Abstract
Background
Cardiac autonomic neuropathy (CAN) is a commonly overlooked complication of Type 2 Diabetes Mellitus (T2DM) characterized by imbalance between sympathetic and parasympathetic supply to the heart. The susceptibility of heart to dysrhythmias and fatal events increases during and after exercise due to a shift in autonomic regulation. Diabetes and hypertension (HTN) frequently occur concurrently and both conditions lead to impaired cardiac autonomic control. However, their impact together on post-exercise autonomic recovery remains to be explored.
Objective
The objective of the study was to investigate the effect of co-existence of HTN on cardiac autonomic recovery (assessed by heart rate recovery and heart rate variability) in patients with T2DM.
Methods
Forty eight type 2 diabetic patients (24 normotensive, 24 hypertensive), 24 non-diabetic patients with essential HTN, and 27 healthy controls, were recruited into the study and assessed for heart rate recovery (HRR) following a graded maximal test. Also, heart rate variability (HRV) was recorded before and following the bout of maximal exercise.
Results
Heart rate recovery at 1 (HRR1min) and 2 (HRR2min) minute(s) showed significant effects for DM (p < 0.001) and HTN (p < 0.001), while DM × HTN interaction was found to be non-significant. Resting HRV showed a significant decline in time-domain variables for the DM group (p < 0.01). Recovery of HRV showed a significant effect of time (p < 0.05) for all indices, the group effect was found significant only for time-domain measures (p < 0.05).
Conclusion
Both HRR and HRV recovery were impaired in DM and HTN. Moreover, the co-existence of HTN had a synergistic effect, causing further worsening of autonomic recovery in T2DM.
Keywords: Heart rate recovery, Heart rate variability, Autonomic function, Cardiac autonomic dysfunction
1. Introduction
Type 2 diabetes mellitus (T2DM) is a growing epidemic associated with a spectrum of complications, making it a multi-faceted metabolic disorder with 2- to 4-fold higher incidence of cardiovascular disease (CVD) 1 Hypertension (HTN) is one such co-morbidity, that is twice as frequent among patients with type 2 diabetes, compared with non-diabetic population. 2 Its prevalence is around 40% among patients with newly diagnosed type 2 diabetes 3 but can be as high as 49–92% in some parts of the world. 4,5 The coexistence of DM and HTN is mainly the consequence of metabolic syndrome, abdominal obesity, diabetic nephropathy, and advanced atherosclerosis.5 About 60%–80% people with type 2 diabetes die of cardiovascular complications, and up to 75% of specific cardiovascular complications have been attributed to high blood pressure (BP).2,6 Moreover, hypertension is also a primary contributing factor to kidney failure and eye ailments in people with diabetes. 7,8
A commonly overlooked complication of T2DM is cardiac autonomic neuropathy (CAN), characterized by imbalance between sympathetic and parasympathetic supply to heart. CAN is found to be associated with severe cardiovascular events such as ventricular tachyarrhythmia and sudden cardiac deaths. It has been reported to occur in 42% of patients with T2DM, with a higher incidence (54–60%) reported in the Indian population 9,10 In addition, it has been found that hypertension independently causes cardiac autonomic dysfunction. Hazari et al 11 examined cardiac autonomic control of diabetics with and without HTN, and found that the presence of HTN in T2DM patients synergistically causes progression of CAN. Pikkujamsa et al 12 proposed that essential hypertension acts synergistically with type 2 diabetes to depress cardiac reflex vagal and sympathetic function, and that insulin resistance may play a pathogenic role in these processes. Liao et al 13 also reported that HTN clustered with diabetes synergistically influenced cardiac autonomic control. More recently, Istenes et al 14 examining the effect of co-existence of diabetes and HTN on autonomic function, compared heart rate variability and ambulatory blood pressure monitoring (ABPM) parameters across the groups, found a similar additive effect. However, all these previous investigations have explored resting autonomic function but the impact of DM and HTN on autonomic modulation following exercise is not known.
Exercise has profound and complex effects on autonomic heart rate modulation 15 Acute physical exertion causes physiological stress affecting the cardiac autonomic modulation. 16 During transition from rest to exercise and the recovery period following exercise, the heart has been suggested to be more vulnerable to dysrhythmias and fatal events, owing to a shift in the autonomic balance from parasympathetic to sympathetic dominance. 17,18 An increased risk of death during and following exercise has been attributed to both the magnitude and subsequent restoration of normal autonomic balance. 19 Thus, exploration of the behavior of autonomic recovery following exercise is warranted. As proposed by previous investigations, 16,20 the spectral analysis of HRV can be used to delineate the autonomic disturbance after exercise cessation and during recovery. Therefore, the present study aimed to investigate the effect of co-existence of hypertension on cardiac autonomic recovery in type 2 diabetics. We hypothesized that autonomic recovery after the maximum exercise bout is compromised, and co-existence of hypertension causes further impairment of autonomic recovery in T2DM patients.
2. Methods
2.1. Study design and setting
A four-arm, cross-sectional, observational study design with comparison of variables across the groups was employed for the present study. The type 2 diabetic and hypertensive patients were recruited from the outpatient department of Ansari Health Centre, Jamia Millia Islamia, New Delhi, India, and age-matched healthy controls were recruited from the community. Assessments for heart rate recovery and variability were performed in the Human Performance Laboratory, Centre for Physiotherapy and Rehabilitation Sciences, Jamia Millia Islamia.
2.2. Participants
We recruited 48 type 2 diabetic patients (24 normotensive, 24 hypertensive), 24 non-diabetic patients with essential HTN, and 27 healthy controls into the study.
Sedentary participants (normotensive diabetics, hypertensive diabetics, hypertensives, and healthy controls) who did not engage in exercise for more than 20 min on 3 or more days a week, between 30–70 years of age, were recruited. Patients in the diabetic group were diagnosed in accordance with American Diabetes Association criteria (HbA1c level 6.5–10%) with type 2 diabetes for a minimum of 12 months and were receiving oral anti-diabetic medication. The diagnosis of HTN was made when the average of 2 or more diastolic BP measurements on at least 2 subsequent visits is ≥90 mmHg or when the average of multiple systolic BP readings on 2 or more subsequent visits is consistently ≥140 mmHg 21 or the use of an anti-hypertensive medication (stage 1 and 2 hypertensives).
Exclusion criteria included participants with BMI > 40 kg/m2, hypertensive crisis (systolic blood pressure, SBP > 180 mmHg; diastolic blood pressure, DBP > 120 mmHg), the use of exogenous insulin, any drugs that can influence heart rate kinetics, such as, beta-blockers and non-dihydropyridine type calcium channel blockers, recent onset of cardiac-origin symptoms, and orthopedic problems limiting physical activity.
2.3. Procedures
The present study was approved by the Institutional Ethical Committee, JMI and was conducted in accordance with the Helsinki Declaration. Participants were recruited based upon the inclusion and exclusion criteria, and informed about the study purpose and methodology. They were given an informed consent form explaining their rights as research subjects and a written consent was obtained. All participants were asked to refrain from the consumption of caffeine, alcohol and tobacco products at least 12 h before testing. In addition, they were also asked to abstain from any kind of exercise 48 h prior to testing. All four groups were assessed for resting heart rate variability, following which, they performed the graded maximal exercise testing on a treadmill and their peak heart rate was recorded. HR response during exercise was evaluated by the chronotropic reserve (CR), as follows: (chronotropic reserve = (peak HR-resting HR/220-age-resting HR) × 100) 22 and chronotropic incompetence (CI) was determined when subjects failed to achieve ≥ 80% of the chronotropic reserve. 22 Immediately after the treadmill test, HRR was recorded for 2 min and post-exercise HRV was recorded for 20 min.
2.3.1. Heart rate recovery (HRR)
Heart rate recovery is a measure of the rate of decline of heart rate during the first few minutes after peak exercise 23 Heart rate was derived from a continuous record obtained via electrocardiography (ECG) with surface electrodes placed in a Lead II arrangement. Lead site preparation and placement were standardized according to American Heart Association standards. 24 HRR was calculated as the absolute difference between heart rate at peak exercise and heart rate recorded at 1- (HRR1min) and 2- minutes (HRR2min) post-exercise.
2.3.2. Heart rate variability (HRV)
Electrocardiographic (ECG) signals were recorded for 20 min in supine position before graded exercise test. Participants were instructed to close their eyes, and avoid any bodily movement or conversation during recording. Since breathing rate has been found to confound the HRV results, breathing pace was controlled at 12 breaths/min with a metronome. Immediately following the maximal exercise using Balke protocol, participants were allowed a 2-minute transition from the treadmill, before comfortably assuming the supine position again. The post-exercise ECG was recorded for 20-minutes. After recording, all the data were stored and analyzed offline. Analysis of HRV was done on a time series of the last five-minutes selected from the 20-min recording 25 both at rest and post-exercise. Data were visually and automatically inspected for ectopic beats (premature, supraventricular, and ventricular) which had to be <10% for each record to be included in the analysis. Time and frequency domain variables of HRV were analyzed by the detection of R waves. AD instruments lab chart version 7.3.7 with HRV module version 1.4.2 using weltch window (Power Lab 8 SP, AD Instruments, Australia) was used as data acquisition software for recording ECG, which calculates the R-R intervals as the measure of difference between successive beats. All data acquisition and post-acquisition analyses was performed in accordance with the guidelines proposed by Task Force of the European Society of Cardiology and North American Society of Pacing and Electrophysiology 26. Time-domain variables of HRV included mean of normalized R-R intervals (AvgNN), standard deviation of N-N intervals (SDNN), square root of the mean squared differences between adjacent R-R intervals (RMSSD) and percentage of interval differences of adjacent R-R intervals greater than 50 ms (pNN50). 26,27 Frequency-domain indices of HRV included total power, Low Frequency (LF: 0.04-0.15 Hz), High Frequency (HF: 0.15-0.40 Hz) and LF/HF ratio. Non-linear analysis included the Poincare parameters: SD1 and SD2. 28 Combination of all these variables of HRV comprehensively provides information regarding the relative contributions from sympathetic and parasympathetic branches of ANS to the heart. 29
2.4. Sample size
The number of subjects (n = 99) was determined using Software G. Power 3.192 using the difference in heart rate variability (LF power) between 4 similar groups, from a study done by Istenes et al 14 and a total of 96 subjects (24 per group) were shown to be necessary based on the effect size of 0.348, alpha level of 0.05 and power (1-beta) of 0.80.
2.5. Statistical analyses
The normality of distribution was examined for all variables using Kolmogorov-Smirnov test, and those found to be non-normal (RMSSD, pNN50, Total power, LFms 2 HFms 2 and SD2) were log transformed for further analyses. Subject demographic characteristics were compared between the groups using One-way ANOVA and those found to be different (BMI) were adjusted. To determine the effect of Diabetes (DM), Hypertension (HTN), and their interaction (DMHTN), univariate analysis was employed with DM and HTN as fixed factors. The HRV recovery was compared between the groups using a 4 × 2 split plot ANCOVA, assessing the effect of group (Healthy, DM, HTN, DMHTN) and time (resting, post-exercise), with the resting values of HRV treated as co-variates. Significance level was set at p < 0.05.
3. Results
The comparison of demographic characteristics using one-way ANOVA revealed no significant differences among the groups except for BMI (p = 0.035). Post-hoc analysis showed a significantly higher BMI in DMHTN than DM group. For this reason, BMI was considered as a covariate in all further analysis. In addition to expected differences in resting BP and HbA1c between the groups, a significant difference was observed for the peak exercise heart rate achieved (Ex HR) (p < 0.001) and the heart rate at 1 min following exercise (HR1min) (p = 0.027) (Table 1). Post-hoc analysis for Ex HR and HR1min revealed significantly lower values in DMHTN as compared to the other groups.
Table 1.
Comparison of the demographic, clinical characteristics and heart rate during exercise test between groups.
| Healthy n = 27 Mean (SD) |
DM n = 24 Mean (SD) |
HTN n = 24 Mean (SD) |
DMHTN n = 24 Mean (SD) |
p-value | |
|---|---|---|---|---|---|
| Age (yrs) | 50.4 (4.1) | 51.2 (5.9) | 49 (3.6) | 48.5 (3.6) | 0.133 |
| Height (cm) | 160.7 (7.8) | 158.6 (5.8) | 161.8 (7.4) | 157.9 (8.6) | 0.237 |
| Weight (kg) | 77.7 (8.7) | 74.2 (8.7) | 72.6 (8.6) | 73.5 (10.1) | 0.19 |
| BMI (kg/m2) | 29.4 (3.1) | 27.8 (2.4) | 29.5 (3.3) | 30.1 (2.5) | 0.035* |
| Sex (M/F) | 15/12 | 11/13 | 9/15 | 10/14 | 0.605 |
| DM duration (yrs) | – | 6.9 (3.4) | – | 7.2 (3.3) | 0.8 |
| HTN duration (yrs) | – | – | 7.1 (3.2) | 8.1 (3.4) | 0.3 |
| SBP (mmHg) | 121.7 (6.4) | 125.3 (8.6) | 144.6 (8.9) | 146.3 (9.7) | <0.001* |
| DBP (mmHg) | 81.3 (4.3) | 79.9 (5.1) | 84.9 (5.4) | 85.2 (5.6) | 0.001* |
| HbA1c (%) | 5.9 (0.2) | 8.2 (1.2) | 6.0 (0.2) | 8.6 (1.4) | <0.001* |
| Ex HR (bpm) | 157.4 (9.3) | 151.5 (6.6) | 150.9 (8.3) | 141.3 (6.3) | <0.001* |
| HR1min (bpm) | 119.8 (9.2) | 120.1 (8.6) | 120.7 (8.5) | 114 (8.0) | 0.027* |
| HR2min (bpm) | 97 (7.4) | 100.8 (7.5) | 100.7 (6.8) | 98.5 (6.2) | 0.181 |
| CR | 88.27 (11.4) | 77.9 (7.4) | 79.8 (10.7) | 66.7 (9.6) | <0.001* |
| CI n (%) | 7 (25.9) | 18 (75) | 13 (54.2) | 21 (87.5) | <0.001* |
| Comorbidities | |||||
| Dyslipidemia | – | 14 | 11 | 10 | – |
| Hypothyroidism | – | 4 | 3 | 5 | – |
| PCOD | – | 4 | – | 3 | – |
| Medications | |||||
| Metformin | – | 11 | – | 9 | – |
| Sulfonylureas | – | 7 | – | 8 | – |
| TZDs | – | 1 | – | 2 | – |
| DPP-4 Inhibitors | – | 3 | – | 4 | – |
| AGIs | – | 1 | – | 3 | – |
| ACE inhibitors | – | – | 14 | 16 | – |
| ARBs | – | – | 11 | 13 | – |
| Statins | – | 8 | 6 | 3 | – |
BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; HbA1c: Glycosylated hemoglobin; Ex HR: Peak exercise heart; HR1min: Heart rate at the end of first minute following maximal exercise; HR2min: Heart rate recovery at the end of 2 min following maximal exercise; CR: Chronotropic Reserve; CI: Chronotropic Incompetence; PCOD: Polycystic Ovarian Disease; TZDs: Thiazolidinediones; DPP-4: Dipeptidyl peptidase-4; AGIs: Alpha-Glucosidase Inhibitors; ACE: Angiotensin-Converting Enzyme; ARBs: Angiotensin-II Receptor Blockers.
significant difference.
The HR response to exercise as determined by CR and CI were found to be significantly different between the groups (Table 1). Post hoc analysis of CR found significant reductions in DM (p = 0.002), HTN (p = 0.002) and DMHTN (p < 0.001) as compared to the healthy controls. Further, CR in DMHTN was also lower in comparison to both DM (p = 0.001) and HTN (p = 0.001), with CI being recorded in 87% of the DMHTN patients (Table 1).
3.1. Heart rate recovery
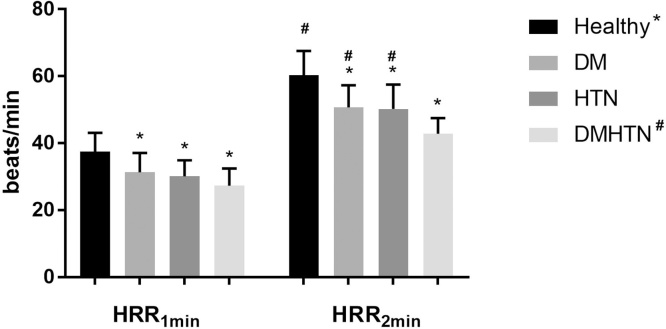
Heart rate recovery at 1(HRR1min) and 2 (HRR2min) minute(s) showed significant effects for DM (p < 0.001) and HTN (p < 0.001), while DM × HTN interaction was found to be non-significant (Table 2). Post hoc analysis revealed that HRR2min in the DMHTN group was significantly lower than the other groups (p < 0.05) (Fig. 1).
Table 2.
Effect of DM and HTN on Heart Rate Recovery.
| Healthy n = 27 Mean(SD) |
DM n = 24 Mean(SD) |
HTN n = 24 Mean(SD) |
DMHTN n = 24 Mean(SD) |
DM main effect |
HTN main effect |
DM × HTN interaction |
|
|---|---|---|---|---|---|---|---|
| HRR1min | 37.5 (5.6) | 31.3 (5.8) | 30.1 (4.8) | 27.3 (5.1) | <0.001* | <0.001* | 0.155 |
| HRR2min | 60.3 (7.3) | 50.7 (6.6) | 50.2 (7.3) | 42.8 (4.7) | <0.001* | <0.001* | 0.487 |
HRR1min: Heart rate recovery in the first minute following maximal exercise; HRR2min: Heart rate recovery in the first 2 min following maximal exercise.
significant difference.
Fig. 1.
HRR at 1- and 2-minutes for Healthy controls vs. patients with Type 2 diabetes Mellitus (DM), Hypertension (HTN) and both (DMHTN).
Note: * denotes significant difference from the healthy controls;
# denotes statistically significant difference when compared with DMHTN group.
3.2. Resting heart rate variability
The results of resting HRV analysis showed a significant effect of DM on all HRV indices (p < 0.05). Hypertension seemed to impact AvgNN (p = 0.045), AvgHR (p = 0.041), SD2 (p = 0.001), and the frequency-domains measures (Total power, LF, HF). The interaction effect was found to be significant for the frequency-domain indices (p < 0.05) (Table 3).
Table 3.
Effect of DM and HTN on Resting Heart Rate Variability.
| Healthy n = 27 Mean (SD) |
DM n = 24 Mean (SD) |
HTN n = 24 Mean (SD) |
DMHTN n = 24 Mean (SD) |
DM main effect |
HTN main effect |
DM × HTN interac- -tion |
|
|---|---|---|---|---|---|---|---|
| AvgNN (ms) | 834.4 (82.4) |
750.6 (94.9) |
772.8 (93.4) | 727.7 (92.3) | 0.001* | 0.045* | 0.207 |
| SDNN (ms) | 45.1 (17.6) |
21.1 (10.9) |
36.1 (16.4) |
27.9 (11.6) |
<0.001* | 0.574 | 0.017* |
| AvgHR (bpm) | 73 (6.8) |
81 (10.4) |
79 (9.2) |
84 (10.9) |
0.001* | 0.041* | 0.291 |
| RMSSD (ms) | 37.4 (15.6) |
19.9 (11.9) |
37.9 (28.1) |
22.0 (15.1) |
<0.001* | 0.896 | 0.984 |
| pNN50 (%) | 16.1 (15.6) |
3.7 (8.5) |
15.9 (18.8) |
2.2 (5.7) |
<0.001* | 0.610 | 0.642 |
| Total power (ms2) | 2377.2 (1938.5) | 1008.9 (1410.2) | 1041.8 (807.6) | 836.9 (781.9) | 0.005* | 0.007* | 0.043* |
| LF (ms2) | 647.3 (473.8) | 207.6 (302.0) | 197.8 (248.1) | 237.6 (230.6) | 0.004* | 0.003* | 0.001* |
| HF (ms2) | 655.5 (592) |
400.3 (559.9) | 522.9 (520.9) | 207.9 (353.2) | 0.008* | 0.025* | 0.759 |
| LF: HF | 1.15 (0.63) |
0.85 (0.84) |
0.75 (0.86) |
1.7 (1.5) |
0.095 | 0.219 | 0.002* |
| LFnu | 48.5 (14.4) |
36.7 (20.4) |
31.9 (23.4) |
55.6 (16.7) |
0.133 | 0.697 | <0.001* |
| HFnu | 49.7 (14.8) |
59.3 (19.2) |
64.7 (21.6) |
42.4 (14.9) |
0.088 | 0.694 | <0.001* |
| SD1 | 26.5 (12.1) |
18.4 (14) |
26.5 (20.6) |
14.8 (7.6) |
0.001** | 0.458 | 0.442 |
| SD2 | 32.1 (18.6) |
40.3 (18.4) |
36.7 (12.9) |
60.5 (22.9) |
<0.001* | 0.001* | 0.221 |
AvgNN: Mean of N-N intervals; SDNN: Standard deviation of N-N intervals; AvgHR: Mean heart rate; RMSSD: Square root of the mean squared differences between adjacent RR intervals; pNN50: Percentage of interval differences of adjacent RR intervals greater than 50 ms derived from differences between consecutive RR intervals; LF nu: Low frequency power normalize units; HF nu: High frequency power normalize units LF/HF ratio: Ratio of low and high frequency power.
Significant difference, p < 0.05.
3.3. Post-Exercise heart rate variability
All HRV indices demonstrated a significant effect of time (p < 0.05). The group effect was found to be significant only for the time-domain indices of HRV (p < 0.05) and signified an altered recovery in the presence of DM or HTN or both (Table 4),(Fig. 2).
Table 4.
Summary of Split-plot ANCOVA.
| Variable | Source | df | F | p-value | Partial eta squared |
|---|---|---|---|---|---|
| AvgNN (ms) | Time | 1 | 65.82 | <0.001* | 0.412 |
| Group | 3 | 5.046 | 0.003* | 0.139 | |
| SDNN (ms) | Time | 1 | 7.419 | 0.008* | 0.073 |
| Group | 3 | 3.398 | 0.021* | 0.098 | |
| AvgHR (bpm) | Time | 1 | 34.882 | <0.001* | 0.271 |
| Group | 3 | 3.667 | 0.015* | 0.105 | |
| lnRMSSD | Time | 1 | 36.46 | <0.001* | 0.279 |
| Group | 3 | 6.509 | <0.001* | 0.172 | |
| lnpNN50 | Time | 1 | 93.65 | <0.001* | 0.639 |
| Group | 3 | 2.054 | 0.117 | 0.104 | |
| lnTotal power | Time | 1 | 51.052 | <0.001* | 0.352 |
| Group | 3 | 1.992 | 0.121 | 0.06 | |
| lnLF | Time | 1 | 76.583 | <0.001* | 0.449 |
| Group | 3 | 3.389 | 0.021* | 0.098 | |
| lnHF | Time | 1 | 28.79 | <0.001* | 0.234 |
| Group | 3 | 1.936 | 0.129 | 0.058 | |
| lnLF: HF | Time | 1 | 70.962 | <0.001* | 0.43 |
| Group | 3 | 0.477 | 0.699 | 0.015 | |
| LFnu | Time | 1 | 64.213 | <0.001* | 0.406 |
| Group | 3 | 0.905 | 0.442 | 0.028 | |
| HFnu | Time | 1 | 97.926 | <0.001* | 0.510 |
| Group | 3 | 0.696 | 0.557 | 0.022 | |
| SD1 | Time | 1 | 5.379 | 0.023* | 0.054 |
| Group | 3 | 1.660 | 0.181 | 0.05 | |
| SD2 | Time | 1 | 4.572 | 0.035* | 0.046 |
| Group | 3 | 1.198 | 0.315 | 0.037 |
AvgNN: Mean of N-N intervals; SDNN: Standard deviation of N-N intervals; AvgHR: Mean heart rate; RMSSD: Square root of the mean squared differences between adjacent RR intervals; pNN50: Percentage of interval differences of adjacent RR intervals greater than 50 ms derived from differences between consecutive RR intervals; LF nu: Low frequency power normalize units; HF nu: High frequency power normalize units LF/HF ratio: Ratio of low and high frequency power; ln: natural log-transformed.
Significant difference, p < 0.05.
Fig. 2.
Comparison of HRV recovery between the groups.
Time domain variables: 1(a) Average Heart Rate (bpm); 1(b) Average NN (ms); 1(c) pNN50 (%), and Frequency domain variables: 1(d) Low-frequency power in normalized units (LFnu); 1(e) High-frequency power in normalized units (HFnu); 1(f) Ratio of low frequency to high frequency power (LF: HF)
4. Discussion
4.1. Purpose and Main findings
The present study was the first to examine cardiac autonomic recovery in patients with T2DM and investigate the effect of co-existing HTN on the same. Our results found that post-exercise cardiac autonomic recovery as assessed by HRR and HRV was found to be slower in the presence of T2DM. Moreover, the simultaneous occurrence of HTN further caused impairment in the autonomic recovery rate.
4.2. Heart rate recovery
HRR is the assessment of the rate at which HR decreases following the cessation of exercise.
HRR derived from a standard exercise stress test provides high diagnostic accuracy for CAN in patients with type 2 diabetes, and its high sensitivity points to utility in identification and preliminary screening of at-risk patients 30 Attenuated HRR after exercise testing, is indicative of decreased parasympathetic nervous system activity 31,32 and is associated with an increased risk of all-cause mortality and risk for CV events in asymptomatic patients with T2DM. 33 However, attributing the slowed HRR in CAN exclusively to vagal dysfunction may be overly simplistic. Indeed, blunting of HRR with hyperglycemia 34,35 and insulin resistance 36 may implicate additional involvement of the sympatho-vagal imbalance.
Kannankeril et al 37 proposed that HRR at 1 min is mediated by enhanced activity of parasympathetic nervous system, whereas, HRR at 2 min is primarily mediated by sympathetic withdrawal, and therefore, HRR reflects the coordinated interaction between parasympathetic re-activation and sympathetic withdrawal. In contrast, Goldberger et al 38 found that during the first 30 s of the recovery phase, most of the changes in heart rate are due to sympathetic withdrawal subsequently overlapped with the effect of parasympathetic reactivation during later part of this phase. Freeman et al 39 suggested that HRR at 1 min post exercise is more reflective of parasympathetic autonomic nervous system function, while HRR at 2 min reflects activity of both branches of the autonomic nervous system.
In the present study, heart rate recovery at 1 (HRR1min) and 2 (HRR2min) minutes showed a significant effect for both DM and HTN, denoting a slower heart rate recovery when compared to the healthy controls. Furthermore, the non-significant interaction effect implies an additive effect of the two conditions. Ng et al 40 compared HRR between diabetics and healthy controls and found HRR1min to be a better discriminator than HRR2min. However, we found HRR2min to be more sensitive to the presence of HTN along with T2DM (Fig. 1). Moreover, in accordance with the diagnostic criteria for autonomic dysfunction proposed by Sacre et al 30 the DMHTN group in the current study demonstrated significantly altered control, with the mean HRR1min and HRR2min values as 27 and 43 bpm, respectively.
4.3. Resting heart rate variability
HRV has been analyzed and presented in the time domain; however, more complex assessments include power spectral (frequency domain) and non-linear analysis. These analyses of HRV provide researchers with direct information on the parasympathetic contributions, and by extension, inferred information on the sympathetic contributions to resting and post-exercise modulation of heart rate (HR). Most commonly used time domain variables of HRV are: SDNN which denotes overall variability in HR, and RMSSD & pNN50 which are indicators of vagal activity. Power spectral measures of HRV are Total power, LF power, HF power, and LF/HF ratio. Total power cumulatively indicates overall HRV, LF power in the power spectrum of HRV is an indicator of both sympathetic and parasympathetic activity, whereas, HF power has been found to be strongly associated with vagal activity, and ratio of LF and HF power (LF/HF ratio) is a measure of sympatho-vagal balance. The Poincare plot analysis is a graphical nonlinear method to assess the dynamics of HRV that provides summary information as well as detailed beat‑to‑beat information on the behavior of the heart. The Poincare plot parameters usually used are SD1 and SD2. SD1 is the standard deviation of projection of the Poincare plot on the line perpendicular to the line of identity, while SD2 is defined as the standard deviation of the projection of the Poincare plot on the line of identity (y = x). The parameter SD1 has been correlated with HF power (vagal activity), whereas SD2 has been correlated with sympathetic activity. 28
The analysis of resting HRV in the current study revealed that both DM and HTN, independently lowered the overall HRV. Resting tachycardia (reflected in the AvgHR) and fixed heart rate (decline in the measures of overall HRV such as AvgNN, SDNN and Total Power) are characteristic late findings in diabetic patients with vagal impairment due to CAN 41 (Fig. 2). There was a depression of the resting vagal activity and an increased sympathetic outflow (indicated by LFnu and SD2) in both these diseased states. LFnu component of HRV is affected by baroreflex activity 42 which explains the significantly increased values in the DMHTN group (Fig. 2). The co-existence, moreover, showed an added effect causing a further lowering of the parasympathetic and upregulation of the sympathetic tone. These findings were similar to those reported by previous studies. An investigation by Takahashi et al 43 exploring the co-existence of hypertension with diabetes suggested that hypertensive diabetics had a lower BRS, lower myocardial uptake, and enhanced clearance of I-metaiodobenzylguanidine (I-MIBG) as compared to normotensive diabetics. Furthermore, Hazari et al 11 also revealed similar findings, and suggested that cardiovascular reflexes (Ewing’s battery of tests), which exemplify cardiac autonomic function are further deteriorated in hypertensive diabetics than normotensive diabetics, and therefore, diabetes and hypertension may cause synergistic progression of CAN. More recently, Istenes et al 14 assessed resting HRV using the frequency domain measures and found an additive effect of HTN on impaired autonomic function in diabetic patients. However, Solanki et al 44,45 found non-significant difference in HRV parameters (frequency domain, time domain and non-linear measures) between diabetics with optimal vs. poor BP control. They recorded no difference in the overall HRV except for LF/HF ratio, which was found to be lower in the hypertensive diabetics. On the contrary, the present study found the LF/HF ratio to be the highest in DMHTN group. LF/HF ratio denotes sympatho-vagal balance, and an increase signifies sympathetic dominance which could result from either damage to cardiac autonomic nerves by hyperglycemia in T2DM or by the sympathetic overactivity as seen in HTN. Findings of Solanki et al 44,45 were in contrast to those of previous studies, where they reported no significant effect of HTN on autonomic function in T2DM. However, they did not include healthy controls or a euglycemic hypertensive group which makes it difficult to compare it with the results of the present study.
4.4. Post-exercise HRV
The results of present study revealed a significant difference in resting and post-exercise autonomic status for all the groups (healthy, hypertensive, normotensive diabetic, and hypertensive diabetic), suggesting a shift in cardiac autonomic modulation after exercise.
Comparison of the trend of recovery between the 4 groups suggested that while no group reached the baseline state, both DM and HTN showed a slower rate of recovery than the healthy group. Moreover, the co-existence of both HTN and DM further delayed the attaining of resting values. The time-domain variables showed a significant effect of group, with impaired recovery in diabetics (both normotensives and hypertensives) as compared to healthy controls. The frequency-domain demonstrated no significant difference between the groups. While the DMHTN group showed a slower rate of recovery in comparison to the other three groups, this difference was not statistically significant. Although, frequency domain variables are considered to be more reliable measures of HRV in short-term recordings, if the modulations are not stable, interpretation of the results of frequency analysis are less well defined 26 It has been recommended that with lower stability of heart rate modulations (during long-term recordings), time-domain methods are ideal for analysis. The post-exercise recovery period might also be speculated to possess lower heart rate stability and therefore, results of time-domain variables may be more meaningful to interpretation. The recovery of time-domain variables was found to be sensitive to the presence of both T2DM and HTN.
Acute physical exertion causes physiological stress affecting the cardiac autonomic modulation 15 During transition from rest to exercise and the recovery period following exercise, the heart has been suggested to be more vulnerable to dysrhythmias and fatal events owing to a shift in the autonomic balance from parasympathetic to sympathetic dominance. 17,18 Results of present study suggested that recovery of autonomic nervous system from sympathetic to parasympathetic was impaired in diabetics and hypertensive patients when compared to healthy controls. Present findings may be explained by the fact that the autonomic nervous system of diabetics as well as hypertensive patients is less responsive to change, which in part may be explained by baroreflex mechanism. Diminished responsiveness of the baroreflex in hypertension 46,47 and diabetes 48 may contribute to autonomic imbalance during recovery from exercise.
4.5. Possible mechanisms for impaired autonomic function in hypertensive diabetics
There exists substantial overlap in the pathophysiological pathways underlying the etiology of both T2DM and HTN. The association of T2DM and HTN has been explained by the presence of common susceptibility genes and single nucleotide polymorphisms (SNPs), oxidative stress-mediated cascades, chronic low-grade inflammation, insulin resistance and obesity 49 The pathogenesis of diabetic CAN is complex and involves a series of pathways activated by hyperglycemia resulting in neuronal ischemia and cellular death. Hyperglycemia in T2DM patients results in increased oxidative stress, which can cause direct neuronal dysfunction or endothelial dysfunction resulting in neuronal ischemia. 50 However, in HTN, owing to the fluctuations in BP, baroreflex function gets hampered and is not able to set itself to a new operating point. Diminished sensitivity of baroreflex is a leading cause of autonomic imbalance in HTN. 46,47 Although, there are some common pathways which are related to development of autonomic dysfunction in diabetes and HTN, CAN in diabetes is primarily hyperglycemia-mediated and autonomic dysfunction in HTN is in part due to changes in baroreflex mechanism. Considering different etiologies behind autonomic dysfunction in diabetes and hypertension, it may be speculated that due to the co-existence of these different etiologies together in DMHTN group, further deterioration of autonomic function at rest and impaired autonomic recovery was found when compared to normotensive diabetic group.
4.6. Limitations and recommendations for future research
The present study was the first to examine post-exercise cardiac autonomic recovery in patients with type 2 diabetes and the effect of co-existence of HTN on the same but since patients with structural abnormalities of the heart were not screened, it is likely that diabetic and hypertensive cardiomyopathies would have contributed to the blunt autonomic recovery. We assessed autonomic recovery using HRR and the change in post-exercise and resting values of HRV. Although impairments in these parameters are suggestive of CAN, the presence of CAN was not objectively assessed by standard cardiovascular autonomic reflex tests. Moreover, the unavailability of echocardiography limits the results of the study since diastolic dysfunction is very common in these patients and might significantly interfere with autonomic function. Future studies should administer definitive screening of CAN using standard tests such as the Ewing’s battery and other objective markers of autonomic function such as baroreflex sensitivity, I-MIBG, chronotropic incompetence, to corroborate these findings and present a more comprehensive conclusion. A comparison of these tests could help provide a sensitive prognostic marker that occurs earlier in the disease process of T2DM leading to improved treatment guidelines and outcomes.
5. Conclusion
The present study found impaired post-exercise autonomic recovery as assessed by HRR and HRV, in patients with T2DM. The co-existence of HTN further worsened the rate of recovery. These findings have clinical implications as graded maximal tests are commonly used for cardiovascular screening, and since diabetics show compromised autonomic recovery, caution must be exercised while administration of stress tests. Moreover, hypertensive diabetics demonstrated greater autonomic dysfunction and slower recovery as compared to normotensive diabetics, making them more susceptible to exercise intolerance. Furthermore, this study gives definitive evidence on the role of post-exercise recovery period for risk stratification in T2DM, HTN and DMHTN patients. Autonomic dysfunction is a common complication in T2DM that can be reversed with strict glycemic control, however, screening in asymptomatic patients is not routinely performed and overt clinical sequelae manifest only in advanced cases. This causes unrecognized subclinical CAN to insidiously contribute to cardiovascular risk. Therefore, the early identification of at-risk patients, such as hypertensive diabetics, is imperative.
6. Conflict of interest
The authors declare no conflicts of interest.
References
- 1.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care. 2012;35:S11. [Google Scholar]
- 2.Sowers J.R., Epstein M., Frohlich E.D. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37:1053–1059. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- 3.Hypertension in Diabetes Study Group Hypertension in Diabetes Study (HDS): I. Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications. J Hypertens. 1993;11:309–317. doi: 10.1097/00004872-199303000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Abougalambou S.S., Abougalambou A.S., Sulaiman S.A., Hassali M.A. Prevalence of hypertension, control of blood pressure and treatment in hypertensive with type 2 diabetes in Hospital University Sains Malaysia. Diabetes Metab Syndr. 2011;5:115–119. doi: 10.1016/j.dsx.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Tiptaradol S., Aekplakorn W. Prevalence, awareness, treatment and control of coexistence of diabetes and hypertension in Thai population. Int J Hypertens. 2012;2012 doi: 10.1155/2012/386453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bild D., Teutsch S.M. The control of hypertension in persons with diabetes: a public health approach. Public Health Rep. 1987;102:522–529. [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner B.M., Cooper M.E., de Zeeuw D. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 8.Lewis E.J., Hunsicker L.G., Clarke W.R. Renoprotective effect of the angiotensin-receptor antagonist Irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 9.Pappachan J.M., Sebastian J., Bino B.C. Cardiac autonomic neuropathy in diabetes mellitus: prevalence, risk factors and utility of corrected QT interval in the ECG for its diagnosis. Postgrad Med J. 2008;84:205–210. doi: 10.1136/pgmj.2007.064048. [DOI] [PubMed] [Google Scholar]
- 10.Basu A.K., Bandyopadhyay R., Chakrabarti S., Paul R., Santra S. A study on the prevalence of cardiac autonomic neuropathy in type-2 diabetes in Eastern India. J Indian Acad Clin Med. 2010;11:190–194. [Google Scholar]
- 11.Hazari M.A., Khan R.T., Reddy B.R., Hassan M.A. Cardiovascular autonomic dysfunction in type 2 diabetes mellitus and essential hypertension in a South Indian population. Neurosciences (Riyadh) 2012;17:173–175. [PubMed] [Google Scholar]
- 12.Pikkujämsä S.M., Huikuri H.V., Airaksinen K.J. Heart rate variability and baroreflex sensitivity in hypertensive subjects with and without metabolic features of insulin resistance syndrome. Am J Hypertens. 1998;11:523–531. doi: 10.1016/s0895-7061(98)00035-1. [DOI] [PubMed] [Google Scholar]
- 13.Liao D., Sloan R.P., Cascio W.E. Multiple metabolic syndrome is associated with lower heart rate variability: the atherosclerosis risk in communities study. Diabetes Care. 1998;21:2116–2122. doi: 10.2337/diacare.21.12.2116. [DOI] [PubMed] [Google Scholar]
- 14.Istenes I., Körei A.E., Putz Z. Heart rate variability is severely impaired among type 2 diabetic patients with hypertension. Diabetes Metab Res Rev. 2014;30:305–312. doi: 10.1002/dmrr.2496. [DOI] [PubMed] [Google Scholar]
- 15.Mohan V., Shah S.N., Joshi S.R. Current status of management, control, complications and psychosocial aspects of patients with diabetes in India: results from the DiabCare India 2011 Study. Indian J Endocrinol Metab. 2014;18:370–378. doi: 10.4103/2230-8210.129715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hautala A., Tulppo M.P., Makikallio T.H., Laukkanen R., Nissila S., Huikuri H.V. Changes in cardiac autonomic regulation after prolonged maximal exercise. Clin Physiol. 2001;21:238–245. doi: 10.1046/j.1365-2281.2001.00309.x. [DOI] [PubMed] [Google Scholar]
- 17.Singer D.H., Martin G.J., Magid N. Low heart rate variability and sudden cardiac death. J Electrocardiol. 1988;21:S46–S55. doi: 10.1016/0022-0736(88)90055-6. [DOI] [PubMed] [Google Scholar]
- 18.Paterson D.J. Antiarrhythmic mechanisms during exercise. J Appl Physiol. 1996;80:1853–1862. doi: 10.1152/jappl.1996.80.6.1853. [DOI] [PubMed] [Google Scholar]
- 19.Dimitropoulos G., Tahrani A.A., Stevens M.J. Cardiac autonomic neuropathy in patients with diabetes mellitus. World J Diabetes. 2014;5:17–39. doi: 10.4239/wjd.v5.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parekh A., Lee C.M. Heart rate variability after isocaloric exercise bouts of different intensities. Med Sci Sports Exerc. 2005;37:599–605. doi: 10.1249/01.mss.0000159139.29220.9a. [DOI] [PubMed] [Google Scholar]
- 21.Carretero O.A., Oparil S. Essential hypertension. Circulation. 2000;101:329–335. doi: 10.1161/01.cir.101.3.329. [DOI] [PubMed] [Google Scholar]
- 22.Brubaker P.H., Kitzman D.W. Chronotropic incompetence: causes, consequences, and management. Circulation. 2011;123:1010–1020. doi: 10.1161/CIRCULATIONAHA.110.940577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Y.J., Lauer M.S., Earnest C.P. Heart rate recovery following maximal exercise testing as a predictor of cardiovascular disease and all-cause mortality in men with diabetes. Diabetes Care. 2003;26:2052–2057. doi: 10.2337/diacare.26.7.2052. [DOI] [PubMed] [Google Scholar]
- 24.Drew B.J., Califf R.M., Funk M. AHA scientific statement: practice standards for electrocardiographic monitoring in Hospital settings: an American heart association scientific statement from the councils on cardiovascular nursing, clinical cardiology, and cardiovascular disease in the young: endorsed by the International society of computerized electrocardiology and the American association of critical‐care nurses. J Cardiovasc Nurs. 2005;20:76–106. doi: 10.1097/00005082-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Soares‐Miranda L., Sandercock G., Vale S. Metabolic syndrome, physical activity and cardiac autonomic function. Diabetes Metab Res Rev. 2012;28:363–369. doi: 10.1002/dmrr.2281. [DOI] [PubMed] [Google Scholar]
- 26.Task Force of the European Society of Cardiology Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 27.Furlan R., Guzzetti S., Crivellaro W. Continuous 24-hour assessment of the neural regulation of systemic arterial pressure and RR variabilities in ambulant subjects. Circulation. 1990;81:537–547. doi: 10.1161/01.cir.81.2.537. [DOI] [PubMed] [Google Scholar]
- 28.Rhaman M., Karim A.H., Hasan M., Sultana J. Successive RR interval analysis of PVC with sinus rhythm using fractal dimension, poincaré plot and sample entropy method. Int J Image Graph Signal Process. 2013;2:17–24. [Google Scholar]
- 29.Malliani A., Pagani M., Lombardi F., Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 30.Sacre J.W., Jellis C.L., Coombes J.S., Marwick T.H. Diagnostic accuracy of heart‐rate recovery after exercise in the assessment of diabetic cardiac autonomic neuropathy. Diabetes Med. 2012;29:e312–20. doi: 10.1111/j.1464-5491.2012.03719.x. [DOI] [PubMed] [Google Scholar]
- 31.Imai K., Sato H., Hori M. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24:1529–1535. doi: 10.1016/0735-1097(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 32.Jae S.Y., Carnethon M.R., Heffernan K.S., Fernhall B., Lee M.K., Park W.H. Heart rate recovery after exercise and incidence of type 2 diabetes in men. Clin Auton Res. 2009;19:189–192. doi: 10.1007/s10286-009-0007-4. [DOI] [PubMed] [Google Scholar]
- 33.Chacko K.M., Bauer T.A., Dale R.A., Dixon J.A., Schrier R.W., Estacio R.O. Heart rate recovery predicts mortality and cardiovascular events in patients with type 2 diabetes. Med Sci Sports Exerc. 2008;40:288–295. doi: 10.1249/mss.0b013e31815c4844. [DOI] [PubMed] [Google Scholar]
- 34.Panzer C., Lauer M.S., Brieke A., Blackstone E., Hoogwerf B. Association of fasting plasma glucose with heart rate recovery in healthy adults: a population-based study. Diabetes. 2002;51:803–807. doi: 10.2337/diabetes.51.3.803. [DOI] [PubMed] [Google Scholar]
- 35.Seshadri N., Acharya N., Lauer M.S. Association of diabetes mellitus with abnormal heart rate recovery in patients without known coronary artery disease. Am J Cardiol. 2003;91:108–111. doi: 10.1016/s0002-9149(02)03014-x. [DOI] [PubMed] [Google Scholar]
- 36.Lind L., Andren B. Heart rate recovery after exercise is related to the insulin resistance syndrome and heart rate variability in elderly men. Am Heart J. 2002;144:666–672. doi: 10.1067/mhj.2002.124836. [DOI] [PubMed] [Google Scholar]
- 37.Kannankeril P.J., Le F.K., Kadish A.H., Goldberger J.J. Parasympathetic effects on heart rate recovery after exercise. J Investig Med. 2004;52:394–401. doi: 10.1136/jim-52-06-34. [DOI] [PubMed] [Google Scholar]
- 38.Goldberger J.J., Le FK Lahiri M., Kannankeril P.J., Ng J., Kadish A.H. Assessment of parasympathetic reactivation after exercise. Am J Physiol Heart Circ Physiol. 2006;290:H2446–H2452. doi: 10.1152/ajpheart.01118.2005. [DOI] [PubMed] [Google Scholar]
- 39.Freeman J.V., Dewey F.E., Hadley D.M., Myers J., Froelicher V.F. Autonomic nervous system interaction with the cardiovascular system during exercise. Prog Cardiovasc Dis. 2006;48:342–362. doi: 10.1016/j.pcad.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Ng F., Wong S., La Cruz A., Hernández M.I., Gomis P., Passariello G. Heart rate recovery in the diagnosis of diabetic cardiovascular autonomic neuropathy. Comput Cardiol. 2007;2007:681–684. [Google Scholar]
- 41.Ewing D.J., Borsey D.Q., Bellavere F., Clarke B.F. Cardiac autonomic neuropathy in diabetes: comparison of measures of RR interval variation. Diabetologia. 1981;21:18–24. doi: 10.1007/BF03216217. [DOI] [PubMed] [Google Scholar]
- 42.Tarvainen M.P., Laitinen T.P., Lipponen J.A., Cornforth D.J., Jelinek H.F. Cardiac autonomic dysfunction in type 2 diabetes–effect of hyperglycemia and disease duration. Front Endocrinol. 2014;5 doi: 10.3389/fendo.2014.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi N., Nakagawa M., Saikawa T. Effect of essential hypertension on cardiac autonomic function in type 2 diabetic patients. J Am Coll Cardiol. 2001;38:232–237. doi: 10.1016/s0735-1097(01)01363-8. [DOI] [PubMed] [Google Scholar]
- 44.Solanki J.D., Basida S.D., Mehta H.B., Panjwani S.J., Gadhavi B.P., Patel P. Impact of disease control and co-existing risk factors on heart rate variability in Gujarati type 2 diabetics: an observational study. J Family Med Prim Care. 2016;5:393–398. doi: 10.4103/2249-4863.192323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solanki J.D., Basida S.D., Mehta H.B., Panjwani S.J., Gadhavi B.P. Comparative study of cardiac autonomic status by heart rate variability between under-treatment normotensive and hypertensive known type 2 diabetics. Indian Heart J. 2017;69:52–56. doi: 10.1016/j.ihj.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bristow J.D., Honour A.J., Pickering G.W., Sleight P., Smyth H.S. Diminished baroreflex sensitivity in high blood pressure. Circulation. 1969;39:48–54. doi: 10.1161/01.cir.39.1.48. [DOI] [PubMed] [Google Scholar]
- 47.Del Colle S., Milan A., Caserta M. Baroreflex sensitivity is impaired in essential hypertensives with central obesity. J Hum Hypertens. 2007;21:473–478. doi: 10.1038/sj.jhh.1002163. [DOI] [PubMed] [Google Scholar]
- 48.Borowik E., Grabowicz W., Grycewicz T., Lubiński A. Clinical usefulness of baroreflex sensitivity test in the detection of cardiovascular autonomic neuropathy in patients with type 2 diabetes mellitus. Polski merkuriusz lekarski: organ Polskiego Towarzystwa Lekarskiego. 2015;39:277–280. [PubMed] [Google Scholar]
- 49.Cheung B.M., Li C. Diabetes and hypertension: is there a common metabolic pathway? Curr. Atheroscler. Rep. 2012;14:160–166. doi: 10.1007/s11883-012-0227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wada R., Yagihashi S. Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Ann N Y Acad Sci. 2005;1043:598–604. doi: 10.1196/annals.1338.067. [DOI] [PubMed] [Google Scholar]