Abstract
Background
Fragmented QRS (FQRS) on surface electrocardiogram (ECG) is associated with the presence of myocardial scar tissue and may have prognostic value after certain ischemic events. We aimed to examine the anatomical correlation of FQRS with the presence of perfusion abnormalities in patients with suspected coronary artery disease (CAD).
Methods
Patients without a known history of CAD, who were referred for myocardial perfusion imaging (MPI) between January 2016 and May 2016, were enrolled. The presence of FQRS on surface ECG was evaluated. The presence of FQRS, number of leads with FQRS, and the location of FQRS as well as patient characteristics were compared in patients with normal versus abnormal MPI. Multivariate model was constructed to identify independent factors associated with perfusion defect.
Results
One hundred four women and 94 men were enrolled. Fragmentation of anterior, lateral, and inferior leads was detected in 13 (6.5%), 17 (8.5%), and 36 (18.1%) subjects, respectively. MPI was normal in 134 (67.6%) patients. FQRS was significantly more common in patients with abnormal MPI (p < 0.001). Age (odds ratio [OR]: 1.05 [1.02–1.08]; p = 0.001), number of the leads presenting FQRS (OR: 1.46 [1.12–1.92] p = 0.006), and diabetes (OR: 2.33 [1.16–4.69]; p = 0.018) were independent predictors of the presence of perfusion defect on MPI.
Conclusion
In the absence of known CAD, FQRS is associated with the presence of perfusion abnormalities. Incorporating FQRS in diagnostic armamentarium may aid us in selecting patients who may benefit from MPI.
Keywords: Electrocardiogram, Coronary artery disease, Myocardial perfusion imaging, Fragmented QRS
1. Introduction
Ischemic heart disease (IHD) is still a major problem and leading cause of death worldwide. Early diagnosis and intervention have dramatically improved the outcomes of patients and decreased the mortality due to IHD over the past decades. Development of various tools that helps with timely diagnosis plays an important role in early intervention for this deadly disease.1 Fragmentation of QRS complex (FQRS) on a 12-lead surface electrocardiogram (ECG) had recently gained increasing attention as a simplified noninvasive ECG marker with diagnostic and prognostic value in various cardiac pathologies associated with myocardial scarring.2, 3 FQRS is defined as the presence of various RSR' patterns including an additional R wave, notching of the R or S waves, or the presence of more than one fragmentation in two contiguous leads.4 FQRS is a sign of depolarization abnormality of the myocardium that corresponds to the region within the territory of a significant coronary artery lesion. Its presence has been associated with significant myocardial scarring.5
Myocardial perfusion imaging (MPI) is considered the diagnostic gold standard in identifying regional abnormalities in myocardial perfusion and myocardial viability with some prognostic value.6 MPI is currently used in routine practice to diagnose and to guide management of patients with coronary artery disease (CAD). It has been shown to be of value for risk stratification of patients with suspected acute coronary syndrome (ACS).7 Therefore, guidelines recommend considering MPI in patients with possible ACS who have normal serial ECG and cardiac troponin level.8
The goal of the present study was to evaluate the significance of incorporating FQRS on the 12-lead ECG as a predictor of a myocardial perfusion defect in patients without known CAD. We hypothesized that even in these patients, the presence of FQRS would associate with perfusion abnormality on MPI.
2. Methods
This was a cross-sectional prospective study performed at a university-affiliated imaging center. Institutional review board approved the study for its scientific and ethical merit. Written informed consent was obtained from participants upon full description of the study. Data were treated carefully and confidentially to ensure patient privacy. Patients who were referred for MPI between January 2016 and May 2016 were screened by a research team member. Patients with paced rhythm, prior history of myocardial infarction (MI), pathological Q wave in surface ECGs without a history of MI, prior coronary revascularization or ACS, bundle branch block, atrial fibrillation, and those with pericardial diseases were excluded. In addition, patients with hypertrophic or restrictive cardiomyopathy and those with a known history of systolic heart failure with left ventricular ejection fraction < 50% were not included.
The study variables including demographic, ECG, and clinical factors were collected for each patient. Demographic data and clinical variables including gender, age, smoking history, family history of CAD; preexisting comorbidities including hypertension, diabetes mellitus (DM), hyperlipidemia; and clinical presentations including chest pain, dyspnea, or positive exercise stress test (EST). The indication for MPI was also recorded for each patient (symptoms suggestive of ischemia, positive findings EST, and preoperative evaluation for noncardiac surgery).
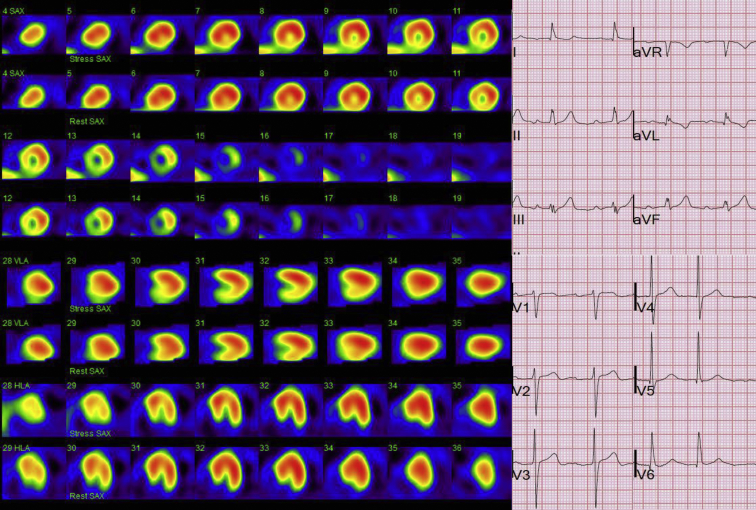
Standard 12-lead surface ECG was obtained on the day of examination and reviewed and evaluated for QRS fragmentation of anterior, lateral, and inferior leads independently by two cardiologists, and a consensus was reached later on. The FQRS was defined as notching of R or S waves or the presence of an additional r wave or QRS fragmentation in two contiguous leads (Fig. 1).9 All patients underwent ECG-gated MPI with single-photon emission computed tomography. For patients, for whom standard EST can not be performed, pharmacologic stress was induced using dipyridamole for nonasthmatic patients and dobutamine for those with reactive airway disease. MPI was performed using technetium methoxy isobutyl isonitrile. An experienced physician specialized in nuclear medicine and blinded to the results of ECG interpreted all images obtained from MPI studies. The number of segments with abnormal perfusion was also recorded using the standard 20-segment model (Fig. 1).
Fig. 1.
An example of myocardial perfusion scan images of a patient who also demonstrated fragmentation of the QRS complexes mainly in lead III and avF.
Normal distribution of the quantitative data was assessed using the Kolmogorov–Smirnov test. Data are expressed as mean ± standard deviation or median (interquartile range), as appropriate. The independent samples t-test and the Mann–Whitney U-test were used to compare independent groups for continuous variables. Fisher's exact chi-square analysis was used as appropriate to compare the frequencies of categorical variables. Odds ratios with 95% confidence intervals were reported for each variable. The significantly different variables with a p-value < 0.05 in univariate analysis (DM, age, and number of leads with FQRS) were entered into a multivariate binary logistic regression model to assess the independent predictive factors of myocardial perfusion defect. Bonferroni adjustment was made for multiple comparisons, and the adjusted p-values were reported. Null hypotheses were rejected if adjusted p-values were less than 0.05.
3. Results
One hundred ninety-eight patients (104 women and 94 men) who fulfilled the inclusion criteria during the aforementioned time period were enrolled. The mean age of the patients was 59.2 ± 13.0 years. FQRS was present in 62 (31.3%) patients. Fragmentation of anterior, lateral, and inferior leads was detected in 13 (6.5%), 17 (8.5%) and 36 (18.1%) patients, respectively. MPI was reported normal in 134 (67.6%) patients, and the remaining 68 patients had varying degrees of perfusion abnormalities of the myocardium. FQRS was significantly more common in patients with abnormal MPI (p < 0.001). Seventy patients (35.3%) had been referred for MPI as for preoperative risk assessment, 10 patients (5.0%) had positive EST results, and the remaining 59.6% had symptoms suggestive of ischemia.
Clinical presentations or positive findings in EST did not correlate with the presence of abnormalities in MPI study. Hypertension and smoking status were not found to be related to myocardial perfusion defects. Table 1 shows the characteristics of patients with normal MPI in comparison with those who were reported to have perfusion defect in their study. In patients with positive findings in MPI, the median number of leads with FQRS was two (interquartile range (IQR) = 2), whereas in patients with normal MPI, the median number of leads with FQRS was 0 (IQR = 0) (p < 0.001). The median number of segments with abnormal perfusion was 1 (ranging 0–5) in patients with positive MPI. Age was significantly higher in patients with positive perfusion scans (p < 0.001). Patients with FQRS in anterior leads were 3.69 times more likely to have abnormal MPI compared with those who did not have this finding (p = 0.030).
Table 1.
Contribution of coronary risk factors, clinical presentation, and QRS fragmentation of the patients to positive perfusion scan.
| Variables in equation | Negative perfusion Scan, N = 134 | Positive perfusion scan, N = 64 | Odds ratio | Lower CI | Upper CI | p-value |
|---|---|---|---|---|---|---|
| Gender (female/male) | 75/59 | 29/35 | 1.58 | 0.87 | 2.86 | 0.173 |
| Age (years) | 56.7 ± 12.7 | 64.2 ± 12.3 | 1.05 | 1.02 | 1.08 | <0.001 |
| Family history of CAD | 17 (12.8%) | 1 (1.6%) | 0.11 | 0.01 | 0.82 | 0.008 |
| Hypertension | 73 (54.9%) | 35 (54.7%) | 0.99 | 0.54 | 1.81 | 1.000 |
| Diabetes mellitus | 29 (21.8%) | 26 (40.6%) | 2.39 | 1.25 | 4.56 | 0.011 |
| Hyperlipidemia | 36 (27.1%) | 26 (40.6%) | 1.84 | 0.98 | 3.46 | 0.071 |
| Current smoking | 12 (9.0%) | 9 (14.1%) | 1.62 | 0.65 | 4.07 | 0.330 |
| Prior smoking | 14 (10.5%) | 9 (14.1%) | 1.37 | 0.56 | 3.35 | 0.488 |
| Chest pain | 43 (32.3%) | 20 (31.3%) | 0.93 | 0.49 | 1.76 | 0.872 |
| Dyspnea | 22 (16.4%) | 9 (14.1%) | 0.83 | 0.36 | 1.93 | 0.835 |
| Positive exercise tolerance test | 3 (14.3%) | 0 (0.0%) | 0.86 | 0.72 | 1.02 | 1.000 |
| Fragmentation of anterior leads | 5 (3.7%) | 8 (12.5%) | 3.69 | 1.15 | 11.76 | 0.030 |
| Fragmentation of lateral leads | 8 (6.0%) | 9 (14.1%) | 2.58 | 0.94 | 7.03 | 0.100 |
| Fragmentation of inferior leads | 19 (14.2%) | 17 (26.6%) | 2.19 | 1.05 | 4.58 | 0.048 |
CAD, coronary artery disease; CI, confidence interval.
Multivariate binary logistic regression analyses demonstrated that the presence of FQRS on ECG was associated with 46% increase in the likelihood of perfusion abnormality in MPI (odds ratio [OR] = 1.46; 95% confidence interval of 1.12–1.92; p = 0.006). For every 1-year increase in age, the risk of positive finding in MPI increased by 5% (OR = 1.05; 95% confidence interval of 1.02–1.08; p = 0.001). In addition, the number of the ECG leads with FQRS and history of DM (OR = 2.33; 95% confidence interval of 1.16–4.69; p = 0.018) were independent predictors of presence of perfusion defect on MPI (Table 2).
Table 2.
Multivariate binary logistic regression of the factors with a p-value < 0.15 in univariate analysis which contribute to development of a positive perfusion scan.
| Variables in equation | Coefficient | SE | Wald | p-value | Odds ratio | Lower CI | Upper CI |
|---|---|---|---|---|---|---|---|
| Age (year) | 0.045 | 0.014 | 10.67 | 0.001 | 1.05 | 1.02 | 1.08 |
| Number of the leads | 0.382 | 0.138 | 7.68 | 0.006 | 1.46 | 1.12 | 1.92 |
| Diabetes mellitus | 0.846 | 0.356 | 5.64 | 0.018 | 2.33 | 1.16 | 4.69 |
| Constant | −4.069 | 0.886 | 21.08 | <0.001 | 0.02 |
CI, confidence interval; SE, standard error.
4. Discussion
The present study demonstrated that the number of ECG leads with FQRS, age, and DM were independent predictors of perfusion defects on MPI in patients with suspected CAD. MPI is routinely performed to determine the extent of ischemia and to differentiate regional perfusion defects from old preexisting myocardial scars. Our patient population was highly selected to have no prior history of CAD, and therefore, there was expectedly a high proportion of normal MPI studies. MPI is accompanied by economic and biological burden because of its use of radioactive material.10 It is reported that almost two-thirds of nuclear stress imaging tests are appropriate according to the criteria set through published guidelines.11 The frequency of FQRS on ECG examinations was interestingly less common in patients with normal MPI in the absence of CAD. Therefore, the presence of FQRS on ECG may be of value in determining the necessity for referring patients with various cardiac diseases to MPI. As such, incorporating FQRS to patient profile could be helpful in risk justification and appropriate referral of patients for more invasive imaging studies.12
Reports have focused on the association of FQRS with myocardial ischemia and scarring. Some studies have shown that the presence of FQRS on ECG is associated with myocardial ischemia.13, 14 Others have confirmed that FQRS could be a sign of myocardial scarring.15 While the exact electrophysiology of this phenomenon is not completely understood, the experimental studies have indicated that the chronic myocardial ischemia causes partial depolarization and depression in upstroke velocities of the action potential. Subsequently, the activation of cardiac myocytes significantly slows down and in conjunction with the abnormal conduction pattern of the tissue surrounding the scar tissue‘ various spikes are generated within QRS complex.9, 16, 17
We demonstrated a relatively higher association between myocardial perfusion defects and FQRS detected in anterior and inferior leads compared with those in lateral leads. Ozdemir et al14 suggested that patients with FQRS had a higher incidence of myocardial ischemia and scarring on MPI compared to patients without FQRS. We also found that the number of ECG leads with FQRS is an independent predictor of perfusion defect on MPI. This is probably due to the involvement of larger areas of myocardium (anterior and inferior regions vs. lateral wall and the total number of leads with FQRS) leading to larger perfusion defects. Various studies have investigated the association between the number of ECG leads and adverse cardiovascular events. It has been reported that the presence of FQRS in two or more ECG leads in patients with CAD is predictive of future coronary events.9 A larger number of leads with FQRS (particularly ≥3), has been shown to be an independent predictor of cardiac death or heart failure–related hospitalization in patients with prior MI.18
Several studies have shown a relationship between the existence of FQRS in patients with CAD and prognosis of cardiac events. Das et al reported higher all-cause mortality and cardiac event rate in the patients with FQRS compared with the non-FQRS group.9 They also confirmed that fragmentation of the QRS complex could be used as an indication of non-Q wave MI and as a predictor of ventricular arrhythmia. They reported higher sensitivity and negative predictive value of FQRS as a marker of prior MI compared with the Q wave.4
In this study, we also found that for every year increase in age, the risk of myocardial perfusion abnormality on MPI increases. We speculated that this observation occurred because of the higher prevalence of CAD in elderly. Although the effect of aging on myocardial perfusion remains controversial, the study by Uren et al19 suggests that a significant reduction occurs in hyperemic flow and myocardial perfusion reserve of individuals beyond the age of 70 years. Comparably, Ozdemir et al14 have shown an increase in the frequency of FQRS with aging.
Finally, we found that DM also independently is associated with perfusion abnormalities on MPI. The predictive role of DM can be explained by vascular dysfunction and postprandial hyperglycemia associated with myocardial perfusion defects in these patients. Hyperglycemia, excessive free fatty acids (ketone bodies), and associated insulin resistance result in endothelial dysfunction and inflammation that both lead to atherogenesis among diabetics.20 Wackers et al21 have reported silent myocardial ischemia among asymptomatic diabetic subjects and found suppressed Valsalva heart rate response due to autonomic neuropathy caused by diabetes as the strongest factor associated with moderate/large perfusion defects.
5. Limitations
The authors have a full understanding that the single-center nature of this study may limit its impact. Although single-center studies may suffer from lower sample size, but the fact that all perfusion examinations are examined by a single nuclear imaging specialist and that basically eliminates any possibility for inter-rater variability in this study. In addition, we do not have the results of coronary angiography for patients who underwent further evaluation for positive MPI and do not report the rate of cardiac events in follow-up, which would have been of value. Although coronary angiogram is widely used to diagnose obstructive coronary lesions in high-risk population, MPI with a sensitivity of close to 80% offers a decisive role on whether the observed lesions carry a significance in tissue level perfusion of the myocardium.22 Therefore, we have opted to use the abnormal findings of any sort in this test as the primary end point to this study with an aim to link these findings to the presence of fragmentation of the QRS complex on surface ECG without focusing on the angiographic findings.
6. Conclusion
In this selected population without known CAD, the number of ECG leads with QRS complex fragmentation, age, and DM were predictive of presence of perfusion defect on MPI. This study illustrates the importance of incorporating noninvasive tools in the identification of at-risk groups. Our study results have led us to conclude that the presence of FQRS on ECG is an inexpensive and readily available marker that can be considered as an indicator of myocardial ischemia and/or subclinical infarction with related ventricular perfusion abnormalities. Therefore, patients with this ECG abnormality may benefit from more invasive testing with myocardial perfusion scanning to confirm the diagnosis of myocardial scaring.
Conflict of interest
All authors have none to declare.
Funding sources
No funding was requested for this study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ihj.2018.09.011.
Contributor Information
Sepideh Hekmat, Email: sepidhekmat@hotmail.com.
Leili Pourafkari, Email: Leili.p@gmail.com.
Mojan Ahmadi, Email: mojan.ahmadi88@gmail.com.
Mohammad Reza Chavoshi, Email: chavoushi.smr@gmail.com.
Bijan Zamani, Email: bijanzamani@gmail.com.
Nader D. Nader, Email: nnader@buffalo.edu.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Cassar A., Holmes D.R., Jr., Rihal C.S., Gersh B.J. Chronic coronary artery disease: diagnosis and management. Mayo Clin Proc. 2009;84:1130–1146. doi: 10.4065/mcp.2009.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadeghi R., Dabbagh V.R., Tayyebi M., Zakavi S.R., Ayati N. Diagnostic value of fragmented qrs complex in myocardial scar detection: systematic review and meta-analysis of the literature. Kardiol Pol. 2016;74:331–337. doi: 10.5603/KP.a2015.0193. [DOI] [PubMed] [Google Scholar]
- 3.Akbarzadeh F., Pourafkari L., Ghaffari S., Hashemi M., Sadeghi-Bazargani H. Predictive value of the fragmented qrs complex in 6-month mortality and morbidity following acute coronary syndrome. Int J Gen Med. 2013;6:399–404. doi: 10.2147/IJGM.S40050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das M.K., Khan B., Jacob S., Kumar A., Mahenthiran J. Significance of a fragmented qrs complex versus a q wave in patients with coronary artery disease. Circulation. 2006;113:2495–2501. doi: 10.1161/CIRCULATIONAHA.105.595892. [DOI] [PubMed] [Google Scholar]
- 5.Dabbagh Kakhki V.R., Ayati N., Zakavi S.R., Sadeghi R., Tayyebi M., Shariati F. Comparison between fragmented qrs and q waves in myocardial scar detection using myocardial perfusion single photon emission computed tomography. Kardiol Pol. 2015;73:437–444. doi: 10.5603/KP.a2014.0242. [DOI] [PubMed] [Google Scholar]
- 6.Cheng W., Zeng M., Arellano C. Detection of myocardial perfusion abnormalities: standard dual-source coronary computed tomography angiography versus rest/stress technetium-99m single-photo emission CT. Br J Radiol. 2010;83:652–660. doi: 10.1259/bjr/82257160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim S.H., Anantharaman V., Sundram F. Stress myocardial perfusion imaging for the evaluation and triage of chest pain in the emergency department: a randomized controlled trial. J Nucl Cardiol. 2013;20:1002–1012. doi: 10.1007/s12350-013-9736-9. [DOI] [PubMed] [Google Scholar]
- 8.Amsterdam E.A., Wenger N.K., Brindis R.G. 2014 AHA/ACC guideline for the management of patients with non-st-elevation acute coronary syndromes: a report of the American college of Cardiology/American heart association task force on practice guidelines. Circulation. 2014;130:e344–e426. doi: 10.1161/CIR.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 9.Das M.K., Saha C., El Masry H. Fragmented qrs on a 12-lead ecg: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007;4:1385–1392. doi: 10.1016/j.hrthm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Picano E. Economic and biological costs of cardiac imaging. Cardiovasc Ultrasound. 2005;3:13. doi: 10.1186/1476-7120-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbons R.J., Miller T.D., Hodge D. Application of appropriateness criteria to stress single-photon emission computed tomography sestamibi studies and stress echocardiograms in an academic medical center. J Am Coll Cardiol. 2008;51:1283–1289. doi: 10.1016/j.jacc.2007.10.064. [DOI] [PubMed] [Google Scholar]
- 12.Pourafkari L., Ghaffari S., Nader N.D. Incorporating fragmented qrs on surface electrocardiogram to exercise stress test. Ann Noninvasive Electrocardiol. 2016;21:435–436. doi: 10.1111/anec.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caliskan B., Korkmaz A.N., Erdem F. Contribution of fragmented qrs on myocardial perfusion imaging in the assessment of functionally significant coronary artery stenoses. Eur Rev Med Pharmacol Sci. 2016;20:1575–1581. [PubMed] [Google Scholar]
- 14.Ozdemir S., Tan Y.Z., Colkesen Y., Temiz A., Turker F., Akgoz S. Comparison of fragmented qrs and myocardial perfusion-gated spect findings. Nucl Med Commun. 2013;34:1107–1115. doi: 10.1097/MNM.0b013e3283653884. [DOI] [PubMed] [Google Scholar]
- 15.Take Y., Morita H. Fragmented qrs: what is the meaning? Indian Pacing Electrophysiol J. 2012;12:213–225. doi: 10.1016/s0972-6292(16)30544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain R., Singh R., Yamini S., Das M.K. Fragmented ecg as a risk marker in cardiovascular diseases. Curr Cardiol Rev. 2014;10:277–286. doi: 10.2174/1573403X10666140514103451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee S., Changawala N. Fragmented qrs complex: a novel marker of cardiovascular disease. Clin Cardiol. 2010;33:68–71. doi: 10.1002/clc.20709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torigoe K., Tamura A., Kawano Y., Shinozaki K., Kotoku M., Kadota J. The number of leads with fragmented qrs is independently associated with cardiac death or hospitalization for heart failure in patients with prior myocardial infarction. J Cardiol. 2012;59:36–41. doi: 10.1016/j.jjcc.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Uren N.G., Camici P.G., Melin J.A. Effect of aging on myocardial perfusion reserve. J Nucl Med. 1995;36:2032–2036. [PubMed] [Google Scholar]
- 20.Beckman J.A., Creager M.A., Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 21.Wackers F.J., Young L.H., Inzucchi S.E. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the diad study. Diabetes Care. 2004;27:1954–1961. doi: 10.2337/diacare.27.8.1954. [DOI] [PubMed] [Google Scholar]
- 22.Rozanski A., Berman D.S. The synergistic use of coronary artery calcium imaging and noninvasive myocardial perfusion imaging for detecting subclinical atherosclerosis and myocardial ischemia. Curr Cardiol Rep. 2018;20:59. doi: 10.1007/s11886-018-1001-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.