Significance
Bacterial infections caused by Streptococcus pneumoniae are responsible for millions of deaths world-wide each year. Currently marketed glycoconjugate vaccines do not cover all serotypes, such that serotype replacement is observed clinically. Our work aimed at improving the licensed vaccines Prevnar13 (13-valent) and Synflorix (10-valent) by adding synthetic glycoconjugates representing serotypes that are not covered by existing vaccines, and developing a pentavalent semisynthetic glycoconjugate vaccine (sPCV5). The sPCV5 as well as coformulation of existing vaccines proved highly efficacious in a rabbit model considering the three most important indicators of vaccine efficacy. A substantial rise in antibody titer between pre- and postimmunization sera was observed and the opsonophagocytic activity of antibodies, and immunological memory were confirmed.
Keywords: synthetic glycans, vaccine, Streptococcus pneumoniae
Abstract
Streptococcus pneumoniae remains a deadly disease in small children and the elderly even though conjugate and polysaccharide vaccines based on isolated capsular polysaccharides (CPS) are successful. The most common serotypes that cause infection are used in vaccines around the world, but differences in geographic and demographic serotype distribution compromises protection by leading vaccines. The medicinal chemistry approach to glycoconjugate vaccine development has helped to improve the stability and immunogenicity of synthetic vaccine candidates for several serotypes leading to the induction of higher levels of specific protective antibodies. Here, we show that marketed CPS-based glycoconjugate vaccines can be improved by adding synthetic glycoconjugates representing serotypes that are not covered by existing vaccines. Combination (coformulation) of synthetic glycoconjugates with the licensed vaccines Prevnar13 (13-valent) and Synflorix (10-valent) yields improved 15- and 13-valent conjugate vaccines, respectively, in rabbits. A pentavalent semisynthetic glycoconjugate vaccine containing five serotype antigens (sPCV5) elicits antibodies with strong in vitro opsonophagocytic activity. This study illustrates that synthetic oligosaccharides can be used in coformulation with both isolated polysaccharide glycoconjugates to expand protection from existing vaccines and each other to produce precisely defined multivalent conjugated vaccines.
Capsular polysaccharides (CPS) surround many deadly human pathogens. Polysaccharide-conjugated vaccines, based on isolated CPS antigens attached to carrier proteins, protect young children and the elderly from deadly bacterial pathogens including Haemophilus influenzae type b (Hib), Neisseria meningitides, and the encapsulated gram-positive bacterium Streptococcus pneumoniae. S. pneumoniae is the leading cause of life-endangering diseases such as pneumonia, septicemia, and meningitis (1), and a major cause of death in children under five in developing countries (2–4). More than 90 S. pneumoniae serotypes can be distinguished based on their CPS (5, 6). Currently available CPS-based pneumococcal vaccines contain the serotypes most frequently associated with invasive pneumococcal diseases (IPDs). Although the licensed 23-valent polysaccharide vaccine (Pneumovax 23) is not effective in younger children (3, 7), the conjugate vaccines Prevnar13 and Synflorix cover 13 and 10 serotypes, respectively, and are highly successful in all age groups (8). Nevertheless, serotype replacement due to vaccination and regional differences in dominant serotypes necessitate the expansion of existing vaccines to include additional serotypes. An additional weak point is that some serotype antigens, such as ST5 and ST1, that are present in existing vaccines undergo undesired chemical modification during production (9, 10); others have limited immunogenicity and lead to protective levels well below those required for herd immunity, such as SP3 (6).
The procurement of polysaccharides for conjugate-vaccine production by the isolation of CPS from cultured bacteria is conceptionally simple but operationally challenging. Antigen heterogeneity, batch-to-batch variation, and poorly defined conjugation to carrier proteins can be overcome when synthetic oligosaccharides are employed (11). The glycoconjugate vaccine QuimiHib, licensed in several countries to protect against Haemophilus influenzae type b, is based on a synthetic oligosaccharide resulting from chemical polymerization and is effective (12). The medicinal chemistry approach to glycoconjugate vaccine development offers an alternative to CPS isolation for a variety of glycan antigens including those for the hospital-acquired infection-causing bacteria Clostridium difficile and Klebsiella pneumoniae (13–16). Recent advances in the chemical synthesis of complex glycans including automated glycan assembly (AGA) have made the synthesis of a variety of diverse oligosaccharides resembling the CPS of different serotypes possible. Active immunization of mice and rabbits with CRM197 conjugates with ST2 (17), ST3 (18), ST5 (9), ST8 (19), and ST14 (20, 21) elicits opsonic antibodies that were shown in some cases to be protective in challenge models of disease.
The production of effective semisynthetic oligosaccharide-based glycoconjugate vaccines relies on the identification and synthesis of well-defined glycotopes. Glycan microarrays containing isolated CPS as well as synthetic glycans enable the rapid screening and identification of protective glycotopes as a basis for the development of highly effective next generation synthetic glycoconjugate vaccines. The medicinal chemistry approach has identified di- to tetrasaccharides as potential vaccine candidates (11). The immunogenicity of the CPS depends among other factors on rare sugars and labile functional groups (9, 17, 19). Stable oligosaccharide analogs can fix production problems such as those encountered for ST5 CPS due to the labile ketone present in the repeating unit (9). Defined oligosaccharide antigens have been identified as vaccine candidates to protect against bacterial infections caused by S. pneumoniae, C. difficile, K. pneumoniae, and N. meningitides via the medicinal chemistry approach (3, 16, 19, 22).
Previously, we had demonstrated that single semisynthetic glycoconjugate vaccine candidates ST2, ST3, and ST8, in which the synthetic antigen is conjugated to carrier protein CRM197, induce opsonic antibodies, kill bacteria in vitro (OPKA), and can protect against lethal infection in an in vivo S. pneumoniae challenge model (17–19). This study was designed to address the question of whether it is possible to improve the licensed polysaccharide-based vaccines Prevnar13 and Synflorix by adding antigens of nonvaccine serotypes, and to formulate a semisynthetic pentavalent glycoconjugate vaccine consisting exclusively of synthetic antigens. Here we demonstrate that conjugate vaccine candidates based on synthetic oligosaccharide antigens resembling different S. pneumoniae serotypes can be coformulated to produce a pentavalent semisynthetic vaccine, and that synthetic glycoconjugates can be added to marketed vaccines such as Prevnar13 and Synflorix to enhance their coverage. These multivalent vaccines induce a strong antibody response and immunological memory in rabbits. The resulting antibodies are capable of killing the respective bacteria.
Materials and Methods
General Methods.
Oligosaccharide antigens were synthesized using standard protocols and conjugated to CRM197 using the bis(4-nitrophenyl) adipate (PNP)-activated ester method. Synthetic antigens were printed on NHS-activated microarray slides. Immunization was carried out using New Zealand White (NZW) rabbits and the immune response analyzed by microarrays and ELISA. The functional attribute of the immune response was monitored by OPKA using HL-60 cells. Detailed materials and methods can be found in SI Appendix.
Study Design and Sample Size.
The aims of this study were (i) to improve existing polysaccharide glycoconjugate vaccines by coformulating them with the oligosaccharide-based glycoconjugates of nonvaccine serotypes and (ii) to formulate the pentavalent semisynthetic vaccine. The sample size was determined based on previous analyses to ensure statistical significance while minimizing animal usage (19). Each experimental group contained three to six animals. P values of <0.05 were considered statistically significant.
Ethical Statements.
Rabbit immunization experiments were carried out by BioGenes GmbH. The animals were housed and handled according to international animal regulations (European Union [EU] Directive 2010/63/EU) and sanctioned by governmental authorities “Landesamt für Landwirtschaft, Lebensmittelsicherheit und Fischerei Mecklenburg-Vorpommern” (LALLF M-V).
Results
A medicinal chemistry approach to glycoconjugate vaccine development is enabled by accelerated access to defined oligosaccharides by AGA (23, 24). Minimal protective glycan epitopes are identified using synthetic homogeneous oligosaccharides that constitute one or more repeating units of bacterial CPS. Glycoconjugates containing such synthetic antigens have been previously tested individually for their antigenicity, immunogenicity, and protective effects in animal challenge models of disease, and have been shown to be effective in some cases (9, 10, 17–19). We aimed to expand S. pneumoniae vaccine coverage by including serotype antigen conjugates (Fig. 1) that are either currently absent from marketed vaccines, such as ST2 and ST8 in the case of Prevnar13 and additionally ST3 in the case of Synflorix, or present in marketed vaccines such as ST3 and ST5 but suffering from low immunogenicity or production inconsistencies. The synthetic tetrasaccharide conjugate ST14 (Fig. 1), resembling the previously identified repeating unit of the widespread S. pneumoniae ST14 serotype (21), was included to show that nonproblematic isolated serotypes can in principle also be substituted by synthetic antigens.
Fig. 1.
CRM197 conjugates of synthetic oligosaccharide antigens resembling the capsular polysaccharides (CPS) of Streptococcus pneumoniae serotypes 2 (ST2), 3 (ST3), 5 (ST5), 8 (ST8), and 14 (ST14).
CRM197 Conjugates of Synthetic Oligosaccharide Antigens Are Immunogenic in Rabbits.
Synthetic oligosaccharide-CRM197 conjugates were obtained by equipping the corresponding antigens with an amine group at the reducing end and coupling them to the carrier protein CRM197 using bis(4-nitrophenyl) adipate. The resulting glycoconjugates were characterized by a gel shift assay (10% SDS/PAGE) (SI Appendix, Fig. S1) and found by MALDI-TOF mass spectrometric analysis to contain between 7 and 11 glycan epitopes on each CRM197 protein molecule (ST2: 8.8; ST3: 7.2; ST5: 9.7; ST8: 11.2; and ST14: 8.7) (SI Appendix, Figs. S2–S6). Immunization of NZW rabbits with the glycoconjugates induces strong anti-glycan antibody titers, as determined by ELISA and glycan microarray (SI Appendix, Fig. S7).
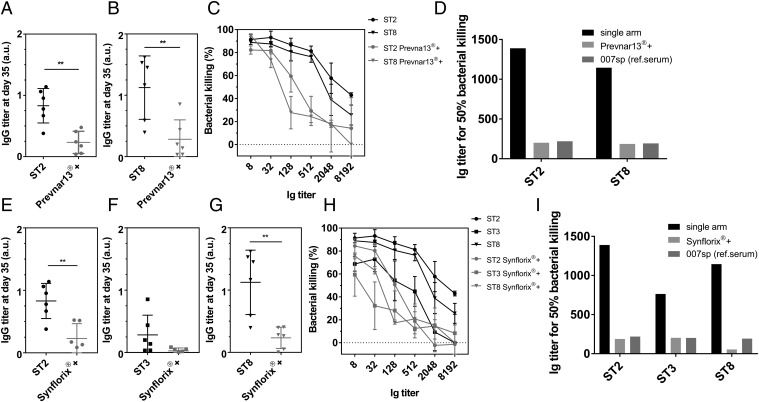
Coformulation of Synthetic Conjugates Representing Nonvaccine Serotypes (ST2, ST3, and ST8) with Marketed Pneumococcal Conjugate Vaccines.
Rabbits were immunized s.c. with 100 µL of coformulated vaccines Prevnar13+ST2+ST8 or Synflorix+ST2+ST3+ST8 (2.2 µg of each synthetic glycan). Antibody titers were analyzed by ELISA (Fig. 2 A, B, and E–G) and glycan microarray (SI Appendix, Fig. S7). The coformulated vaccines elicit significant immune responses, but as expected the serological effects are weaker than those observed after immunization with single serotypes. The sera of animals that received the coformulated vaccines contain antibodies that perform well in in vitro OPKA (Fig. 2 C, D, H, and I). The higher antibody titer responsible for 50% bacteria killing for coformulated vaccines compared with single serotypes is caused by an overall weaker serotype-specific antibody response.
Fig. 2.
Coformulation of synthetic glycoconjugates of nonvaccine serotypes with licensed polysaccharide-based conjugate vaccines. NZW rabbits (n = 4–6) were vaccinated s.c. with synthetic glycoconjugates ST2, ST3, or ST8 individually or in combination with licensed vaccines (Prevnar13+ST2+ST8 and Synflorix+ST2+ST3+ST8). (A and B) ELISA analysis of ST2 (A) and ST8 (B) polysaccharide-specific antibodies in rabbit sera at the endpoint titer dilution (1:1,000) from animals immunized with ST2 or ST8 individually or in coformulation with Prevnar13. Each dot represents the individual animal response and data were analyzed by an unpaired t test. (C) In vitro OPKA of pooled sera raised against synthetic glycoconjugate ST2 or ST8 individually or in coformulation with Prevnar13. Data presented as mean ± SD of three independent experiments. (E–G) ELISA analysis of ST2 (E), ST3 (F), and ST8 (G) polysaccharide-specific antibodies in rabbit sera at the endpoint titer dilution from animals immunized with ST2, ST3, and ST8 individually or in coformulation with Synflorix. Each dot represents the individual animal response and data were analyzed by an unpaired t test. (H) OPKA of pooled sera raised against glycoconjugates ST2, ST3 or ST8 individually or in combination with Synflorix. Data presented as mean ± SD of three independent experiments. (D and I) Comparison of antibody titers of pooled sera responsible for 50% killing of bacteria in the OPKA. IgG titers are expressed as the reciprocal serum dilution mediating 50% bacterial killing, estimated through nonlinear interpolation of the dilution-killing OPKA data. Human reference serum 007sp was used as a control. Each dot represents an individual rabbit immune response; data were analyzed by an ANOVA test, **P < 0.01.
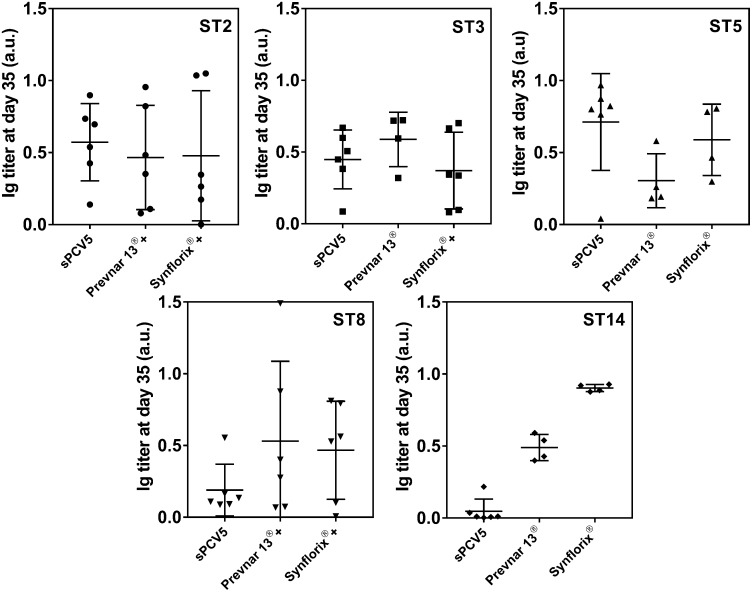
Formulation of Semisynthetic Pentavalent Conjugated Vaccine sPCV5.
The semisynthetic pentavalent-conjugated vaccine sPCV5 containing all five of the above synthetic glycoconjugates (2.2 µg of each sugar antigen adsorbed onto 125 µg of Al[OH]3 alum in PBS) was evaluated based on its immunogenicity and ability to boost production of protective antibodies. A group of six female NZW rabbits were immunized s.c. with 0.5 mL of sPCV5. The placebo group received CRM197 with Al(OH)3 while the positive control groups received Prevnar13 or Synflorix with identical immunization schedules. Individual rabbit sera were analyzed for specific CPS antibodies by ELISA (Fig. 3) and glycan microarrays (SI Appendix, Fig. S7). Overall, sPCV5 elicits a strong, polysaccharide-specific antibody response compared with marketed vaccines. Antibody titers against nonvaccine ST2 CPSs elicited by sPCV5 are comparable to coformulated Prevnar13+ST2+ST8 and Synflorix+ST2+ST3+ST8 and in the case of ST8 the antibody level is slightly lower. The ST3 antigen already present in Prevnar13 triggers an antibody response similar to that of sPCV5 and coformulated Synflorix+ST2+ST3+ST8. The ST5 antigen present in licensed vaccines is problematic as the ketone moiety in the ketoamino sugar 2-acetamido-2,6-dideoxy-d-xylose-hexos-4-ulose (Sugp) becomes reduced during vaccine production, yielding a mixture of different antigens and decreased immunogenicity (9). On the other hand, the reduced ST5 antigen used here with reducing end 2-N-acetyl-2-deoxy-β-d-fucopyranoside (Fig. 1), identified by the medicinal chemistry approach, yields a better immune response in rabbits immunized with sPCV5 compared with the licensed vaccines Prevnar13 and Synflorix. Surprisingly, a lower IgG titer was observed in the case of ST14 as incorporated into sPCV5 compared with the marketed vaccines.
Fig. 3.
Immune response of pentavalent synthetic glycoconjugate vaccine sPCV5 in rabbits. NZW rabbits (n = 3–6) were vaccinated s.c. with sPCV5, the marketed vaccines Prevnar13 or Synflorix, or the coformulated vaccines Prevnar13+ST2+ST8 or Synflorix+ST2+ST3+ST8. Polysaccharide-specific ST2, ST3, ST5, ST8, and ST14 antibody levels in rabbit sera were evaluated by ELISA at the endpoint titer dilution. Each dot represents an individual rabbit immune response; data were analyzed by an ANOVA test.
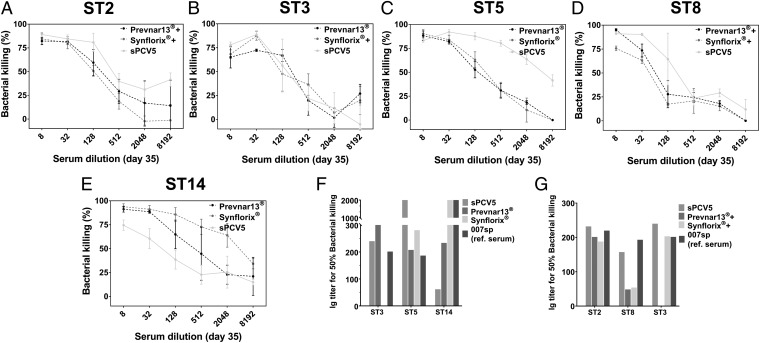
Antibodies in Sera of Animals Immunized with sPCV5 and Coformulated Vaccines Show in Vitro Opsonophagocytic Activity.
Polysaccharide-specific antibodies are the major protective mechanism against pneumococcal bacteria. To determine antibody-mediated bacterial killing in vitro, OPKAs were performed with pooled sera of rabbits immunized with (i) serotype-specific synthetic glycoconjugates alone; (ii) the combination of semisynthetic glycoconjugates (sPCV5); (iii) coformulations of synthetic oligosaccharide conjugates with licensed vaccines (Prevnar13+ST2+ST8 or Synflorix+ST2+ST3+ST8); (iv) the licensed vaccines (Prevnar13 or Synflorix); and (v) placebo (CRM197 plus alum) (Fig. 4). Sera were heat inactivated and compared with the Human Anti-Pneumococcal capsule Reference Serum (007sp) that is the established WHO standard serum (25). The OPKA results confirm that the synthetic glycoconjugate vaccines elicit opsonic antibodies that are able to kill the respective bacterial serotypes (Fig. 4 A–E). Overall, the serum dilutions necessary for 50% bacterial killing, estimated by nonlinear interpolation of the dilution-killing OPKA data, are considered biologically significant for successful vaccines (Fig. 4 F–H). These values vary from serotype to serotype and are substantially higher for the vaccine-present ST5 serotype (Fig. 4F) and the nonvaccine ST8 serotype (Fig. 4G) compared with Prevnar13 and human reference serum 007sp. The OPKA results are consistent with the serological data (Fig. 3).
Fig. 4.
In vitro opsonophagocytic activity of sPCV5 formulation. (A–E) Comparison of pooled sera from rabbit immunized with sPCV5, Prevnar13+ST2+ST8, Synflorix+ST2+ST3+ST8 as well as positive controls Prevnar13 and Synflorix for opsonophagocytic killing of S. pneumoniae serotypes (A) ST2, (B) ST3, (C) ST5, (D) ST8, and (E) ST14. Data are means ± SD of colony-forming units reduction relative to negative control wells (samples lacking either antibody or complement) of three independent experiments (F and G). Comparison of antibody titer of pooled serum responsible for 50% killing of bacteria in opsonophagocytic killing assay. IgG titers are expressed as the reciprocal serum dilution mediating 50% bacterial killing, estimated through nonlinear interpolation of the dilution-killing OPKA data. Human reference serum 007sp was used as a control.
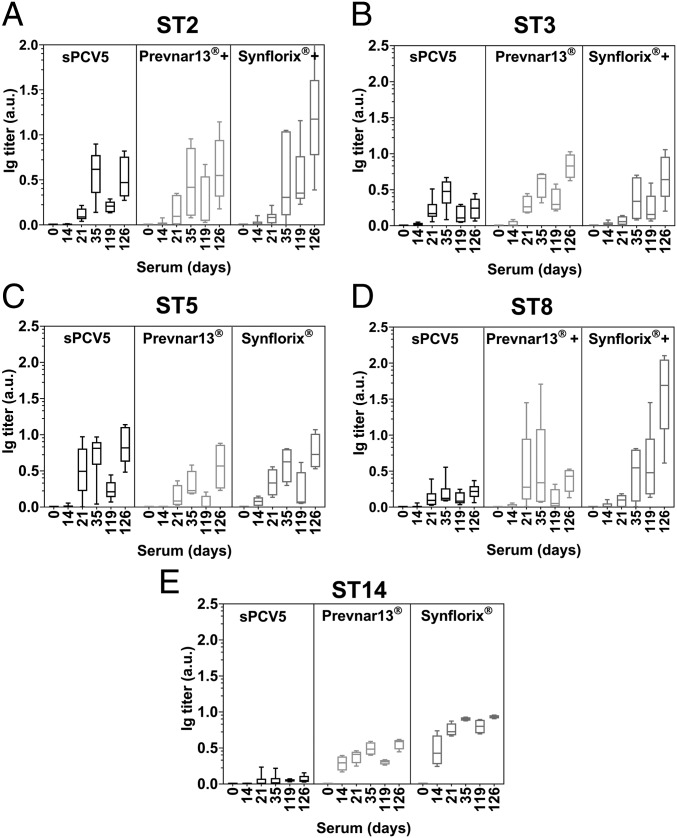
Synthetic Glycoconjugate Vaccine Induces Memory Response.
NZW rabbits (n = 3–6) immunized s.c. with sPCV5, Prevnar13+ST2+ST8, or Synflorix+ST2+ST3+ST8 as well as appropriate controls with Prevnar13 or Synflorix were boosted on day 119 (91 d after the last scheduled immunization on day 28). Over the 13 wk resting period, IgG titers had decreased as expected before the booster immunization at day 119, which resulted in high antibody titers at day 126 that are associated with memory B cells as assessed by ELISA (Fig. 5). The serum antibodies on day 126 are highly active as confirmed by OPKA analysis (SI Appendix, Fig. S8).
Fig. 5.
Synthetic glycoconjugate vaccine sPCV5 induces long-term memory response. NZW rabbits (n = 3–6) were immunized four times (days 0, 14, 28, and 119) s.c. with sPCV5, Prevnar13+ST2+ST8, Synflorix+ST2+ST3+ST8 as well as positive controls Prevnar13 and Synflorix. Serum was collected 1 wk after each immunization. Polysaccharide-specific antibody titers were analyzed by ELISA. Plates coated with CPSs corresponding to (A) ST2, (B) ST3, (C) ST5, (D) ST8, and (E) ST14. Data represented as mean ± SD, duplicate determinations.
Discussion
Induction of protective antibodies upon active immunization is crucial for the efficacy of bacterial vaccines. Capsular polysaccharides are abundant surface molecules of many pathogenic microorganisms such as bacteria, protozoa, and fungi (26). Active immunization with carbohydrate antigens elicits glycan-specific protective immune responses. Poorly immunogenic carbohydrate antigens and ill-defined conjugation render the production of carbohydrate-based vaccines challenging (27). Licensed polysaccharide-based 7-, 10-, and 13-valent pneumococcal conjugate vaccines are effective in significantly reducing the burden of IPD-related mortality and morbidity (8). Despite the success of glycoconjugate pneumococcal vaccines, limited serotype coverage and infections by nonvaccine serotypes cause problems globally (28, 29). Serotype replacement, the observation that infection is caused by serotypes not included in marketed vaccines, has been widely reported (30).
S. pneumoniae serotypes that are not included in current marketed vaccines such as ST2 and ST8 are responsible for IPDs in risk groups globally (31–33). Coformulation of licensed polysaccharide conjugate vaccines and oligosaccharide-based glycoconjugate vaccine candidates of nonvaccine serotypes would increase serotype coverage. Expansion would be particularly important in regions where serotype distribution differs from those included in the polysaccharide conjugate vaccines. With our coformulated vaccines (Prevnar13+ST2+ST8 and Synflorix+ST2+ST3+ST8) we were able to demonstrate that it is possible to broaden the serotype spectrum of marketed vaccines by adding synthetic oligosaccharide conjugates. The antibody response against serotype-specific CPS observed with single glycoconjugate vaccines was somewhat higher than that resulting from the coformulated vaccines. This observation may be explained by considering a few key factors. First, the coformulations, as with Prevnar13 and Synflorix, with numerous antigens may burden the immune system by engaging multiple parallel responses to the different serotype epitopes and reducing the efficiency of the response to any given single one; whereas, in the case of the single glycoconjugates, this burden is not present and the response is more efficient. Second, the preadsorbed longer polysaccharides from the licensed vaccine may compete with the shorter synthetic oligosaccharide examined here; that is, longer CPSs may mask shorter oligosaccharides. Additionally, synthetic glycoconjugates were added to the preformulated vaccines containing 13 isolated polysaccharides with aluminum phosphate AlPO4 as an adjuvant; however, we have found our constructs to be better adsorbed on aluminum hydroxide Al(OH)3 (SI Appendix, Table S1). This critical aspect of vaccine development has been discussed for other carbohydrate conjugate vaccines such as Hib-CRM197 and MenC-CRM197 conjugates which adsorb weakly or not at all to aluminum phosphate (0–11% and 0%, respectively) and strongly to aluminum hydroxide (88–100% and 90–100%, respectively) (34, 35).
To overcome this problem with mixing chemically precisely defined synthetic oligosaccharides with the poorly defined native polysaccharide antigens isolated from bacterial culture present in the formulated marketed vaccines, we further designed the completely semisynthetic glycoconjugate sPCV5. Homogeneous synthetic antigens that constitute pharmaceuticals with virtually no batch-to-batch variability activate the desired immune response and are cost-effective compared with biologicals. Modifications could be implemented down to the atomic level to increase both immunogenicity and protective effects as has been illustrated for ST5 where a ketone was replaced by a hydroxyl group to improve stability while maintaining immunogenicity and antigenicity. One dominant criterium used to assess an adequate vaccination response in adults is a two- to fourfold change in specific antibody levels after vaccination as measured by ELISA (36, 37). The semisynthetic pentavalent conjugated vaccine sPCV5 reached this standard for all serotypes except ST14 CPS. We are able to achieve high levels of specific antibodies, even though the adsorption ratio of glycoconjugates on alum particles, one of the key factors that influences immune responses, was lower than expected (SI Appendix, Table S1). The function of the vaccine is characterized by the opsonophagocytic activity of antibodies against the capsular polysaccharide as determined by an OPKA (38). Protection in infants vaccinated with anti-pneumococcal vaccines is expected when an opsonic titer of at least 1:8 is reached in a mouse model (39, 40). The pentavalent sPCV5 triggers the production of highly active opsonophagocytic antibodies, that are more efficient than Prevnar13, Synflorix, and the coformulations of these licensed vaccines with synthetic glycoconjugates, again with the exception of serotype ST14. The comparatively poor performance of ST14 as present in sPCV5 cannot be simply explained on the basis of the data reported here and is currently under investigation. It is possible that the physicochemical properties of this highly branched and compact tetrasaccharide hinder its efficient adsorption onto the alum particles.
Conjugation of the saccharides to the protein carrier induces a T cell dependent immune response and the formation of memory B cells (41). The antibody level gradually diminishes after a series of primary immunizations, and immunological memory is enhanced by a final booster dose with the vaccine. Large increases in IgG concentration within 5 to 7 d after the final booster immunization on day 119 proved the formation of immunological memory. The IgG produced after the final boosting dose proved to be protective (SI Appendix, Fig. S8).
The semisynthetic sPCV5 as well as coformulation of existing vaccines with synthetic glycans proved highly efficacious in a rabbit model considering the three most important indicators of vaccine efficacy. A substantial rise in antibody titer between pre- and postimmunization sera was observed and the opsonophagocytic activity of antibodies, and immunological memory were confirmed. The medicinal chemistry approach to carbohydrate-conjugate vaccine design based on AGA and glycan arrays is advantageous to the formulation of multivalent synthetic vaccine candidates and to extending coverage with existing vaccines (42, 43).
Supplementary Material
Acknowledgments
We thank Dr. Allison Berger and Tom Monroe for carefully revising the manuscript; Eva Settels and Olaf Niemeyer for technical support and help with NMR analyses. We gratefully acknowledge financial support from the Max Planck Society. This work was supported in part by grants from the German Research Foundation (SFB/TR 84 “Innate Immunity of the Lung,” C3, C6, C8; to M.W. and P.H.S.) and from the German Federal Ministry of Education and Research (e:Med CAPSyS-FKZ 01ZX1304B/E; to M.W.). We also appreciate the support of Zentrum für Infektionsbiologie und Immunität (ZIBI) Graduate School and International Max Planck Research School for Infectious Diseases and Immunology program (IMPRS-IDI).
Footnotes
Conflict of interest statement: Glycoconjugates containing the synthetic glycan structures of all five Streptococcus pneumoniae serotypes (type 2, 3, 5, 8, and 14) capsular polysaccharide conjugate elicit opsonic antibodies and is included in patent “Pneumococcal oligosaccharide-protein conjugate composition” no. EP 16 179 133.0 filed by the inventors P.H.S., C.L.P., N.K., M.E., S.G.P., A.D.J.C., M.P.L., B.S., F.-F.X., P.K., and P.H.S. has a significant financial interest in “Vaxxilon,” a spinoff company that is developing vaccines based on synthetic oligosaccharide antigens.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1811862115/-/DCSupplemental.
References
- 1.Centers for Disease Control and Prevention 2016 Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pneumoniae, 2016. Available at https://www.cdc.gov/abcs/reports-findings/survreports/spneu16.pdf. Accessed November 30, 2018.
- 2.Rodrigues CMC, Groves H. Community-acquired pneumonia in children: The challenges of microbiological diagnosis. J Clin Microbiol. 2018;56:e01318-17. doi: 10.1128/JCM.01318-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein Klouwenberg P, Bont L. Neonatal and infantile immune responses to encapsulated bacteria and conjugate vaccines. Clin Dev Immunol. 2008;2008:628963. doi: 10.1155/2008/628963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dretler AW, Rouphael NG, Stephens DS. Progress toward the global control of Neisseria meningitidis: 21st century vaccines, current guidelines, and challenges for future vaccine development. Hum Vaccin Immunother. 2018;14:1146–1160. doi: 10.1080/21645515.2018.1451810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Weinberger DM, Thompson CM, Trzciński K, Lipsitch M. Surface charge of Streptococcus pneumoniae predicts serotype distribution. Infect Immun. 2013;81:4519–4524. doi: 10.1128/IAI.00724-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruber WC, Scott DA, Emini EA. Development and clinical evaluation of Prevnar 13, a 13-valent pneumocococcal CRM197 conjugate vaccine. Ann N Y Acad Sci. 2012;1263:15–26. doi: 10.1111/j.1749-6632.2012.06673.x. [DOI] [PubMed] [Google Scholar]
- 7.Hayward S, Thompson LA, McEachern A. Is 13-valent pneumococcal conjugate vaccine (PCV13) combined with 23-valent pneumococcal polysaccharide vaccine (PPSV23) superior to PPSV23 alone for reducing incidence or severity of pneumonia in older adults? A Clin-IQ. J Patient Cent Res Rev. 2016;3:111–115. doi: 10.17294/2330-0698.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Principi N, Esposito S. Development of pneumococcal vaccines over the last 10 years. Expert Opin Biol Ther. 2018;18:7–17. doi: 10.1080/14712598.2018.1384462. [DOI] [PubMed] [Google Scholar]
- 9.Lisboa MP, et al. Semisynthetic glycoconjugate vaccine candidate against Streptococcus pneumoniae serotype 5. Proc Natl Acad Sci USA. 2017;114:11063–11068. doi: 10.1073/pnas.1706875114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schumann B, et al. Development of an efficacious, semisynthetic glycoconjugate vaccine candidate against Streptococcus pneumoniae serotype 1. ACS Cent Sci. 2018;4:357–361. doi: 10.1021/acscentsci.7b00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schumann B, Anish C, Pereira CL, Seeberger PH. Carbohydrate vaccines. In: Jones L, McKnight AJ, editors. Biotherapeutics: Recent Developments using Chemical and Molecular Biology. Royal Society of Chemistry; Cambridge, UK: 2013. pp. 68–104. [Google Scholar]
- 12.Aguilar-Betancourt A, et al. Safety and immunogenicity of a combined hepatitis B virus-Haemophilus influenzae type B vaccine comprising a synthetic antigen in healthy adults. Hum Vaccin. 2008;4:54–59. doi: 10.4161/hv.4.1.5257. [DOI] [PubMed] [Google Scholar]
- 13.Anish C, Schumann B, Pereira CL, Seeberger PH. Chemical biology approaches to designing defined carbohydrate vaccines. Chem Biol. 2014;21:38–50. doi: 10.1016/j.chembiol.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Broecker F, et al. Multivalent display of minimal Clostridium difficile glycan epitopes mimics antigenic properties of larger glycans. Nat Commun. 2016;7:11224. doi: 10.1038/ncomms11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broecker F, et al. Synthetic lipoteichoic acid glycans are potential vaccine candidates to protect from Clostridium difficile infections. Cell Chem Biol. 2016;23:1014–1022. doi: 10.1016/j.chembiol.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Seeberger PH, et al. A semi-synthetic glycoconjugate vaccine candidate for Carbapenem-resistant Klebsiella pneumoniae. Angew Chem Int Ed Engl. 2017;56:13973–13978. doi: 10.1002/anie.201700964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emmadi M, et al. A Streptococcus pneumoniae type 2 oligosaccharide glycoconjugate elicits opsonic antibodies and is protective in an animal model of invasive pneumococcal disease. J Am Chem Soc. 2017;139:14783–14791. doi: 10.1021/jacs.7b07836. [DOI] [PubMed] [Google Scholar]
- 18.Parameswarappa SG, et al. A semi-synthetic oligosaccharide conjugate vaccine candidate confers protection against Streptococcus pneumoniae serotype 3 infection. Cell Chem Biol. 2016;23:1407–1416. doi: 10.1016/j.chembiol.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schumann B, et al. A semisynthetic Streptococcus pneumoniae serotype 8 glycoconjugate vaccine. Sci Transl Med. 2017;9:eaaf5347. doi: 10.1126/scitranslmed.aaf5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polonskaya Z, et al. T cells control the generation of nanomolar-affinity anti-glycan antibodies. J Clin Invest. 2017;127:1491–1504. doi: 10.1172/JCI91192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurbatova EA, et al. Neoglycoconjugate of tetrasaccharide representing one repeating unit of the Streptococcus pneumoniae type 14 capsular polysaccharide induces the production of opsonizing IgG1 antibodies and possesses the highest protective activity as compared to hexa- and octasaccharide conjugates. Front Immunol. 2017;8:659. doi: 10.3389/fimmu.2017.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anish C, Guo X, Wahlbrink A, Seeberger PH. Plague detection by anti-carbohydrate antibodies. Angew Chem Int Ed Engl. 2013;52:9524–9528. doi: 10.1002/anie.201301633. [DOI] [PubMed] [Google Scholar]
- 23.Weishaupt MW, Hahm HS, Geissner A, Seeberger PH. Automated glycan assembly of branched β-(1,3)-glucans to identify antibody epitopes. Chem Commun (Camb) 2017;53:3591–3594. doi: 10.1039/c7cc00520b. [DOI] [PubMed] [Google Scholar]
- 24.Naresh K, Schumacher F, Hahm HS, Seeberger PH. Pushing the limits of automated glycan assembly: Synthesis of a 50mer polymannoside. Chem Commun (Camb) 2017;53:9085–9088. doi: 10.1039/c7cc04380e. [DOI] [PubMed] [Google Scholar]
- 25.Goldblatt D, et al. Establishment of a new human pneumococcal standard reference serum, 007sp. Clin Vaccine Immunol. 2011;18:1728–1736. doi: 10.1128/CVI.05252-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreira SS, Passos CP, Madureira P, Vilanova M, Coimbra MA. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr Polym. 2015;132:378–396. doi: 10.1016/j.carbpol.2015.05.079. [DOI] [PubMed] [Google Scholar]
- 27.Sun L, Middleton DR, Wantuch PL, Ozdilek A, Avci FY. Carbohydrates as T-cell antigens with implications in health and disease. Glycobiology. 2016;26:1029–1040. doi: 10.1093/glycob/cww062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hicks LA, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis. 2007;196:1346–1354. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 29.Yildirim I, Stevenson A, Hsu KK, Pelton SI. Evolving picture of invasive pneumococcal disease in Massachusetts children: A comparison of disease in 2007-2009 with earlier periods. Pediatr Infect Dis J. 2012;31:1016–1021. doi: 10.1097/INF.0b013e3182615615. [DOI] [PubMed] [Google Scholar]
- 30.Huang SS, et al. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009;124:e1–e11. doi: 10.1542/peds.2008-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaiswal N, et al. Distribution of serotypes, vaccine coverage, and antimicrobial susceptibility pattern of Streptococcus pneumoniae in children living in SAARC countries: A systematic review. PLoS One. 2014;9:e108617. doi: 10.1371/journal.pone.0108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaensbauer JT, et al. Guatemala Pediatric Bacterial Surveillance Working Group Pediatric invasive pneumococcal disease in Guatemala city: Importance of serotype 2. Pediatr Infect Dis J. 2016;35:e139–e143. doi: 10.1097/INF.0000000000001067. [DOI] [PubMed] [Google Scholar]
- 33.Vanderkooi OG, Church DL, MacDonald J, Zucol F, Kellner JD. Community-based outbreaks in vulnerable populations of invasive infections caused by Streptococcus pneumoniae serotypes 5 and 8 in Calgary, Canada. PLoS One. 2011;6:e28547. doi: 10.1371/journal.pone.0028547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otto RB, Burkin K, Amir SE, Crane DT, Bolgiano B. Patterns of binding of aluminum-containing adjuvants to Haemophilus influenzae type b and meningococcal group C conjugate vaccines and components. Biologicals. 2015;43:355–362. doi: 10.1016/j.biologicals.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He P, Zou Y, Hu Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum Vaccin Immunother. 2015;11:477–488. doi: 10.1080/21645515.2014.1004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daly TM, Pickering JW, Zhang X, Prince HE, Hill HR. Multilaboratory assessment of threshold versus fold-change algorithms for minimizing analytical variability in multiplexed pneumococcal IgG measurements. Clin Vaccine Immunol. 2014;21:982–988. doi: 10.1128/CVI.00235-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaccaro DJ, Wagener DK, Whisnant CC, Staats HF. Evaluation of vaccine-induced antibody responses: Impact of new technologies. Vaccine. 2013;31:2756–2761. doi: 10.1016/j.vaccine.2013.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jódar L, et al. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine. 2003;21:3265–3272. doi: 10.1016/s0264-410x(03)00230-5. [DOI] [PubMed] [Google Scholar]
- 39.Rose CE, et al. Multilaboratory comparison of Streptococcus pneumoniae opsonophagocytic killing assays and their level of agreement for the determination of functional antibody activity in human reference sera. Clin Vaccine Immunol. 2011;18:135–142. doi: 10.1128/CVI.00370-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romero-Steiner S, et al. Multilaboratory evaluation of a viability assay for measurement of opsonophagocytic antibodies specific to the capsular polysaccharides of Streptococcus pneumoniae. Clin Diagn Lab Immunol. 2003;10:1019–1024. doi: 10.1128/CDLI.10.6.1019-1024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pletz MW, Maus U, Krug N, Welte T, Lode H. Pneumococcal vaccines: Mechanism of action, impact on epidemiology and adaption of the species. Int J Antimicrob Agents. 2008;32:199–206. doi: 10.1016/j.ijantimicag.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 42.Seeberger PH. The logic of automated glycan assembly. Acc Chem Res. 2015;48:1450–1463. doi: 10.1021/ar5004362. [DOI] [PubMed] [Google Scholar]
- 43.Geissner A, Anish C, Seeberger PH. Glycan arrays as tools for infectious disease research. Curr Opin Chem Biol. 2014;18:38–45. doi: 10.1016/j.cbpa.2013.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.