Abstract
Purpose
Kallikrein (KLK) proteases are hormone-like signaling molecules with critical functions in different cancers. This study investigated the expression of KLK6 in gastric cancer and its potential role in the growth, migration, and invasion of gastric cancer cells.
Materials and Methods
In this study, we compared protein levels of KLK6, vascular endothelial growth factor (VEGF), and matrix metallopeptidase (MMP) 9 in normal gastric epithelial and gastric cancer cell lines by western blot. Fluorescence-activated cell sorting was employed to sort 2 clones of SGC-7901 cells with distinct KLK6 expression, namely, KLK6-high (KLK6high) and KLK6-low (KLK6low), which were then expanded. Lastly, immunohistochemical analysis was performed to investigate KLK6 expression in gastric cancer patients.
Results
The expression levels of KLK6, VEGF, and MMP 9, were significantly higher in the gastric cancer cell lines SGC-7901, BGC-823, MKN-28, and MGC-803 than in the normal gastric epithelial cell line GES-1. Compared to KLK6low cells, KLK6high cells showed enhanced viability, colony-forming ability, migration, and invasion potential in vitro. Importantly, immunohistochemical analysis of a human gastric cancer tissue cohort revealed that the staining for KLK6, VEGF, and MMP9 was markedly stronger in the cancerous tissues than in the adjacent normal tissues. KLK6 expression also correlated with that of VEGF and MMP9 expression, as well as several key clinicopathological parameters.
Conclusions
Together, these results suggest an important role for KLK6 in human gastric cancer progression.
Keywords: Gastric cancer; KLK6 protein, human; MMP9 protein, human; VEGF
INTRODUCTION
The human tissue kallikreins (KLKs), consisting of 15 members, are involved in tissue-specific proteolysis of numerous endogenous substrates [1,2]. It is an important family of serine proteases that are involved in various processes in both healthy and diseased states, including a number of cancers [1,2]. For instance, KLK4 plays a critical role in promoting prostate cancer progression by integrating androgen and mammalian target of rapamycin signaling network [3]. KLK3, better known as prostate specific antigen (PSA), has been well-established as a clinical marker for prostate cancer screening, diagnosis, and management [4].
KLK, another key member of the family, is upregulated in several types of cancers, including gastric cancer [5,6]. Its expression has also been associated with shorter overall survival (OS) and/or disease-free survival in colon, lung, and ovarian cancers [7,8,9]. Functionally, KLK6 was found to contribute to cancer progression by degrading extracellular matrix proteins leading to angiogenesis, tumor invasion, and metastasis [9,10]. KLK6 downregulates E-cadherin expression in ovarian and colon cancer cells and interrupts E-cadherin-mediated cell-cell adhesion, a prerequisite for tumor invasion [11,12]. In gastric cancer, KLK6 has been shown to promote chemotherapeutic resistance by inducing protective autophagy and p53 expression [13]. Despite these findings, the role of KLK6 in the growth, migration, and invasion of gastric cancer cells is still largely elusive.
In this study, we first observed that the expression level of KLK6 in 4 different gastric cancer cell lines was significantly higher than that in the normal gastric epithelial cells. A similar expression pattern was also observed for vascular endothelial growth factor (VEGF) and matrix metallopeptidase (MMP) 9, 2 critical players known to promote invasion and metastasis [14,15]. We then sorted SGC-7901 cells into clones with distinct KLK6 expression and selected 2 clones KLK6-high (KLK6high) and KLK6-low (KLK6low). Functional assays revealed that KLK6high SGC-7901 cells displayed higher potential for migration and invasion in vitro, relative to KLK6low cells. Lastly, immunostaining of a large human gastric cancer tissue cohort revealed that the expression of KLK6 positively correlated with that of VEGF, MMP9, as well as several clinicopathological parameters. Together, these data suggest an important role for KLK6 in promoting growth, migration, and invasion of gastric cancer cells.
MATERIALS AND METHODS
Cell culture
Gastric cancer cell lines SGC-7901, BGC-823, MGC-803, and MKN-28 and the normal gastric epithelial cell line GES-1 were routinely cultured in Roswell Park Memorial Institute 1640 medium containing 10% fetal calf serum supplemented with 50 U/mL penicillin, 50 lg/mL streptomycin, and 2 mM L-glutamine in a humidified chamber with 5% CO2 at 37°C. All reagents were purchased from Hyclone (GE Healthcare, Chicago, IL, USA). All cell lines were routinely tested and were negative for mycoplasma contamination.
Clinical samples
Gastric cancer tissue samples from 326 patients were collected at the People's Hospital of Zhengzhou between March 2005 and March 2011. Prior to surgery, all patients were free of distant metastases, and had not received chemotherapy, radiotherapy, or immunotherapy. A part of the collected tissues was fixed in 4% paraformaldehyde for immunohistochemical analysis, and the other part was preserved at −80°C. The adjacent normal tissues were collected from sites at least 5 cm away from the tumor margins, which were further confirmed by pathological analysis. The detailed information of patients is shown in Table 1. All patients provided informed consent under the guidelines approved by the Ethical Committee of People's Hospital of Zhengzhou.
Table 1. Patient distribution according to different characteristics.
| Characteristics | No. of patients (%) | |
|---|---|---|
| Gender | ||
| Male | 234 | |
| Female | 92 | |
| Age (yr) | ||
| ≥54.5 | 210 | |
| <54.5 | 116 | |
| Tumor differentiation | ||
| Low | 113 | |
| Moderate–high | 213 | |
| TNM stage | ||
| Phase I | 82 | |
| Phase II | 114 | |
| Phase III | 130 | |
| Lymph node metastases | ||
| Yes | 136 | |
| No | 190 | |
TNM = tumor, node, and metastasis.
RNA extraction, complementary DNA (cDNA) synthesis, and quantitative polymerase chain reaction (qPCR)
Total RNA from cells or frozen gastric tissues was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. RNA concentration was measured using the Nanodrop ND-1000 spectrophotometer and RNA quality was verified by agarose gel electrophoresis. The cDNA synthesis was performed using SuperScript II Reverse Transcriptase (Invitrogen), which was then used as a template for qPCR analysis performed using SYBR green dye (Applied Biosystems, Foster City, CA, USA) and LightCycler 480 system (Roche, Basel, Switzerland). The sequences of the primers used are available upon request. GAPDH gene expression was used for normalization.
Western blot analysis
Cell or tissue extracts were prepared, and protein concentration was measured using the bicinchoninic acid assay. Primary antisera against KLK6 (ab190924), MMP9 (ab137867), and VEGFA (ab51745) were all purchased from Abcam (Cambridge, UK) and used at a dilution of 1:1,000. The secondary antiserum (Thermo Fisher Scientific, Waltham, MA, USA) was used according to the manufacturer's instructions at a dilution of 1:4,000. ECL substrate kit (ZSBio, Beijing, China) was used for the detection of proteins.
Fluorescence-activated cell sorting (FACS) assay
Flow cytometry was performed on BD FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) using CellQuest (Largo, FL, USA) and the data were analyzed with FlowJo V10.2 software (Tree Star Inc., San Carlos, CA, USA). KLK6 antibody was diluted in phosphate-buffered saline containing 0.1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA).
Immunohistochemistry
Immunohistochemistry was performed using the SP kit (SPN-9002) (ZSBio) according to the manufacturer's guidelines. Primary antibodies against KLK6 (ab190924), MMP9 (ab137867), and VEGFA (ab51745) were all purchased from Abcam, and used at a dilution of 1:50. The immunohistochemical images were quantified. Five fields of view were randomly selected on each section (with at least 200 cells per view), and the quantification was performed as follows:
Score A was assigned according to the proportion of positively-stained cells: 0 for <5%, 1 for 6%–25%, 2 for 26%–50%, 3 for 51%–75%, and 4 for >75%. An independent score B was assigned according to the intensity of staining: 0 for negative, 1 for moderate, 2 for strong, and 3 for very strong. A final score was then calculated according to the formula, score C = score A × score B. Eventually, samples with a score C above 5 were considered positive, while samples with a score C below 5 were considered negative.
Methyl thiazolyl tetrazolium (MTT) assay
Cells were seeded in 96-well plates (5×103 cells per well) and cultured in normal growth media up to 72 hours. The MTT reagent (Sigma-Aldrich) was used according to the manufacturer's instructions. Three experiments were performed separately in quadruplicates and the representative results are shown.
Colony formation assay
Approximately 2,000 cells were seeded in 6-cm plates and cultured in normal growth media. After 2 weeks, the colonies were fixed with cold methanol, and visualized using 0.5 mL of 0.01% crystal violet and photographed. The experiment was repeated 3 times in triplicate.
Wound healing assay
SGC-7901 cells (5×105) were cultured as confluent monolayers in a 6-well plate. After attaining 100% confluency, the cell layer was scratched by removing a strip of cells across the well using a 200 µL pipette tip. The scratched monolayers were then washed twice to remove non-adherent cells. The wells were photographed using a cell imaging system at 4× magnification, at 0, 24, and 48 hours after the scratch. The experiment was performed in triplicates and the images were analyzed using Image J software. Error bars in the figure represent the standard deviation. Statistical significance was determined by Student's t-test, with P<0.05 considered statistically significant.
Invasion assay
Cells were cultured in complete culture medium, grown to 70% confluency, harvested, and suspended in serum-free medium. Inserts were pre-coated with 100 µL Matrigel (Corning Inc., New York, NY, USA) 24 hours prior to conducting the assay. Cells (2×104) were seeded in each insert and media was added in the lower chamber. After 48 hours of incubation, the inserts were fixed and stained, and the number of invaded tumor cells was counted under a microscope. Ten fields of view were randomly counted for each insert and the assay was performed in triplicate.
Statistical analysis
Statistical comparisons were analyzed using SPSS 17.0 software (IBM Corp., Armonk, NY, USA). Immunohistochemical results were analyzed using χ2 test. Spearman's rank correlation was used for concordant expression analysis and other data was analyzed using analysis of variance. A value of P<0.05 was considered as statistically significant.
RESULTS
Expression of KLK6, VEGF, and MMP9 in gastric cancer and normal gastric epithelial cells
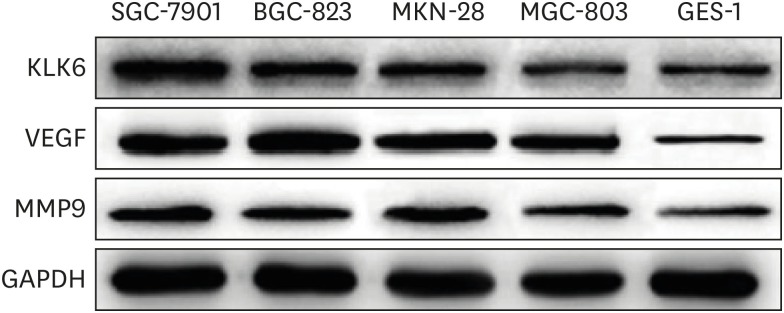
We first assessed the protein expression of KLK6 in a panel of 4 different gastric cancer cell lines SGC-7901, BGC-823, MKN-28, and MGC-803, and 1 normal gastric epithelial cell line GES-1, by western blotting. Our results showed that KLK6 expression was markedly higher in the cancer cells compared to the normal cell line, with the highest expression in SGC-7901 cells (Fig. 1). Meanwhile, the expression levels of VEGF and MMP9, 2 well-established pro-metastasis factors, displayed a similar pattern and largely correlated with that of KLK6 (Fig. 1).
Fig. 1. Protein expression of KLK6, VEGF, and MMP9 in various gastric cancer and normal gastric epithelial cells.
KLK6 = kallikrein 6; VEGF = vascular endothelial growth factor; MMP9 = matrix metallopeptidase 9.
Comparison of in vitro growth, migration, and invasion potential between KLK6high and KLK6low gastric cancer cells
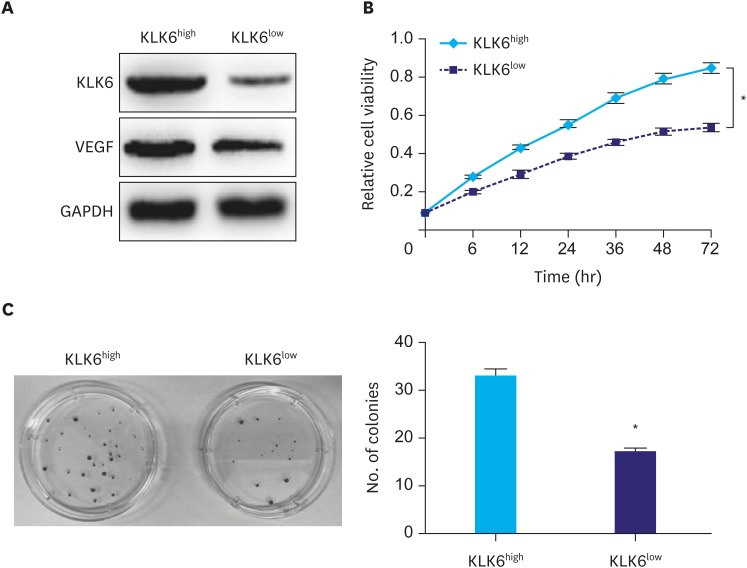
Considering the heterogeneity of cancer cells, we first tried to sort SGC-7901 cells according to KLK6 expression using FACS. Both KLK6high and KLK6low expressing clones were obtained. This was validated by western blot analysis, where a marked difference was observed in KLK6 and VEGF expression (Fig. 2A). We then expanded these clones and performed the MTT assay, where KLK6high cells showed drastically higher viability and proliferation rate relative to KLK6low cells over a period of 72 hours (Fig. 2B). Simultaneously, KLK6high cells also exhibited a 2-fold increase in the clonogenic ability compared to KLK6low cells (Fig. 2C). These results suggested that KLK6 promotes gastric cancer cell growth in vitro.
Fig. 2. (A) Western blot analysis of KLK6 and VEGF levels in KLK6high and KLK6low SGC-7901 cells. (B) The MTT assay was conducted to assess cell viability of KLK6high and KLK6low cells over a duration of 72 hours. (C) Colony formation assay was performed to compare the ability of KLK6high and KLK6low cells to form colonies. The number of colonies was then quantified.
KLK6 = kallikrein 6; VEGF = vascular endothelial growth factor; MTT = methyl thiazolyl tetrazolium; KLK6high = kallikrein 6-high; KLK6low = kallikrein 6-low.
*Indicated value means statistical significance.
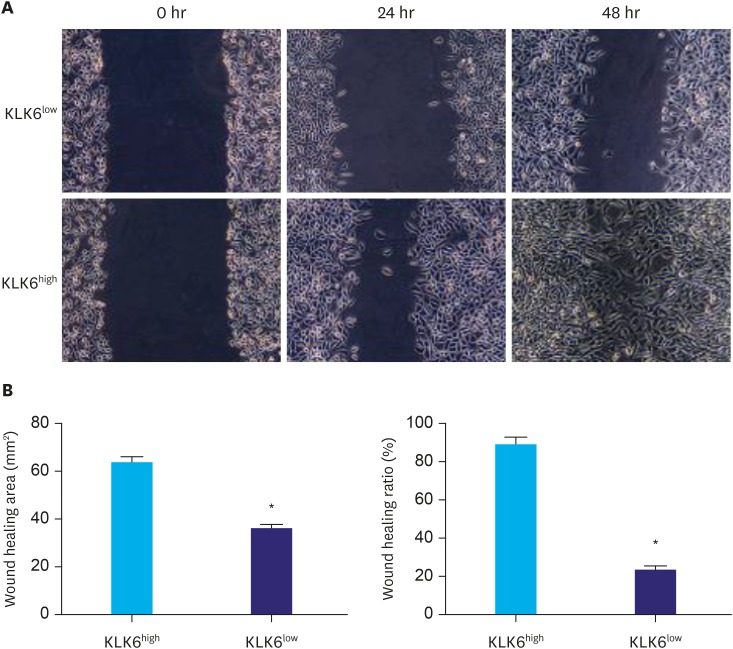
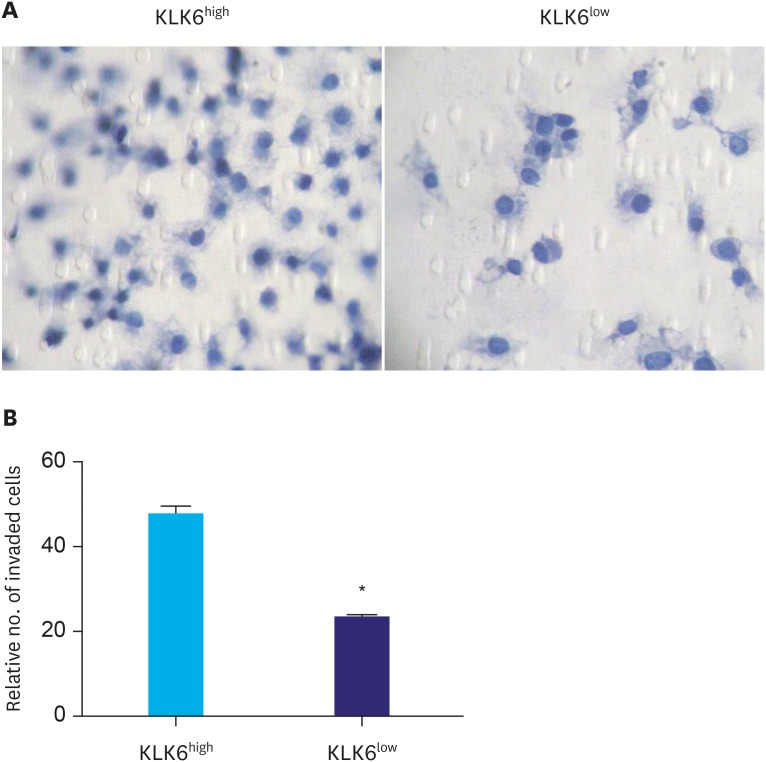
To compare the migration ability of these cells, the wound healing assay was performed. As shown in Fig. 3, KLK6high cells migrated significantly faster than KLK6low cells and the wound of KLK6high cells healed almost completely after 48 hours. To further assess the invasive potential of these cells, we conducted transwell invasion assay, where significantly more KLK6high cells invaded through the matrigel-coated insert within 24 hours compared to the KLK6low cells (Fig. 4). These data suggested that KLK6high SGC-7901 cells have greater migration and invasion potential in vitro, compared to the KLK6low cells.
Fig. 3. (A) Wound healing assay was performed using KLK6low and KLK6high cells. The wound was imaged at 0, 24, and 48 hours after the scratch. (B) Quantification of wound healing area and wound healing ratio (%) in (A).
KLK6high = kallikrein 6-high; KLK6low = kallikrein 6-low.
*Indicated value means statistical significance.
Fig. 4. (A) Transwell invasion assay was performed to assess the invasive potential of KLK6high and KLK6low cells. Cells were allowed to invade for 24 hours, and the inserts were then fixed and stained. (B) Quantification of the invaded cells in (A).
KLK6high = kallikrein 6-high; KLK6low = kallikrein 6-low.
*Indicated value means statistical significance.
Expression analysis of KLK6, VEGF, and MMP9 in gastric cancer tissues and normal tissues
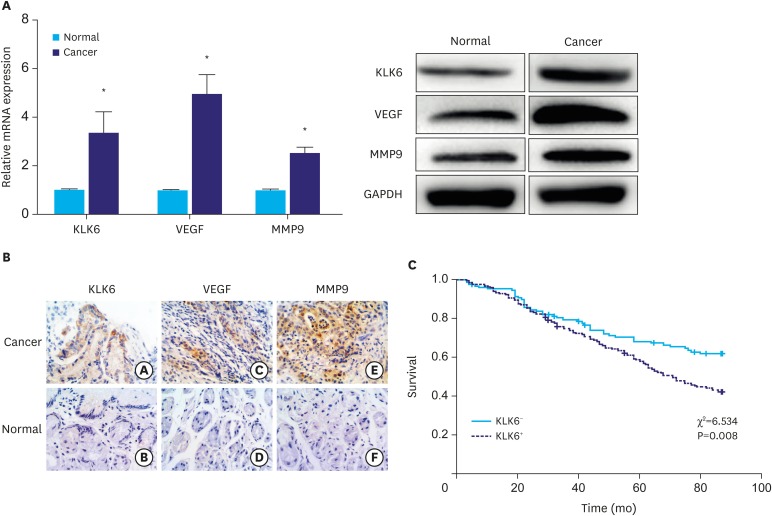
To further gain insight into the role of KLK6 in human gastric cancer, we first randomly selected 10 patient samples from a cohort consisting of 326 gastric tumor tissues and their matching neighboring normal tissues. The qPCR and western blot analyses showed that both messenger RNA (mRNA) and protein expression levels of KLK6, VEGF, and MMP9 were significantly higher in cancer tissues relative to their adjacent normal tissues (Fig. 5A).
Fig. 5. (A) qPCR and western blot analyses of KLK6, VEGF, and MMP9 expression in gastric cancer and adjacent normal tissues. (B) Immunohistochemical staining of KLK6, VEGF, and MMP9 in gastric cancer and adjacent normal tissues. The a, c, and e are tumor tissues; b, d, and f are normal tissues. (C) Kaplan–Meier curves suggesting that KLK6− patients had a significantly better chance of survival than KLK6+ patients. The χ2 and P-values are indicated in the plot.
qPCR = quantitative polymerase chain reaction; KLK6 = kallikrein 6; VEGF = vascular endothelial growth factor; MMP9 = matrix metallopeptidase 9; mRNA = messenger RNA.
*Indicated value means statistical significance.
Next, we performed immunohistochemical analysis of KLK6, VEGF, and MMP9 in the same cohort. As shown in Fig. 5B, KLK6, VEGF, and MMP9 were all predominantly expressed in the cytoplasm of cancer cells, whereas membrane and extracellular staining of KLK6 and MMP9 could also be observed occasionally. Consistent with the qPCR and western blot results, all of these proteins displayed stronger staining intensity in cancer tissues compared to that in the normal tissues. Table 2 represents significant difference (P<0.01) in the number of positively-stained samples between tumor and normal tissues.
Table 2. Comparison of KLK6, VEGF, and MMP9 staining between gastric cancer and normal tissues.
| Tissue type | Number of positively-stained samples | KLK6 | VEGF | MMP9 |
|---|---|---|---|---|
| Tumor | 326 | 142 (43.56)* | 173 (53.07)* | 238 (73.01)* |
| Normal | 326 | 26 (7.98) | 57 (17.48) | 44 (13.50) |
Values are presented as number (%).
KLK6 = kallikrein 6; VEGF = vascular endothelial growth factor; MMP9 = matrix metallopeptidase 9.
*Indicated value means significant difference (P<0.05).
Correlation of KLK6 expression with that of VEGF and MMP9 in human gastric cancer
In this cohort, 100 patient samples showed positive staining for both KLK6 and VEGF, while 111 patient samples were negatively stained for both; 125 patient samples showed positive staining for both KLK6 and MMP9, while 71 patient samples were negative for both. According to Spearman's correlation analysis, the expression of KLK6 was significantly associated with the expression levels of VEGF and MMP9 in this cohort (P<0.01; Table 3).
Table 3. Concordant expression of KLK6 with VEGF and MMP9 expression in human gastric cancer samples.
| KLK6 | VEGF | r-value | P-value | MMP9 | r-value | P-value | ||
|---|---|---|---|---|---|---|---|---|
| + | − | + | − | |||||
| + | 100 | 73 | 0.305 | <0.01 | 125 | 17 | 0.297 | <0.01 |
| − | 42 | 111 | 113 | 71 | ||||
Values are presented as number (%).
KLK6 = kallikrein 6; VEGF = vascular endothelial growth factor; MMP9 = matrix metallopeptidase 9.
Association of KLK6 expression with clinicopathological parameters of gastric cancer
When cross-referencing the immunostaining results of KLK6 expression with patient survival data, we found that patients with negative KLK6 expression (KLK6−) had significantly better survival rate compared to those patients with positive KLK6 expression (KLK6+) (χ2=6.534, P=0.008; Fig. 5C). Further analyses of KLK6 expression with respect to pathological tumor, node, and metastasis (TNM) staging showed that 50% of the phase III patients (65/130) were KLK6+, which was significantly higher compared to patients either in phase II (48/114) or phase I (27/82) (P=0.027). In addition, the probability of KLK6+ patients to develop lymph node metastases was 52.94% (72/136), significantly higher than that of lymph node metastases-free patients (36.84%, 70/190) (P=0.004). In contrast, no significant correlation was observed between KLK6 expression and age, gender, or the degree of tumor differentiation. These results are summarized in Table 4.
Table 4. Correlation of KLK6 expression with major clinicopathological parameters.
| Parameters | Number of patients | KLK6+ | r-value | P-value | |
|---|---|---|---|---|---|
| Gender | 1.586 | 0.208 | |||
| Female | 92 | 35 (38.04) | |||
| Male | 234 | 107 (45.73) | |||
| Age | 0.677 | 0.410 | |||
| <54.5 | 116 | 47 (40.52) | |||
| ≥54.5 | 210 | 95 (45.24) | |||
| Tumor differentiation | 0.364 | 0.834 | |||
| High | 104 | 47 (45.19) | |||
| Medium | 109 | 45 (41.28) | |||
| Low | 113 | 50 (44.25) | |||
| TNM stage | 7.235 | 0.027 | |||
| Phase I | 82 | 27 (32.93) | |||
| Phase II | 114 | 48 (42.11) | |||
| Phase III | 130 | 67 (51.54) | |||
| Lymph node metastases | 8.356 | 0.004 | |||
| Yes | 136 | 72 (52.94) | |||
| No | 190 | 70 (36.84) | |||
Values are presented as number (%).
KLK6 = kallikrein 6; TNM = tumor, node, and metastasis.
DISCUSSION
Gastric cancer is one of the most aggressive cancer types that continues to have a daunting impact on global health [16]. Despite an overall decline in incidence over the past decades, gastric cancer remains the fourth most common type of cancer and the second leading cause of cancer-related death worldwide [17]. To date, surgery remains the only curative treatment. For locally advanced disease, adjuvant or neoadjuvant therapy is usually implemented in combination with surgery. Unfortunately, treatment for advanced or metastatic gastric cancer has seen little progress over the years, with the median survival being approximately 1 year [16,17]. Therefore, understanding the functions of new genes, unravelling novel therapeutic targets and developing drugs accordingly, especially for patients with advanced gastric cancer, is the current mainstay for gastric cancer research.
KLKs are an important family of proteases and numerous studies have characterized the functions of KLKs in different aspects of cancer and have explored their potential value as diagnostic or prognostic markers, as well as therapeutic targets [1,2]. The best-known example is KLK3 (PSA), which is applied for the diagnosis and management of prostate cancer [17]. KLK6 is another key member of the family whose expression is upregulated in a variety of cancers, thereby influencing critical functional roles [11,18,19,20]. Studies on ovarian and colon cancer model systems also showed that KLK6 promotes metastasis by inhibiting E-cadherin expression [11,12]. Despite this knowledge, the functional role of KLK6 in gastric cancer cells has not been fully elucidated.
A couple of previous studies have compared KLK6 expression in human gastric cancerous tissues and normal tissues and both studies revealed a higher level of KLK6 expression in cancerous gastric tissues than in normal tissues, shedding light on the expression and significance of KLK6 in this malignant disease [5,19]. To further validate the nature of these findings, we first examined KLK6 expression in a panel of 4 different gastric cancer cell lines and 1 normal gastric epithelial cell line. KLK6 expression was markedly higher in gastric cancer cells relative to normal cells (Fig. 1). Interestingly, 2 pro-metastasis factors VEGF and MMP9 showed a similar expression pattern across these cell lines (Fig. 1). In line with these in vitro observations, both mRNA and protein expression levels of KLK6 were drastically elevated in gastric cancer tissues compared to their adjacent normal counterparts (Fig. 5A), which showed elevated VEGF and MMP9 expression levels (Fig. 5B and Table 2). Therefore, our results confirmed previous findings on both mRNA and protein levels in a large cohort of Chinese gastric cancer patients, and suggest that increased KLK6 expression is a common phenomenon in human gastric cancer and that it might serve as a diagnostic marker.
To investigate the functional significance of KLK6 in gastric cancer, we first utilized FACS to sort the gastric cancer cell line SGC-7901 into clones that expressed KLK6 in various degrees. We then expanded 2 clones with distinct KLK6 expression confirmed by Western blot analysis (Fig. 2A). Compared to KLK6low cells, KLK6high cells exhibited significantly higher growth rate and colony forming ability (Fig. 2B and C), as well as stronger migration and invasion potential (Figs. 3 and 4). These data were in line with the roles of KLK6 in other cancer types. For example, KLK6 was shown to promote breast cancer cell invasiveness, which could be suppressed by a specifically engineered inhibitor of KLK6 [21,22]. Meanwhile, in ovarian and colon cancer cells, KLK6 was found to decrease E-cadherin expression and cell adhesion, thereby promoting tumor invasion and metastasis [11,12]. In squamous skin cancer, KLK6 was found to degrade extracellular matrix proteins, stimulate angiogenesis, and promote proliferation, migration, and invasion [10]. In our study, the fact that the expression of KLK6 was closely associated with the expression levels of VEGF and MMP9 in cell lines and in human gastric cancer tissues (Fig. 1 and Table 3) indicated that the effect of KLK6 in gastric cancer cells was possibly mediated, at least in part, by VEGF and MMP9.
An early report has linked KLK6 mRNA level with lymphatic invasion and OS rate in a cohort of Japanese gastric cancer patients [5], whereas a recent study observed no significant difference in serum KLK6 level among patients at various tumor stages [19]. Taking advantage of our cohort, we also examined the correlation of KLK6 expression with several key clinicopathological parameters in gastric cancer. Importantly, our results revealed that positive KLK6 expression was significantly correlated with increased incidence of lymph node metastasis, higher grade of tumor differentiation and TNM staging, and poor OS in gastric cancer patients (Fig. 5C and Table 4). These findings, together with in vitro functional results, suggested that enhanced expression of KLK6 might play an important role in the progression of gastric cancer.
In conclusion, our study demonstrated that KLK6 promoted the growth, migration, and invasion of gastric cancer cells in vitro. We also showed that KLK6 expression was elevated in gastric cancers compared to normal tissues, and it was significantly correlated with several critical clinicopathological parameters. Future studies will address whether KLK6 can serve as a potential therapeutic target for gastric cancer by targeting its expression in both in vitro and in vivo models.
ACKNOWLEDGMENTS
The authors would like to thank the colleagues at The First Affiliated Hospital and School of Basic Medicine of Zhengzhou University for their help, and the pathologists at the People's Hospital of Zhengzhou for their assistance.
Footnotes
Funding: This study was funded by the Key Scientific Research Projects for Universities (18A320058), Education Department of Henan Province, China.
- Conceptualization: Z.S., L.Z.
- Funding acquisition: L.Z.
- Investigation: Z.S., Z.S.
- Resources: S.J.
- Writing - original draft: Z.S., L.Z.
- Writing - review & editing: Z.S., L.Z.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Yousef GM, Diamandis EP. The new human tissue kallikrein gene family: structure, function, and association to disease. Endocr Rev. 2001;22:184–204. doi: 10.1210/edrv.22.2.0424. [DOI] [PubMed] [Google Scholar]
- 2.Borgoño CA, Diamandis EP. The emerging roles of human tissue kallikreins in cancer. Nat Rev Cancer. 2004;4:876–890. doi: 10.1038/nrc1474. [DOI] [PubMed] [Google Scholar]
- 3.Jin Y, Qu S, Tesikova M, Wang L, Kristian A, Mælandsmo GM, et al. Molecular circuit involving KLK4 integrates androgen and mTOR signaling in prostate cancer. Proc Natl Acad Sci U S A. 2013;110:E2572–E2581. doi: 10.1073/pnas.1304318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317:909–916. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- 5.Nagahara H, Mimori K, Utsunomiya T, Barnard GF, Ohira M, Hirakawa K, et al. Clinicopathologic and biological significance of kallikrein 6 overexpression in human gastric cancer. Clin Cancer Res. 2005;11:6800–6806. doi: 10.1158/1078-0432.CCR-05-0943. [DOI] [PubMed] [Google Scholar]
- 6.Schuster R, Max N, Mann B, Heufelder K, Thilo F, Gröne J, et al. Quantitative real-time RT-PCR for detection of disseminated tumor cells in peripheral blood of patients with colorectal cancer using different mRNA markers. Int J Cancer. 2004;108:219–227. doi: 10.1002/ijc.11547. [DOI] [PubMed] [Google Scholar]
- 7.Nathalie HV, Chris P, Serge G, Catherine C, Benjamin B, Claire B, et al. High kallikrein-related peptidase 6 in non-small cell lung cancer cells: an indicator of tumour proliferation and poor prognosis. J Cell Mol Med. 2009;13(9B):4014–4022. doi: 10.1111/j.1582-4934.2009.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shan SJ, Scorilas A, Katsaros D, Diamandis EP. Transcriptional upregulation of human tissue kallikrein 6 in ovarian cancer: clinical and mechanistic aspects. Br J Cancer. 2007;96:362–372. doi: 10.1038/sj.bjc.6603556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vakrakou A, Devetzi M, Papachristopoulou G, Malachias A, Scorilas A, Xynopoulos D, et al. Kallikrein-related peptidase 6 (KLK6) expression in the progression of colon adenoma to carcinoma. Biol Chem. 2014;395:1105–1117. doi: 10.1515/hsz-2014-0166. [DOI] [PubMed] [Google Scholar]
- 10.Klucky B, Mueller R, Vogt I, Teurich S, Hartenstein B, Breuhahn K, et al. Kallikrein 6 induces E-cadherin shedding and promotes cell proliferation, migration, and invasion. Cancer Res. 2007;67:8198–8206. doi: 10.1158/0008-5472.CAN-07-0607. [DOI] [PubMed] [Google Scholar]
- 11.Kim JT, Song EY, Chung KS, Kang MA, Kim JW, Kim SJ, et al. Up-regulation and clinical significance of serine protease kallikrein 6 in colon cancer. Cancer. 2011;117:2608–2619. doi: 10.1002/cncr.25841. [DOI] [PubMed] [Google Scholar]
- 12.Sawada K, Mitra AK, Radjabi AR, Bhaskar V, Kistner EO, Tretiakova M, et al. Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res. 2008;68:2329–2339. doi: 10.1158/0008-5472.CAN-07-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim TW, Lee SJ, Kim JT, Kim SJ, Min JK, Bae KH, et al. Kallikrein-related peptidase 6 induces chemotherapeutic resistance by attenuating auranofin-induced cell death through activation of autophagy in gastric cancer. Oncotarget. 2016;7:85332–85348. doi: 10.18632/oncotarget.13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masoumi Moghaddam S, Amini A, Morris DL, Pourgholami MH. Significance of vascular endothelial growth factor in growth and peritoneal dissemination of ovarian cancer. Cancer Metastasis Rev. 2012;31:143–162. doi: 10.1007/s10555-011-9337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 16.Carcas LP. Gastric cancer review. J Carcinog. 2014;13:14. doi: 10.4103/1477-3163.146506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 18.Lenga Ma Bonda W, Iochmann S, Magnen M, Courty Y, Reverdiau P. Kallikrein-related peptidases in lung diseases. Biol Chem. 2018;399:959–971. doi: 10.1515/hsz-2018-0114. [DOI] [PubMed] [Google Scholar]
- 19.Kim JJ, Kim JT, Yoon HR, Kang MA, Kim JH, Lee YH, et al. Upregulation and secretion of kallikrein-related peptidase 6 (KLK6) in gastric cancer. Tumour Biol. 2012;33:731–738. doi: 10.1007/s13277-011-0267-1. [DOI] [PubMed] [Google Scholar]
- 20.Oikonomopoulou K, Diamandis EP, Hollenberg MD. Kallikrein-related peptidases: proteolysis and signaling in cancer, the new frontier. Biol Chem. 2010;391:299–310. doi: 10.1515/BC.2010.038. [DOI] [PubMed] [Google Scholar]
- 21.Sananes A, Cohen I, Shahar A, Hockla A, De Vita E, Miller AK, et al. A potent, proteolysis-resistant inhibitor of kallikrein-related peptidase 6 (KLK6) for cancer therapy, developed by combinatorial engineering. J Biol Chem. 2018;293:12663–12680. doi: 10.1074/jbc.RA117.000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sidiropoulos KG, Ding Q, Pampalakis G, White NM, Boulos P, Sotiropoulou G, et al. KLK6-regulated miRNA networks activate oncogenic pathways in breast cancer subtypes. Mol Oncol. 2016;10:993–1007. doi: 10.1016/j.molonc.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]