Abstract
Background
TRAIL, tumor necrosis factor-related apoptosis-inducing ligand, can selectively kill cancer cells with little or no cytotoxicity toward normal human cells and is regarded as a potential relatively safe antitumor drug. However, some cancer cells are resistant to TRAIL-induced apoptosis. Thus, reagents that potentiate TRAIL-induced cytotoxicity are needed. Herein, we investigated whether shikonin, a natural compound from the root of Lithospermum erythrorhizon, can sensitize TRAIL-resistant cells to TRAIL-induced cytotoxicity.
Results
The viability of A549 cells, which were resistant to TRAIL, was significantly decreased after treatment with TRAIL followed by shikonin. The underlying mechanisms by which shikonin sensitizes cells to TRAIL-induced cytotoxicity were also examined. Combined treatment with shikonin and TRAIL activated the caspase and JNK pathways, inhibited the STAT3 and AKT pathways, downregulated the expression of Mcl-1, Bcl-2, Bcl-xL, c-FLIP and XIAP and upregulated the expression of Bid.
Conclusions
In conclusion, the results indicated that shikonin sensitized resistant cancer cells to TRAIL-induced cytotoxicity via the modulation of the JNK, STAT3 and AKT pathways, the downregulation of antiapoptotic proteins and the upregulation of proapoptotic proteins.
Keywords: Shikonin, TRAIL, Resistant cancers, Sensitization
Background
Tumor necrosis factor-related apoptosis-inducing ligand, TRAIL, is a promising antitumor drug because it can induce apoptosis in cancer cells but is minimally cytotoxic to normal cells [1, 2]. However, intrinsic or acquired resistance to TRAIL-induced apoptosis has limited the further utilization of TRAIL in clinical trials [3]. Thus, researchers are studying ways to overcome this resistance, but combination treatments to sensitize resistant cancer cells to TRAIL have been shown effective. Therefore, seeking reagents that can sensitize resistant cancer cells to TRAIL-induced apoptosis is greatly important.
Shikonin, a natural compound isolated from the Chinese herbal plant Lithospermum erythrorhizon, has been widely used for thousands of years for the treatment of diverse diseases [4, 5]. Shikonin has recently been demonstrated to have tumoricidal [6, 7] and antiproliferative [8, 9] abilities, to reverse drug resistance to chemotherapy [10–12] and to enhance the cytotoxicity of chemotherapy [11–13]. Moreover, studies have shown that shikonin may be similar to TRAIL in its ability to selectively kill cancer cells while causing minimal cytotoxicity to normal cells [7, 10, 14]. The present study investigated whether shikonin can sensitize TRAIL-resistant cells to TRAIL-induced cytotoxicity and whether combined treatment with shikonin and TRAIL is cytotoxic to normal cells. Furthermore, the underlying mechanism was studied.
The above findings suggest that combined treatment with shikonin and TRAIL may be synergistic in effectively enhancing cytotoxicity and destroying tumors. The mechanisms may operate via processes such as the activation of proapoptotic pathways and the inhibition of prosurvival pathways, with a decrease in the expression of antiapoptotic proteins. The present study is devoted to exploring a reagent that can synergistically promote TRAIL-induced cytotoxicity with minimal toxicity to normal cells; this reagent might be a safe and effective sensitizer useful in TRAIL-based therapy in clinical applications.
Methods
Chemicals and reagents
Shikonin (Sigma, USA) was dissolved in dimethyl sulfoxide (DMSO) and stored as a stock solution (50 mM) in aliquots at − 20 °C. Soluble recombinant human TRAIL was purchased from Peprotech. Primary antibodies specific to caspase-3, caspase-8, caspase-9, Mcl-1, Bcl-2, Bcl-xL, Bax, JNK, STAT3, Akt and the phosphorylated forms of JNK, STAT3 and Akt (Ser 473) were purchased from Abcam. Primary antibodies specific to β-actin, c-FLIP, XIAP, Bid, and cleaved caspase-3, − 8, and − 9 were purchased from Cell Signaling Technology.
Cell culture and treatment
A549 human lung cancer cells (CCL-185, ATCC, USA) were cultured in RPMI-1640, and HEK-293 human embryonic kidney cells (CRL-1573, ATCC, USA) were cultured in Eagle’s minimum essential medium (EMEM). The two cells strains purchase need not ethical approval. All types of media were supplemented with 10% heat-inactivated fetal bovine serum. Cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2/95% air.
Cell viability assay
A549 and HEK-293 cells were seeded into two sets of 96-well plates in triplicate (2 × 104 cells in 100 μl/well). After incubation for 12 h, filter-sterilized shikonin was added to the culture medium in one plate at concentrations of 0, 1, 2, 4, 6 and 8 μM, and 50 ng/ml or 5 ng/ml of soluble TRAIL (sTRAIL) was added to the culture medium in the other plate, with the appropriate controls. After incubation for 12 h, the cell viability in the plate treated with only sTRAIL was assessed by a Cell Counting Kit-8 (CCK8) assay; the shikonin-pretreated plate was then treated with 50 ng/ml or 5 ng/ml sTRAIL for another 12 h, and the cell viability was determined by a CCK8 assay. Furthermore, a long-term cytotoxicity experiment was performed as follows: shikonin (4 μM), alone or supplemented with TRAIL (50 ng/ml), was added to A549 and HEK-293 cells. Cell viability was detected over 96 h at 24 h intervals via a CCK8 assay. Moreover, the pan-caspase inhibitor Z-VAD-FMK (Sigma, USA) was employed for the assessment of the effects of shikonin and TRAIL on A549 cells. After pretreatment with Z-VAD-FMK (50 μM) for 60 min, shikonin and TRAIL were added.
Annexin V/PI assay
Early indicators of apoptosis were detected by using an annexin V/PI binding kit (Biolegend) and a flow cytometer (Accuri C6, BD Biosciences). In brief, A549 cells were seeded in 6-well plates (4 × 105 cells/well) and treated with shikonin (4 μM) or TRAIL (50 ng/ml) for 12 h. TRAIL-treated cells were collected by trypsinization and then analyzed following the indicated protocol. Flow cytometry was performed within 1 h of cell harvesting. After pretreatment with shikonin (4 μM) for 12 h, A549 cells were then incubated in the presence or absence of 50 ng/ml TRAIL for another 12 h. Then, all the cells were collected and analyzed as above.
Western blotting
A549 cells were seeded and incubated overnight in a 10 mm culture dish (2.5 × 106/well). Next, shikonin (4 μM) and TRAIL (50 ng/ml) were added to the culture medium. After incubation for 12 h, the TRAIL-treated A549 cells were harvested, and whole cell lysates were prepared with lysis buffer. The shikonin-treated A549 cells were then treated or not treated with 50 ng/ml TRAIL for another 12 h, after which whole cell lysates were prepared.
Statistical analysis
The data are expressed as the means ± SDs and were analyzed by Student’s t-test using SPSS software to determine the significance of differences between groups. p < 0.05 was considered statistically significant.
Results
Shikonin enhances TRAIL-induced cytotoxicity
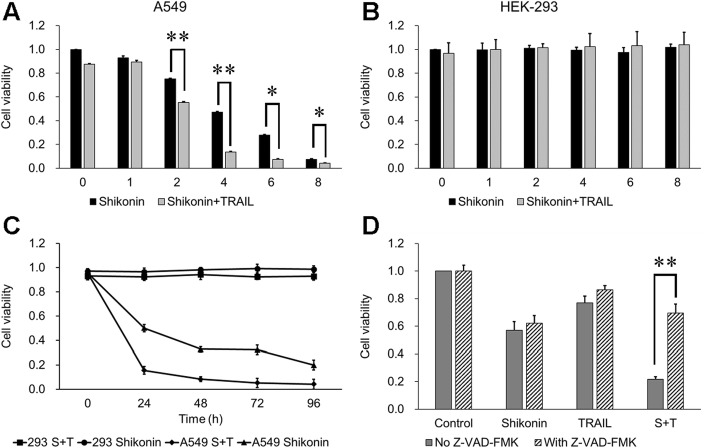
After treatment with TRAIL alone, the viability of TRAIL-resistant A549 cells was almost 95%. However, in cells pretreated with shikonin, TRAIL noticeably decreased the viability of A549 cells over time. In addition, a synergistic effect of shikonin and TRAIL on A549 cell killing was observed (Fig. 1a). In addition, the cytotoxicity of shikonin and TRAIL in the normal human renal cell line HEK-293 was assessed. Surprisingly, although shikonin was cytotoxic to the human lung cancer cell line A549, it was almost nontoxic to HEK-293 cells. Moreover, shikonin and TRAIL cotreatment was not cytotoxic to HEK-293 cells (Fig. 1b). The long-term cytotoxicity experiment demonstrated that the concentrations of shikonin and TRAIL were safe in normal cells (Fig. 1c). Z-VAD-FMK, the caspase inhibitor, significantly increased cell viability in the groups cotreated with shikonin and TRAIL (Fig. 1d). Nevertheless, Z-VAD-FMK did not noticeably influence cell viability when shikonin and TRAIL were administered as single reagents.
Fig. 1.
Effect of shikonin on TRAIL-induced cell cytotoxicity. The viability of (a) A549 and (b) HEK-293 cells after treatment were determined by a CCK8 assay. Cells were pretreated with different concentrations of shikonin for 12 h and then further incubated in the presence or absence of TRAIL (50 ng/ml) for another 12 h. c In the long-term cytotoxicity experiment, HEK-293 cell viability was not influenced by shikonin and TRAIL over the 96 h incubation period; however, the viability of A549 cells was significantly inhibited by shikonin, especially with TRAIL cotreatment. d These data show the efficacy of shikonin (4 μM) and TRAIL (50 ng/ml) on A549 cells pretreated with Z-VAD-FMK (50 μM). The data are presented as the means ± SDs of at least three independent experiments, *p < 0.05 and **p < 0.01. S + T: Shikonin + TRAIL
Synergistic effect of shikonin and TRAIL on apoptosis induction in A549 cells
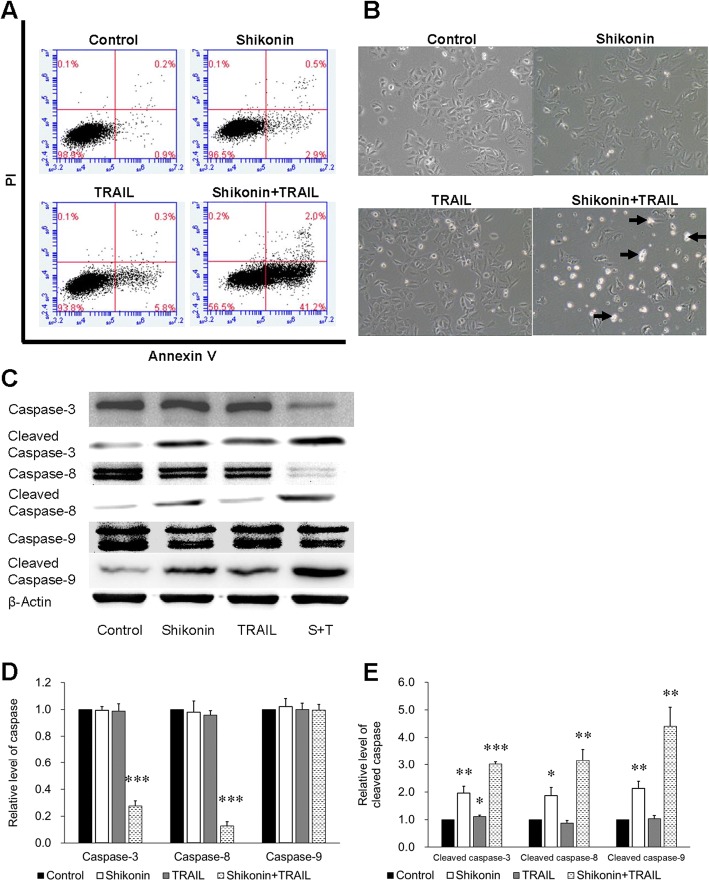
After the cell viability experiment, a concentration of shikonin (4 μM) was selected for subsequent experiments because this concentration demonstrated the most apparent synergistic effect on TRAIL-induced cytotoxicity in A549 cells. To determine whether shikonin enhanced TRAIL-induced cytotoxicity via apoptosis, apoptosis was quantified by annexin V-PI flow cytometry. After pretreatment with shikonin (4 μM) for 12 h followed by treatment with 50 ng/ml TRAIL for 12 h, the adherent and suspended A549 cells were collected for flow cytometric analysis. The results indicated that treatment with either shikonin (4 μM) or TRAIL (50 ng/ml) alone induced an apoptosis rate of only 3.4% or 6.1%, respectively, while cotreatment with shikonin and TRAIL induced an apoptosis rate of 43.2%; most of the apoptotic cells were in early apoptosis stages (Fig. 2a). Morphological changes in A549 cells after the treatments described above were observed by microscopy; as the arrows in the figure indicated, cellular suspension, shrinkage and blebbing were observed after cotreatment with shikonin and TRAIL, whereas no obvious morphological changes were observed in the groups treated with only shikonin or TRAIL. In addition, the proliferation of A549 cells was inhibited after shikonin treatment. Caspase-3, − 8 and − 9 expression, the hallmarks of cells undergoing apoptosis, was also investigated by Western blotting to further confirm the apoptosis phenomenon. The levels of both pro-caspase-3 and pro-caspase-8 were appreciably decreased in A549 cells after cotreatment with shikonin and TRAIL, while treatment with either shikonin or TRAIL alone had almost no effect on caspase-3 or caspase-8 expression. The Western blotting results showed that cotreatment with shikonin and TRAIL did not activate caspase-9 (Fig. 2c). Taken together, our results indicated that TRAIL-induced apoptosis in A549 cells was augmented after shikonin pretreatment.
Fig. 2.
Synergistic effect of shikonin and TRAIL on apoptosis induction in TRAIL-resistant A549 cells. a A549 cells were pretreated with shikonin (4 μM) for 12 h and then exposed to TRAIL (50 ng/ml) for another 12 h. Cells were harvested to quantify the induction of apoptosis by annexin V/PI staining and flow cytometry. The data in each plot indicated the percentage of apoptotic cells. b Cell morphology was analyzed under an inverted microscope (magnification × 100) after cells were treated as described in (a). c Whole cell lysates were prepared after cells were treated as described above and were analyzed by Western blotting using antibodies specific to caspase-3, − 8, − 9 and cleaved caspase-3, − 8, − 9. β-Actin was used as the loading control. d-e The data are presented as the means ± SDs of at least three independent experiments, and significant differences between the control group and the other groups in each experiment are shown as*p < 0.05, ** p < 0.01 and ***p < 0.001. S + T: Shikonin + TRAIL
Shikonin potentiates TRAIL-induced apoptosis by inhibiting the expression of antiapoptotic proteins and enhancing the expression of proapoptotic proteins
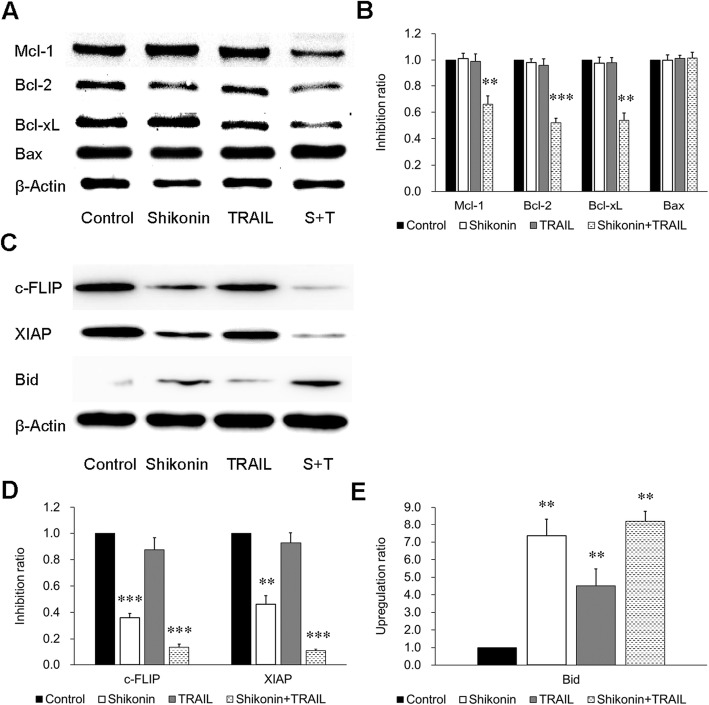
Next, the mechanisms by which TRAIL-induced apoptosis is enhanced by shikonin were explored. Various anti- or proapoptotic proteins regulate TRAIL-induced apoptosis, so the antiapoptotic proteins Mcl-1, Bcl-2, Bcl-xL, c-FLIP, XIAP and the proapoptotic proteins Bax and Bid were investigated by Western blotting. Cotreatment with shikonin and TRAIL inhibited the expression of the antiapoptotic proteins Mcl-1, Bcl-2, Bcl-xL, c-FLIP and XIAP, while the combination treatment enhanced the expression of the proapoptotic protein Bid but had no effect on Bax expression (Fig. 3). Thus, these results indicated that the downregulation of antiapoptotic proteins and upregulation of proapoptotic proteins were the mechanisms by which shikonin enhanced TRAIL-induced apoptosis.
Fig. 3.
Effect of shikonin and TRAIL on antiapoptotic and proapoptotic protein expression in A549 cells. a and c A549 cells were pretreated with shikonin (4 μM) for 12 h and were then incubated with TRAIL (50 ng/ml) for another 12 h. Whole cell lysates were prepared and analyzed by Western blotting using the indicated antibodies. β-Actin was used as the loading control. b, d and e The data are presented as the means ± SDs of at least three independent experiments, and significant differences between the control group and other groups in each experiment are shown as **p < 0.01 and ***p < 0.001. S + T: Shikonin + TRAIL
JNK, STAT3 and AKT pathways are involved in shikonin-mediated TRAIL sensitization
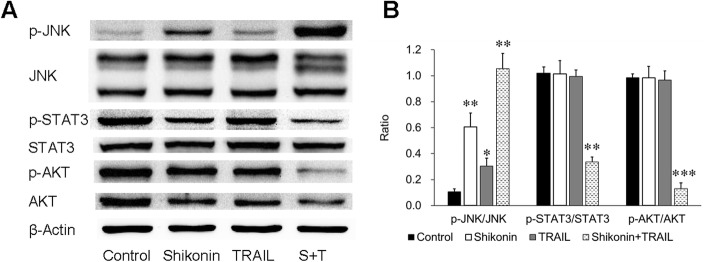
Other signaling pathways, including the JNK, STAT3 and AKT pathways, which were reported to be responsible for resistance to TRAIL, were further investigated. In cells pretreated with shikonin (4 μM) for 12 h followed by treatment with TRAIL (50 ng/ml) for 12 h, Western blotting showed that shikonin upregulated the phosphorylation of JNK (p-JNK); more specifically, the expression of p-JNK was higher after cotreatment than after either treatment alone. On the other hand, shikonin downregulated the phosphorylation of STAT3 and AKT, and this downregulation was more apparent after cotreatment with shikonin and TRAIL (Fig. 4). Therefore, the upregulation of p-JNK and the suppression of p-STAT3 and p-AKT might be the mechanisms by which shikonin sensitizes cells to TRAIL-induced apoptosis.
Fig. 4.
Phosphorylation effect of shikonin and TRAIL on JNK, STAT3 and AKT. a Activation of JNK and inhibition of STAT3 and AKT were associated with the induction of apoptosis in A549 cells by shikonin and TRAIL cotreatment as described above. The expression of the total and phosphorylated forms of JNK, STAT3 and AKT were assessed using the corresponding antibodies via Western blotting. b The data are presented as the means ± SDs of at least three independent experiments, and significant differences between the control group and the other groups are shown as *p < 0.05, **p < 0.01 and ***p < 0.001. S + T: Shikonin + TRAIL
Discussion
Cancer has always been one of the most lethal causes of death among human beings. Human beings have to battle cancer for extended periods; surgical excision is the first-line treatment, but many cancers recur after excision. Radiotherapy and chemotherapy are also common treatments for cancers; however, their considerable side effects decrease patients’ quality of life. Thus, relatively safe antitumor drugs that inhibit cancer but have weak or no side effects are urgently needed.
In this study, the role of caspase was the first concern. Cysteinyl aspartate specific protease, or caspase, is a kind of proteolytic enzyme closely related to apoptosis. In apoptosis, the caspase family has two main functions: apoptosis initiation and apoptosis execution [15, 16]. The activated apoptosis-initiating caspase molecule, which is cleaved and activated by an external protein signal, activates the apoptosis-executing caspase molecule and hydrolyzes the target protein, leading to programmed cell death. Therefore, cleaved caspases were investigated, and the results demonstrated that the shikonin + TRAIL drug combination promoted caspase-8 and caspase-3 cleavage. Caspase-8, an apoptosis-initiating molecule, undergoes self-activation through oligomerization and activates downstream caspases, resulting in irreversible apoptosis [17, 18]. Caspase-8 is the key protease in the death receptor pathway. When cells are stimulated by apoptotic factors, TRAIL binds the corresponding death receptor, causing the activation of caspase-8. Then, the downstream caspases can induce a “waterfall” activation process. This action leads to the transfer of the apoptosis signal from the nondependent mitochondrial pathway to the mitochondrial pathway, linking the death receptor pathway with the mitochondrial pathway and amplifying the apoptotic signal [19, 20]. The observation of cleaved caspase-3, − 8, and − 9 was consistent with this theory. Moreover, the pan-caspase inhibitor enhanced cell viability in the shikonin + TRAIL group, proving that shikonin increased the apoptosis-inducing ability of TRAIL via the caspase pathway.
TRAIL, because it can selectively destroy tumor cells but is minimally toxic to normal cells, is regarded as one of the most promising cancer therapeutics [21]. However, drug resistance substantially hinders the efficacy of chemical medicines, and resistance to TRAIL has been reported [22]. Some patients may develop resistance to TRAIL [23, 24], which limits the utilization of TRAIL as a therapeutic reagent in cancer. The mechanism of resistance to TRAIL is not completely understood but may be related to deregulation of the components in the TRAIL-induced apoptotic pathway. Deregulation of TRAIL receptors, including downregulation of the death receptors DR4 and DR5 and overexpression of the decoy receptors DcR1 and DcR2, at the membrane level and deregulation of apoptosis-related proteins, including overexpression of the antiapoptotic proteins Bcl-2, Bcl-xL, and Mcl-1 and downregulation of the proapoptotic proteins Bax, Bid and Bak, at the intracellular level, are responsible for resistance to TRAIL-induced apoptosis [7]. The present results indicated that Bid, a proapoptotic protein, was upregulated, which was believed advantageous to tumor suppression. Bid is activated by Caspase-8 [19]. Our experimental results were consistent with this theory. On the other hand, activation of antiapoptotic pathways, such as the NF-κB, MAPK, PI3K/AKT, and signal transducers and activators of transcription (STAT) pathways, might also confer resistance to TRAIL-induced apoptosis [25]. The role of these pathways is reflected in the experimental results.
Moreover, the caspase, c-FLIP and XIAP proteins play an important regulatory role. c-FLIP is an inhibitor of apoptotic proteins that can inhibit tumor cell apoptosis at high expression levels [26]. The carboxyl terminus of c-FLIP has a structural domain similar to that of caspase-8; thus, c-FLIP competitively binds FADD. Then, c-FLIP regulates multiple apoptotic pathways, including the TRAIL pathway. Therefore, downregulation of c-FLIP was reported to be an effective method for enhancing the sensitivity of tumor cells to TRAIL [27]. Our results indicated that the activity of caspase-3, − 8, and − 9 was increased after c-FLIP expression was suppressed. XIAP, which inhibits apoptotic proteins, mainly mediates protein-protein interactions [28]. Tumor cells can survive and proliferate under adverse conditions, such as nutritional deficiency, hypoxia, DNA damage and chromosomal aberrations. In contrast, these adverse conditions are sufficient to activate apoptosis in normal cells [29]. The process of tumor escape from apoptosis is believed to be the abnormal expression of XIAP in cells. XIAP is an inhibitor of caspase and can selectively bind to caspase-3 and -9 to inhibit cell apoptosis [30]. XIAP can increase the apoptosis threshold, thus playing an important role in mediating tumor amplification and chemotherapeutic drug resistance [31]. In the present study, the expression of XIAP decreased significantly in cells treated with shikonin, especially those cotreated with TRAIL, suggesting that XIAP downregulation may increase tumor sensitization to TRAIL.
Another exciting phenomenon observed was the induction of minimal cytotoxicity in the human embryonic kidney cell line HEK-293 by treatment with shikonin (1–8 μM) either alone or in combination with TRAIL (Fig. 1b). This result is consistent with the report by Gong K et al. [7], in which HEK-293 T and normal human hepatic cells did not undergo cell death after treatment with shikonin (1–8 μM) for 24 h. In addition, several reports indicated that shikonin may be able to selectively kill tumor cells [10, 14] because it can induce intrinsic reactive oxygen species (ROS) upregulation [32, 33]. In addition, compared with normal cells, tumor cells have a higher ROS level and are under oxidative stress due to an imbalanced redox status [34–36], so they are more vulnerable than normal cells to reagents that increase ROS [35]. Thus, the ROS mechanism can be a potential future research direction to better understand the shikonin pathway.
In studying the mechanisms by which shikonin sensitizes cells to TRAIL-induced cytotoxicity, we found that treatment with shikonin followed by TRAIL inhibited the expression of antiapoptotic proteins in the Bcl-2 family: Mcl-1, Bcl-2 and Bcl-xL (Fig. 3). In addition, JNK was activated, and STAT3 and AKT were inhibited. STAT3 is a cytoplasmic transcription factor that plays a critical role in the regulation of genes involved in cell proliferation and survival. Activated STAT3 has been reported to prevent tumor cell apoptosis by regulating associated genes, such as Bcl-2, Bcl-xL and Fas [37, 38]. Furthermore, STAT3 inhibition has been shown to prevent cell proliferation and induce apoptosis in several cancer cell types [39–41]. AKT is a protein kinase involved in multiple cellular processes, including cell survival, proliferation, metabolism, apoptosis and tumorigenesis. AKT suppression has been reported to inhibit proliferation and induce apoptosis in multiple tumor cells [42–44]. As Fig. 2c shows, we observed a phenomenon of growth and proliferation inhibition in the shikonin-treated group; this result might be related to the decreases in activated STAT3 and JNK. Among the above three pathways, the inhibition of the STAT3 and AKT pathways is generally considered beneficial for promoting tumor killing. However, the function of the JNK pathway in tumor killing is controversial [45, 46]. Some findings support the pro-oncogenic function of JNK, while others suggest that JNK is a tumor suppressor [47]. The results of the present study support the latter view.
Conclusions
The present study demonstrated that shikonin potentiates TRAIL-induced apoptosis by activating JNK and suppressing the activation of STAT3 and AKT, which leads to decreases in antiapoptotic proteins and finally induces extrinsic apoptotic pathways. Importantly, both shikonin and TRAIL selectively kill cancer cells with minimal or no cytotoxicity to normal cells; therefore, combined treatment with shikonin and TRAIL may be an effective and safe strategy for cancer therapy.
Acknowledgments
The authors thank Professor Lei Yan for assistance.
Funding
This research is supported by the National Natural Science Foundation of China (31170829, 81171762, 81550017 and 81473418), the Beijing Natural Science Foundation (7172150) and the Research Project of CACMS (ZZ2018006, HX2017001).
Availability of data and materials
Harvard Dataverse doi:10.7910/DVN/VD8ZBV
Consent to publish
Not applicable.
Authors’ contributions
CZL conceptualized the study. JZL, ZXS, ZLG, PH, BP and YW participated in designing experiments. ZLG, JZL, DQ, JYZ, MMJ, PH and LYC performed experiments. CZL, ZXS, ZLG, JZL, PH, BP and YW analyzed data. CZL, ZLG, JZL and ZXS wrote the manuscript. CZL supervised the project. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhi Lan Guo, Email: gzl20141019@163.com.
Jing Zhe Li, Email: superatp@hotmail.com.
Yan Yan Ma, Email: yanyan_m@sina.com.
Dan Qian, Email: qiandan2104@163.com.
Ju Ying Zhong, Email: zhongjuying@hotmail.com.
Meng Meng Jin, Email: jinmengmeng301@163.com.
Peng Huang, Email: harryhp@vip.sina.com.
Lu Yang Che, Email: geu_cheluyang@aliyun.com.
Bing Pan, Email: stone_panbing@126.com.
Yi Wang, Email: prof.wangyi@foxmail.com.
Zhen Xiao Sun, Phone: +86-10-84738646, Email: sunzxcn@hotmail.com.
Chang Zhen Liu, Phone: +86-10-64089526, Email: lcz0220@163.com.
References
- 1.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8(10):782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 2.Szliszka E, Jaworska D, Ksek M, Czuba ZP, Krol W. Targeting death receptor TRAIL-R2 by chalcones for TRAIL-induced apoptosis in cancer cells. Int J Mol Sci. 2012;13(11):15343–15359. doi: 10.3390/ijms131115343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14(3–4):337–348. doi: 10.1016/S1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 4.Dai X, Zhang J, Arfuso F, Chinnathambi A, Zayed ME, Alharbi SA, Kumar AP, Ahn KS, Sethi G. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp Biol Med (Maywood) 2015;240(6):760–773. doi: 10.1177/1535370215579167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan D, Zhang B, Yao J, Liu Y, Fang J. Shikonin targets cytosolic thioredoxin reductase to induce ROS-mediated apoptosis in human promyelocytic leukemia HL-60 cells. Free Radic Biol Med. 2014;70:182–193. doi: 10.1016/j.freeradbiomed.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Dyer MJ, MacFarlane M, Cohen GM. Barriers to effective TRAIL-targeted therapy of malignancy. J Clin Oncol. 2007;25(28):4505–4506. doi: 10.1200/JCO.2007.13.1011. [DOI] [PubMed] [Google Scholar]
- 7.Goncharenko-Khaider N, Lane D, Matte I, Rancourt C, Piche A. The inhibition of bid expression by Akt leads to resistance to TRAIL-induced apoptosis in ovarian cancer cells. Oncogene. 2010;29(40):5523–5536. doi: 10.1038/onc.2010.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong K, Li W. Shikonin, a Chinese plant-derived naphthoquinone, induces apoptosis in hepatocellular carcinoma cells through reactive oxygen species: a potential new treatment for hepatocellular carcinoma. Free Radic Biol Med. 2011;51(12):2259–2271. doi: 10.1016/j.freeradbiomed.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12(12):931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 10.He G, He G, Zhou R, Pi Z, Zhu T, Jiang L, Xie Y. Enhancement of cisplatin-induced colon cancer cells apoptosis by shikonin, a natural inducer of ROS in vitro and in vivo. Biochem Biophys Res Commun. 2016;469(4):1075–1082. doi: 10.1016/j.bbrc.2015.12.100. [DOI] [PubMed] [Google Scholar]
- 11.Heasley LE, Han SY. JNK regulation of oncogenesis. Mol Cells. 2006;21(2):167–173. [PubMed] [Google Scholar]
- 12.Huang S. Regulation of metastases by signal transducer and activator of transcription 3 signaling pathway: clinical implications. Clin Cancer Res. 2007;13(5):1362–1366. doi: 10.1158/1078-0432.CCR-06-2313. [DOI] [PubMed] [Google Scholar]
- 13.Lee DH, Sung KS, Bartlett DL, Kwon YT, Lee YJ. HSP90 inhibitor NVP-AUY922 enhances TRAIL-induced apoptosis by suppressing the JAK2-STAT3-Mcl-1 signal transduction pathway in colorectal cancer cells. Cell Signal. 2015;27(2):293–305. doi: 10.1016/j.cellsig.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Liu J, Jackson K, Shi R, Zhao Y. Sensitizing the therapeutic efficacy of taxol with shikonin in human breast cancer cells. PLoS One. 2014;9(4):e94079. doi: 10.1371/journal.pone.0094079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Algeciras-Schimnich A, Barnhart BC, Peter ME. Apoptosis-independent functions of killer caspases. Curr Opin Cell Biol. 2002;14(6):721–726. doi: 10.1016/S0955-0674(02)00384-8. [DOI] [PubMed] [Google Scholar]
- 16.Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson DW, Briand C, Grutter MG. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem. 2002;277(47):45162–45171. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- 17.Werner AB, de Vries E, Tait SW, Bontjer I, Borst J. Bcl-2 family member Bfl-1/A1 sequesters truncated bid to inhibit is collaboration with pro-apoptotic Bak or Bax. J Biol Chem. 2002;277(25):22781–22788. doi: 10.1074/jbc.M201469200. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Shi HY, Zhang M. Maspin overexpression modulates tumor cell apoptosis through the regulation of Bcl-2 family proteins. BMC Cancer. 2005;5:50. doi: 10.1186/1471-2407-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94(4):491–501. doi: 10.1016/S0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 20.Nagata S. Apoptosis by death factor. Cell. 1997;88(3):355–365. doi: 10.1016/S0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Zhang C, Ren A, Li T, Jin R, Li G, Gu X, Shi R, Zhao Y. Shikonin suppresses skin carcinogenesis via inhibiting cell proliferation. PLoS One. 2015;10(5):e0126459. doi: 10.1371/journal.pone.0126459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Fan XX, Jiang ZB, Loo WT, Yao XJ, Leung EL, Chow LW, Liu L. Shikonin inhibits gefitinib-resistant non-small cell lung cancer by inhibiting TrxR and activating the EGFR proteasomal degradation pathway. Pharmacol Res. 2017;115:45–55. doi: 10.1016/j.phrs.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Ma X, Wang Y, Li G. miR-489 inhibits proliferation, cell cycle progression and induces apoptosis of glioma cells via targeting SPIN1-mediated PI3K/AKT pathway. Biomed Pharmacother. 2017;93:435–443. doi: 10.1016/j.biopha.2017.06.058. [DOI] [PubMed] [Google Scholar]
- 24.Liang W, Cai A, Chen G, Xi H, Wu X, Cui J, Zhang K, Zhao X, Yu J, Wei B, et al. Shikonin induces mitochondria-mediated apoptosis and enhances chemotherapeutic sensitivity of gastric cancer through reactive oxygen species. Sci Rep. 2016;6:38267. doi: 10.1038/srep38267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao X, Yu CR, Li WH, Li WX. Induction of apoptosis by shikonin through a ROS/JNK-mediated process in Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell Res. 2008;18(8):879–888. doi: 10.1038/cr.2008.86. [DOI] [PubMed] [Google Scholar]
- 26.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388(6638):190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 27.Shirley S, Micheau O. Targeting c-FLIP in cancer. Cancer Lett. 2013;332(2):141–150. doi: 10.1016/j.canlet.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Sun C, Cai M, Meadows RP, Xu N, Gunasekera AH, Herrmann J, Wu JC, Fesik SW. NMR structure and mutagenesis of the third Bir domain of the inhibitor of apoptosis protein XIAP. J Biol Chem. 2000;275(43):33777–33781. doi: 10.1074/jbc.M006226200. [DOI] [PubMed] [Google Scholar]
- 29.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 30.Nachmias B, Ashhab Y, Ben-Yehuda D. The inhibitor of apoptosis protein family (IAPs): an emerging therapeutic target in cancer. Semin Cancer Biol. 2004;14(4):231–243. doi: 10.1016/j.semcancer.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 31.LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG. IAP-targeted therapies for cancer. Oncogene. 2008;27(48):6252–6275. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]
- 32.Min R, Tong J, Wenjun Y, Wenhu D, Xiaojian Z, Jiacai H, Jian Z, Wantao C, Chenping Z. Growth inhibition and induction of apoptosis in human oral squamous cell carcinoma Tca-8113 cell lines by Shikonin was partly through the inactivation of NF-kappaB pathway. Phytother Res. 2008;22(3):407–415. doi: 10.1002/ptr.2340. [DOI] [PubMed] [Google Scholar]
- 33.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475(7355):231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Rushworth SA, Micheau O. Molecular crosstalk between TRAIL and natural antioxidants in the treatment of cancer. Br J Pharmacol. 2009;157(7):1186–1188. doi: 10.1111/j.1476-5381.2009.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277(5327):818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 36.Shodeinde AL, Barton BE. Potential use of STAT3 inhibitors in targeted prostate cancer therapy: future prospects. Onco Targets Ther. 2012;5:119–125. doi: 10.2147/OTT.S32559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song J, Zhao Z, Fan X, Chen M, Cheng X, Zhang D, Wu F, Ying X, Ji J. Shikonin potentiates the effect of arsenic trioxide against human hepatocellular carcinoma in vitro and in vivo. Oncotarget. 2016;7(43):70504–70515. doi: 10.18632/oncotarget.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan W, Zhu S, Cao J, Zhang L, Li W, Liu K, Zhong J, Shang C, Chen Y. Inhibition of MMP-2 expression enhances the antitumor effect of Sorafenib in hepatocellular carcinoma by suppressing the PI3K/AKT/mTOR pathway. Oncol Res. 2017;25(9):1543–1553. doi: 10.3727/096504017X14886444100783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorburn A, Behbakht K, Ford H. TRAIL receptor-targeted therapeutics: resistance mechanisms and strategies to avoid them. Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy. 2008;11(1–2):17–24. doi: 10.1016/j.drup.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tournier C. The 2 faces of JNK signaling in Cancer. Genes Cancer. 2013;4(9–10):397–400. doi: 10.1177/1947601913486349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8(7):579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 42.Wang S. The promise of cancer therapeutics targeting the TNF-related apoptosis-inducing ligand and TRAIL receptor pathway. Oncogene. 2008;27(48):6207–6215. doi: 10.1038/onc.2008.298. [DOI] [PubMed] [Google Scholar]
- 43.Wei Y, Li M, Cui S, Wang D, Zhang CY, Zen K, Li L. Shikonin Inhibits the Proliferation of Human Breast Cancer Cells by Reducing Tumor-Derived Exosomes. Molecules (Basel, Switzerland). 2016;21(6). [DOI] [PMC free article] [PubMed]
- 44.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3(6):673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 45.Zhang H, Zhang D, Luan X, Xie G, Pan X. Inhibition of the signal transducers and activators of transcription (STAT) 3 signalling pathway by AG490 in laryngeal carcinoma cells. J Int Med Res. 2010;38(5):1673–1681. doi: 10.1177/147323001003800512. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Z, Lv B, Zhang L, Zhao N, Lv Y. miR-202 functions as a tumor suppressor in non-small cell lung cancer by targeting STAT3. Mol Med Rep. 2017;16(2):2281–2289. doi: 10.3892/mmr.2017.6841. [DOI] [PubMed] [Google Scholar]
- 47.Trivedi R, Maurya R, Mishra DP. Medicarpin, a legume phytoalexin sensitizes myeloid leukemia cells to TRAIL-induced apoptosis through the induction of DR5 and activation of the ROS-JNK-CHOP pathway. Cell Death Dis. 2014;5:e1465. doi: 10.1038/cddis.2014.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Harvard Dataverse doi:10.7910/DVN/VD8ZBV