ABSTRACT
Pharyngeal pouches, a series of outpocketings derived from the foregut endoderm, are essential for craniofacial skeleton formation. However, the molecular mechanisms underlying endodermal pouch-regulated head cartilage development are not fully understood. In this study, we find that zebrafish dmrt2b, a gene encoding Doublesex- and Mab-3-related transcription factor, is specifically expressed in endodermal pouches and required for normal pharyngeal cartilage development. Loss of dmrt2b doesn't affect cranial neural crest (CNC) specification and migration, but leads to prechondrogenic condensation defects by reducing cxcl12b expression after CNC cell movement into the pharyngeal arches. Moreover, dmrt2b inactivation results in reduced proliferation and impaired differentiation of CNC cells. We also show that dmrt2b suppresses crossveinless 2 expression in endodermal pouches to maintain BMP/Smad signaling in the arches, thereby facilitating CNC cell proliferation and chondrogenic differentiation. This work provides insight into how transcription factors expressed in endodermal pouches regulate pharyngeal skeleton development through tissue–tissue interactions.
KEY WORDS: dmrt2b, Endodermal pouch, Craniofacial cartilage, cxcl12b, crossveinless 2
Summary: Transcription factor dmrt2b facilitates cxcl12b expression in endodermal pouches to accelerate cranial neural crest cell condensation, but suppresses crossveinless 2 expression for supporting cell growth and chondrogenic differentiation.
INTRODUCTION
Craniofacial malformations, which occur due to developmental issues of the head, face and neck, account for approximately one-third of congenital birth defects (DeLuke, 2014). Owing to the ceaseless efforts of scientists, more than 700 distinct craniofacial syndromes have been described (Johnson and Wilkie, 2011; Lisa and Elden, 2014; Schutte and Murray, 1999). The neurocranium is derived from both the cranial neural crest (CNC) and mesoderm, while the pharyngeal skeleton, including the jaw and branchial arches, is solely derived from CNC cells (Yelick and Schilling, 2002). CNC cells emerge from the dorsal and lateral regions of the neural ectoderm when the epidermal ectoderm interacts with the neural plate to induce formation of the neural plate border (Donoghue et al., 2008). Subsequently, bilateral CNC cells migrate medially with the developing head and then from the midbrain and hindbrain as three streams of collective cell populations (mandibular, hyoid and branchial) into the pharyngeal arches to form the pharyngeal cartilages (Couly et al., 1993; Köntges and Lumsden, 1996; Lumsden et al., 1991; Schilling and Kimmel, 1994).
The craniofacial complex comprises cells from all three germ layer origins: ectodermal, endodermal and mesodermal, and craniofacial morphogenesis requires continuous and reciprocal tissue–tissue interactions (Chai and Maxson, 2006). In particular, in the pharyngeal arches, the CNC with mesoderm core is separated with endodermal pouch inner and covered with epidermal ectoderm outer (Noden, 1988). Endodermal pouches are a series of outpocketings developed in an anterior–posterior wave from the pharyngeal endoderm. Interestingly, although CNC cells are not required for the formation of endodermal pouches (Veitch et al., 1999), these pouches have signaling functions important for the development of the pharyngeal skeleton. Zebrafish mutants, such as casanova/sox32 and faust/gata5, which lack early endoderm and the pharyngeal pouches, exhibit severe defects in craniofacial chondrogenesis, suggesting the endodermal requirements in pharyngeal skeletal development (Dickmeis et al., 2001; Kikuchi et al., 2001; Reiter et al., 1999). Endodermal pouch-derived FGF and BMP ligands have been shown to be required for the survival, proliferation and differentiation of postmigratory CNC cells that give rise to branchial cartilages (Crump et al., 2004; David et al., 2002; Holzschuh et al., 2005; Ning et al., 2013; Nissen et al., 2003). In addition, the T-box transcription factor Tbx1 is a key molecule in the regulation of tissue–tissue interactions (Choe and Crump, 2014; Huh and Ornitz, 2010; Kopinke et al., 2006; Okada et al., 2016; Okubo et al., 2011; Piotrowski et al., 2003). tbx1 is expressed in the endodermal pouches as well as the mesodermal core of the pharyngeal arches (Piotrowski et al., 2003). In vgo/tbx1 mutants, the pharyngeal pouches are largely absent, and the pharyngeal cartilages are misshapen and fused together (Piotrowski and Nüsslein-Volhard, 2000). Yet while mesodermal Tbx1 has been shown to function in shaping the lower jaw (Aggarwal et al., 2010), transplantation of wild-type endoderm into vgo/tbx1 mutants partial rescue the formation of pharyngeal cartilages, indicating that tbx1 acts non-autonomously in the endoderm (Piotrowski et al., 2003). The identification of new chondrogenic regulators with endodermal expression will promote our understanding of the tissue–tissue interactions during craniofacial skeleton development.
The doublesex/mab-3 related (Dmrt) gene family consists of transcription factors with a DSX/MAB-3 (DM) domain, which is a zinc finger-like DNA binding motif first identified in the sexual regulatory proteins Doublesex (DSX) and MAB-3 (Erdman and Burtis, 1993; Raymond et al., 1998). There are multiple dmrt paralogs in the animal kingdom, but most Dmrt proteins display little similarity with the exception of their DM domain (Volff et al., 2003). These dmrt genes have different spatial-temporal expression, suggesting they could have additional functions besides sex determination (Hodgkin, 2002; Hong et al., 2007; Lints and Emmons, 2002; Volff et al., 2003). There are five dmrt genes, designated drmt1, dmrt2a, dmrt2b, dmrt3 and dmrt5, in zebrafish. The dmrt2a and dmrt2b genes originated from the second round of genome duplication, and dmrt2a is the homolog of Dmrt2 that is involved in somitogenesis in vertebrates (Lu et al., 2017; Matsui et al., 2012; Meng et al., 1999; Sato et al., 2010; Saúde et al., 2005; Seo et al., 2006). Interestingly, dmrt2b is expressed in the pharyngeal region (Johnsen and Andersen, 2012; Zhou et al., 2008), indicating its potential role in the development of the branchial skeleton.
In this study, we find that zebrafish dmrt2b is uniquely expressed in endodermal pouches and reveal a function for this gene in regulating endodermal expression of cxcl12b and crossveinless 2 (cv2) to promote pharyngeal CNC cell condensation, proliferation and differentiation. Therefore, this study uncovers a molecular mechanism for regulation of craniofacial cartilage development through tissue–tissue interactions mediated by endodermal pouch-expressed transcription factor Dmrt2b.
RESULTS
Zebrafish dmrt2b is specifically expressed in pharyngeal pouches
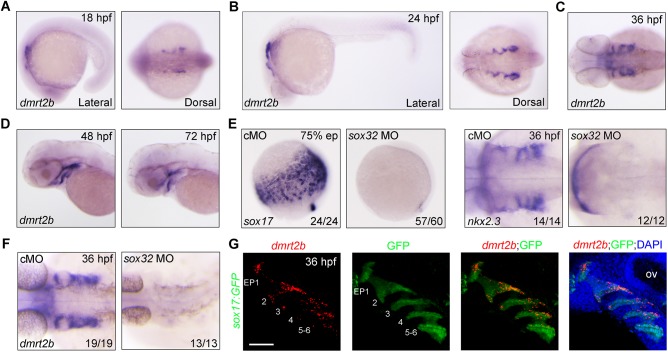
To evaluate the developmental functions of dmrt2b, we first examined its spatiotemporal expression during zebrafish embryonic development using in situ hybridizations with an anti-sense probe targeting the cDNA sequence downstream the coding region of DM domain. We found that dmrt2b was uniquely expressed in the pharyngeal region as early as 18 hours post-fertilization (hpf), when the first endodermal pouch budded (Fig. 1A). During later stages, dmrt2b expression spread to the bilateral side of the head in a thread-like manner (Fig. 1B–D), suggesting that dmrt2b is expressed in the endodermal pouches. To examine this, 8 ng sox32 morpholino (MO) was injected into embryos at the one-cell stage, which resulted in the elimination of the entire endoderm and endoderm-derived pouches as indicated by sox17 and nkx2.3 expression (Fig. 1E). As expected, dmrt2b expression disappeared from the sox32 morphants (Fig. 1F). Furthermore, RNAscope in situ hybridization combined with immunofluorescence was employed to figure out the exact expression pattern of dmrt2b in the Tg(sox17:GFP) transgenic fish embryos (Chung and Stainier, 2008). As shown in Fig. 1G, dmrt2b transcripts co-localized with GFP-expressing endodermal pouches at 36 hpf. These observations strongly suggest that dmrt2b is expressed specifically in the pharyngeal pouches.
Fig. 1.
Expression of dmrt2b in the developing endodermal pouches. (A–D) Analysis of dmrt2b expression at different stages. (E,F) Endodermal cells were absent from sox32 morphants. Expression of endodermal marker sox17 (E), endodermal pouch marker nkx2.3 (E) and dmrt2b (F) were examined by in situ hybridizations at the indicated stages in wild-type embryos injected with 8 ng control MO (cMO) or sox32 MO. (G) Expression of dmrt2b in endodermal pouches. At 36 hpf, Tg(sox17:GFP) transgenic embryos were stained for dmrt2b mRNA with Dr-dmrt2b-C3 probe (red), and then immunostained with anti-GFP antibody (green). Nuclei were counterstained with DAPI (blue). The six endodermal pouches are labeled in the left two panels. EP, endodermal pouch; ov, otic vesicle. Scale bar: 50 µm.
Loss of dmrt2b causes malformation of pharyngeal cartilages
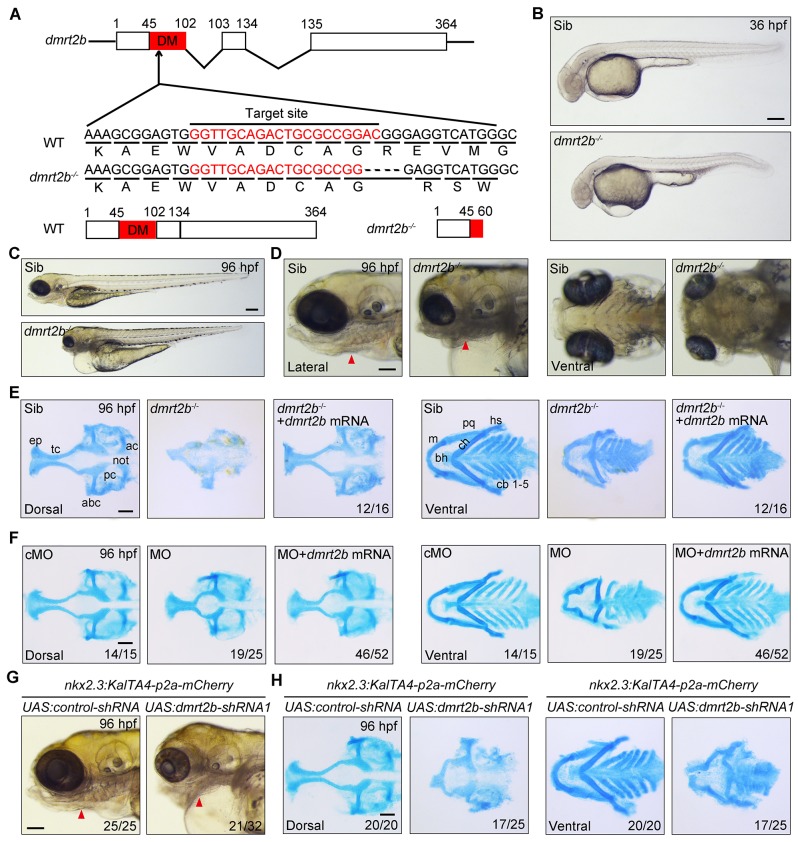
To investigate whether dmrt2b has functions in pharyngeal pouch formation and craniofacial cartilage development, we mutated the dmrt2b gene using the CRISPR-Cas9 system. Because the DM domain enables dmrt2b to act as a transcription factor, we targeted this domain and obtained one mutant with a four base frameshift deletion in dmrt2b, which led to a premature stop codon (Fig. 2A; Fig. S1A). Furthermore, the obvious reduction of dmrt2b transcripts in dmrt2b homozygous mutants confirmed the loss of function of this gene (Fig. S1B). In comparison to wild-type and heterozygous siblings, dmrt2b homozygous mutants had shrunken heads, pericardial edema and smaller jaws (Fig. 2B,C). In addition, the dmrt2b mutants had restricted protruding jaws due to poor pharyngeal arch outgrowth (Fig. 2D). Alcian Blue staining revealed severe reduced and dysmorphic neurocranium cartilages and pharyngeal arch-derived chondrogenic elements in these mutants, suggesting chondrogenic differentiation defects (Fig. 2E). Importantly, these cartilages were recovered by injection of 475 pg dmrt2b mRNA into dmrt2b mutants (Fig. 2E).
Fig. 2.
Depletion of dmrt2b impairs cranial cartilage development. (A) Generation of dmrt2b mutant using the CRISPR/Cas9 system. The dmrt2b mutant has a four base deletion that results in expression of a truncated protein lacking the DM domain. (B,C) Morphological defects in dmrt2b mutants at the indicated stages. (D) Anatomy of the pharyngeal arches and head skeleton in dmrt2b mutants. Red arrowheads indicate branchial arches. (E,F) Alcian Blue staining of head cartilages at 96 hpf. Cartilage defects in dmrt2b mutants and morphants were abrogated by injection of dmrt2b mRNA (F). (G) Anatomy of the pharyngeal arches and head skeleton in embryos injected with indicated shRNA expression plasmids. Red arrowheads indicate branchial arches. (H) Alcian Blue staining of head cartilages in shRNA expression plasmid injected embryos. Scale bar: 100 µm. ac, auditory capsule; not, notochord; pc, parachordal; abc, anterior basicranial commissure; ep, ethmoid plate; tc, trabeculae cranii; m, Meckel's cartilage; bh, basihyal; ch, ceratohyal; pq, palatoquadrate; hs, hyosymplectic; cb, ceratobranchial. Scale bars: 200 µm (B,C), 100 µm (D–H).
To further confirm the role of dmrt2b in these pharyngeal cartilage defects, knockdown experiments were performed using antisense MOs. Specifically, 4 ng of dmrt2b MO targeting the intron-exon boundary of the first intron and the second exon of the dmrt2b gene was injected into one-cell stage embryos. This resulted in the elimination of endogenous mature dmrt2b mRNA and the emergence of interfered mRNA products in the morphants (Fig. S2A), indicating the dmrt2b MO is specific and effective. Similar to the dmrt2b mutants, the knockdown morphants had obvious defects in head cartilage formation, which were abrogated by co-injection of dmrt2b mRNA (Fig. 2F; Fig. S2B). Interestingly, the pericardial edema in the morphants was also alleviated by dmrt2b mRNA injection, indicating that dmrt2b might function in cardiac development (Fig. S2B). miR30-based short hairpin RNAs (shRNAs) from tissue specific promoters displayed very efficient knockdown of gene expression in eucaryotic organisms (Stegmeier et al., 2005; Zeng et al., 2005). To explore tissue-specific roles of dmrt2b, we utilized the KalTA4-UAS system to drive the expression of miR30-based shRNAs (dmrt2b-shRNA1 and dmrt2b-shRNA2) against two different regions of dmrt2b transcripts. By using the Tol2 transposon, we generated a Tg(nkx2.3:KalTA4-p2a-mCherry) transgenic zebrafish line with a 5.5 kb nkx2.3 promoter that could specifically drive the expression of KalTA4 activators and red fluorescent proteins in endodermal pouches (Choe et al., 2013), which were indicated by Tg(sox17:GFP) embryos at 36 hpf (Fig. S3A). As shown in Fig. S3B, co-injection of 50 pg UAS:dmrt2b-shRNA1 plasmid with 100 pg Tol2 transposase mRNA into one-cell stage Tg(nkx2.3:KalTA4-p2a-mCherry) embryos led to an obvious decrease of dmrt2b expression in endodermal pouches compared with control embryos (Fig. S3B). Importantly, the inactivation of dmrt2b in pouches resulted in obvious defects in head cartilage formation (Fig. 2G,H). Thus, endodermal pouch-expressed dmrt2b is important for craniofacial cartilage development. In addition, tissue specific depletion of dmrt2b by injection of UAS:dmrt2b-shRNA1 plasmid into Tg(nkx2.3:KalTA4-p2a-mCherry) embryos resulted in obvious pericardial edema, implying a non-cell autonomous role of pharyngeal endodermal dmrt2b during heart development (Fig. 2G). Interestingly, more severe pharyngeal cartilage defects were observed in dmrt2b MO or UAS:dmrt2b-shRNA1 plasmid injected embryos than dmrt2b mutants, indicating that a compensatory protective response against the loss of dmrt2b may be to some extent activated in the mutants (Rossi et al., 2015; Wei et al., 2017).
Inactivation of dmrt2b results in disorganized pharyngeal arches
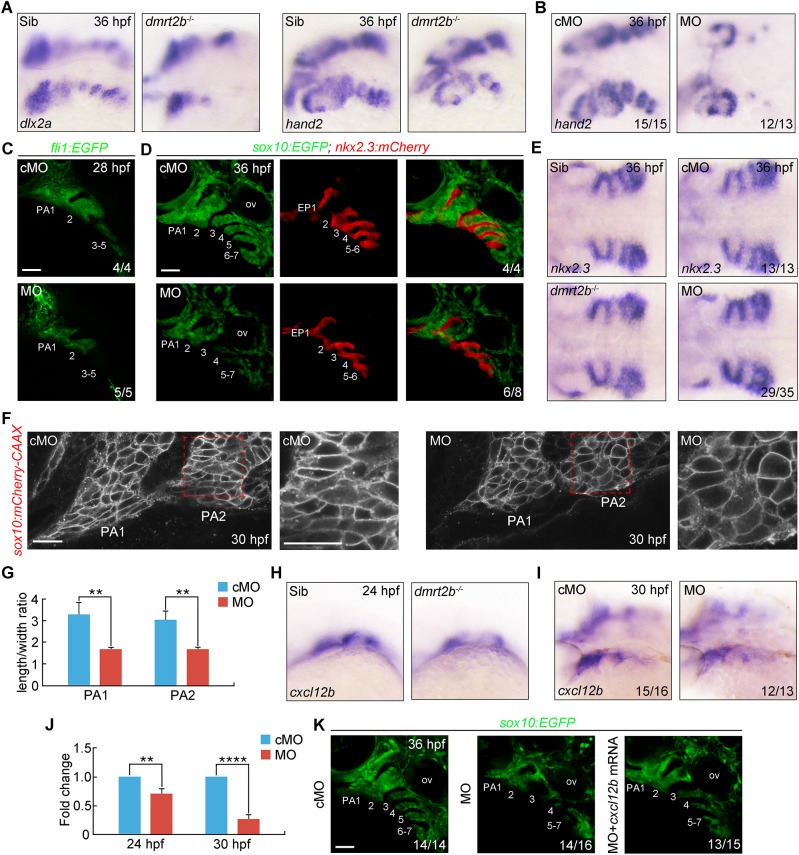
To delineate the mechanisms underlying pharyngeal cartilage defects in the absence of functional dmrt2b, we examined the expression of several different markers over the course of embryonic development. In situ hybridization revealed the CNC specification marker foxd3 was expressed in a similar manner in control embryos and dmrt2b mutants at the five somite stage (Fig. S4A). This demonstrates that dmrt2b is not required for the specification of the CNC. In control embryos, dlx2a was expressed in the three CNC groups at 18 and 24 hpf. A slight decrease of dlx2a expression was observed at 18 hpf in dmrt2b mutants, but subsequently recovered at 24 hpf, indicating that dmrt2b is not essential for CNC cell migration into the pharyngeal arches (Fig. S4B,C).
Subsequently, the pharyngeal endoderm migrates laterally to form pouches that interdigitate each of the pharyngeal arches (Crump et al., 2004), then the postmigratory CNC cells in the arches start condensation and proliferation processes and finally differentiate into chondrocytes (Clouthier and Schilling, 2004; Hall and Miyake, 1995; Hall and Miyake, 2000). As dmrt2b continues to express in the endodermal pouches after CNC cells reach their destination in the arches, we then examined the arch morphology in dmrt2b depleted embryos via the expression of two markers of postmigratory crest, dlx2a and hand2, at 36 hpf. Compared with control embryos, there were notably fewer CNC cells in dmrt2b mutants and morphants (Fig. 3A,B). Similar phenotypes were observed in dmrt2b MO-injected Tg(fli1:GFP) embryos expressing GFP in CNC derivatives at 28 hpf (Fig. 3C) (Lawson and Weinstein, 2002). Furthermore, dmrt2b morphants exhibited loose and disorganized anterior arch structures and the CNC cells failed to aggregate toward certain centers (Fig. 3C). Moreover, injection of 4 ng dmrt2b MO into Tg(nkx2.3:mCherry; sox10:EGFP) embryos (Carney et al., 2006) resulted in no obvious defects in mCherry-expressing pharyngeal pouches at 36 hpf, and the endodermal pouch marker nkx2.3 was normally expressed in both dmrt2b mutants and morphants (Fig. 3D,E). In contrast, dmtr2b depletion gave rise to a similar disorganized arch phenotype and notably fewer GFP-positive CNC cells (Fig. 3D). Moreover, the CNC cells in the pharyngeal region were flattened and elongated, reflecting condensation defects in 4 ng dmrt2b MO injected Tg(sox10:mCherry-CAAX) embryos, in which cell shape was outlined through plasma membrane-bound mCherry (Fig. 3F,G). Taken together, these findings reveal that dmrt2b is dispensable for endodermal pouch morphogenesis, but plays an important role in controlling prechondrogenic condensation in the pharyngeal arches.
Fig. 3.
dmrt2b functions in CNC cell condensation. (A,B) Depletion of dmrt2b resulted in fewer CNC cells in the pharyngeal arches. dmrt2b mutants (A) and morphants (B) were harvested at 36 hpf for in situ hybridization with dlx2a and hand2 probes. Lateral views of embryos presented with anterior to the left. (C) Live confocal images of Tg(fli1:EGFP) transgenic embryos injected with 4 ng cMO or dmrt2b MO at 24 hpf. The pharyngeal arches are numbered. (D) Live confocal images of endodermal pouches and CNC cells in the pharyngeal regions of Tg(nkx2.3:mCherry; sox10:EGFP) transgenic embryos at 36 hpf. (E) The expression of endodermal pouch marker nkx2.3 in dmrt2b mutants and morphants. Dorsal views with anterior to the left. (F) Changes in cell shape in the leading edge of the first and second pharyngeal arches in Tg(sox10:mCherry-CAAX) embryos injected with 4 ng dmtr2b MO. The boxed areas are presented at a higher magnification in the right panels. (G) Quantitation of length/width ratio of CNC cells in the leading edge of the pharyngeal arches. All data are presented as the mean of three independent experiments. Error bars represent s.d. Significance was analyzed using unpaired t-tests. **, P<0.01. (H,I) The expression of cxcl12b in the developing pouches of dmrt2b mutants (H) and morphants (I) at the indicated stages. (J) The expression of cxcl12b in the head of dmrt2b morphants were examined by qRT-PCR at the indicated stages. All data are presented as the mean of three independent experiments. Error bars represent s.d. Significance was analyzed using unpaired t-tests. **P<0.01; ****P<0.0001. (K) Live confocal images of CNC cells in the pharyngeal regions of Tg(sox10:EGFP) transgenic embryos at 36 hpf. EP, endodermal pouch; ov, otic vesicle; PA, pharyngeal arch. Scale bars: 50 µm (C,D,K), 20 µm (F).
Because Dmrt2b is a transcription factor specifically expressed in the pharyngeal endoderm, the non-autonomous activity of dmrt2b should be mediated by some secretory molecules that are derived from endodermal pouches and able to regulate pharyngeal arch development. It has been shown that chemokine Cxcl12b signaling from the endodermal pouches is required for the proper condensation of Cxcr4a expressing CNC cells in pharyngeal arches (Boer et al., 2015; Olesnicky Killian et al., 2009). Therefore, we speculate that dmrt2b might regulate the expression of cxcl12b in pouches to control the prechondrogenic condensation. In support of this hypothesis, after CNC cells migrating from the brain into the pharyngeal arches, both genetic depletion and knockdown of dmrt2b resulted in a significant reduction of cxcl12b expression in the pharyngeal region (Fig. 3H–J). In addition, the condensation defects of GFP-positive CNC cells were partially rescued by co-injection of 20 pg cxcl12b mRNA into dmrt2b morphants (Fig. 3K). Therefore, we conclude that dmrt2b is a positive regulator of cxcl12b in endodermal pouches and thereby drives CNC cell compaction in the pharyngeal arches.
The proliferation and chondrogenic differentiation of CNC cells require dmrt2b
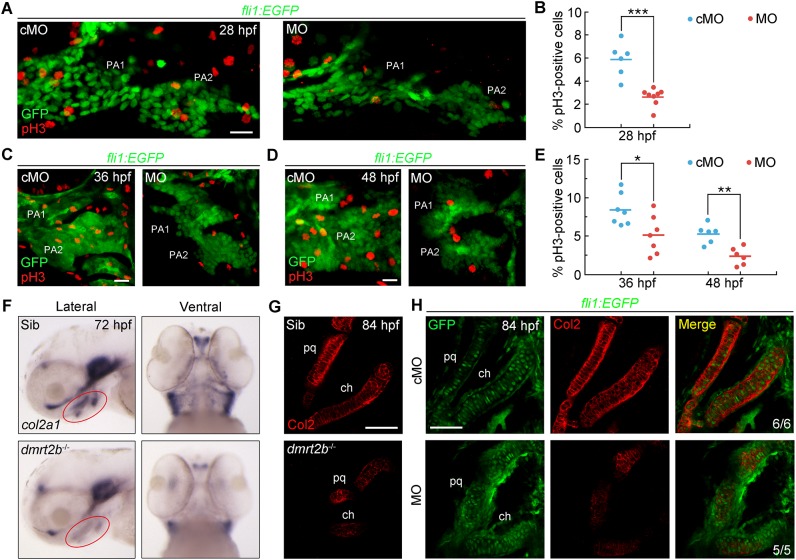
After migrating into the pharyngeal arches, CNC cells proliferate and differentiate into chondrocytes (Hall and Miyake, 2000; Ning et al., 2013). To dynamically observe pharyngeal cartilage defects induced by dmrt2b inactivation, in vivo time-lapse imaging of CNC cells was performed on Tg(fli1:EGFP) embryos. In the control MO (cMO)-injected embryos, GFP-positive CNC cells aggregated as prechondrogenic condensations at 48 hpf, differentiated into chondrocytes at 60 hpf, and organized into chondrocyte stacks from 72 to 84 hpf (Fig. S5). However, in addition to the condensation defects in the first and second pharyngeal arches in dmrt2b morphants, there were significantly fewer CNC cells at 48 hpf (Fig. S5). Moreover, only a few CNC cells were observed in pharyngeal arches 3–7 (Fig. S5), consistent with the previously noted cell number reduction in the dmrt2b morphants (Fig. 3C,D). These posterior segments emerged at 60 hpf, but were much smaller (Fig. S5). At 72 and 84 hpf, the palatoquadrate and ceratohyal cartilages were shorter and the chondrocytes failed to stack (Fig. S5). These observations are consistent with the Alcian Blue staining results (Fig. 2E).
The reduction in the size of the pre-chondrogenic segments in the dmrt2b morphants raises the possibility that cell proliferation and/or survival are inhibited. Therefore, Tg(fli1:EGFP) embryos were immunostained for phosphorylated histone 3 (pH3) to assess CNC cell proliferation at 28, 36 and 48 hpf. We found notably fewer mitotic pH3-positive CNC cells in dmrt2b morphants than control embryos (Fig. 4A–E). Conversely, TUNEL revealed a lack of apoptotic cells in the pharyngeal region in dmrt2b mutants and morphants (Fig. S6A,B). These findings support that loss of dmrt2b impairs CNC cell proliferation, which contributes to the reduction in chondrocyte number within the pharyngeal arches.
Fig. 4.
dmrt2b facilitates CNC cell proliferation and differentiation. (A,C,D) Representative confocal sections of pH3-positive cells in the first and second pharyngeal arches at the indicated stages. (B,E) Percentage of pH3-positive cells among GFP-positive CNC cells. n≥6 embryos for each condition. Significance was analyzed using unpaired t-tests. *P<0.05; **P<0.01; ***P<0.001. (F) Expression levels of col2a1 mRNA at 72 hpf. Red circles indicate the pharyngeal region. The left panels are lateral views with anterior to the left and the right panels are ventral views with the anterior to the top. (G,H) Immunostaining of Col2 protein in pharyngeal cartilages at 84 hpf. dmrt2b mutants (G) and Tg(fli1:EGFP) embryos injected with 4 ng dmrt2b MO (H) were stained with the indicated fluorescent antibodies. PA, pharyngeal arch; pq, palatoquadrate; ch, ceratohyal. Scale bars: 20 µm.
Due to the disordered arrangement of the chondrocytes in the absence of dmrt2b, we inferred that dmrt2b also has an essential role in CNC cell differentiation into chondrocytes. To test this, we assessed the expression of col2a1, the gene encoding type II collagen, the primary cartilage matrix protein produced by mature chondrocytes (Vandenberg et al., 1991; Yan et al., 2002). There were dramatically fewer col2a1 transcripts in the pharyngeal region of dmrt2b mutants (Fig. 4F). Meanwhile, Col2 protein levels were also reduced and displayed discontinuous distribution in dmrt2b-depleted embryos (Fig. 4G,H). Therefore, loss of dmrt2b disrupts chondrogenic differentiation of CNC cells.
dmrt2b maintains BMP signaling through inhibiting crossveinless 2 to facilitate CNC cell proliferation and chondrogenic differentiation
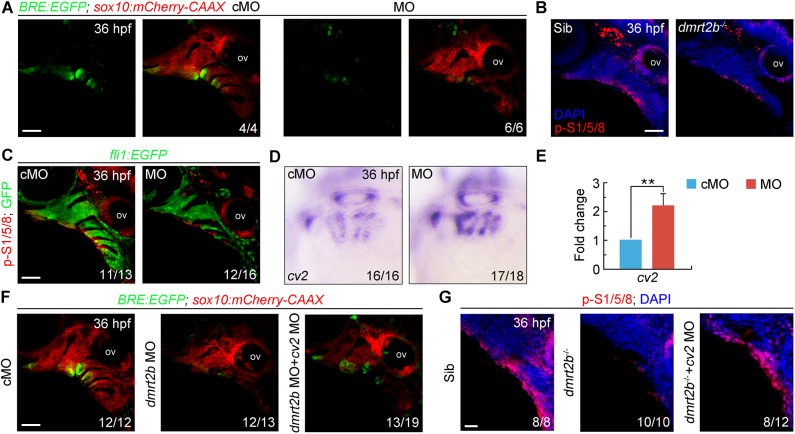
BMP signaling is essential for CNC cell proliferation and chondrogenic differentiation during pharyngeal cartilage development (Ning et al., 2013; Retting et al., 2009; Yoon et al., 2005). Therefore, we mated Tg(sox10:mCherry-CAAX) with Tg(BRE:EGFP), a BMP signaling reporter transgenic line (Laux et al., 2011), to examine whether inactivation of dmrt2b affects BMP signaling. In the dmrt2b morphants, there was decreased fluorescence intensity in the pharyngeal arches, implying loss of dmrt2b attenuates BMP signaling in CNC cells (Fig. 5A). To further confirm this result, we examined the expression level of endogenous phosphorylated Smad1/5/8 (p-Smad1/5/8), the intracellular effectors of BMP signaling, in dmrt2b mutants and Tg(fli1:EGFP) embryos injected with 4 ng dmrt2b MO, and found a significantly decrease of p-Smad1/5/8 level in pharyngeal region when dmrt2b was depleted (Fig. 5B,C). Thus, we established that dmrt2b is required to maintain BMP signaling in CNC cells.
Fig. 5.
dmrt2b maintains BMP signaling in the pharyngeal region by inhibiting cv2 expression. (A) Expression of BRE-driven EGFP in the pharyngeal region of Tg(BRE:EGFP;sox10:mCherry-CAAX) transgenic embryos injected with 4 ng cMO or dmrt2b MO at 36 hpf. (B,C) p-Smad1/5/8 levels were decreased in the pharyngeal region of dmrt2b mutants (B) and morphants (C) at 36 hpf. Embryos were stained with the indicated antibodies. Nuclei were counterstained with DAPI (blue). (D,E) The expression of cv2 in dmrt2b morphants were examined by in situ hybridization (D) and qRT-PCR (E). All data are presented as the mean of three independent experiments. Error bars represent s.d. Significance was analyzed using unpaired t-tests. **P<0.01. (F) Co-injection of 100 pg cv2 MO with 4 ng dmrt2b MO partially rescued the EGFP expression in Tg(BRE:EGFP;sox10:mCherry-CAAX) transgenic embryos. (G) p-Smad1/5/8 levels were partially rescued in the pharyngeal region of dmrt2b mutants co-injected with 100 pg cv2 MO at 36 hpf. Embryos were stained with the indicated antibodies. Nuclei were counterstained with DAPI (blue). ov, otic vesicle; p-S1/5/8, phosphorylated Smad1/5/8. Scale bars: 50 µm (A–C,F), 20 µm (G).
BMP genes, including bmp2a, bmp2b, bmp4 and bmp5, are expressed in the endodermal pouches during pharyngeal arch development (Holzschuh et al., 2005). However, the expression of these BMP genes was not obviously decreased in dmrt2b-depleted embryos (data not shown). Another possibility is that dmrt2b promotes BMP signaling in the pharyngeal region by inhibiting the expression of some BMP antagonists. In zebrafish embryos, several BMP antagonist genes like noggin3, follistatin, chordin and chordin-like 2, have been found to be expressed in the pharyngeal region, but only crossveinless 2 (cv2) is specifically expressed in the endodermal pouches during cartilage development (Ning et al., 2013; Rentzsch et al., 2006). It has been reported that Cv2 functions as a secreted BMP inhibitory protein during human chondrogenic and osteogenic differentiation (Binnerts et al., 2004). In the loss-of-function experiments, we observed that the expression of cv2 in the pharyngeal pouches was significantly higher in dmrt2b morphants compared to control embryos (Fig. 5D,E). Importantly, BMP signaling in the CNC cells was partially restored by co-injecting 100 pg cv2 MO into the dmrt2b morphants or mutants (Fig. 5F,G). Together, these data indicate that dmrt2b inhibits cv2 expression in the pharyngeal pouches, thereby maintaining BMP signaling and facilitating CNC cell proliferation and differentiation.
DISCUSSION
The interaction of different tissues plays vital roles in organogenesis during embryo developemt (Zorn and Wells, 2009). For example, in zebrafish, wnt2bb is expressed in restricted bilateral domains in the lateral plate mesoderm and directly induces the adjacent endoderm to form liver anlage (Ober et al., 2006). Recent evidence suggests that trachea-derived Decapentaplegic, the main bone morphogenetic protein ligand in Drosophila, is required for adult midgut homeostasis (Li et al., 2013). During craniofacial cartilage development, once the CNC cells reach their destination in the arches, signals from surrounding endodermal pouches such as FGF, BMP and CXCL12, direct the final cell fate (Crump et al., 2004; David et al., 2002; Holzschuh et al., 2005; Ning et al., 2013; Nissen et al., 2003; Boer et al., 2015; Olesnicky Killian et al., 2009). In this study, we find that dmrt2b is expressed in the endodermal pouches and loss of dmrt2b impairs pharyngeal cartilage formation. dmrt2b plays a critical role in the condensation of postmigratory CNC cells by promoting cxcl12b expression. We also provide evidence that dmrt2b is required for CNC cell proliferation and chondrogenic differentiation due to its ability to suppress cv2 expression and, thus, maintain BMP signaling in pharyngeal regions. Therefore, this study demonstrates dmrt2b-mediated tissue–tissue interactions are essential for pharyngeal skeleton development. dmrt2b mutants also exhibit severe neurocranial defects and pericardial edema, indicating the possibility that dmrt2b is expressed at relatively low levels in other regions beyond endodermal pouches.
Members of the Dmrt family are generally associated with sex determination, but mouse Dmrt2 is not essential for sexual differentiation (Seo et al., 2006). Mouse Dmrt2 and its homologue gene, zebrafish terra/dmrt2a, have shown to be expressed specifically in developing smites and function in somitogenesis (Meng et al., 1999; Saúde et al., 2005; Seo et al., 2006). Zebrafish dmrt2a is also required for left–right asymmetric organ positioning (Matsui et al., 2012; Saúde et al., 2005), while this left–right function is not conserved in mouse (Lourenço et al., 2010). Interestingly, in mouse Dmrt2 mutants, the somite patterning defects were gradually recovered during embryonic development, but the axial skeletal and rib malformations were evidently induced by the lacking of Fgf4 and Fgf6 expression in the myotome, suggesting a non-cell autonomous role of Dmrt2 in controlling skeletal development (Seo et al., 2006). In our experiments, dmrt2b is found to be essential for pharyngeal skeleton development. Multiple lines of evidence support the idea that dmrt2b functions in a similar non-cell-autonomous manner by transferring developmental signals from pharyngeal endoderm to postmigratory CNC cells. (1) The expression of dmrt2b can be specifically detected in endodermal pouches by in situ hybridization experiments during pharyngeal cartilage development. (2) The endodermal pouches in dmrt2b depleted embryos are normally developed, indicating that the skeletal defects in the pharyngeal region are not the secondary effects of abnormalities of endodermal pouch development. (3) The experimental inhibition of dmrt2b function gene in pouches gives rise to obvious defects in head cartilage formation. (4) Inactivation of dmrt2b leads to obviously altered expression of cxcl12b and cv2 in endodermal pouches, which results in disorganized arches and proliferation and differentiation defects in CNC cells. (5) Importantly, co-injection of cxcl12b mRNA or cv2 MO into the dmrt2b morphants can partially recover the condensation defects or the decrease of BMP signaling in CNC cells. All these observations imply that dmrt2b regulates craniofacial skeleton development through tissue–tissue interactions. Although mouse Dmrt2 mutant does not exhibit obvious craniofacial abnormalities (Seo et al., 2006), our findings will help to understand the developmental differences of craniofacial skeletons between lower vertebrates and mammals.
CXCL12, also known as stromal derived factor 1 (SDF-1), signals via its cognate receptor CXCR4 and plays a key role in many cellular processes including hematopoiesis, organogenesis and vascularization (Cheng et al., 2014; Teicher and Fricker, 2010). In mouse and chick embryos, Cxcl12 is expressed in the lateral ectoderm and pharyngeal endoderm at early stages of CNC cell migration, while Cxcr4 is expressed in migrating pharyngeal NC cells (Escot et al., 2016). Defective CXCR4 signaling impedes the migration of CNC cells into pharyngeal arches and leads to anomalies of the lower jaw and hyoid bone (Escot et al., 2016). In zebrafish embryos, cxcl12b, but not cxcl12a, is expressed within the domain of CNC cell migration from 14-17 hpf and in the endodermal pouches during pharyngeal arch morphogenesis (Olesnicky Killian et al., 2009). Unlike mouse mutants, cxcl12b or cxcr4a morphants display only a mild migration phenotype as most CNC cells arrive at the pharyngeal arches. Disruption of Cxcl12b/Cxcr4a signaling in zebrafish also results in the failure of CNC cells to fully condense within the pharyngeal arches, which is thought to be secondarily caused by the aberrant migration of CNC cells (Olesnicky Killian et al., 2009). Interestingly, loss of dmrt2b gives rise to decreased expression of cxcl12b and disorganized cells within the arches that resemble the defects observed in cxcl12b morphants, suggesting that cxcl12b is genetically downstream of dmrt2b during pharyngeal NC development. In addition, the expression of dmrt2b in the pharyngeal region is not detected until 18 hpf, and the expression of cxcl12b starts to decrease at 24 hpf, when the CNC cells have already migrated into pharyngeal arches. These observations would explain the lack of CNC cell migration defects in dmrt2b mutants, and raise the provocative idea that Cxcl12b/Cxcr4a signaling may function directly in pharyngeal NC condensation. Moreover, the disorganized arch phenotype could be rescued by injecting cxcl12b mRNA into dmrt2b morphants, suggesting that cxcl12b is the major downstream target of dmrt2b for regulating CNC cell condensation.
Cv2 displays opposing effects on BMP signaling depending on the biological context. Cv2 has been shown to potentiate BMP signaling during mouse organogenesis (Ikeya et al., 2006), and crossvein formation in the fly wing (Conley et al., 2000; O'Connor et al., 2006; Ralston and Blair, 2005), but functions as a BMP antagonist during endothelial cell differentiation (Moser et al., 2003), frog embryogenesis (Ambrosio et al., 2008; Coles et al., 2004) and human chondrogenic and osteogenic differentiation (Binnerts et al., 2004). In zebrafish, loss of cv2 via MO-mediated knockdown results in reduced BMP signaling and dorsalized phenotypes during gastrulation (Rentzsch et al., 2006). In contrast, in our experiments, co-injection of cv2 MO into dmrt2b morphants could partially recover the reduction of BMP activity in the pharyngeal arches, suggesting that Cv2 antagonizes BMP activity during lower jaw development. Indeed, the full-length zebrafish Cv2 protein acts as an inhibitor of BMP signaling and can be converted from an anti- to a pro-Bmp factor by proteolytic cleavage (Rentzsch et al., 2006). However, whether Cv2 protein can be cleaved in the pharyngeal region remains to be determined.
BMP signaling has long been recognized as an essential signal for neural crest cell specification and migration (Kanzler et al., 2000; Nie et al., 2006; Tribulo et al., 2003). During early craniofacial development, BMP signaling is required for the dorsal-ventral (DV) patterning of the pharyngeal arches (Alexander et al., 2011; Bonilla-Claudio et al., 2012; Zuniga et al., 2011). Previous studies show that, in zebrafish, the requirement of BMP activity for ventral arch development only occurs within a narrow time window from 17 to 24 hpf, a period just after CNC cell migration and before the establishment of arch primordia (Alexander et al., 2011). Not surprisingly, in dmrt2b mutants, no obvious defects of CNC cell specification, migration and DV patterning were found, as dmrt2b is expressed in the pharyngeal endoderm as early as 18 hpf and regulates cv2 expression after 24 hpf. By contrast, our studies reveal a significant role of dmtr2b in CNC cell proliferation and chondrogenic differentiation by maintaining BMP activity in pharyngeal arches via suppressing cv2 expression. This is supported by previous findings that, after arch primordia are established, inactivation of BMP signaling leads to poor proliferation and impaired differentiation of pharyngeal chondrogenic progenitors (Ning et al., 2013).
MATERIALS AND METHODS
Zebrafish lines
Wild-type (Tuebingen), Tg(sox17:GFP), Tg(fli1:EGFP), Tg(sox10:EGFP), Tg(sox10:mCherry-CAAX), Tg(BRE:EGFP), Tg(nkx2.3:mCherry) and Tg(nkx2.3:KalTA4-p2a-mCherry) zebrafish lines were maintained under standard laboratory conditions. Embryos were obtained from natural zebrafish matings, raised in Holtfreter's solution at 28.5°C, and staged by morphology as previously described (Kimmel et al., 1995). All zebrafish experiments were approved by and carried out in accordance with the Animal Care Committee at the Institute of Zoology, Chinese Academy of Sciences (Permission number: IOZ-13048).
Generation of dmrt2b mutants
The zebrafish dmrt2b mutant was generated using the CRISPR/Cas9 system. The dmrt2b gRNA was designed using ZiFiT Targeter (http://zifit.partners.org/ZiFiT/ChoiceMenu.aspx) and the targeting sequence was 5′-GGTTGCAGACTGCGCCGGAC-3′. The Cas9 mRNA and gRNA were prepared as previously described (Wei et al., 2017) and co-injected into one-cell stage wild-type embryos. For genotyping analysis, the genomic DNA was isolated and used as template for amplification of gRNA targeted sequences with the forward primer 5′-CAATCACTGCTGCATTCCGAC-3′ and the reverse primer 5′-TGTCTCCGTAGGGCGACTTGA-3′. Then the amplified fragments were identified with Sanger DNA sequencing.
Constructs
Total RNA was extracted from wild-type embryos at 36 hpf using TRIzol reagent (15596018, Invitrogen) and reverse transcribed using the Rever Tra kit (Toyobo). The resulting total cDNAs were used to amplify required segments of dmrt2b (NM_001079976.1), nkx2.3 (NM_131423.1), and cv2 (NM_001020487.2) transcripts by using the primers listed below and then cloned into the EZ-TTM vector (T168-101, GenStar).
Primers: dmrt2b, forward primer 5′-CGCTGTCAGACCCAATCATG-3′ and reverse primer 5′-CTTTACTAGCACCCTCC-3′; nkx2.3, forward primer 5′-GATTTCAGGCACCATCGTGG-3′ and reverse primer 5′-GCTGGGTTGCACTGGCACTA-3′; and cv2, forward primer 5′- AGGCAAAGACAACCGGACATCTA-3′ and reverse primer 5′- AAAGTCATTCTGTAATCCCAGTC-3′.
For rescue studies, the full length cDNAs of dmrt2b and cxcl12b were amplified by RT-PCR using the following primers and then cloned into the pCS2-Flag vector.
Primers: dmrt2b, forward primer 5′-CCGGAATTCATGTCCACTAAAGCGGATAG-3′ and reverse primer 5′-CGCGGATCCTTATCTCATGAGCAGTGCCT-3′; cxcl12b, forward primer 5′-GCCACCATGGATAGCAAAGTAGTAG-3′ and reverse primer 5′-TATCTCGAGCTCTGAGCGTTTCTTC-3′.
RNA synthesis, MOs, microinjections and whole-mount in situ hybridization
Digoxigenin-UTP-labeled RNA probes were synthesized in vitro from linearized plasmids using the MEGAscript® Kit (Ambion) according to the manufacturer's instructions. In vitro synthesis of dmrt2b mRNA was performed from linearized plasmids using the mMESSAGE mMACHINE Kit (Ambion). The standard control morpholino (cMO) (5′-CCTCTTACCTCAGTTACAATTTATA-3′), sox32 MO (5′-CAGGGAGCATCCGGTCGAGATACAT-3′) (Dickmeis et al., 2001) and cv2 MO (5′-TTACTGGAGGAGACAGACACAGCAT-3′) (Rentzsch et al., 2006) were used as previously described. The dmrt2b MO (5′-CTTTCTTACCCTGTTGATGAGAACA-3′) was designed and synthesized by Gene Tools. Microinjections and whole-mount in situ hybridization were performed as previously described (Jia et al., 2009).
RNAscope assay combined with immunofluorescence staining
RNAscope assay was conducted by using the RNAscope Flurescent Multiplex Reagent Kit [320850, Advanced Cell Diagnostics (ACD)]. Embryos were treated with Pretreat 3 buffer for 10 min at room temperature before hybridization. For the hybridization, dmrt2b RNAscope probe (510211-C3, ACD) and Amp4 Alt A-FL (320855, ACD) were used. Immediately following this, immunofluorescence staining was performed to detect proteins with GFP antibody (1/1000; A-11122, Invitrogen) and DAPI (1/3000; 10236276001, Sigma-Aldrich) as previously reported (Gross-Thebing et al., 2014; Wang et al., 2012). Finally, the embryos were washed in PBST and images were taken using a Nikon A1R+ confocal microscope.
Whole-mount immunofluorescent staining and TUNEL assays
Whole-mount immunofluorescent staining was performed as previously reported (Ning et al., 2013). Briefly, embryos were fixed with 4% phosphate-buffered paraformaldehyde and washed with 0.3% Triton X-100 and 0.1% Tween-20 in PBS for 20 min before immunostaining. The embryos were stained with the indicated antibodies, including anti-GFP (1/1000; A-11122, Invitrogen), anti-GFP (1/1000; A-11120, Invitrogen), anti-Collagen type II (Col2) (1/100; II-116B3, Developmental Studies Hybridoma Bank), anti-pH3 (1/1000; 3377, Cell Signaling Technology) and anti-p-Smad1/5/8 (1/200; 9511, Cell Signaling Technology). All immunofluorescent images were captured using a Nikon A1R+ confocal microscope with the same settings for all experiments.
TUNEL assays were performed using the In Situ Cell Death Detection Kit, TMR red (12156792910, Roche) according to the manufacturer's instructions. DAPI was used to visualize nuclei.
Alcian Blue staining
Embryos were fixed in 4% paraformaldehyde overnight at 4°C. Immediately following, fixed embryos were washed in distilled water with 0.1% Tween-20 for 8 h. The embryos were then stained with Alcian Blue staining buffer (0.015% Alcian Blue, 80% ethanol, and 20% acetic acid) overnight at room temperature and then de-stained in 70% ethanol/30% acetic acid. Next, the embryos were rehydrated through a graded series of alcohols to distilled water and then treated with 0.5% trypsin (0458, AMRESCO) in supersaturated borax at room temperature until the tissues were soft enough to dissect. The embryos were then transferred to 1% KOH for 30 min and then washed in distilled water with 0.1% Tween-20 twice for 5 min each. Finally, the embryos were dehydrated with a graded series of glycerol solutions and dissected for imaging.
Time-lapse imaging
Embryos were anaesthetized and embedded in 0.8% low-melt agarose (0815, AMRESCO) at the indicated time points for live imaging with a Nikon A1R+ confocal microscope (20× dry, 40× dry or 60× oil objectives). All confocal stack pictures were processed using Nikon NIS-Elements AR 4.13.00 software.
Semi-quantitative and quantitative RT-PCR
Semi-quantitative RT-PCR and quantitative RT-PCR were performed as previously described (Wei et al., 2017). For semi-quantitative RT-PCR analysis, dmrt2b MO interfered products were amplified using forward primer 5′-AGCCTTTGTTAGACAGATA-3′ and reverse primer 5′-ACGGGAAGAAATACGG-3′. To detect endogenous dmrt2b mRNA, forward primer 5′-AGTCGCCTTCTAGGAAACATC-3′ and reverse primer 5′-CAGTATTGGAGGAATGTCTTG-3′ were used. For quantitative analysis, cxcl12b and cv2 were amplified using SYBR® Premix Ex TaqTM dye (Takara) in Analytic Jena PCR qTOWER 2.2 system using the following primers: 5′- CTCCACCCTCAACACCG-3′ and 5′-TTTAGATACTGCTGAAGCCATT-3′ for cxcl12b; 5′-CCAAACGCCACAATCAAC-3′ and 5′-CACTTCTCCTGCTTACACTCC-3′ for cv2. In both experiments, β-actin were amplified as internal controls using primers as previously described (Wei et al., 2017).
Statistical analysis
The cell shape of CNC cells was indicated by length/width ratio and the front eight rows of CNC cells in the first and second pharyngeal arches were analyzed. ImageJ software was used to measure the distance. All experiments were performed in triplicate and unpaired t-test was employed to analyze all data sets. Results were considered statistically significant at P<0.05.
Supplementary Material
Acknowledgements
We are grateful to members of the Qiang Wang Laboratory for assistance and discussion.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: Q.W.; Validation: L.L., Q.W.; Formal analysis: Q.W.; Investigation: L.L., A.M., P.W., G.N., Y.C.; Writing - original draft: L.L.; Writing - review & editing: Q.W.; Supervision: Q.W.; Project administration: Q.W.; Funding acquisition: Q.W.
Funding
This work was supported by grants from the National Key Research and Development Program of China (2016YFA0100503), the National Natural Science Foundation of China (31571501 and 91739101) and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16000000).
Supplementary information
Supplementary information available online at http://bio.biologists.org/lookup/doi/10.1242/bio.035444.supplemental
References
- Aggarwal V. S., Carpenter C., Freyer L., Liao J., Petti M. and Morrow B. E. (2010). Mesodermal Tbx1 is required for patterning the proximal mandible in mice. Dev. Biol. 344, 669-681. 10.1016/j.ydbio.2010.05.496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander C., Zuniga E., Blitz I. L., Wada N., Le Pabic P., Javidan Y., Zhang T., Cho K. W., Crump J. G. and Schilling T. F. (2011). Combinatorial roles for BMPs and Endothelin 1 in patterning the dorsal-ventral axis of the craniofacial skeleton. Development 138, 5135-5146. 10.1242/dev.067801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio A. L., Taelman V. F., Lee H. X., Metzinger C. A., Coffinier C. and De Robertis E. M. (2008). Crossveinless-2 Is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning. Dev. Cell 15, 248-260. 10.1016/j.devcel.2008.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnerts M. E., Wen X., Cante-Barrett K., Bright J., Chen H. T., Asundi V., Sattari P., Tang T., Boyle B., Funk W. et al. (2004). Human Crossveinless-2 is a novel inhibitor of bone morphogenetic proteins. Biochem. Biophys. Res. Commun. 315, 272-280. 10.1016/j.bbrc.2004.01.048 [DOI] [PubMed] [Google Scholar]
- Boer E. F., Howell E. D., Schilling T. F., Jette C. A. and Stewart R. A. (2015). Fascin1-dependent Filopodia are required for directional migration of a subset of neural crest cells. PLoS Genet. 11, e1004946 10.1371/journal.pgen.1004946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla-Claudio M., Wang J., Bai Y., Klysik E., Selever J. and Martin J. F. (2012). Bmp signaling regulates a dose-dependent transcriptional program to control facial skeletal development. Development 139, 709-719. 10.1242/dev.073197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney T. J., Dutton K. A., Greenhill E., Delfino-Machin M., Dufourcq P., Blader P. and Kelsh R. N. (2006). A direct role for Sox10 in specification of neural crest-derived sensory neurons. Development 133, 4619-4630. 10.1242/dev.02668 [DOI] [PubMed] [Google Scholar]
- Chai Y. and Maxson R. E. Jr. (2006). Recent advances in craniofacial morphogenesis. Dev. Dyn. 235, 2353-2375. 10.1002/dvdy.20833 [DOI] [PubMed] [Google Scholar]
- Cheng J. W., Sadeghi Z., Levine A. D., Penn M. S., von Recum H. A., Caplan A. I. and Hijaz A. (2014). The role of CXCL12 and CCL7 chemokines in immune regulation, embryonic development, and tissue regeneration. Cytokine 69, 277-283. 10.1016/j.cyto.2014.06.007 [DOI] [PubMed] [Google Scholar]
- Choe C. P. and Crump J. G. (2014). Tbx1 controls the morphogenesis of pharyngeal pouch epithelia through mesodermal Wnt11r and Fgf8a. Development 141, 3583-3593. 10.1242/dev.111740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe C. P., Collazo A., Trinh le A., Pan L., Moens C. B. and Crump J. G. (2013). Wnt-dependent epithelial transitions drive pharyngeal pouch formation. Dev. Cell 24, 296-309. 10.1016/j.devcel.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W.-S. and Stainier D. Y. R. (2008). Intra-endodermal interactions are required for pancreatic beta cell induction. Dev. Cell 14, 582-593. 10.1016/j.devcel.2008.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouthier D. E. and Schilling T. F. (2004). Understanding endothelin-1 function during craniofacial development in the mouse and zebrafish. Birth Defects Res. C Embryo Today 72, 190-199. 10.1002/bdrc.20007 [DOI] [PubMed] [Google Scholar]
- Coles E., Christiansen J., Economou A., Bronner-Fraser M. and Wilkinson D. G. (2004). A vertebrate crossveinless 2 homologue modulates BMP activity and neural crest cell migration. Development 131, 5309-5317. 10.1242/dev.01419 [DOI] [PubMed] [Google Scholar]
- Conley C. A., Silburn R., Singer M. A., Ralston A., Rohwer-Nutter D., Olson D. J., Gelbart W. and Blair S. S. (2000). Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development 127, 3947-3959. [DOI] [PubMed] [Google Scholar]
- Couly G. F., Coltey P. M. and Le Douarin N. M. (1993). The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development 117, 409-429. [DOI] [PubMed] [Google Scholar]
- Crump J. G., Maves L., Lawson N. D., Weinstein B. M. and Kimmel C. B. (2004). An essential role for Fgfs in endodermal pouch formation influences later craniofacial skeletal patterning. Development 131, 5703-5716. 10.1242/dev.01444 [DOI] [PubMed] [Google Scholar]
- David N. B., Saint-Etienne L., Tsang M., Schilling T. F. and Rosa F. M. (2002). Requirement for endoderm and FGF3 in ventral head skeleton formation. Development 129, 4457-4468. [DOI] [PubMed] [Google Scholar]
- DeLuke D. M. (2014). Syndromes of the Head and Neck, 1 edn Atlas of the Oral & Maxillofacial Surgery Clinics, Elsevier. [Google Scholar]
- Dickmeis T., Mourrain P., Saint-Etienne L., Fischer N., Aanstad P., Clark M., Strahle U. and Rosa F. (2001). A crucial component of the endoderm formation pathway, CASANOVA, is encoded by a novel sox-related gene. Genes Dev. 15, 1487-1492. 10.1101/gad.196901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue P. C. J., Graham A. and Kelsh R. N. (2008). The origin and evolution of the neural crest. BioEssays 30, 530-541. 10.1002/bies.20767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdman S. E. and Burtis K. C. (1993). The Drosophila doublesex proteins share a novel zinc finger related DNA binding domain. EMBO J. 12, 527-535. 10.1002/j.1460-2075.1993.tb05684.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escot S., Blavet C., Faure E., Zaffran S., Duband J.-L. and Fournier-Thibault C. (2016). Disruption of CXCR4 signaling in pharyngeal neural crest cells causes DiGeorge syndrome-like malformations. Development 143, 582-588. 10.1242/dev.126573 [DOI] [PubMed] [Google Scholar]
- Gross-Thebing T., Paksa A. and Raz E. (2014). Simultaneous high-resolution detection of multiple transcripts combined with localization of proteins in whole-mount embryos. BMC Biol. 12, 55 10.1186/s12915-014-0055-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. K. and Miyake T. (1995). Divide, accumulate, differentiate: Cell condensation in skeletal development revisited. Int. J. Dev. Biol. 39, 881-893. [PubMed] [Google Scholar]
- Hall B. K. and Miyake T. (2000). All for one and one for all: condensations and the initiation of skeletal development. BioEssays 22, 138-147. [DOI] [PubMed] [Google Scholar]
- Hodgkin J. (2002). The remarkable ubiquity of DM domain factors as regulators of sexual phenotype: ancestry or aptitude? Genes Dev. 16, 2322-2326. 10.1101/gad.1025502 [DOI] [PubMed] [Google Scholar]
- Holzschuh J., Wada N., Wada C., Schaffer A., Javidan Y., Tallafuss A., Bally-Cuif L. and Schilling T. F. (2005). Requirements for endoderm and BMP signaling in sensory neurogenesis in zebrafish. Development 132, 3731-3742. 10.1242/dev.01936 [DOI] [PubMed] [Google Scholar]
- Hong C.-S., Park B.-Y. and Saint-Jeannet J.-P. (2007). The function of Dmrt genes in vertebrate development: it is not just about sex. Dev. Biol. 310, 1-9. 10.1016/j.ydbio.2007.07.035 [DOI] [PubMed] [Google Scholar]
- Huh S.-H. and Ornitz D.-M. (2010). Beta-catenin deficiency causes DiGeorge syndrome-like phenotypes through regulation of Tbx1. Development 137, 1137-1147. 10.1242/dev.045534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya M., Kawada M., Kiyonari H., Sasai N., Nakao K., Furuta Y. and Sasai Y. (2006). Essential pro-Bmp roles of crossveinless 2 in mouse organogenesis. Development 133, 4463-4473. 10.1242/dev.02647 [DOI] [PubMed] [Google Scholar]
- Jia S., Wu D., Xing C. and Meng A. (2009). Smad2/3 activities are required for induction and patterning of the neuroectoderm in zebrafish. Dev. Biol. 333, 273-284. 10.1016/j.ydbio.2009.06.037 [DOI] [PubMed] [Google Scholar]
- Johnsen H. and Andersen Ø. (2012). Sex dimorphic expression of five dmrt genes identified in the Atlantic cod genome. The fish-specific dmrt2b diverged from dmrt2a before the fish whole-genome duplication. Gene 505, 221-232. 10.1016/j.gene.2012.06.021 [DOI] [PubMed] [Google Scholar]
- Johnson D. and Wilkie A. O. M. (2011). Craniosynostosis. Eur. J. Hum. Genet. 19, 369-376. 10.1038/ejhg.2010.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzler B., Foreman R. K., Labosky P. A. and Mallo M. (2000). BMP signaling is essential for development of skeletogenic and neurogenic cranial neural crest. Development 127, 1095-1104. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Agathon A., Alexander J., Thisse C., Waldron S., Yelon D., Thisse B. and Stainier D. Y. (2001). casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 15, 1493-1505. 10.1101/gad.892301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B. and Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253-310. 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- Köntges G. and Lumsden A. (1996). Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development 122, 3229-3242. [DOI] [PubMed] [Google Scholar]
- Kopinke D., Sasine J., Swift J., Stephens W. Z. and Piotrowski T. (2006). Retinoic acid is required for endodermal pouch morphogenesis and not for pharyngeal endoderm specification. Dev. Dyn. 235, 2695-2709. 10.1002/dvdy.20905 [DOI] [PubMed] [Google Scholar]
- Laux D. W., Febbo J. A. and Roman B. L. (2011). Dynamic analysis of BMP-responsive smad activity in live zebrafish embryos. Dev. Dyn. 240, 682-694. 10.1002/dvdy.22558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson N. D. and Weinstein B. M. (2002). In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 248, 307-318. 10.1006/dbio.2002.0711 [DOI] [PubMed] [Google Scholar]
- Li Z., Zhang Y., Han L., Shi L. and Lin X. (2013). Trachea-derived dpp controls adult midgut homeostasis in Drosophila. Dev. Cell 24, 133-143. 10.1016/j.devcel.2012.12.010 [DOI] [PubMed] [Google Scholar]
- Lints R. and Emmons S. W. (2002). Regulation of sex-specific differentiation and mating behavior in C. elegans by a new member of the DM domain transcription factor family. Genes Dev. 16, 2390-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisa M. and Elden K. B. Z. (2014). Congenital Malformations of the Head and Neck. Springer Science+Business Media. [Google Scholar]
- Lourenço R., Lopes S. S. and Saúde L. (2010). Left-right function of dmrt2 genes is not conserved between zebrafish and mouse. PLoS ONE 5, e14438 10.1371/journal.pone.0014438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C., Wu J., Xiong S., Zhang X., Zhang J. and Mei J. (2017). MicroRNA-203a regulates fast muscle differentiation by targeting dmrt2a in zebrafish embryos. Gene 625, 49-54. 10.1016/j.gene.2017.05.012 [DOI] [PubMed] [Google Scholar]
- Lumsden A., Sprawson N. and Graham A. (1991). Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development 113, 1281-1291. [DOI] [PubMed] [Google Scholar]
- Matsui T., Sasaki A., Akazawa N., Otani H. and Bessho Y. (2012). Celf1 regulation of dmrt2a is required for somite symmetry and left-right patterning during zebrafish development. Development 139, 3553-3560. 10.1242/dev.077263 [DOI] [PubMed] [Google Scholar]
- Meng A., Moore B., Tang H., Yuan B. and Lin S. (1999). A Drosophila doublesex-related gene, terra, is involved in somitogenesis in vertebrates. Development 126, 1259-1268. [DOI] [PubMed] [Google Scholar]
- Moser M., Binder O., Wu Y., Aitsebaomo J., Ren R., Bode C., Bautch V. L., Conlon F. L. and Patterson C. (2003). BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol. Cell. Biol. 23, 5664-5679. 10.1128/MCB.23.16.5664-5679.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X. G., Luukko K. and Kettunen P. (2006). BMP signalling in craniofacial development. Int. J. Dev. Biol. 50, 511-521. [DOI] [PubMed] [Google Scholar]
- Ning G., Liu X., Dai M., Meng A. and Wang Q. (2013). MicroRNA-92a upholds Bmp signaling by targeting noggin3 during pharyngeal cartilage formation. Dev. Cell 24, 283-295. 10.1016/j.devcel.2012.12.016 [DOI] [PubMed] [Google Scholar]
- Nissen R. M., Yan J., Amsterdam A., Hopkins N. and Burgess S. M. (2003). Zebrafish foxi one modulates cellular responses to Fgf signaling required for the integrity of ear and jaw patterning. Development 130, 2543-2554. 10.1242/dev.00455 [DOI] [PubMed] [Google Scholar]
- Noden D. M. (1988). Interactions and fates of avian craniofacial mesenchyme. Development 103 Suppl, 121-140. [DOI] [PubMed] [Google Scholar]
- O'Connor M. B., Umulis D., Othmer H. G. and Blair S. S. (2006). Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development 133, 183-193. 10.1242/dev.02214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober E. A., Verkade H., Field H. A. and Stainier D. Y. R. (2006). Mesodermal Wnt2b signalling positively regulates liver specification. Nature 442, 688-691. 10.1038/nature04888 [DOI] [PubMed] [Google Scholar]
- Okada K., Inohaya K., Mise T., Kudo A., Takada S. and Wada H. (2016). Reiterative expression of pax1 directs pharyngeal pouch segmentation in medaka. Development 143, 1800-1810. 10.1242/dev.130039 [DOI] [PubMed] [Google Scholar]
- Okubo T., Kawamura A., Takahashi J., Yagi H., Morishima M., Matsuoka R. and Takada S. (2011). Ripply3, a Tbx1 repressor, is required for development of the pharyngeal apparatus and its derivatives in mice. Development 138, 339-348. 10.1242/dev.054056 [DOI] [PubMed] [Google Scholar]
- Olesnicky Killian E. C., Birkholz D. A. and Artinger K. B. (2009). A role for chemokine signaling in neural crest cell migration and craniofacial development. Dev. Biol. 333, 161-172. 10.1016/j.ydbio.2009.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski T. and Nüsslein-Volhard C. (2000). The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio). Dev. Biol. 225, 339-356. 10.1006/dbio.2000.9842 [DOI] [PubMed] [Google Scholar]
- Piotrowski T., Ahn D. G., Schilling T. F., Nair S., Ruvinsky I., Geisler R., Rauch G. J., Haffter P., Zon L. I., Zhou Y. et al. (2003). The zebrafish van gogh mutation disrupts tbx1, which is involved in the DiGeorge deletion syndrome in humans. Development 130, 5043-5052. 10.1242/dev.00704 [DOI] [PubMed] [Google Scholar]
- Ralston A. and Blair S. S. (2005). Long-range Dpp signaling is regulated to restrict BMP signaling to a crossvein competent zone. Dev. Biol. 280, 187-200. 10.1016/j.ydbio.2005.01.018 [DOI] [PubMed] [Google Scholar]
- Raymond C. S., Shamu C. E., Shen M. M., Seifert K. J., Hirsch B., Hodgkin J. and Zarkower D. (1998). Evidence for evolutionary conservation of sex-determining genes. Nature 391, 691-695. 10.1038/35618 [DOI] [PubMed] [Google Scholar]
- Reiter J. F., Alexander J., Rodaway A., Yelon D., Patient R., Holder N. and Stainier D. Y. (1999). Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 13, 2983-2995. 10.1101/gad.13.22.2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzsch F., Zhang J., Kramer C., Sebald W. and Hammerschmidt M. (2006). Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation. Development 133, 801-811. 10.1242/dev.02250 [DOI] [PubMed] [Google Scholar]
- Retting K. N., Song B., Yoon B. S. and Lyons K. M. (2009). BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development 136, 1093-1104. 10.1242/dev.029926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A., Kontarakis Z., Gerri C., Nolte H., Hölper S., Krüger M. and Stainier D. Y. (2015). Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 524, 230-233. 10.1038/nature14580 [DOI] [PubMed] [Google Scholar]
- Sato T., Rocancourt D., Marques L., Thorsteinsdóttir S. and Buckingham M. (2010). A Pax3/Dmrt2/Myf5 regulatory cascade functions at the onset of myogenesis. PLoS Genet. 6, e1000897 10.1371/journal.pgen.1000897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saúde L., Lourenço R., Gonçalves A. and Palmeirim I. (2005). terra is a left-right asymmetry gene required for left-right synchronization of the segmentation clock. Nat. Cell Biol. 7, 918-920. 10.1038/ncb1294 [DOI] [PubMed] [Google Scholar]
- Schilling T. F. and Kimmel C. B. (1994). Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development 120, 483-494. [DOI] [PubMed] [Google Scholar]
- Schutte B. C. and Murray J. C. (1999). The many faces and factors of orofacial clefts. Hum. Mol. Genet. 8, 1853-1859. 10.1093/hmg/8.10.1853 [DOI] [PubMed] [Google Scholar]
- Seo K. W., Wang Y., Kokubo H., Kettlewell J. R., Zarkower D. A. and Johnson R. L. (2006). Targeted disruption of the DM domain containing transcription factor Dmrt2 reveals an essential role in somite patterning. Dev. Biol. 290, 200-210. 10.1016/j.ydbio.2005.11.027 [DOI] [PubMed] [Google Scholar]
- Stegmeier F., Hu G., Rickles R. J., Hannon G. J. and Elledge S. J. (2005). A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. P Natl. Acad. Sci. USA 102, 13212-13217. 10.1073/pnas.0506306102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher B. A. and Fricker S. P. (2010). CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res. 16, 2927-2931. 10.1158/1078-0432.CCR-09-2329 [DOI] [PubMed] [Google Scholar]
- Tribulo C., Aybar M. J., Nguyen V. H., Mullins M. C. and Mayor R. (2003). Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development 130, 6441-6452. 10.1242/dev.00878 [DOI] [PubMed] [Google Scholar]
- Vandenberg P., Khillan J. S., Prockop D. J., Helminen H., Kontusaari S. and Ala-Kokko L. (1991). Expression of a partially deleted gene of human type II procollagen (COL2A1) in transgenic mice produces a chondrodysplasia. Proc. Natl. Acad. Sci. USA 88, 7640-7644. 10.1073/pnas.88.17.7640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veitch E., Begbie J., Schilling T. F., Smith M. M. and Graham A. (1999). Pharyngeal arch patterning in the absence of neural crest. Curr. Biol. 9, 1481-1484. 10.1016/S0960-9822(00)80118-9 [DOI] [PubMed] [Google Scholar]
- Volff J.-N., Zarkower D., Bardwell V. J. and Schartl M. (2003). Evolutionary dynamics of the DM domain gene family in metazoans. J. Mol. Evol. 57 Suppl. 1, S241-S249. 10.1007/s00239-003-0033-0 [DOI] [PubMed] [Google Scholar]
- Wang F., Flanagan J., Su N., Wang L.-C., Bui S., Nielson A., Wu X., Vo H.-T., Ma X.-J. and Luo Y. (2012). RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn. 14, 22-29. 10.1016/j.jmoldx.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Dai M., Liu Z., Ma Y., Shang H., Cao Y. and Wang Q. (2017). The guanine nucleotide exchange factor Net1 facilitates the specification of dorsal cell fates in zebrafish embryos by promoting maternal beta-catenin activation. Cell Res. 27, 202-225. 10.1038/cr.2016.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y. L., Miller C. T., Nissen R. M., Singer A., Liu D., Kirn A., Draper B., Willoughby J., Morcos P. A., Amsterdam A. et al. (2002). A zebrafish sox9 gene required for cartilage morphogenesis. Development 129, 5065-5079. [DOI] [PubMed] [Google Scholar]
- Yelick P. C. and Schilling T. F. (2002). Molecular dissection of craniofacial development using zebrafish. Crit. Rev. Oral Biol. Med. 13, 308-322. 10.1177/154411130201300402 [DOI] [PubMed] [Google Scholar]
- Yoon B. S., Ovchinnikov D. A., Yoshii I., Mishina Y., Behringer R. R. and Lyons K. M. (2005). Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc. Natl. Acad. Sci. USA 102, 5062-5067. 10.1073/pnas.0500031102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Cai X. Z. and Cullen B. R. (2005). Use of RNA polymerase II to transcribe artificial microRNAs. Method Enzymol 392, 371-380. 10.1016/S0076-6879(04)92022-8 [DOI] [PubMed] [Google Scholar]
- Zhou X., Li Q., Lu H., Chen H., Guo Y., Cheng H. and Zhou R. (2008). Fish specific duplication of Dmrt2: characterization of zebrafish Dmrt2b. Biochimie 90, 878-887. 10.1016/j.biochi.2008.02.021 [DOI] [PubMed] [Google Scholar]
- Zorn A. M. and Wells J. M. (2009). Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 25, 221-251. 10.1146/annurev.cellbio.042308.113344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga E., Rippen M., Alexander C., Schilling T. F. and Crump J. G. (2011). Gremlin 2 regulates distinct roles of BMP and Endothelin 1 signaling in dorsoventral patterning of the facial skeleton. Development 138, 5147-5156. 10.1242/dev.067785 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.