used to identify samples.
| Acronym | Description of the sample |
|---|---|
| AZ | Mechanical lapped and chemical etched |
| AZD | As AZ and PDOPA coated |
| AZE | As AZ and epoxy resin coated |
| AZDE | As AZ, coated by PDOPA and epoxy resin |
The corrosion behaviour of the various sample was investigated by Electrochemical Impedance Spectroscopy (EIS). The samples were soaked in Hank's solution for 0, 3, 7, 15 days at 37 °C. A conventional three-electrode cell was used, with saturated calomel electrode (SCE) as the reference electrode, a platinum electrode as the counter electrode and the tested sample as the working electrode. The exposed samples area was 2.83 cm2. EIS measurements were carried out imposing a sinusoidal perturbation of 10 mV over the frequency range between 100 KHz and 2 mHz. Before the EIS analysis, the open circuit potential (OCP) was recorded for 90 min. For each electrochemical test, three measurements were carried out and good reproducibility was noted.
The surface morphologies and the chemical compositions of each tested sample were observed by Scanning Electron Microscope (SEM) equipped with the Energy Dispersive Spectroscopy (EDS) probe. The SEM micrographs and EDS analysis were performed before EIS measurements and after the immersion time.
3. Results and discussion
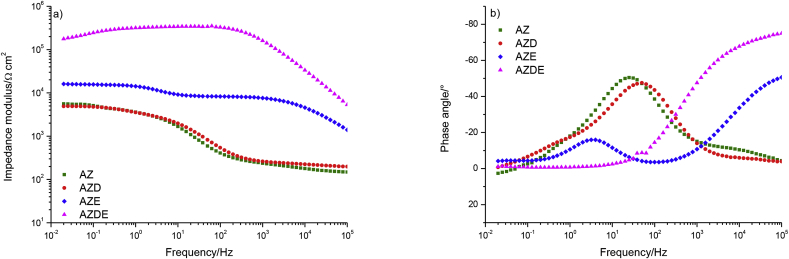
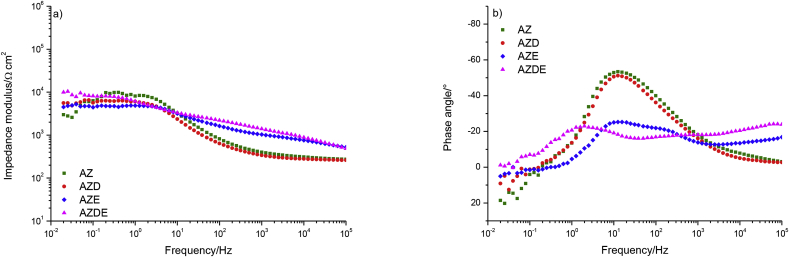
In Fig. 1 a direct comparison of the impedance modulus and phase angle of the tested samples at the beginning of the immersion in Hank's solution is presented.
Fig. 1.
Comparison of EIS spectra of investigated samples at the beginning of the immersion period in Hank's solution.
The AZ and AZD samples showed the quite similar behaviour of both the impedance modulus and the phase angle, in fact, both displayed low values of impedance modulus, at low frequencies, of about 5 × 103 Ohm⋅cm2 and the shape of curves was quite similar too, further, both spectra exhibited a single time constant. In addition, at the high-frequency range, the phase angles of AZ and AZD samples were close to 0° while the phase angles of AZE and AZDE, in the same frequency range, were −50° and −75° respectively. This evidence demonstrated that the PDOPA layer, coating the AZD sample, offered negligible protection against corrosion.
At low-frequency range the AZE sample exhibited a very low total impedance modulus showing a poor corrosion resistance due to the absence of corrosion inhibitors and/or any kind of anticorrosive pigments inside the epoxy resin. The impedance modulus was about than 2 × 104 Ohm⋅cm2 that could indicate the poor quality of the coating. On the other hand, in previous papers [30,31], has been demonstrated that an organic layer, made of the same type of commercial product and retaining the same thickness, was fully saturated by the water in about 2.7 h. So that, the low values of the modulus of impedance can be explained by taking into account that the epoxy resin had absorbed about the 80% of the saturation amount of water, during the preparation and execution of the EIS test (taking in to account that the OCP monitoring was required before EIS measurement requiring about 90 min). The shape of curves reported in Fig. 1, allowed stating that the sample exhibited a well-developed degradation process at interface showing two-time constants.
The AZDE sample exhibited the highest impedance modulus value of 1.7 × 105 Ω cm2, so this highlighted the effect due to the use of the PDOPA in contributing to improving the system corrosion resistance. The spectra recorded examining AZDE sample showed one time constant demonstrating that electrolyte had saturated the coating but there was no corrosion at the metallic substrate/coating interface. The slight decay of modulus of impedance, registered at low frequencies, was explained taking into account that electrolytes still penetrate the coating during the test, decreasing the modulus of impedance value.
To sum up, the AZ and AZD specimens displayed a similar shape of impedance modulus and, considering the phase angles (Fig. 1b), both exhibited one peak at the medium frequency region. In light of these observations, concluding that polydopamine had no protective effect. At the beginning of the immersion test, AZDE sample showed one time constant and presented the impedance modulus of one order of magnitude higher than that shown by the AZE sample. So in this condition, AZDE showed better protective behaviour than that displayed by AZE sample. The ameliorate protective properties of the AZDE sample compared to that of the AZE one has also been confirmed observing the phase angle values that are very sensitive indicators than the impedance modulus values, in fact, AZDE sample did not exhibit the second time constant which instead was present for the AZE specimen (Fig. 1b).
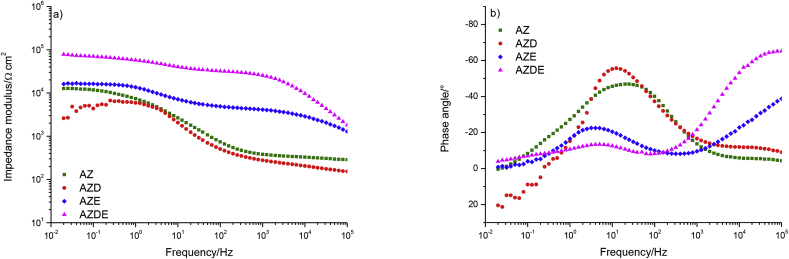
EIS spectra reported in Fig. 2 represent the comparison of all samples analysed after 3 days of immersion in the test solution.
Fig. 2.
Comparison of EIS spectra of investigated samples after 3 days of immersion in Hank's solution.
The AZ sample impedance modulus increased from 5.6 × 103 Ohm⋅cm2 to 1.3 × 104 Ohm⋅cm2. This effect could be addressed to the precipitation of calcium phosphate crystals and the formation of corrosion products, at the interface, that protected the surface from further degradation. The AZD sample showed a lower impedance modulus if compared to the mechanically lapped substrate but the shape of the curve was similar than AZ sample. The AZE sample did not display variation in impedance modulus value if compared to the value recorded at t = 0. The AZDE sample showed lower impedance modulus value than to that registered at the beginning of immersion but it was, still, highest if compared to that of the other specimens, while, a second time constant seems to appear in the impedance modulus and in the phase angle plots, demonstrating the development of degradation activity of the interface.
Therefore, after 3 days of immersion in Hank's solution, the AZDE sample began to display a second time constant, as evident by the phase angle plot. The total impedance modulus decreased over time, remaining of the order of 105 Ohm⋅cm2, which was still higher if compared to that of the other samples. In addition, the AZE sample revealed a low-frequency impedance modulus similar to that displayed by the bare sample, then the AZE sample showed corrosion below the organic resin. In conclusion, the sample with the polydopamine intermediate layer between the substrate and the external epoxy coating exhibited better protective properties than those shown by the other samples.
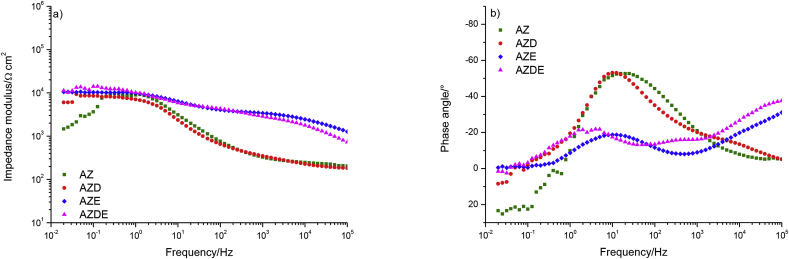
In Fig. 3 Bode plots recorded from the comparison of all samples tested in Hank's solution after 7 days of immersion is reported.
Fig. 3.
Comparison of EIS spectra of investigated samples after different treatments after 7 days of immersion in Hank's solution.
A completely new scenario can be observed at this time of samples immersion in the test solution. The corrosion triggered at the beginning of the test on the AZ sample proceeded as well as the precipitation/dissolution phenomena of the salts on the surface. Even if, the formed layer was considered as a passivating film, it was porous allowing the direct contact between the metal substrate and the solution. The instability of the formed layer was highlighted by the noisy signal recorded at low frequencies in the Bode modulus and phase diagrams. At this stage, the modulus impedance values of AZD, AZE and AZDE samples at low frequencies were comparable among them. In particular, AZDE sample had undergone a greater decrease of the impedance modulus than that suffered by other analysed specimens, in fact, the value was reduced by an order of magnitude, starting from the value of 1.7 × 105 Ω cm2 and reaching the value of 1.1 × 104 Ω cm2. This behaviour was observed in the phase angle shape too. In fact, at the medium frequencies, AZ and AZD showed a single peak while AZE and AZDE displayed more time constant.
In Fig. 4 the EIS spectra of all samples analysed after 15 days of immersion in the test solution are reported.
Fig. 4.
Comparison of EIS spectra of investigated samples after different treatments after 15 days of immersion in Hank's solution.
The impedance modulus of AZ sample increased if compared to the value recorded after 7 days of immersion. This fluctuation was due to the continuous formation and dissolution of the oxide layer on the surface that protected the surface against of the corrosion, as said before.
The shape of impedance modulus curve of AZD sample was similar to that of AZ sample even if the variation of the impedance modulus value was occurred in a restricted range of values, highlighting the stability due to the polydopamine coating on the surface.
The impedance modulus value of AZE sample was similar to that exhibited by AZD sample. As mentioned before, the resin used in this work did not contain the anticorrosion pigments. This absence determined the rapid penetration of the electrolyte toward the coating, as noted at the beginning of the test (Fig. 1), so the corrosion phenomena started in the early hours of immersion, and the penetration remained uniform in the whole organic coating, as corroborated from the shape of the impedance modulus that remained unaltered during the test.
The impedance modulus of AZDE sample was highest if compared to that the other samples. This behaviour highlighted that the combination of PDOPA and epoxy resin provided a good corrosion resistance in comparison to the all tested samples.
Although the impedance modulus values of all samples were the same, the shapes curves at high frequencies were different. In addition, the phase angles of AZ and AZD samples exhibited one high peak at medium frequencies that were not present for AZE and AZDE samples. Hence, while the impedance modulus at low frequencies assumed about the same value for all tested samples, the degradation processes should be quite different. In other terms, the coatings covering AZE and AZDE samples are highly porous so that the resistive effect exhibited by the layer prevailed over its capacitive behaviour since the pores are saturated with the electrolytes. Based on these considerations, we could assume that the entire coating (PDOPA and epoxy) possessed residual protective behaviour due to the strength of adhesion caused by the use of the PDOPA layer. In fact, as reported in the paper by Lee H et al. [25], “the polydopamine coating was found to be an extremely versatile platform for secondary reactions” thanks the presence of catechols that allow the deposition of adherent and uniform metal and organic coatings.
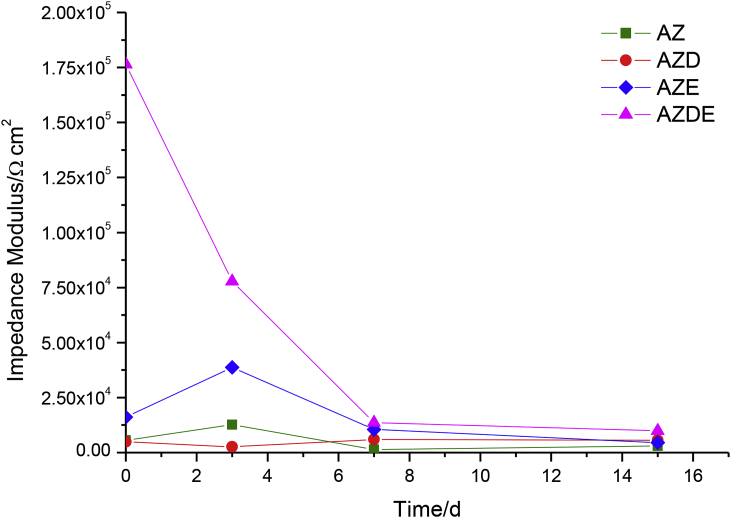
In the light of the above, the values of the total impedance (at the frequency of 0.02 Hz) of the systems under study have been reported in Fig. 5.
Fig. 5.
Time evolution of impedance modulus of all samples at the same frequency of 0.02 Hz.
The AZ sample showed a certain instability of the impedance modulus values during the immersion time. This behaviour was due to the sample was uncoated and a continuous formation and dissolution of the oxide layer on the surface were displayed. The AZD sample also exhibited a certain instability of the impedance modulus values but visibly less than the bare sample, due to the effect of the porous layer.
The AZE sample displayed similar behaviour to that shown by the previous samples and a very low impedance modulus, due to the absence of any kind of inhibitors inside the epoxy resin. This manner highlighted that the sample exhibited a degradation process at the interface between the metal substrate and the external coating. The slight increase of the impedance modulus, after 3 days of immersion, could be addressed to the corrosion products that were nor dissolved in the electrolytic solution but remained under the coating.
Reminding that at the beginning of the test, the AZDE sample showed the impedance modulus value higher than one order of magnitude compared to other specimens and that the all samples will tend to the same value if the duration of the test is sufficiently long, considering the fast diffusion of water and electrolytes, one can affirm that the AZDE sample clearly showed better protective properties related to the other samples.
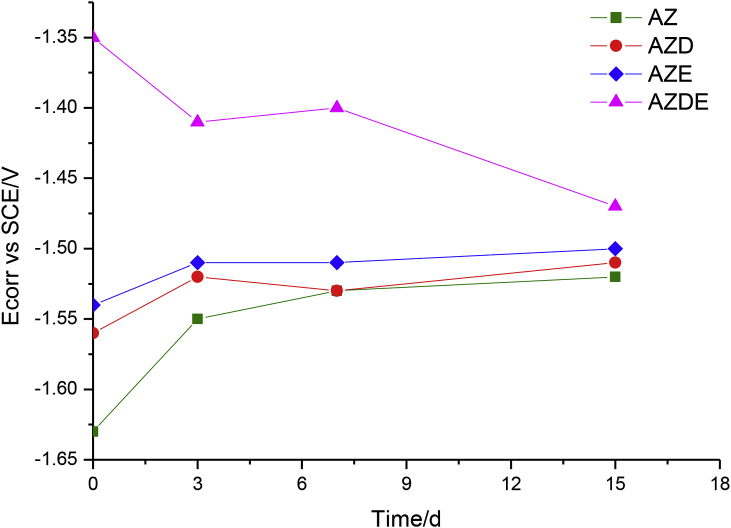
The Ecorr values measured before each EIS test are reported in Fig. 6.
Fig. 6.
Time evolution of corrosion potential of all samples during corrosion tests.
The AZ sample showed the lowest corrosion potential value than of all the tested samples. On the other hand, the measured value should be expected due to the fact that the AZ specimen was not coated [32]. Its increase over time was due to the precipitation of calcium phosphate crystals and the formation of corrosion products, that were deposited on the surface. The AZD sample differed from the bare sample at the beginning of the immersion in solution test. However, the polydopamine layer covering the sample, as corroborated by the EIS spectra, had not a protective effect over time, in fact, the behaviour and therefore the values of Ecorr of AZD sample were similar to those recorded for the AZ sample.
Higher corrosion potential was instead provided by the epoxy resin deposited on the sample if compared to the AZ sample. In fact, it was noted that the AZE specimen showed a value of corrosion potential, at the beginning of the immersion test, greater than 90 mV. After 3 days of immersion, the measured Ecorr values were similar to those recorded for the AZ sample, as confirmed by the degradation behaviour exhibited by the EIS spectra.
When both, the polydopamine and the epoxy resin, were applied to the magnesium substrate, the Ecorr values changes were substantial. In fact, the corrosion potential of AZDE sample, recorded at the beginning of the immersion in the test solution, assumed the highest value than all those registered for the other samples. Even if the difference of the Ecorr values of the different samples was reduced during the exposure time, the AZDE sample retained always the highest one. This indicated that there was an effect due to the use of the PDOPA layer. The substantial difference between the AZDE sample and the other tested specimens was its protective properties for 7 days. If it waits for a sufficiently long time, all samples (coated by different types of coating) must reach the same modulus impedance value.
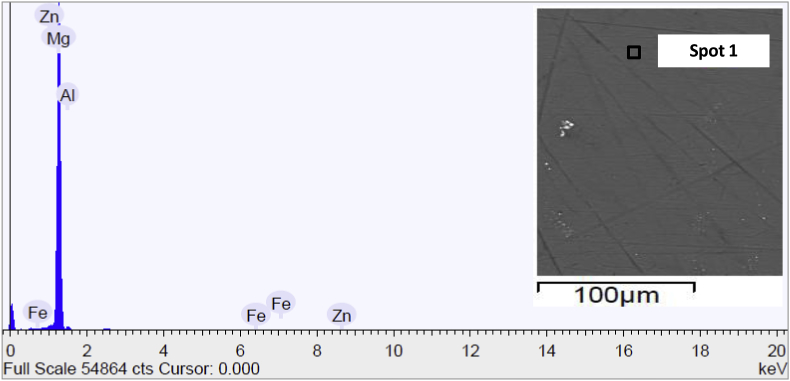
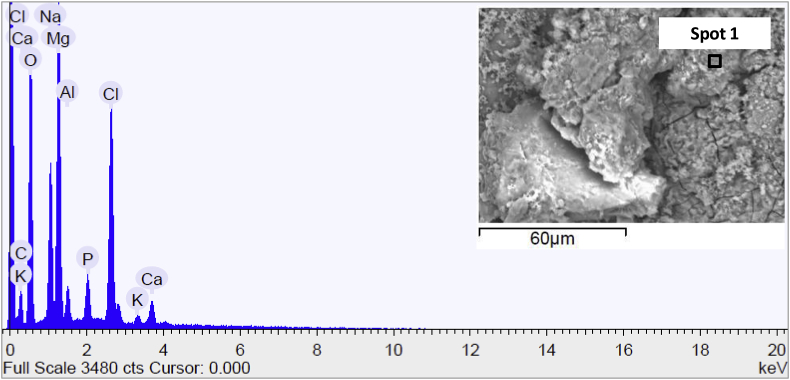
SEM micrograph and EDS spectrum of AZ surface sample are present in Fig. 7, in which the spot number, inside the SEM micrograph, indicated the position in which the spot of the EDS has been addressed to detect the surface chemical composition. As evidenced by the micrography of the specimen and its surface chemical composition, the sample was cleaned in an appropriate manner, in fact, the spectra recorded reflected the composition of the magnesium alloy used in this work. The little quantity of Fe was attributed to the presences of surface contaminations. More points of analysis were detected (herein were not reported for brevity) and the composition was not changed if other spots were considered.
Fig. 7.
Scanning electron micrograph and EDS spectrum of the surface of AZ sample before immersion in Hank's solution.
Fig. 8 showed the SEM surface micrograph and EDS chemical composition of AZ sample after 15 days of soaking in Hank's solution. Also, in this case, one sampling spot was reported and the EDS spectra highlighted the deposition of calcium phosphate on the surface that covered heterogeneously its surface. So the micrograph clearly showed that the surface changed significantly if compared to that of the sample before of soaking in the test solution.
Fig. 8.
Scanning electron micrograph and EDS spectrum of the surface of AZ sample at the end of corrosion test in Hank's solution.
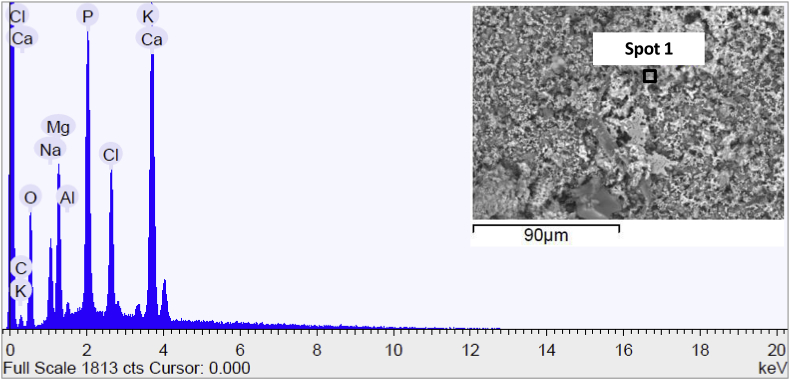
SEM micrograph and the chemical composition of the AZD surface sample before immersion in Hank's solution are reported in Fig. 9. The topography of the sample clearly highlighted the cracks presented in the coating. EDS was carried out in various loci (herein reported only one spot due to the fact that the chemical surface composition was homogeneous), the spectrum showed the presence of C, O and N typically of the PDOPA coating.
Fig. 9.
Scanning electron micrograph and EDS spectrum of the surface of AZD sample before immersion in Hank's solution.
Fig. 10 reported the surface micrograph and EDS spectrum analysis of AZD sample after 15 days of immersion in the test solution. The sample surface was covered by a layer more homogeneous if compared to the surface of AZ sample and the chemical species composing the salts precipitated from Hank's solution were similar to that detected on AZ sample surface.
Fig. 10.
Scanning electron micrograph and EDS spectrum of the surface of AZD sample at the end of the corrosion test in Hank's solution.
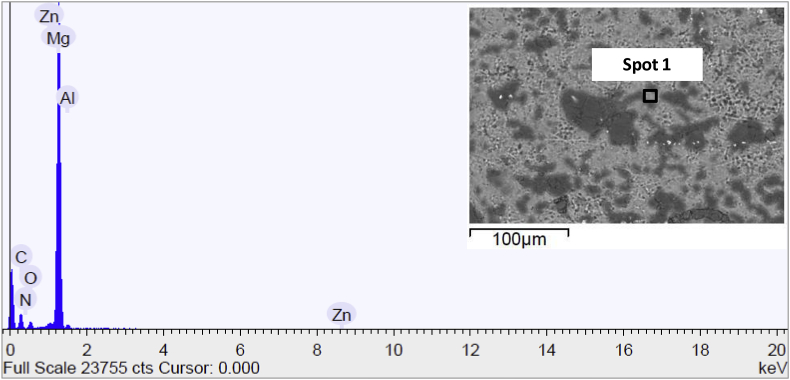
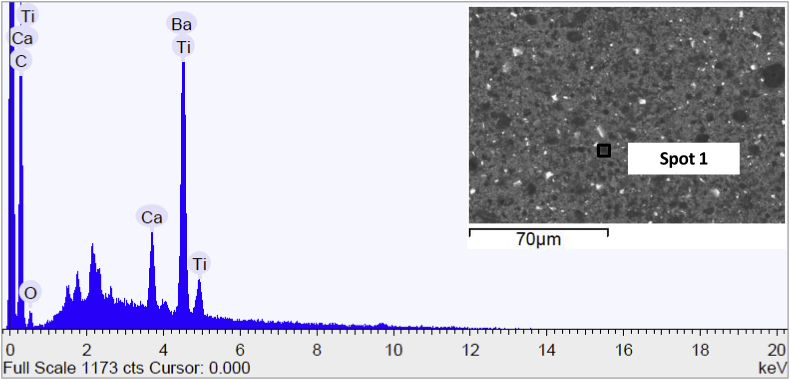
Fig. 11 discloses the SEM image and the elementary surface composition of AZE sample before immersion in the test solution. SEM micrograph showed a surface with the presence of pigments presented in the epoxy resin and this was corroborated to EDS spectra that detected the presence of Ti, Ba and Ca typical of the coating composition. Moreover, the surface did not change at the end of soaking (data not reported), demonstrating that the epoxy resin prevented the formation of calcium phosphate crystals.
Fig. 11.
Scanning electron micrograph and EDS spectrum of the surface of AZE sample before immersion in Hank's solution.
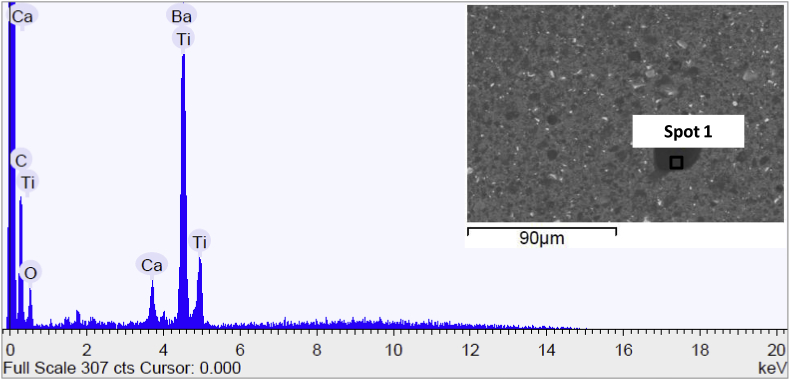
SEM micrograph and EDS spectra of AZDE sample before immersion in Hank's solution are reported in Fig. 12. The metal substrate was coated with PDOPA and epoxy resin and the organic upper layer was visible from the presence of Ti, Ba and Ca, as seen in the EDS spectra of the AZE sample. Also for this sample, no deposit was found after the exposure in Hank's solution at the end of the test.
Fig. 12.
Scanning electron micrograph and EDS spectrum of the surface of AZDE sample before immersion in Hank's solution.
4. Conclusions
The corrosion behaviour of AZ31 magnesium alloy samples naked, coated with polydopamine or epoxy resin or polydopamine/epoxy multilayer, was tested when immersed in Hank's solution for 15 days.
Electrochemical measurements revealed that the PDOPA, when used as interlayer, deeply affected the degradation behaviour of the sample.
Experimental results, in fact, showed that the AZ and AZD samples displayed low corrosion resistance during the exposure time demonstrating that the PDOPA layer, by itself, did not offer any protection against corrosion. AZE sample exhibited a little bit improvement in the corrosion behaviour if compared to that shown by previous ones, even if it revealed the presence of corrosion products at the interface, since the begging of the immersion test. While AZDE sample showed the best corrosion performance among the tested samples.
SEM micrographs and EDS chemical compositions showed that AZ and AZD surface samples were covered with calcium phosphate at the end of the immersion test. On contrary, AZE and AZDE samples did not show the deposition of calcium phosphates, demonstrating that the organic coating prevented the formation of precipitated salts.
Conflicts of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Zartner P., Cesnjevar R., Singer H., Weyand M. First successful implantation of a biodegradable metal stent into the left pulmonary artery of a preterm baby. Cathet. Cardiovasc. Interv. 2005;66:590–594. doi: 10.1002/ccd.20520. [DOI] [PubMed] [Google Scholar]
- 2.Schranz D., Zartner P., Michel‐Behnke I., Akintürk H. Bioabsorbable metal stents for percutaneous treatment of critical recoarctation of the aorta in a newborn. Cathet. Cardiovasc. Interv. 2006;67:671–673. doi: 10.1002/ccd.20756. [DOI] [PubMed] [Google Scholar]
- 3.Di Mario C., Griffiths H., Goktekin O., Peeters N., Verbist J., Bosiers M., Deloose K., Heublein B., Rohde R., Kasese V. Drug‐eluting bioabsorbable magnesium stent. J. Intervent. Cardiol. 2004;17:391–395. doi: 10.1111/j.1540-8183.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- 4.Shin H., Jo S., Mikos A.G. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353–4364. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 5.Chua K.-N., Chai C., Lee P.-C., Tang Y.-N., Ramakrishna S., Leong K.W., Mao H.-Q. Surface-aminated electrospun nanofibers enhance adhesion and expansion of human umbilical cord blood hematopoietic stem/progenitor cells. Biomaterials. 2006;27:6043–6051. doi: 10.1016/j.biomaterials.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y., Zhao S., Chen M., Zhang W., Mao J., Zhao Y., Maitz M.F., Huang N., Wan G. Sandwiched polydopamine (PDA) layer for titanium dioxide (TiO2) coating on magnesium to enhance corrosion protection. Corros. Sci. 2015;96:67–73. [Google Scholar]
- 7.Wang Y., Wei M., Gao J., Hu J., Zhang Y. Corrosion process of pure magnesium in simulated body fluid. Mater. Lett. 2008;62:2181–2184. [Google Scholar]
- 8.Li L., Gao J., Wang Y. Evaluation of cyto-toxicity and corrosion behavior of alkali-heat-treated magnesium in simulated body fluid. Surf. Coating. Technol. 2004;185:92–98. [Google Scholar]
- 9.Monetta T., Acquesta A., Carangelo A., Bellucci F. TiO2 nanotubes on Ti dental implant. Part 1: formation and aging in Hank's solution. Metals. 2017;7:167. [Google Scholar]
- 10.Monetta T., Acquesta A., Carangelo A., Bellucci F. TiO2 nanotubes on Ti dental implant. Part 2: EIS characterization in Hank's solution. Metals. 2017;7:220. [Google Scholar]
- 11.Acquesta A., Carangelo A., Monetta T. TiO2 nanotubes on Ti dental implant. Part 3: electrochemical behavior in Hank's solution of titania nanotubes formed in ethylene glycol. Metals. 2018;8:489. [Google Scholar]
- 12.Xiao X., Xu Y., Fu J., Gao B., Huo K., Chu P.K. Enhanced hydroxyapatite growth and osteogenic activity on polydopamine coated Ti implants. Nanosci. Nanotechnol. Lett. 2015;7:233–239. [Google Scholar]
- 13.Lin B., Zhong M., Zheng C., Cao L., Wang D., Wang L., Liang J., Cao B. Preparation and characterization of dopamine-induced biomimetic hydroxyapatite coatings on the AZ31 magnesium alloy. Surf. Coating. Technol. 2015;281:82–88. [Google Scholar]
- 14.Wilke B.M., Zhang L. Electrochemical investigations of polycaprolactone-coated AZ31 Mg alloy in Earle's balance salt solution and conventional simulated body fluid. JOM. 2016;68:1701–1710. [Google Scholar]
- 15.Zomorodian A., Santos C., Carmezim M., Silva T. M. e, Fernandes J., Montemor M. d. F. “In-vitro” corrosion behaviour of the magnesium alloy with Al and Zn (AZ31) protected with a biodegradable polycaprolactone coating loaded with hydroxyapatite and cephalexin. Electrochim. Acta. 2015;179:431–440. [Google Scholar]
- 16.Chen Y., Song Y., Zhang S., Li J., Zhao C., Zhang X. Interaction between a high purity magnesium surface and PCL and PLA coatings during dynamic degradation. Biomed. Mater. 2011;6 doi: 10.1088/1748-6041/6/2/025005. [DOI] [PubMed] [Google Scholar]
- 17.Waite J.H. Surface chemistry: mussel power. Nat. Mater. 2008;7:8. doi: 10.1038/nmat2087. [DOI] [PubMed] [Google Scholar]
- 18.Bourmaud A., Riviere J., Le Duigou A., Raj G., Baley C. Investigations of the use of a mussel-inspired compatibilizer to improve the matrix-fiber adhesion of a biocomposite. Polym. Test. 2009;28:668–672. [Google Scholar]
- 19.Waite J.H. Adhesion a la moule. Integr. Comp. Biol. 2002;42:1172–1180. doi: 10.1093/icb/42.6.1172. [DOI] [PubMed] [Google Scholar]
- 20.Liu X., Cao J., Li H., Li J., Jin Q., Ren K., Ji J. Mussel-inspired polydopamine: a biocompatible and ultrastable coating for nanoparticles in vivo. ACS Nano. 2013;7:9384–9395. doi: 10.1021/nn404117j. [DOI] [PubMed] [Google Scholar]
- 21.Tsai W.-B., Chen W.-T., Chien H.-W., Kuo W.-H., Wang M.-J. Poly (dopamine) coating to biodegradable polymers for bone tissue engineering. J. Biomater. Appl. 2014;28:837–848. doi: 10.1177/0885328213483842. [DOI] [PubMed] [Google Scholar]
- 22.Huang L., Yi J., Gao Q., Wang X., Chen Y., Liu P. Carboxymethyl chitosan functionalization of CPED-treated magnesium alloy via polydopamine as intermediate layer. Surf. Coating. Technol. 2014;258:664–671. [Google Scholar]
- 23.Singer F., Schlesak M., Mebert C., Höhn S., Virtanen S. Corrosion properties of polydopamine coatings formed in one-step immersion process on magnesium. ACS Appl. Mater. Interfaces. 2015;7:26758–26766. doi: 10.1021/acsami.5b08760. [DOI] [PubMed] [Google Scholar]
- 24.Wang C., Shen J., Xie F., Duan B., Xie X. A versatile dopamine-induced intermediate layer for polyether imides (PEI) deposition on magnesium to render robust and high inhibition performance. Corros. Sci. 2017;122:32–40. [Google Scholar]
- 25.Lee H., Dellatore S.M., Miller W.M., Messersmith P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318:426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geels K., Fowler D.B., Kopp W.-U., Rückert M. ASTM International West Conshohocken; 2007. Metallographic and Materialographic Specimen Preparation, Light Microscopy, Image Analysis, and Hardness Testing. [Google Scholar]
- 27.Monetta T., Acquesta A., Carangelo A., Donato N., Bellucci F. Durability of AZ31 magnesium biodegradable alloys polydopamine aided: Part 1. J. Magnes. Alloy. 2017;5:412–422. [Google Scholar]
- 28.Ho C.-C., Ding S.-J. Structure, properties and applications of mussel-inspired polydopamine. J. Biomed. Nanotechnol. 2014;10:3063–3084. doi: 10.1166/jbn.2014.1888. [DOI] [PubMed] [Google Scholar]
- 29.Ye Q., Zhou F., Liu W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011;40:4244–4258. doi: 10.1039/c1cs15026j. [DOI] [PubMed] [Google Scholar]
- 30.Monetta T., Acquesta A., Carangelo A., Bellucci F. The effect of graphene loading on the corrosion resistance of organic coatings. Metall. Ital. 2017:91–94. [Google Scholar]
- 31.Monetta T., Acquesta A., Carangelo A., Bellucci F. The effect of graphene on the protective properties of water-based epoxy coatings on Al2024-T3. Int. J. Corros. 2017:2017. [Google Scholar]
- 32.Xin Y., Liu C., Zhang X., Tang G., Tian X., Chu P.K. Corrosion behavior of biomedical AZ91 magnesium alloy in simulated body fluids. J. Mater. Res. 2007;22:2004–2011. [Google Scholar]