Significance
Biological dinitrogen (N2) fixation (BNF) is an important source of nitrogen in marine systems. Until recently, it was believed to be primarily limited to subtropical open oceans. Marine BNF is mainly attributed to cyanobacteria. However, recently an unusual N2-fixing unicellular cyanobacteria (UCYN-A)/haptophyte symbiosis was reported with a broader temperature range than other N2-fixing cyanobacteria. We report that the UCYN-A symbiosis is present and fixing N2 in the Western Arctic and Bering Seas, further north than any previously reported N2-fixing marine cyanobacteria. Nanoscale secondary ion mass spectrometry enabled us to directly show that the symbiosis was fixing N2. These results show that N2-fixing cyanobacteria are not constrained to subtropical waters and challenge commonly held ideas about global marine N2 fixation.
Keywords: nitrogen fixation, marine microbiology, Arctic, cyanobacteria, nanoSIMS
Abstract
Biological dinitrogen (N2) fixation is an important source of nitrogen (N) in low-latitude open oceans. The unusual N2-fixing unicellular cyanobacteria (UCYN-A)/haptophyte symbiosis has been found in an increasing number of unexpected environments, including northern waters of the Danish Straight and Bering and Chukchi Seas. We used nanoscale secondary ion mass spectrometry (nanoSIMS) to measure 15N2 uptake into UCYN-A/haptophyte symbiosis and found that UCYN-A strains identical to low-latitude strains are fixing N2 in the Bering and Chukchi Seas, at rates comparable to subtropical waters. These results show definitively that cyanobacterial N2 fixation is not constrained to subtropical waters, challenging paradigms and models of global N2 fixation. The Arctic is particularly sensitive to climate change, and N2 fixation may increase in Arctic waters under future climate scenarios.
Biological N2 fixation, the reduction of atmospheric N2 to biologically available nitrogen, is an important source of nitrogen (N) in oligotrophic tropical and subtropical oceans (1). Historically, studies of marine N2 fixation focused on the well-known cyanobacterium Trichodesmium and the diatom symbiont Richelia, which were reported primarily from warm (>20 °C) waters (2) with low concentrations of fixed N (nitrate and ammonium) (3). The discovery of a unicellular cyanobacterial symbiont (UCYN-A) of a haptophyte alga (4, 5) expanded the geographic distribution of marine N2-fixers to waters with lower temperatures and higher concentrations of fixed inorganic N (6, 7). These regions include the high-latitude waters of the Danish Strait (8) and Western Arctic (9, 10). The presence of N2-fixers does not confirm N2 fixation activity because N2 fixation is a highly regulated process inhibited by multiple environmental factors. Here, we demonstrate that the cyanobacterial symbiont UCYN-A fixes N2 in the cold, high latitude waters of the Western Arctic.
UCYN-A is an unusual cyanobacterium lineage that has lost many of the typical cyanobacterial metabolic pathways, including the ability to fix CO2 and evolve O2 in photosynthesis (5). The organism is a symbiont with a small planktonic unicellular haptophyte alga, related to Braarudosphaera bigelowii (4, 11). UCYN-A is uncultivated and can only be detected by its DNA or through visualization with catalyzed reporter deposition fluorescence in situ hybridization (CARD-FISH). UCYN-A comprises multiple closely related lineages with distinct haptophyte hosts that can be differentiated by distinct DNA sequences (12). Generally, haptophytes are geographically widespread including cold, high-latitude waters (13). The symbiosis between a N2-fixing cyanobacterium and a haptophyte may facilitate a unique adaptation to N2 fixation in colder waters, such as the Arctic Ocean, where other N2-fixing marine cyanobacteria have not been found.
Little is known about marine N2 fixation in polar regions, partially because low temperatures are believed to inhibit the growth and activity of N2-fixing cyanobacteria, such as in Trichodesmium and Crocosphaera (2, 14). Trichodesmium has occasionally been reported in high latitudes (62°N) (15) but does not appear to fix N2 when advected into cold waters (16). However, temperature alone does not necessarily preclude N2 fixation because microorganisms can fix N2 in ice-covered Antarctic lakes to near boiling temperatures at hydrothermal vents (17, 18). A few studies have reported low but measurable N2 fixation rates in the Arctic (0.02–7.7 nmol N L−1 d−1) (19, 20). Recent studies in the Bering and Chukchi Seas (9, 10) reported bulk water N2 fixation rates of 2.3–3.6 nmol N L−1d−1 and detected DNA from UCYN-A and other Bacteria but could not link N2 fixation to specific microorganisms.
Results and Discussion
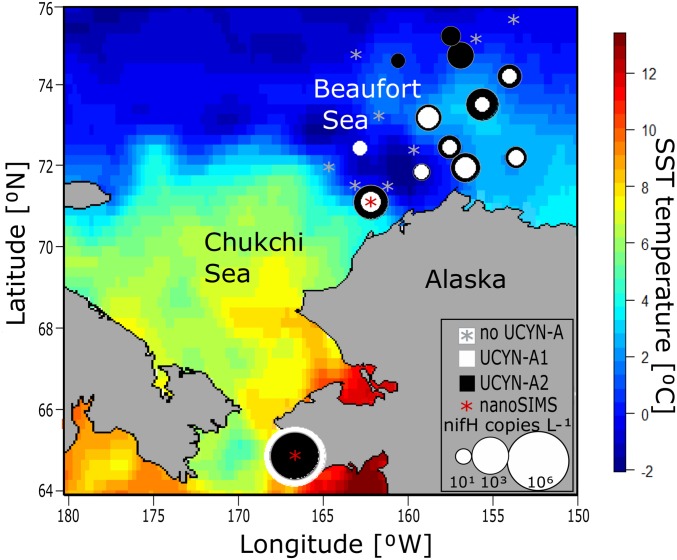
We collected water samples in the Bering, Chukchi, and Beaufort Seas during September 2016 (SI Appendix, Materials and Methods and Fig. S1) and identified N2-fixing microorganisms. The nifH gene, a widely used proxy for N2-fixing microorganisms, was amplified by PCR and sequenced. UCYN-A nifH sequences were the only marine cyanobacterial nifH sequences found and were present in Bering, Chukchi, and Beaufort Sea samples (SI Appendix, Fig. S2). The abundances of the two strains identified, UCYN-A1 and UCYN-A2, were estimated using qPCR (Fig. 1 and SI Appendix, Fig. S3 and Table S2). In the Bering Sea near Nome, AK, UCYN-A abundances (105 to 106 nifH copies L−1) were comparable to those at subtropical latitudes (6, 11, 21) but were considerably lower in the Chukchi Sea, on the north eastern Chukchi shelf and in the Beaufort Seas (Fig. 1 and SI Appendix, Fig. S3 and Table S2). Both UCYN-A lineages were primarily found in surface samples in low salinity ice-melt waters (SI Appendix, Fig. S4 and Table S2). These results verified that two strains of the N2-fixing cyanobacterium UCYN-A are present in polar waters, consistent with recent findings (9, 10).
Fig. 1.
UCYN-A lineages are distributed throughout surface waters of the Western Arctic Ocean. Background colors represent sea-surface temperature on September 10, 2016 (www.esrl.noaa.gov). UCYN-A was quantified with qPCR. UCYN-A2 (black circles) is present at more stations, but UCYN-A1 (white circles) had the highest maximum abundance. Red stars indicate nanoSIMS measurement locations for 15N-uptake rates.
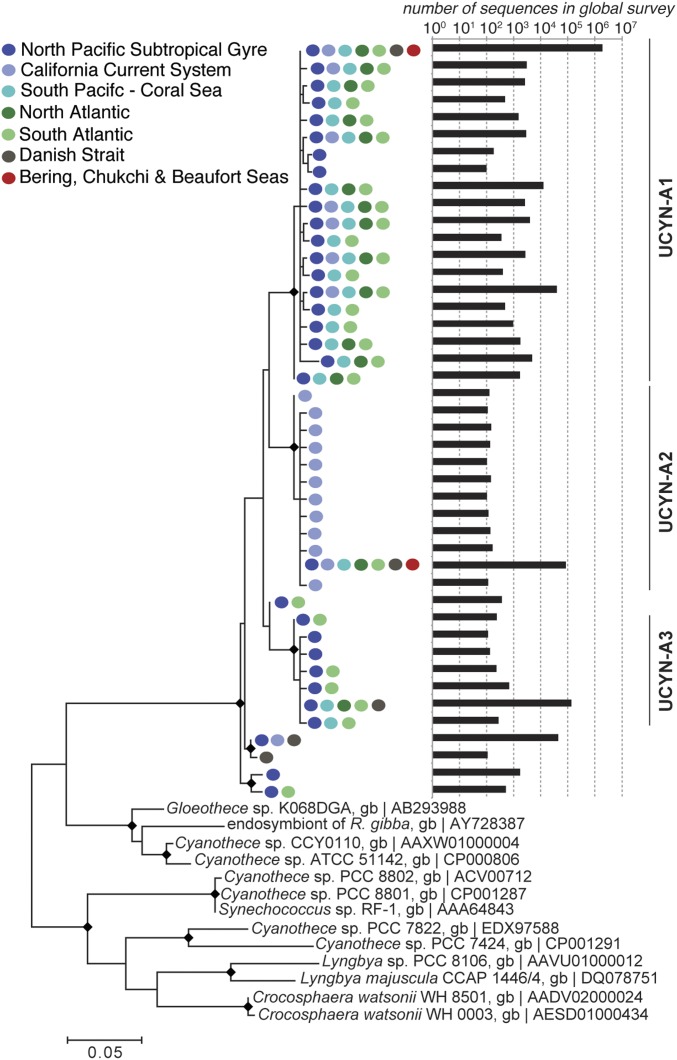
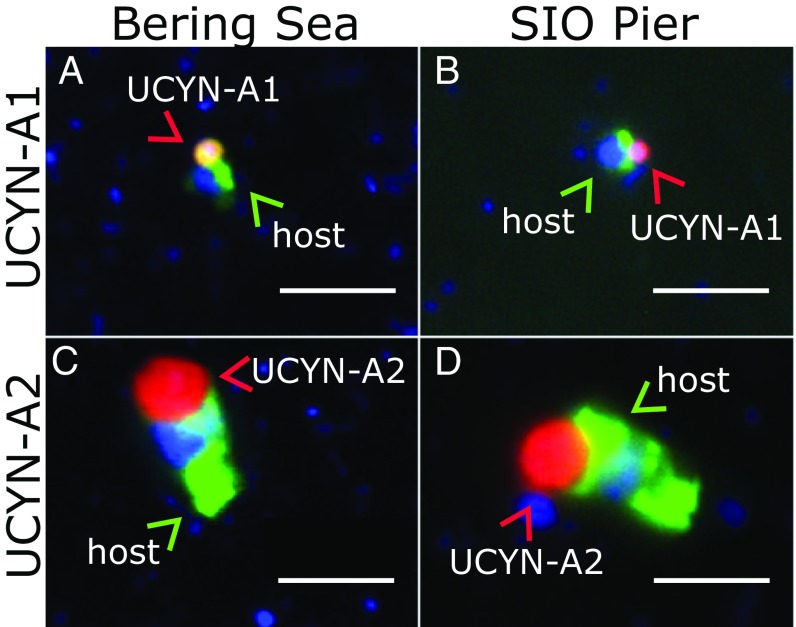
Our genomic and morphological characterization of the high-latitude UCYN-A symbioses show these strains are indistinguishable from those reported in subtropical oceans. The UCYN-A1 and UCYN-A2 nifH sequences from the Arctic samples were identical to the most common sequences reported from subtropical oceans (Fig. 2 and SI Appendix, Fig. S2). Visualization of the different haptophyte hosts and their respective UCYN-A1 and UCYN-A2 cyanobacterial symbionts using lineage-specific CARD-FISH probes (22, 23) (Fig. 3) showed that the symbioses were similar in size and morphology (∼3 and 5 µm, respectively) to the symbioses in tropical and subtropical oceans (4, 7, 23, 24). Other globally distributed eukaryotic picoplankton have specific cold-adapted strains (25, 26), so the discovery of the subtropical strains of the UCYN-A symbiosis in the Arctic was unexpected.
Fig. 2.
Arctic UCYN-A nifH sequences are identical to broadly distributed and abundant sequence types. Shown is a maximum likelihood phylogenetic tree of UCYN-A microdiversity based on partial nifH nucleotide sequences from a recent global survey (12). UCYN-A sequences from the Bering and Arctic Seas (red dots) are identical to dominant sequence types found in all major ocean basins. Regions where each sequence type has been found are specified by colored dots according to the legend; sequence counts from the global survey are also plotted. Nodes with bootstrap support >70 are identified with a diamond. Data from ref. 12.
Fig. 3.
Morphologies of Arctic UCYN-A symbioses are indistinguishable from subtropical strains. Double CARD-FISH comparison of UCYN-A lineages from the Bering Sea (A and C) and water collected at the Scripps Institute of Oceanography Pier in La Jolla, CA (B and D) show similar sizes and morphologies. CARD-FISH images show the symbiosis is intact, with both the haptophyte host (green and blue) and cyanobacteria (red). (Scale bar, 5 µm.)
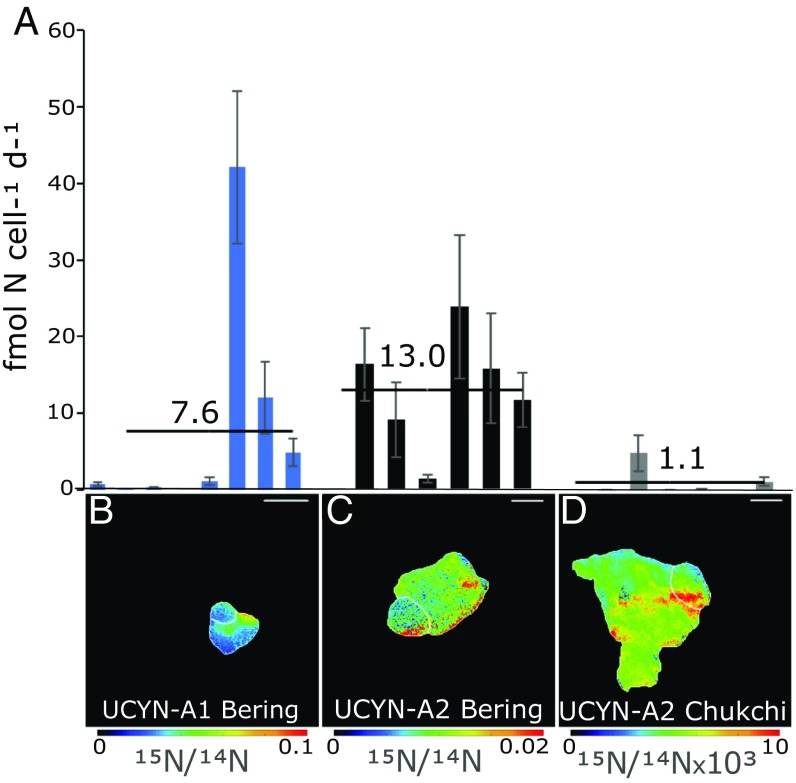
15N2 uptake experiments coupled with CARD-FISH and nanoscale secondary ion mass spectrometry (nanoSIMS) showed that the UCYN-A symbiosis was actively fixing N2. While previous Arctic research has identified diazotrophs and detected bulk rates of N fixation, single cell rates of a marine cyanobacteria (or any marine microorganism) in the Arctic region (72°N) and with low water temperatures (4 °C) has been lacking. The UCYN-A1 and UCYN-A2 symbioses in the Bering Sea had mean cell-specific N2 fixation rates of 7.6 ± 14.5 fmol N cell−1d−1 (n = 6) and 13.0 ± 7.7 fmol N cell−1d−1(n = 8), respectively (Fig. 4). In the Chukchi Sea, the cell-specific N2 fixation rates of the UCYN-A2 symbiosis were considerably lower, but two out of six cells were detectable with an average of 1.1 ± 2.0 fmol N cell−1d−1. Surprisingly, the UCYN-A1 cell-specific N2 fixation rates measured in the Bering Sea at 10.1 °C are similar to rates reported from the warmer waters (>25 °C) of the subtropical North Atlantic [0.45–12 fmol N cell−1d−1 (27, 28)]. UCYN-A N2 fixation in polar waters shows that low temperature does not limit the distribution or activity of N2-fixing cyanobacteria.
Fig. 4.
UCYN-A symbioses fix 15N2 in western Arctic waters. UCYN-A cell-specific 15N2 fixation rates (A) and 15N enrichment (B–D) from nanoSIMS measurements after incubating natural populations in seawater with 15N2. Bars of the same color (A) represent rates measured in individual symbioses (UCYN-A with host alga) from a single station and lineage (noted in underlying cell image). (Scale bar, 2 µm.) Averages are shown by a horizontal black line. Error bars are the SD of the cell-specific rate between the host and UCYN-A. Note the colored axes differ in scale on nanoSIMS images (B–D).
N2 fixation by UCYN-A accounted for the total measured N2 fixation rates in the Bering Sea but not in the Chukchi Sea. UCYN-A N2 fixation rates were estimated to be 10.5 ± 18.4 nmol N L−1d−1 and 0.004 ± 0.007 nmol N L−1d−1 in the Bering Sea and Chukchi Sea, respectively, when per-cell rates were scaled to volumetric rates (SI Appendix, Materials and Methods and Table S3). Measured N2 fixation rates in the bulk water sample from the Bering Sea (Station 1) were 6.9 ± 3.8 nmol N L−1d−1, indicating that N2 fixation by UCYN-A accounted for total bulk rates (within error). In contrast, extrapolated rates from UCYN-A2 cellular N2 fixation in the Chukchi Sea were two orders of magnitude less than the measured bulk N2 fixation of 0.2 ± 0.2 nmol N L−1d−1 (detected, not quantified). More research is needed to determine the quantitative significance of N2 fixation by UCYN-A in this region, but the presence of actively N2-fixing unicellular cyanobacteria in Arctic waters is surprising from an ecological perspective and important for mathematical models that predict global N2 fixation.
It is unclear whether the UCYN-A symbioses in the Bering Sea and Western Arctic are advected into the Western Arctic Seas through the Bering Strait or are endemic populations. Microbial community structure in Arctic waters is heavily influenced by the originating water mass (29), and Shiozaki et al. (10) suggests the UCYN-A symbiosis detected by DNA assays originates from Pacific waters transported to the Arctic in the Alaskan Coastal Current, as has been reported for other species (30). However, UCYN-A1, which is widespread in the North Pacific Subtropical Gyre, disappears at the front between the North Pacific Subtropical Gyre and the North Pacific Subarctic Gyre (9, 21), and the UCYN-A2 symbiosis is not commonly found in the oligotrophic North Pacific (12). This finding suggests that the UCYN-A populations may be maintained throughout the year in the Arctic and may be endemic populations.
Our results provide support for resource ratio theory-based predictions that Bering Sea waters would be favorable for N2-fixers (31) and extend the biogeographical range of active UCYN-A symbioses into the Chukchi and Beaufort Seas. New models are needed for predicting the biogeography of N2-fixing microorganisms and N2 fixation in the world ocean, including other Arctic regions and the Southern Ocean. The results of this study also have implications for global N2 fixation and global environmental change. Arctic ecosystems are rapidly changing. Predicted effects include increased Pacific inflow, phytoplankton growing season, stratification, nutrient limitation, and sea-surface temperatures (32), all of which may select for UCYN-A and other N2-fixing species that are commonly found in warm, oligotrophic waters. The results of this study change the paradigm that N2 fixation and N2-fixing cyanobacteria are common only in warm tropical or subtropical waters, and these results are critical for understanding and predicting global patterns of N2 fixation.
Materials and Methods
Samples were taken in the Bering Sea, Chukchi Sea, on the Chukchi Shelf, and in the Beaufort Sea in September 2016.
DNA Extraction, nifH Amplification, and qPCR.
Samples (2–4 L) were filtered by peristaltic pump onto sequential 3 and 0.2 µm polyphenylene ether filters (0.2 µm, 25 mm; Supor-200; Pall Life Sciences) in Swinnex filter holders. DNA was extracted using a modified DNeasy Plant Mini Kit (Qiagen) protocol, described in detail in ref. 33. PCR amplification of the nifH gene used degenerate universal nifH primers YANNI/450 and up/down in a nested reaction (34), with the second round primers (up/down) modified to contain common sequence linkers (35). Library preparation was carried out by the DNA Sequencing Core Facility at the University of Illinois at Chicago (rrc.uic.edu/cores/genome-research/sequencing-core/). Amplicons were sequenced using Illumina MiSeq, to a sequencing depth of 40,000 sequences per sample.
UCYN-A1 and UCYN-A2 abundances were estimated using TaqMan qPCR chemistry and primers and probes specific for UCYN-A1 (36) and UCYN-A2 (11) and their respective haptophyte partners, UCYN-A1 host (SI Appendix, Materials and Methods) and UCYN-A2 host (11), in samples positive for nifH amplification.
15N2 Rate Measurement Incubations.
N2 fixation was assessed using a modified version of the 15N-bubble method (39). Water samples for rate-measurement incubations were collected from Niskin bottles into gas-tight 1-L glass media bottles (KIMAX model no. 611001000) capped with black open-top caps with gray butyl septa (model no. 240680). The caps and septa were preconditioned in saltwater brine for 60 d before use. The media bottles and caps were acid-washed (10% HCl) and rinsed with copious amounts of high-purity water (18.2 MΩ cm−1). The glass media bottles were also combusted at 500 °C for 4 h before use.
Measuring Cell-Specific N2 Fixation Rates Using NanoSIMS.
To visualize and map both strains and their respective hosts (UCYN-A1/UCYN-A1 host and UCYN-A2/UCYN-A2 host), a double CARD-FISH protocol was used according to the protocols detailed in refs. 22 and 23. The full suite of HRP probes, competitor oligonucleotides, and helper probes are given in SI Appendix, Table S1. Before nanoSIMS analysis, cells were transferred to a gridded silicon chip (1.2 cm × 1.2 cm with a 1 mm × 1 mm raster; Pelcotec SFG12 Finder Grid Substrate) and imaged and mapped under epifluorescence on a Zeiss Axioplan epifluorescence microscope equipped with digital imaging at the University of California, Santa Cruz (UCSC). 15N measurements of individual cells were determined by NanoSIMS analyses performed at Stanford Nano Shared Facilities (https://snsf.stanford.edu) on a Cameca NanoSIMS 50L at Stanford University. Image planes were accumulated after first being aligned. Isotope data were taken as a sum of counts in each plane per pixel. Cell outlines and regions of interest (ROIs) were determined as the best fit based on original CARD-FISH image, electron microscopy image, and accumulated images in 12C14N− and 12C−. Cell size was determined based on ROIs of the defined haptophyte or UCYN-A cell. Cell-specific N2 fixation rates were determined by calculating the carbon content per cell based on a spherical cell volume (V) from the measured cell diameter determined by the ROI following the calculations of ref. 27. The C:N ratio of 6.3 was measured in UCYN-A from the tropical North Atlantic (28) and was used in our calculation to estimate N content of the cell. The limit of detection (LOD) was determined to be three times the SD of 15N in unenriched samples (0.02 At%), similar to the LOD determination described by Jayakumar et al. (37). More detailed methods and calculations can be found in SI Appendix.
Bulk N2 Fixation Rate Measurements.
Bottles were filled in triplicate and capped with ambient air bubbles removed. All bottles were immediately placed in mesh bags to mimic the light intensity at collection depth. Different depths received different levels of screening. The bottles were then amended with 1.1 or 2.5 mL of enriched (>99%) 15N2 gas purchased from Cambridge Isotope Laboratories, Inc. (lot no. I-199168A). Higher volumes of 15N2 gas were used in all samples after Station 1 to obtain enrichment levels closer to 10% (average 15N2 enrichment of 5.8 ± 2.1%). Samples were incubated for 24 h in flow-through incubators on deck (surface samples) or environmental chambers (deep samples) set to 0 °C ± 1 °C. Before use in the incubations, subsamples of the 15N2 gas stocks were assessed for 15NH4+, 15NO3−, and 15NO2− contamination according to the methods described in ref. 38. No contamination was measured. Incubations were terminated after 24 h. A membrane inlet mass spectrometer (MIMS) was used to assess the level of 15N enrichment in each sample immediately upon incubation termination. The MIMS data for each individual bottle were used to calculate uptake rates.
Size-fractionated bulk N2 fixation rates were determined by filtering in series through 3.0-µm silver filters and then precombusted (450 °C for 2 h) glass fiber filter (GF-75) with a nominal pore size of 0.3 µm. Filters were stored frozen at −20 °C in sterile microcentrifuge tubes until analysis. Filters were thawed and dried overnight at 40 °C and analyzed on a Sercon Integra2 SL isotope ratio mass spectrometer tuned to low mass samples. The mass range of calibration standards was 1–10 µg N (low range) or 5–20 µg N (high range) of Sigma-Aldrich ammonium sulfate salt (0.366022/−0.77), which was calibrated against the NIST RM 8573, USGS40 (0.36465/−4.52) with a precision of 0.315 parts per thousand. The LOD for the mass of N was 0.51 µg N. The mass range of samples analyzed was 2.66–19.91 µg N. The LOD (i.e., 3x mass of the 15N blank) was 0.103 At%, and the average minimum quantifiable rate (MQR) of the bulk N2 fixation rates was 0.4 ± 0.7 nmol N L−1 d−1. The LOD and MQRs were calculated according to Montoya et al. (39) and Gradoville et al. (40) for each size fraction and propagated as error to represent total N2 fixation. Controls (natural abundance) samples were collected from the Niskin in dedicated, acid-washed (10% HCl), high-density polyethylene bottles and filtered in a separate laboratory on a filtration unit designated for no isotope use. Blank natural abundance samples were analyzed on an Integra2 combined Isotope ratio mass spectrometer with an SL autosampler that had not been exposed to enriched samples. The average δ15N was 7.8 ± 4.2 (0.37 At%) for the >3-µm size fraction and 7.2 ± 2.9 (0.37 At%) for the 0.3- to 3-µm size fraction.
Data and Materials Availability.
All data are provided in this article and SI Appendix, with the exception of the raw UCYN-A nifH sequences, which have been deposited in the Sequence Read Archive (www.ncbi.nlm.nih.gov/sra) under Bioproject ID PRJNA476143.
Supplementary Material
Acknowledgments
We gratefully acknowledge Mary-Kate Rogener (University of Georgia) for providing MIMS analysis, Quinn Roberts (The Virginia Institute of Marine Science) for providing IMRS analysis, Rosie Gradoville (UCSC) for discussions about N2 fixation rate measurements, as well as Laurie Juranek (Oregon State University) and the captain and crew of the Research Vessel Sikuliaq for field logistical support. We also thank Chuck Hitzman (Stanford Nano Shared Facility) for nanoSIMS consultation, Lubos Polerecky for look@nanoSIMS consulting, and Stefan Green and his staff (DNA Services Facility and the University of Illinois, Chicago) for sequencing consultation. We greatly appreciate Mick Follows (Massachusetts Institute of Technology) and Kevin Arrigo (Stanford University) for helpful discussions. This research was funded by National Science Foundation Award from the Office of Polar Programs (OPP) 1503614 (to J.P.Z.); Division of Ocean Sciences Awards 1241093 and 1559152 (to J.P.Z.) and OPP-1504307 (to R.E.S.); and Simons Foundation Simons Collaboration on Ocean Processes and Ecology (SCOPE) Award ID 329108 (to J.P.Z.). Part of this work was performed at the Stanford Nano Shared Facility under Award ECCS-1542152.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The raw UCYN-A nifH sequences reported in this paper have been deposited in the Sequence Read Archive, www.ncbi.nlm.nih.gov/sra (Bioproject ID PRJNA476143).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1813658115/-/DCSupplemental.
References
- 1.Falkowski PG, Barber RT, Smetacek V. Biogeochemical controls and feedbacks on ocean primary production. Science. 1998;281:200–207. doi: 10.1126/science.281.5374.200. [DOI] [PubMed] [Google Scholar]
- 2.Breitbarth E, Oschlies A, LaRoche J. Physiological constraints on the global distribution of Trichodesmium–Effect of temperature on diazotrophy. Biogeosciences. 2007;4:53–61. [Google Scholar]
- 3.Tyrrell T. The relative influences of nitrogen and phosphorus on oceanic primary production. Nature. 1999;400:525–531. [Google Scholar]
- 4.Thompson AW, et al. Unicellular cyanobacterium symbiotic with a single-celled eukaryotic alga. Science. 2012;337:1546–1550. doi: 10.1126/science.1222700. [DOI] [PubMed] [Google Scholar]
- 5.Tripp HJ, et al. Metabolic streamlining in an open-ocean nitrogen-fixing cyanobacterium. Nature. 2010;464:90–94. doi: 10.1038/nature08786. [DOI] [PubMed] [Google Scholar]
- 6.Moisander PH, et al. Unicellular cyanobacterial distributions broaden the oceanic N2 fixation domain. Science. 2010;327:1512–1514. doi: 10.1126/science.1185468. [DOI] [PubMed] [Google Scholar]
- 7.Farnelid H, Turk-Kubo K, Munoz-Marin MD, Zehr JP. New insights into the ecology of the globally significant uncultured nitrogen-fixing symbiont UCYN-A. Aquat Microb Ecol. 2016;77:125–138. [Google Scholar]
- 8.Bentzon-Tilia M, et al. Significant N2 fixation by heterotrophs, photoheterotrophs and heterocystous cyanobacteria in two temperate estuaries. ISME J. 2015;9:273–285. doi: 10.1038/ismej.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiozaki T, et al. Basin scale variability of active diazotrophs and nitrogen fixation in the North Pacific, from the tropics to the subarctic Bering Sea. Global Biogeochem Cycles. 2017;31:996–1009. [Google Scholar]
- 10.Shiozaki T, et al. Diazotroph community structure and the role of nitrogen fixation in the nitrogen cycle in the Chukchi Sea (western Arctic Ocean) Limnol Oceanogr. 2018;63:2191–2205. [Google Scholar]
- 11.Thompson A, et al. Genetic diversity of the unicellular nitrogen-fixing cyanobacteria UCYN-A and its prymnesiophyte host. Environ Microbiol. 2014;16:3238–3249. doi: 10.1111/1462-2920.12490. [DOI] [PubMed] [Google Scholar]
- 12.Turk-Kubo KA, Farnelid HM, Shilova IN, Henke B, Zehr JP. Distinct ecological niches of marine symbiotic N2 -fixing cyanobacterium Candidatus Atelocyanobacterium thalassa sublineages. J Phycol. 2017;53:451–461. doi: 10.1111/jpy.12505. [DOI] [PubMed] [Google Scholar]
- 13.Comeau AM, Li WKW, Tremblay JE, Carmack EC, Lovejoy C. Arctic Ocean microbial community structure before and after the 2007 record sea ice minimum. PLoS One. 2011;6:e27492. doi: 10.1371/journal.pone.0027492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brauer VS, et al. Low temperature delays timing and enhances the cost of nitrogen fixation in the unicellular cyanobacterium Cyanothece. ISME J. 2013;7:2105–2115. doi: 10.1038/ismej.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivero-Calle S, et al. Interdecadal Trichodesmium variability in cold North Atlantic waters. Global Biogeochem Cycles. 2016;30:1620–1638. [Google Scholar]
- 16.Rees AP, Gilbert JA, Kelly-Gerreyn BA. Nitrogen fixation in the western English channel (NE Atlantic Ocean) Mar Ecol Prog Ser. 2009;374:7–12. [Google Scholar]
- 17.Mehta MP, Baross JA. Nitrogen fixation at 92 degrees C by a hydrothermal vent archaeon. Science. 2006;314:1783–1786. doi: 10.1126/science.1134772. [DOI] [PubMed] [Google Scholar]
- 18.Olson JB, Steppe TF, Litaker RW, Paerl HW. N2-Fixing microbial consortia associated with the ice cover of Lake Bonney, Antarctica. Microb Ecol. 1998;36:231–238. doi: 10.1007/s002489900110. [DOI] [PubMed] [Google Scholar]
- 19.Sipler RE, et al. Preliminary estimates of the contribution of Arctic nitrogen fixation to the global nitrogen budget. Limnol Oceanogr Lett. 2017;2:159–166. [Google Scholar]
- 20.Blais M, et al. Nitrogen fixation and identification of potential diazotrophs in the Canadian Arctic. Global Biogeochem Cycles. 2012;26:GB3022. [Google Scholar]
- 21.Church MJ, Bjorkman KM, Karl DM, Saito MA, Zehr JP. Regional distributions of nitrogen-fixing bacteria in the Pacific Ocean. Limnol Oceanogr. 2008;53:63–77. [Google Scholar]
- 22.Cornejo-Castillo FM, et al. Cyanobacterial symbionts diverged in the late Cretaceous towards lineage-specific nitrogen fixation factories in single-celled phytoplankton. Nat Commun. 2016;7:11071. doi: 10.1038/ncomms11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabello AM, et al. Global distribution and vertical patterns of a prymnesiophyte-cyanobacteria obligate symbiosis. ISME J. 2016;10:693–706. doi: 10.1038/ismej.2015.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krupke A, et al. Distribution of a consortium between unicellular algae and the N2 fixing cyanobacterium UCYN-A in the North Atlantic Ocean. Environ Microbiol. 2014;16:3153–3167. doi: 10.1111/1462-2920.12431. [DOI] [PubMed] [Google Scholar]
- 25.Lovejoy C, et al. Distribution, phylogeny, and growth of cold-adapted picoprasinophytes in arctic seas. J Phycol. 2007;43:78–89. [Google Scholar]
- 26.Paulsen ML, et al. Synechococcus in the Atlantic gateway to the Arctic Ocean. Front Mar Sci. 2016;3:191. [Google Scholar]
- 27.Krupke A, et al. The effect of nutrients on carbon and nitrogen fixation by the UCYN-A-haptophyte symbiosis. ISME J. 2015;9:1635–1647. doi: 10.1038/ismej.2014.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez-Pérez C, et al. The small unicellular diazotrophic symbiont, UCYN-A, is a key player in the marine nitrogen cycle. Nat Microbiol. 2016;1:16163. doi: 10.1038/nmicrobiol.2016.163. [DOI] [PubMed] [Google Scholar]
- 29.Galand PE, et al. Archaeal diversity and a gene for ammonia oxidation are coupled to oceanic circulation. Environ Microbiol. 2009;11:971–980. doi: 10.1111/j.1462-2920.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 30.Lovejoy C, Potvin M. Microbial eukaryotic distribution in a dynamic Beaufort Sea and the Arctic Ocean. J Plankton Res. 2011;33:431–444. [Google Scholar]
- 31.Ward BA, Dutkiewicz S, Moore CM, Follows MJ. Iron, phosphorus, and nitrogen supply ratios define the biogeography of nitrogen fixation. Limnol Oceanogr. 2013;58:2059–2075. [Google Scholar]
- 32.Michel C, et al. Arctic Ocean outflow shelves in the changing Arctic: A review and perspectives. Prog Oceanogr. 2015;139:66–88. [Google Scholar]
- 33.Moisander PH, Beinart RA, Voss M, Zehr JP. Diversity and abundance of diazotrophic microorganisms in the South China Sea during intermonsoon. ISME J. 2008;2:954–967. doi: 10.1038/ismej.2008.51. [DOI] [PubMed] [Google Scholar]
- 34.Zehr JP, McReynolds LA. Use of degenerate oligonucleotides for amplification of the nifH gene from the marine cyanobacterium Trichodesmium thiebautii. Appl Environ Microbiol. 1989;55:2522–2526. doi: 10.1128/aem.55.10.2522-2526.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moonsamy PV, et al. High throughput HLA genotyping using 454 sequencing and the Fluidigm Access Array™ system for simplified amplicon library preparation. Tissue Antigens. 2013;81:141–149. doi: 10.1111/tan.12071. [DOI] [PubMed] [Google Scholar]
- 36.Church MJ, Short CM, Jenkins BD, Karl DM, Zehr JP. Temporal patterns of nitrogenase gene (nifH) expression in the oligotrophic North Pacific Ocean. Appl Environ Microbiol. 2005;71:5362–5370. doi: 10.1128/AEM.71.9.5362-5370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jayakumar A, et al. Biological nitrogen fixation in the oxygen-minimum region of the eastern tropical North Pacific Ocean. ISME J. 2017;11:2356–2367. doi: 10.1038/ismej.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dabundo R, et al. The contamination of commercial 15N2 gas stocks with 15N-labeled nitrate and ammonium and consequences for nitrogen fixation measurements. PLoS One. 2014;9:e110335. doi: 10.1371/journal.pone.0110335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montoya JP, Voss M, Kahler P, Capone DG. A simple, high-precision, high-sensitivity tracer assay for N2 fixation. Appl Environ Microbiol. 1996;62:986–993. doi: 10.1128/aem.62.3.986-993.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gradoville MR, et al. Diversity and activity of nitrogen‐fixing communities across ocean basins. Limnol Oceanogr. 2017;62:1895–1909. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.