Abstract
Purpose
We aimed to discuss the roles of radiation and chemotherapy as adjuvant treatment in patients with staged IB GC who were enrolled in the adjuvant chemoradiotherapy in stomach tumors (ARTIST) trial.
Materials and Methods
Among the 458 patients who were enrolled in the ARTIST trial, 99 had stage IB disease. The patients were randomly assigned to receive either adjuvant chemoradiotherapy with capecitabine plus cisplatin (XP, n=50) or chemoradiotherapy (XPRT, n=49). Survival analyses were performed in accordance with the AJCC 2010 staging system.
Results
According to the AJCC 2010 system, stage migration from IB to II occurred in 71% of the patients; 98% of the T2 N0 cases were reclassified as T3 N0, and 42% of the T1 N1 cases were reclassified as T1 N2. When comparing survival outcomes between the XPRT and XP arms for stage IB cancer (AJCC 2002), no significant difference in 5-year disease-free survival (DFS) between the 2 arms was found. (median 5-year DFS, not reached, P=0.256). The patients classified as having stage IB cancer (AJCC 2002) and reclassified as having stage II cancer (AJCC 2010) exhibited worse prognoses than those who remained in stage IB, although the difference was not statistically significant (5-year DFS rate, 83% vs. 93%). When we compared 5-year DFS in 70 patients with stage II (AJCC 2010), the addition of radiotherapy to XP chemotherapy did not show better outcome than XP alone (P=0.137).
Conclusions
The role of adjuvant chemoradiotherapy in the treatment of stage IB GC (AJCC 2002) warrants further investigation.
Keywords: Gastric cancer; Chemoradiotherapy, Adjuvant
INTRODUCTION
Gastric cancer (GC) remains the second leading cause of death from malignancy worldwide [1]. Although surgical resection with regional lymph node dissection is the only curative treatment for patients with GC, a significant percentage of patients undergoing curative surgery will experience a recurrence, which leads to a poor prognosis. Furthermore, a significant geographic variation in incidence exists, with the highest rates being reported in East Asia [2].
In patients with pathologically staged IB to IV (M0) disease according to the American Joint Committee on Cancer (AJCC) 2002 staging system, adjuvant chemoradiotherapy is considered one of the standard treatments. A prospective intergroup phase III trial (INT 0116) evaluated the usefulness of postoperative chemoradiotherapy in comparison with surgery alone in 556 patients with pathological stage IB to IV (M0) adenocarcinoma of the stomach and gastroesophageal junction, and reported a significant survival benefit with adjuvant chemoradiotherapy [3]. However, the INT 0116 trial has been criticized for including only 54 patients (10%) who had undergone a formal D2 dissection. Although D2 surgery has failed to demonstrate a survival benefit in randomized trials [4,5,6], it has already become the standard surgical approach for GC in Asia and Europe [7]. In another large-scale randomized phase III adjuvant chemoradiotherapy in stomach tumors (ARTIST) trial [8], although we failed to demonstrate an additional benefit from the addition of concurrent radiotherapy to adjuvant chemotherapy, a contradictory finding to the results of INT 0116 [3], we confirmed that D2 resection in patients with GC can achieve long-term disease-free survival (DFS) and possible cures with both adjuvant chemotherapy and chemoradiotherapy.
Currently, we may conclude that patients with GC who have received suboptimal surgery might benefit from adjuvant chemoradiotherapy in terms of reduced risk of locoregional failure, whereas those with stage II or III disease after D2 surgery should receive adjuvant chemotherapy [9]. However, the usefulness of adjuvant therapy for stage IB cancer, either with node-positive (T1 N1) or muscle-invasive (T2 N0) GC, remains controversial. The current National Comprehensive Cancer Center Network (NCCN) guidelines still recommend chemoradiotherapy for medically fit patients with stage IB GC [10]. As the prognosis is relatively favorable for patients with completely resected stage IB disease, the effectiveness of adjuvant chemoradiotherapy for this group is less clear.
Furthermore, the INT 0116 and ARTIST trials included patients with stage IB to IV (M0) GC according to the sixth edition of the AJCC staging system. Since 2010, the seventh edition of the AJCC tumor, node, metastasis (TNM) staging system differs from the previous version regarding some aspects of the T and N parameters [11]. The aim of this study was to compare survival differences between adjuvant chemotherapy and chemoradiotherapy groups of patients with stage IB GC to investigate the prognostic impact of this change in the staging system.
MATERIALS AND METHODS
We reviewed the records and tumor specimens of 99 patients with stage IB GC treated with either adjuvant chemotherapy or chemoradiotherapy in the phase III ARTIST trial. Details on patient eligibility and treatment have been previously published [8]. In brief, after curative gastrectomy with D2 lymph node dissection, the patients with pathologically staged IB to IV (M0) GC were randomly assigned to receive either adjuvant capecitabine plus cisplatin (XP) chemotherapy or concurrent XP chemoradiotherapy (xanthine phosphoribosyltransferase [XPRT]). Patients were required to provide written informed consent before inclusion in the study. The study was approved by the Institutional Review Board of the Samsung Medical Center (Seoul, Korea) and conducted in accordance with the principles of the Declaration of Helsinki (IRB number;2004-08-010).
The primary end point was DFS duration, defined as the time from the date of surgery until death, relapse, or second primary tumor, whichever occurred first. Secondary end points included overall survival (OS), safety, and the pattern of relapse. All data were prospectively recorded in case report forms, and only the outcome results and TNM staging according to the AJCC 2010 system were updated at the time of analyses. The median follow-up duration was 8 years.
Survival curves were estimated using the Kaplan-Meier method. All P-values were 2-sided, with P-values of <0.05 indicating statistical significance.
RESULTS
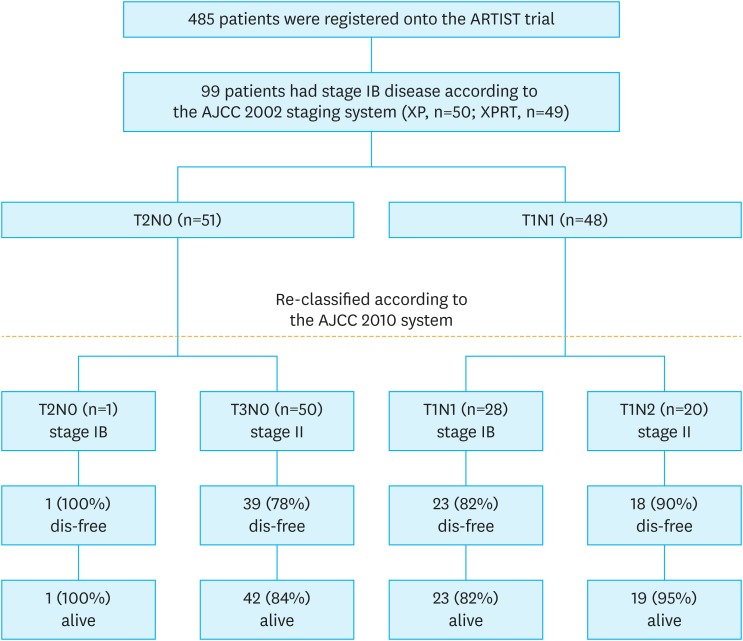
The study population consisted of 99 patients with stage IB GC staged in accordance with the AJCC 2002 staging system. The clinical and pathological characteristics are shown in Table 1. Only 1 patient had an human epidermal growth factor receptor2-positive (i.e., 3+immunohistochemistry) tumor. In this study, 51 patients belonged to the T2 N0 group and 48 patients belonged to the T1 N1 group according to the 2002 AJCC TNM staging system.
Table 1. Patients' baseline characteristics.
| Patient characteristics | No. of patients (n=99) | |
|---|---|---|
| Age (yr) | 54 (39–72) | |
| Sex | ||
| Male | 66 (67) | |
| Female | 33 (33) | |
| ECOG PS | ||
| 0 | 40 (40) | |
| 1 | 59 (60) | |
| Stage according to the AJCC 2002 system | ||
| T2 N0 | 51 (52) | |
| T1 N1 | 48 (48) | |
| Type of surgery | ||
| Total gastrectomy | 14 (14) | |
| Subtotal gastrectomy | 85 (86) | |
| WHO histologic classification | ||
| Well | 3 (3) | |
| Moderate | 19 (19) | |
| Poorly or signet ring cell type | 77 (78) | |
| Lauren's classification | ||
| Intestinal | 32 (32) | |
| Diffuse | 63 (64) | |
| Mixed or indeterminate | 4 (4) | |
| Lymphovascular invasion | 41 (41) | |
| Perineural invasion | 35 (35) | |
| No. of regional lymph nodes | ||
| Resected | 37 (15–84) | |
| Positive | 0 (0–6) | |
| Lymph node ratio | 0 (0–0.25) | |
| Adjuvant treatment | ||
| Chemoradiotherapy (XPRT arm) | 49 (49) | |
| Chemotherapy (XP arm) | 50 (51) | |
Values are presented as median (interquartile range) or number (%).
ECOG PS = Eastern Cooperative Oncology Group performance status; AJCC = American Joint Committee on Cancer; WHO = World Health Organization; XPRT = xanthine phosphoribosyltransferase; XP = capecitabine plus cisplatin.
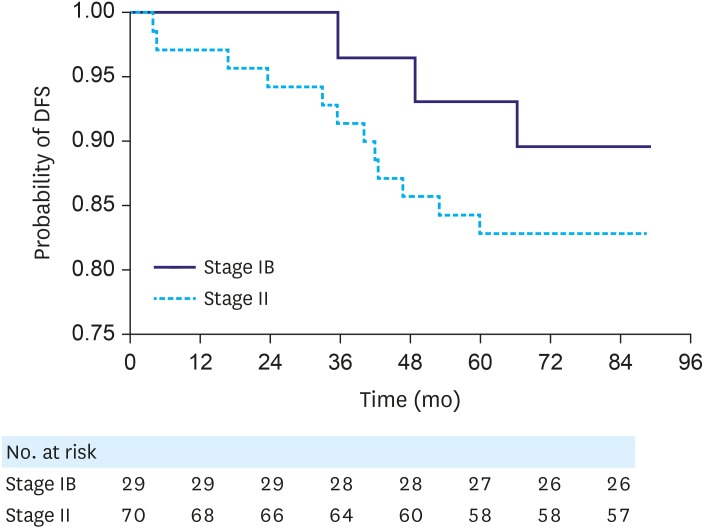
When we reviewed the pathological stages of the patients according to the AJCC 2010 system, the numbers of patients with T1, T2, and T3 tumors were 48, 1, and 50, respectively. Stage migration from IB to II occurred in 71% of the patients (Fig. 1); 98% (50/51) of the T2 N0 cases were reclassified as T3 N0, and 42% (20/48) of the T1 N1 cases were reclassified as T1 N2. The patients classified as having stage IB cancer according to the AJCC 2002 system and reclassified as having stage II cancer exhibited worse, though statistically insignificant, prognoses than the patients who remained in stage IB (5-year DFS: 83% vs. 93%; P=0.183; hazard ratio [HR], 1.178; 95% confidence interval [CI], 0.420–3.311; P=0.158) (Fig. 2). Likewise, the 5-year OS was 97% for the stage IB group and 90% for the stage II group. Among the 29 patients who maintained a stage IB disease, all but 1 had T1 N1 GC. The patient with T2 N0 disease remained disease-free for 7 years after surgery.
Fig. 1. Flow diagram of 99 patients with stage IB disease.
ARTIST = adjuvant chemoradiotherapy in stomach tumors; AJCC = American Joint Committee on Cancer; XP = capecitabine plus cisplatin; XPRT = xanthine phosphoribosyltransferase.
Fig. 2. DFS according to pathological staging using the AJCC 2010 staging system.
DFS = disease-free survival; AJCC = American Joint Committee on Cancer.
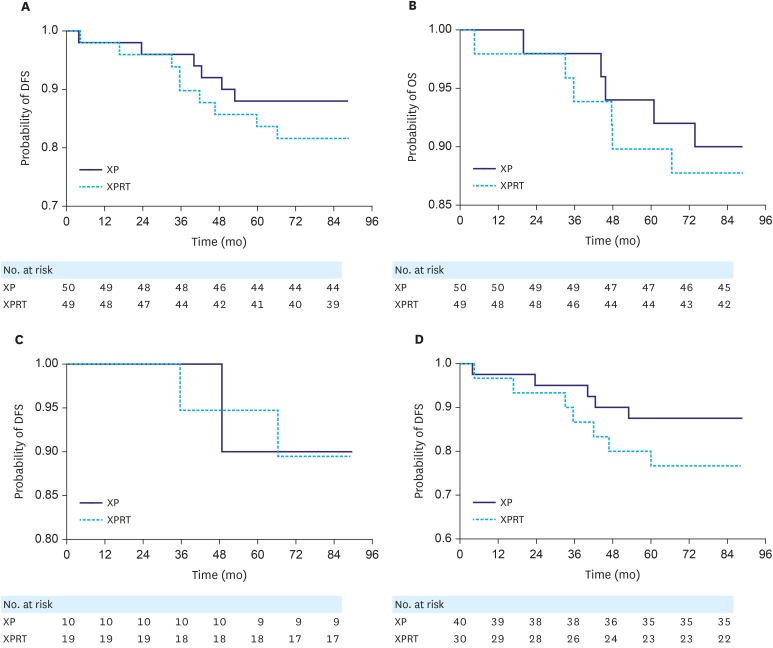
We compared 5-year DFS and OS between the XPRT (n=49) and XP arms (n=50) of the stage IB group (n=99) according to pathological staging using the AJCC 2002 system. Both median DFS and OS were not reached. DFS analysis suggested a trend toward increased DFS for the XP group, but the results were not statistically significant in the stage IB group (P=0.256) (Fig. 3A). No significant difference in OS was found between the XPRT and XP arms (P=0.680) (Fig. 3B). Within the 7-year follow-up, 18 recurrences occurred. The 5-year DFS rates were 88% and 84% in the XP and XPRT arms, respectively (P=0.537). We also compared the 5-year DFS of 29 patients with stage IB GC (Fig. 3C) or 70 patients with stage II GC (Fig. 3D) according to AJCC 2010 system between the XPRT and XP arms. We found that the addition of radiotherapy to XP chemotherapy did not show better outcome than the XP arm in both the stage IB and II groups (stage IB, P=0.900; stage II, P=0.137).
Fig. 3. (A) DFS in the XPRT and XP arms of the stage IB group according to pathological staging using the AJCC 2002 system (P=0.256). (B) Comparison of OS between the XPRT and XP arms of the stage IB group according to pathological staging using the AJCC 2002 staging system (P=0.683). (C) Comparison of DFS between the XPRT and XP arms of the stage IB group according to pathological staging using the AJCC 2010 staging system (P=0.900). (D) Comparison of DFS between the XPRT and XP arms of the stage II group according to pathological staging using the AJCC 2010 system (P=0.137).
DFS = disease-free survival; XP = capecitabine plus cisplatin; XPRT = xanthine phosphoribosyltransferase; OS = overall survival; AJCC = American Joint Committee on Cancer.
DISCUSSION
Postoperative chemoradiation was established as the standard of care for resected GC in the United States at a time when D2 lymphadenectomy was not routinely performed. In one of the most influential studies, known as the INT 0116 randomized controlled trial (Southwest Oncology Group 9008), the usefulness of postoperative chemoradiotherapy was investigated and compared with that of surgery alone [3]. With a median follow-up of 5 years, the 3-year survival rate was 50% in the chemoradiation group and 4% in the surgery-alone group (P=0.005) [3]. Although adjuvant chemoradiotherapy has been adopted as the standard of care in North America and some parts of the world, it is still not commonly used in other countries. This relates mainly to the criticism of INT 0116 regarding surgical quality, as 54% of the patients enrolled underwent less than a D1 lymph node dissection despite the recommendation for a D2 dissection. Although the INT 0116 trial reported an OS benefit from adjuvant chemoradiotherapy after resection in a study of 603 patients with stage IB–IV (M0) GC, the numbers of patients with stage IB and II disease were small, and the benefits in this subgroup remain uncertain. Modified absorption of granisetron in the prevention of chemotherapy-induced nausea and vomiting, another clinical trial of perioperative chemotherapy for GC treatment, tested the efficacy of perioperative chemotherapy (n=250) in comparison with that of surgery alone (n=253) [12]. The patients in the treatment arm received 3 cycles of preoperative and postoperative chemotherapy (epirubicin, cisplatin, and 5-fluorouracil [5-FU] [ECF]) [12]. The perioperative chemotherapy group also demonstrated a higher likelihood of progression-free (HR, 0.66; 95% CI, 0.53–0.81; P<0.001) and OS (HR, 0.75; 95% CI, 0.60–0.93; P=0.009). The 5-year survival rate was 36.3% (95% CI, 29.5%–43.0%) in the perioperative chemotherapy group and 23% (95% CI, 16.6%–29.4%) in the surgery-alone group [12]. Given the positive results of the 2 previous studies using different perioperative treatments, the optimal management of GC remains unclear. The Cancer and Leukemia Group B (CALGB) 80101 trial compared a standard group treated with 5-FU and leucovorin and an experimental group treated with the ECF regimen before and after chemoradiation. Results revealed no improvement in 3-year DFS (47% vs. 46%) or OS (52% vs. 50%) with the addition of an anthracycline and platinum compound to 5-FU [13]. On the basis of the positive results of the North American Intergroup Southwest Oncology Group 9008/INT 0116 trial [14] and the negative results of the CALGB 80101 study [13], fluorouracil-based chemoradiotherapy is the preferred postoperative treatment option for patients with pT3–4 and T1–2N+GC after R0 resection with less than a D2 lymphadenectomy, in accordance with the NCCN guidelines [10].
Previously, we reported the negative results of the phase III trial (ARTIST), which examined whether the addition of radiotherapy to adjuvant chemotherapy improved DFS in patients with D2-resected GC. The ARTIST trial is the only randomized phase III trial to date that was performed in Asian patients who underwent D2 lymph node dissection and compared the efficacy of XP with that of XP with concurrent capecitabine radiotherapy (XPRT) [8]. On the basis of the results of this phase III trial (ARTIST), European Society for Medical Oncology guidelines suggested that postoperative chemoradiotherapy can be avoided by optimum surgery such as modified D2 resection [15]. In our subgroup analysis, we had a sufficient number of subjects (n=99) to investigate the impact of the treatment on stage IB GC. The survival analysis result in this study suggests that adjuvant chemoradiotherapy in the context of stage IB disease did not improve patient survival, perhaps because this disease stage is highly treatable by surgery alone that the toxicity from treatment outweighs the benefit. This study also indicates that the adequacy of surgical resection is an important issue. D0 lymphadenectomy is the most common type of lymph node dissection performed in the United States during GC resection. Adjuvant treatment with fluorouracil plus leucovorin and radiation should be considered for all patients with high-risk GC. Although D2 surgery failed to confer a survival benefit in randomized trials, it has already become the standard surgical approach for GC in Asia and Europe. In the ARTIST trial, we confirmed that patients with D2-resected GC can attain long-term DFS and possibly a cure with both adjuvant chemotherapy and chemoradiotherapy. The results of our analyses suggest that this adjuvant therapy may not confer survival benefits in patients with stage IB disease but is more clearly appropriate for stage III and IV (M0) GC.
The present study evaluated the prognostic impact according to the revised AJCC staging system. In brief, subserosa tumor invasion, which was previously classified as T2b, is now classified as T3 according to the seventh edition of the AJCC staging system. Regarding the N parameter, tumors classified as N1 in the sixth edition with >2 positive lymph nodes are now classified as N2 in the seventh edition. The prognosis of the patients reclassified as having stage II disease was worse than that of the patients who remained in stage IB, although the difference was not statistically significant.
Our study has the following limitations: 1) the number of patients with stage IB (AJCC 2002) was relatively too small for obtaining statistically significant results between stages IB (n=29) and II (n=70) according to the AJCC 2010 staging system, and 2) the generalizability of our study findings must be considered.
In conclusion, we demonstrated no survival benefit after adjuvant chemoradiotherapy for stage IB (AJCC 2002) after D2 dissection. Further studies with large populations will be needed to confirm these results before they can be recommended definitively as the standard of care.
ACKNOWLEDGMENTS
The authors thank the Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, for their substantial work in this research.
Footnotes
- Data curation: K.Y., L.S.J., P.S.H.
- Formal analysis: K.Y., L.S.J., P.S.H.
- Funding acquisition: P.Y.S., K.W.K.
- Investigation: K.K.M., C.M.G., S.T.S., B.J.M., K.S., L.J.H., L.J., P.J.O., P.Y.S., L.H.Y., K.W.K., P.S.H.
- Methodology: K.K.M., C.M.G., L.J.H., S.T.S., B.J.M., K.S., K.S.T., P.J.O., P.Y.S., L.H.Y., K.W.K., P.S.H.
- Project administration: K.S., L.H.Y., K.W.K., P.S.H.
- Supervision: L.J., P.J.O., P.Y.S., L.H.Y., K.W.K., P.S.H.
- Visualization: K.Y., L.S.J., K.S.T., P.S.H.
- Writing - original draft: K.Y., P.S.H.
- Writing - review & editing: K.Y., P.S.H
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald JS. Gastric cancer: Nagoya is not New York. J Clin Oncol. 2011;29:4348–4350. doi: 10.1200/JCO.2011.37.5691. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 4.Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522–1530. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908–914. doi: 10.1056/NEJM199903253401202. [DOI] [PubMed] [Google Scholar]
- 6.Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, et al. Extended lymph node dissection for gastric cancer: who may benefit? final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069–2077. doi: 10.1200/JCO.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–462. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Lim DH, Kim S, Park SH, Park JO, Park YS, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268–273. doi: 10.1200/JCO.2011.39.1953. [DOI] [PubMed] [Google Scholar]
- 9.Park SH, Sohn TS, Lee J, Lim DH, Hong ME, Kim KM, et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol. 2015;33:3130–3136. doi: 10.1200/JCO.2014.58.3930. [DOI] [PubMed] [Google Scholar]
- 10.Ajani JA, Bentrem DJ, Besh S, D'Amico TA, Das P, Denlinger C, et al. Gastric cancer, version 2.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11:531–546. doi: 10.6004/jnccn.2013.0070. [DOI] [PubMed] [Google Scholar]
- 11.Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21:556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 12.Choi AH, Kim J, Chao J. Perioperative chemotherapy for resectable gastric cancer: MAGIC and beyond. World J Gastroenterol. 2015;21:7343–7348. doi: 10.3748/wjg.v21.i24.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leong T, Smithers BM, Michael M, Gebski V, Boussioutas A, Miller D, et al. TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG) BMC Cancer. 2015;15:532. doi: 10.1186/s12885-015-1529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glinski K, Wasilewska-Tesluk E, Rucinska M, Cieslak-Zeranska E, Czeremszynska B, Osowiecka K, et al. Clinical outcome and toxicity of 3D-conformal radiotherapy combined with chemotherapy based on the Intergroup SWOG 9008/INT0116 study protocol for gastric cancer. J BUON. 2015;20:428–437. [PubMed] [Google Scholar]
- 15.Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v38–v49. doi: 10.1093/annonc/mdw350. [DOI] [PubMed] [Google Scholar]