Abstract
The site of crucial damage that causes acquired apraxia of speech (AOS) has been debated in the literature. This study presents five in-depth cases that offer insight into the role of brain areas involved in AOS. Four of the examined participants had a primary impairment of AOS either with (n=2) or without concomitant mild aphasia (n=2). The fifth participant presented with a lesion relatively isolated to the left anterior insula (AIns-L), damage that is rarely reported in the literature, but without AOS. Taken together, these cases challenge the role of the AIns-L and implicate the left motor regions in AOS.
Keywords: Aphasia, apraxia of speech, inferior frontal gyrus, insula, motor cortex, motor speech assessment, rolandic operculum
Introduction
Overview of AOS
Apraxia of speech (AOS) is a motor speech disorder characterized by impaired planning and programming of sensorimotor movements for speech. Speech characteristics include off-target articulation, visible and audible articulatory groping, atypical prosody (e.g. decreased rate of speech, prolongations), and variable attempts to self-correct incorrect productions, with error rates increasing upon utterance length and complexity (Darley, 1982, Darley, Aronson, & Brown, 1975; Duffy, 2005; Wertz, LaPointe, & Rosenbek, 1984).
AOS is a distinct impairment that can occur independent of language disturbances (aphasia) and/or neuromuscular involvement (dysarthria) (Darley et al., 1975; Duffy, 2005; Duffy, 2006; McNeil, Robin, & Schmidt, 1997). Estimates suggest that approximately 4% of individuals diagnosed with an acquired neurological communication disorder present with AOS as the primary disorder, although individuals with stroke-induced AOS as their only communication impairment are rarely reported in the literature (Duffy, 2005; Graff-Radford et al., 2014). In fact, in patients with AOS, there is an estimated co-occurrence of aphasia in 81%, dysarthria in 29–47%, and nonverbal oral apraxia in 48–75% of cases (Duffy, 2005; McNeil, Doyle, & Wambaugh, 2000). To emphasize the difficulty of studying patients with post-stroke AOS as the primary presenting impairment, Graff-Radford et al. (2014) reported that in a chart review of patients who received speech-language evaluations at the Mayo Clinic over a period of 14 years, only seven patients were identified with “pure” AOS (defined as “absent or equivocal aphasia,” Graff-Radford et al., 2014, p.44). Accordingly, because acquired AOS commonly co-occurs with other speech and/or language disturbances, it is difficult to isolate the brain-behavior relationship specific to AOS. For example, if a common lesion site is found in patients with AOS and concomitant aphasia, it is impossible to determine if damage to this region is associated with AOS, aphasia, or both. Similarly, in the case of large lesions affecting multiple regions, identifying the exact damage responsible for the behavioral deficits is difficult. Thus, attempts to determine the characteristic behaviors for differential diagnosis, as well as the neuroanatomical localization of AOS, have generated significant debate (Ballard, Granier, & Robin, 2000; Haley, Jacks, de Riesthal, Abou-Khalil, & Roth, 2012; Ogar et al., 2005; Ziegler, Aichert, & Staiger, 2012; Mumby, Bowen, & Hesketh, 2007; Strand et al., 2014).
Neuroanatomical correlates
Acquired AOS typically occurs due to stroke affecting the language-dominant hemisphere (Duffy, 2005), but may also be caused by a degenerative process (Josephs et al., 2006; Josephs et al., 2012; Whitwell et al., 2013), tumor, or traumatic injury (Duffy, 2005). Several studies have attempted to identify the location of crucial brain damage that results in AOS, arguing for primary involvement of the left anterior insula (AIns-L; Dronkers, 1996; Marien, Pickut, Engelborghs, Martin, & De Deyn, 2001; Nagao, Takeda, Komori, Isozaki, & Hirai, 1999; Ogar et al., 2005), Broca’s area/left inferior frontal gyrus (IFG-L; Hillis et al., 2004; Richardson, Fillmore, Rorden, LaPointe, & Fridriksson, 2012; Schiff, Alexander, Naeser, & Galaburda, 1983; Shuren, 1993; Trupe et al., 2013), or the left motor, premotor and supplementary motor areas (Josephs et al., 2006; Josephs et al., 2012; Graff-Radford et al., 2014; Whitwell et al., 2013).
Initially, the role of the insula in motor speech production received substantial attention following Dronkers’ seminal study (1996), where 100% lesion overlap was found in the AIns-L, specifically the superior precentral gyrus of the insula (SPGI), in post-stroke individuals with chronic AOS. A follow-up study by Ogar and colleagues (2006) found that lesions were restricted to the SPGI in individuals with mild AOS; but extended into the middle frontal gyrus, Broca’s area, basal ganglia, and the internal/external capsule in individuals with moderate AOS, and into the fibers of the superior longitudinal fasciculus in those with severe AOS.
Although the findings of Dronkers (1996) and Ogar et al. (2006) are compelling, there are some inherent limitations in interpreting these data. Notably, lesion overlap methods do not account for the fact that due to the nature of cerebral blood flow, certain areas are more likely than others to experience damage from stroke (Rorden & Karnath, 2004). The location of the insula makes it highly susceptible to hypoperfusion following large middle cerebral artery (MCA) strokes (Payabvash et al., 2011), and it is often damaged in individuals where the inferior frontal lobe is also damaged (Finley et al., 2003; Hillis et al., 2004). This is a phenomenon that could explain Dronkers’ (1996) findings of 100% lesion overlap in insula in those with chronic AOS. Furthermore, to our knowledge, cases of isolated insula damage are rarely reported in the literature. Cereda et al. (2002) reported that in a chart review of 4,800 strokes over a nine-year period, only four individuals experienced isolated infarct to the insula (two with left insula and two with right insula infarct). Additionally, Nagao et al. (1999) provided a case study of a patient with isolated insula infarct. However, neither provided sufficient detail regarding the extent to which patients were indeed diagnosed with AOS. For example, Nagao et al. (1999) reported that their patient demonstrated “difficulty in initiating utterances and inconsistency in repeated production…” (p. 41), but these behaviors could also be attributed to aphasia. Cereda et al. (2002) described production impairments in the two patients with left insula damage, but only one presented with non-fluent aphasia. Again, there were insufficient details reported to adjudicate whether this patient had AOS or if the difficulties were attributable to aphasia.
In a study of acquired AOS during the acute phase, Hillis and colleagues (2004) utilized structural images as well as perfusion weighted and diffusion weighted imaging to examine areas that may have been structurally intact, but functionally impaired due to hypoperfusion. In contrast to the findings of Dronkers (1996) and Ogar et al. (2006), AOS was associated with structural and/or functional (hypoperfusion) damage to Broca’s area, but not the AIns-L.
Building upon these discrepant findings, Richardson and colleagues (2012) compared a lesion overlap approach to voxel-wise lesion-symptom mapping (VLSM) in 26 individuals with chronic AOS and 24 without AOS to determine if different methodologies would yield the same results. Findings differed based on the methodology employed. Using lesion overlap, greatest overlap was found in the left middle insula for the individuals with AOS, while VLSM results revealed that damage and/or hypoperfusion to the left inferior prefrontal cortex pars opercularis (IPCpo-L) and premotor cortex were the greatest predictors of AOS. These findings suggest that the lesion overlap method (Dronkers, 1996; Ogar et al., 2006) may over-represent the involvement of the AIns-L in AOS and motor speech production. However, it should be noted that both methods are subject to the inherent problems; and therefore, have limitations in analyzing stroke-induced lesions.
Because stroke-induced lesions that lead to AOS are often large and involve multiple brain areas (Hillis et al., 2004), it is challenging to isolate its precise neurological correlates. However, when AOS occurs secondary to degenerative disease, the damage is not constrained by vasculature and is often much more isolated, particularly in mild cases and/or early in the progression of the disease. Progressive AOS (PAOS) has been described as a neurodegenerative process in which AOS (defined by current diagnostic criteria; Duffy & Josephs, 2012) is the primary presenting impairment (Duffy & Josephs, 2012; Josephs et al., 2006, Josephs et al., 2012; Whitwell et al., 2013). Neuroimaging findings show that PAOS is associated with hypometabolism and grey/white matter loss in the left premotor cortex and supplementary motor areas, without damage to the insula (Josephs et al., 2006, 2012; Whitwell et al., 2013). Interestingly, there are several studies that have reported damage to the left hemisphere premotor areas in post-stroke individuals with AOS and no aphasia (e.g., Graff-Radford et al., 2014; Ogar et al., 2006; Seddoh et al., 1996); however, damage to other structures (e.g., insula, Broca’s area) in these studies makes it difficult to infer the role that each location plays in AOS. Notably, in a study involving 7 persons with post-stroke AOS (with no or equivocal aphasia), Graff-Radford and colleagues (2014) found damage to the premotor cortex in 6/7 cases, while damage to primary motor cortex, Broca’s area, and the left insula occurred in 7/7, 3/7, and 2/7 cases, respectively.
Discrepancies remain regarding the site of damage responsible for disrupted speech motor planning in post-stroke AOS, and coexisting behavioral diagnoses can confound attempts to establish brain-behavior relationships. It is imperative that cases of stroke-induced AOS as a primary impairment, although rare, be examined to help adjudicate areas implicated in AOS and motor speech production (Duffy & Josephs, 2012). In the current study, five in-depth case studies are explored to examine the role of the neuroanatomical correlates of AOS. We sought to isolate variables either behaviorally, based on the presence of either pure AOS (no concomitant aphasia) or prominent AOS (presenting primarily with AOS symptoms, with minor symptoms of anomic aphasia); or anatomically, based on the presence of a lesion primarily isolated to the insula. Given conflicting results from previous group studies, this study strategically selected and examined individual participants that may inform the localization of AOS.
Method
Participants
This study included five participants (three females, all native English speakers and premorbidly right-handed) with brain damage secondary to a single-event left hemisphere ischemic stroke (mean age =55; SD=15.7; range=41–77). Time post-stroke ranged between 10–69 months (mean=33; SD=25), and participants’ education level ranged from 14–18 years (mean = 16.2; SD=1.8). Participant characteristics are listed in Table 1. Participants were recruited from a database of over 75 individuals with left hemisphere stroke who participated in research studies at the University of South Carolina over the past 10 years. Inclusion for the current study required participants to either: (a) present with AOS as their primary deficit, or (b) have a lesion isolated to the insula. Of the 75 individuals in the database, only these five participants fulfilled the inclusion criteria. This low rate of eligibility (less than 7%) reflects a fundamental challenge in conducting research in this population. All procedures complied with the regulations of the University of South Carolina Institutional Review Board. Participants provided informed consent prior to testing.
Table 1.
Participant demographic information.
| AOS1 | AOS2 | AOS+AA1 | AOS+AA2 | AA1 | Median (Range) | |
|---|---|---|---|---|---|---|
| Gender | F | F | M | M | F | F:M (3:2)+ |
| Age at Testing | 46 | 41 | 77 | 66 | 45 | 46 (41–77) |
| Handedness (Pre-Morbid) | R | R | R | R | R | R:L (5:0)+ |
| Education | 18 | 18 | 16 | 14 | 15 | 16 (15–18) |
| Stroke Age | 45 | 40 | 76 | 62 | 39 | 45 (39–76) |
| Months Post-Stroke | 10 | 19 | 18 | 49 | 69 | 19 (10–69) |
Indicates ratio instead of median and range
Speech & language assessment
Participants completed a battery of speech and language assessments administered by two American Speech-Language-Hearing Association (ASHA) certified speech-language pathologists (CCC-SLPs). Testing was recorded to allow for scoring offline. For the purpose of differential diagnosis, the Apraxia of Speech Rating Scale (ASRS; Strand et al., 2014), a relatively new tool for differential diagnosis, served as the primary measure to distinguish speech errors related to AOS, dysarthria, and aphasia. The speech characteristics included on this scale delineate speech abnormalities as features that can occur (a) exclusively in AOS; (b) due to AOS and/or dysarthria; (c) due to AOS and/or aphasia, and (d) due to AOS/dysarthria/aphasia. Ratings are based on a 5-point scale (0=not present; 1 = detectable but not frequent; 2 = frequent but not pervasive; 3 = nearly always evident but not marked in severity; 4 = nearly always evident and marked in severity).
The ASRS was scored offline from video recordings of all testing. The motor speech evaluation (MSE; Duffy, 2005) was used as the primary assessment to evaluate speech production from easy to complex articulatory demands. The same ASHA-certified SLPs who administered the assessments independently completed ASRS ratings for each participant. Intraclass correlation coefficients (ICC) were computed for each of the 16 items rated on the ASRS for each participant. The two raters demonstrated high reliability (average measure ICC = .954). In cases of disagreement, videos were reviewed and the raters came to a forced agreement.
Additionally, the Western Aphasia Battery-Revised (WAB-R) Part 1 (Kertesz, 2007), and the Frenchay Dysarthria Assessment, 2nd Edition (FDA-2; Enderby & Palmer, 2007) were given as a supplement to the MSE and to provide standardized measures for assessing language abilities (WAB-R) and the integrity of the oral mechanism (FDA-2). The FDA-2 was the primary means for characterizing dysarthria characteristics. It should be noted that some domains (i.e. respiration, palate, laryngeal) on the FDA-2 are frequently affected in dysarthria, but fall within normal limits for people with AOS while other domains can be affected in AOS as well (i.e. lips, tongue, intelligibility). Therefore, additional tests were given to thoroughly assess speech and language. Scores for these additional tests are presented in the Supplemental Materials. While these psychometric tools independently fail to provide valid and reliable measures for differentially diagnosing AOS versus aphasia versus dysarthria, collectively, they provide substantial information to inform clinical judgment.
Neuroimaging
MRI Data Collection
MRI data were acquired using a Siemens 3T system with a 12-element head coil. Participants were scanned with high-resolution T1 and T2 MRI sequences. The T1 sequence utilized a turbo field echo sequence (MP-RAGE) with the following parameters: FOV = 256 × 256 mm, 160 sagittal slices, 15 degree flip angle, TI = 900 ms, TR = 9.5 ms, TE = 5.7 ms. The T2 sequence utilized a SPACE (Sampling Perfection with Application optimized Contrasts by using different flip angle Evolutions) protocol with the following parameters: field of view (FOV) = 256 × 256 mm, 160 sagittal slices, variable degree flip angle, TR = 3200 ms, TE = 352 ms.
Preprocessing & analyses
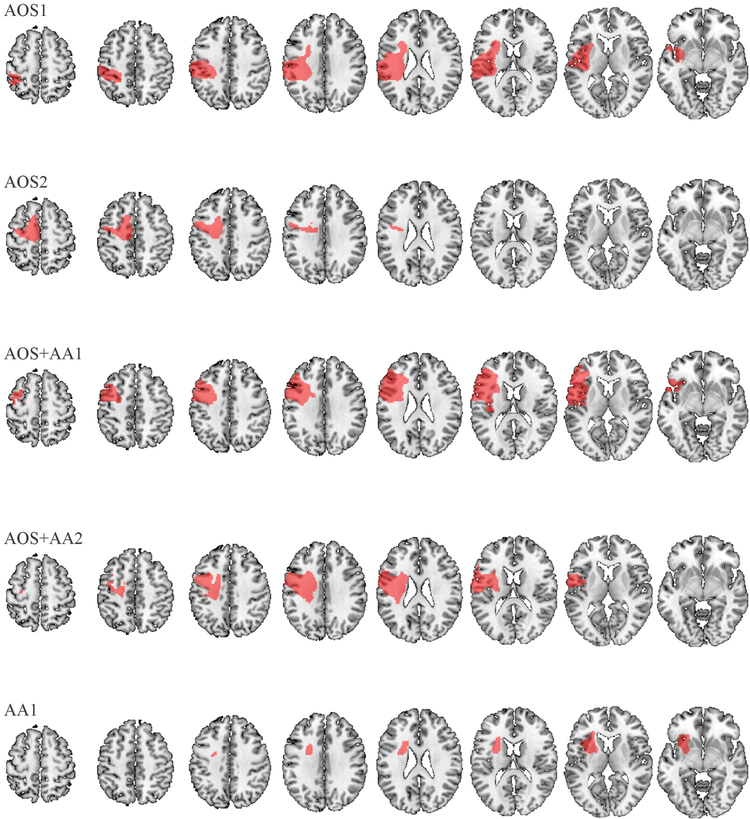
The high-resolution MR images were preprocessed for analyses using the Clinical Toolbox (Rorden et al., 2012), a custom toolbox for the Statistical Parametric Mapping software package (SPM8, Friston et al., 1995). Brain lesions were demarcated by a neurologist on the native T2-weighted MRI for each participant using MRIcron (Rorden & Brett, 2009). T2-weighted and lesion images were coregistered to the T1-weighted image, which was then segmented and normalized to the MNI152 template using SPM’s standard unified-segmentation normalization procedures and cost function masking (2 mm isotropic voxels). Images were visually inspected after normalization to ensure accuracy. Normalized lesion images were then individually overlaid onto the MNI152 template using MRIcron to visually highlight regions of damage in each participant (as presented in Figure 2).
Figure 2.
Lesion maps for each participant. Each normalized lesion (red) is displayed in axial slices (superior to inferior) on a standard brain template (MNI152).
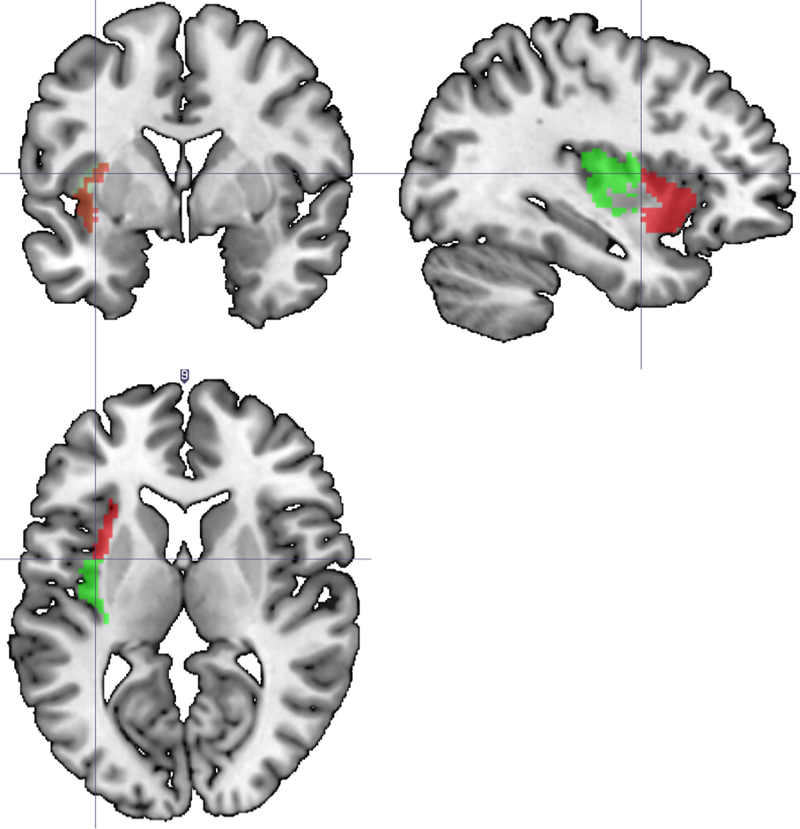
The proportion of damage to specific neuroanatomical locations, defined by the Automated Anatomical Labeling (AAL; Tzourio-Mazoyer et al., 2000) map, was computed using the batch descriptives function in MRICron (Rorden & Brett, 2009). Further analysis between the anterior and posterior insula was completed by dividing the insula along the anterior-posterior axis at its midpoint (see Figure 1), with proportional damage of these regions computed using a customized code in Matlab (The Mathworks, 2007). Each participant’s damage was reviewed on a case-by-case basis to interpret behavioral profiles with respect to lesion locations.
Figure 1.
Locations of left anterior (red) and left posterior (green) insula used for localization of insula damage. The insula was divided along the anterior-posterior axis at its midpoint.
Results
Summary of behavioral results
Categorization of Participants Based on Behavioral Presentation
Participants were classified based on the presence/absence of AOS and/or anomic aphasia (AA). These groups are as follows: two participants presented with AOS in the absence of aphasia (AOS1 & AOS2); two participants with both AOS and AA (AOS+AA1 & AOS+AA2); and one participant with only AA (AA1).
AOS was the primary impairment in four of the five participants (AOS1, AOS2, AOS+AA1, AOS+AA2). For the participants with concomitant aphasia (AOS+AA1, AOS+AA2), AOS was more severe relative to language deficits. Testing results from AA1 are consistent with mild anomic aphasia, without concomitant AOS. Overall patterns and behavioral profiles for each participant are presented in the sections that follow.
Overall speech and language assessment
Four of the five participants received a diagnosis of AOS based on their performance on the ASRS (Strand et al., 2014). On the ASRS, each of these four participants presented with at least four of the six behaviors attributed to AOS only (i.e. speech disturbances that would not be attributable to aphasia and/or dysarthria). Although these four participants additionally demonstrated behaviors that may occur in either dysarthria or AOS, only one of these participants (AOS+AA2) had mild concomitant dysarthria based on ASRS ratings and evidence from further testing (See Supplemental Materials for more detail). ASRS ratings for each participant are presented in Table 4.
Table 4.
Apraxia of Speech Rating Scale (ASRS) ratings for each participant.
| AOS1 | AOS2 | AOS+AA1 | AOS+AA2 | AA1 | |||
|---|---|---|---|---|---|---|---|
| AOS | |||||||
| Distorted sound substitutions | 2 | 2 | 2 | 4 | 0 | ||
| Distorted sound additions (not intrusive schwa) | 1 | 2 | 0 | 2 | 0 | ||
| Increased distortions/distorted sound substitutions with increased utterance length or increased syllable/word articulatory complexity | 2 | 3 | 3 | 4 | 0 | ||
| Increased sound distortions/distorted sound substitutions with increased speech rate | 2 | 3 | 3 | 4 | 0 | ||
| Inaccurate AMR’s | 2 | 0 | 0 | 2 | 0 | ||
| Reduced words/breath group | 3 | 2 | 1 | 4 | 0 | ||
| Total (24) | 12 | 11 | 9 | 20 | 0 | ||
| AOS/Dysarthria | |||||||
| Syllable segmentation within words >1 syllable | 3 | 2 | 1 | 4 | 0 | ||
| Syllable segmentation across words in phrases/sentences | 3 | 2 | 2 | 4 | 0 | ||
| Sound distortions | 2 | 2 | 2 | 4 | 0 | ||
| Slow overall rate | 3 | 1 | 1 | 4 | 0 | ||
| Lengthened vowel &/or consonant segments | 2 | 1 | 1 | 4 | 0 | ||
| Lengthened intersegment durations | 3 | 2 | 0 | 4 | 0 | ||
| Total (24) | 16 | 10 | 7 | 24 | 0 | ||
| AOS/Aphasia | |||||||
| Deliberate, slowly sequenced, segmented, &/or distorted SMRs compared to AMRs | 3 | 0 | 3 | 4 | 2 | ||
| Audible/visual articulatory groping; initiation difficulty; false starts/restarts | 3 | 2 | 2 | 4 | 0 | ||
| Total (8) | 6 | 2 | 5 | 8 | 2 | ||
| AOS/Dysarthria/Aphasia | |||||||
| Sound/syllable repetitions | 2 | 1 | 1 | 2 | 0 | ||
| Sound prolongations (beyond lengthened segments) | 0 | 0 | 0 | 0 | 0 | ||
| Total (8) | 2 | 1 | 1 | 2 | 0 | ||
Note: The presence/severity of each speech characteristics are rated based on a 5-point scale (0=not present; 1 = detectable but not frequent; 2 = frequent but not pervasive; 3 = nearly always evident but not marked in severity; 4 = nearly always evident and marked in severity).
Three of the five participants presented with mild anomic aphasia (average WAB-R AQ = 90, S.D.=4.3). Overall, participants presented with relatively intact Information Content, Fluency, and Comprehension subtest scores, consistent with their aphasia classification (i.e., absent or anomic). High performance on WAB-R Repetition subtest was noted across all, ruling out possible conduction aphasia. See Table 2 for WAB-R Part 1 scores for all participants.
Table 2.
Performance on the Western Aphasia Battery (WAB) Part 1.
| AOS1 | AOS2 | AOS+AA1 | AOS+AA2 | AA1 | Median (Range) | |
|---|---|---|---|---|---|---|
| Aphasia Presence (y/n; severity) | N | N | Y; Mild | Y; Mild | Y; Mild | Y:N (3:2)+ |
| Aphasia Type | N/A | N/A | Anomic | Anomic | Anomic | Anomic: N/A (3:0)+ |
| Aphasia Quotient (/100) | 94.6 | 94.2 | 88.2 | 84.4 | 88.6 | 88.6 (84.4–94.6) |
| Information Content (/10) | 9 | 9 | 9 | 8 | 9 | 9 (8–9) |
| Fluency (/10) | 9 | 9 | 8 | 6 | 9 | 9 (6–9) |
| Spontaneous Speech (/20) | 18 | 18 | 17 | 14 | 18 | 18 (12–18) |
| Comprehension (/10) | 10 | 10 | 8.5 | 9 | 9.2 | 9.2 (8.5–10) |
| Repetition (/10) | 9.7 | 9.6 | 9.8 | 9.4 | 8.2 | 9.6 (8.2–9.8) |
| Naming/Word Finding (/10) | 9.6 | 9.5 | 8.8 | 8.5 | 8.9 | 8.9 (8.5–9.6) |
Indicates ratio instead of median and range
Detailed behavioral results
AOS1 (Participant with apraxia of speech without aphasia #1)
AOS1’s impairment was specific to AOS. This participant did not present with aphasia, as her Aphasia Quotient (AQ) of 94.6 is above the 93.8 cutoff for WAB-R aphasia diagnosis. Additionally, the errors noted on the WAB-R could be attributed to AOS instead of aphasia. For example, this participant’s performance on the picture description task was complete and grammatically intact, but a reduced score was received on the Fluency scale due to a decreased rate of speech and to dysfluencies that could be attributed to increased demands in speech production, rather than problems with conceptualization or word finding. This participant’s naming performance on the BNT and PNT further support this assertion (Supplemental Table 1). AOS1’s speech production was moderately impaired and most notably characterized by a slow overall rate, reduced number of words per breath, and altered prosody (syllable segmentation within single words and across words in phrases/sentences, lengthened durations of phonemes and between word segments). These are speech characteristics that are not associated with aphasia. Difficulties with speech production were noted across tasks, except at the single, monosyllabic word level. Overall production was characterized by altered prosody, voicing errors, visible and audible groping, and initiation difficulty. Sound substitutions were more evident as articulatory complexity increased (e.g., for “statistical analysis”: /stɪsɾkļɪs ənælɪsɪs/). ASRS ratings for specific AOS characteristics across tasks are presented in Table 4. The FDA-2 (see Table 3) revealed mild impairment in speech intelligibility, as well as mild impairment in motor tasks involving the lips and tongue. However, this participant did not demonstrate motor weakness and/or spasticity in these articulators (which would be indicative of dysarthria), but rather, the manifestation of these errors was indicative of difficulties with the coordination of the articulators (e.g. distorted sound substitutions and increased errors with increased utterance length, articulatory complexity, or speech rate). Thus, these errors are better explained by AOS, rather than difficulties with motor execution.
Table 3.
Frenchay Dysarthria Assessment-2nd Edition (FDA-2) ratings for each participant.
| FDA-2 Domain | AOS1 | AOS2 | AOS+AA1 | AOS+AA2 | AA1 |
|---|---|---|---|---|---|
| Reflexes | WNL | WNL | WNL | Mild drooling | Occasional difficulty swallowing |
| Respiration | WNL | WNL | WNL | Mild difficulty in speech | WNL |
| Lips | Mild in alternating, occasional omissions in speech | Mild asymmetry at rest/spread, inconsistent rounding/spreading | Mild asymmetry at rest/spread | Mild in alternating & speech; Mod. asymmetry at rest/spread | Slight asymmetry at rest, spread, seal |
| Palate | WNL | WNL | WNL | WNL | WNL; Cleft lip/palate repair at 1 yr, 16 yrs |
| Laryngeal | WNL | WNL | WNL | Mild impairment with time, pitch, volume, speech | WNL |
| Tongue | Mild in alternating, occasional misarticulations in speech | Moderate difficulty with elevation; mild impairment in speech | Mild difficulty with protrusion, misarticulations in speech | Mild at rest/protrusion, moderate with alternating, severe during speech | WNL |
| Intelligibility | Mild in conversation; normal for words/sentences | Moderate in words, mild in conversation | Mild in conversation; moderate in words/sentences | Moderate in conversation, severe in words/sentences | WNL |
Note: Domains frequently affected in dysarthria, and typically WNL in AOS, are presented in italics.
AOS2 (Participant with apraxia of speech without aphasia #2)
Similar to AOS1, AOS2’s difficulties were specific to AOS with moderate severity. WAB-R scores were within normal limits (AQ=94.2), and the production difficulties that were noted (i.e., in the Content, Fluency, and Repetition subscores) can be more accurately explained by disturbed speech (e.g. altered prosody, syllable segmentation, and distortions/distorted sound substitutions) rather than disturbed language processing. This participant’s naming performance on the BNT and PNT further support this assertion (Supplemental Table 1). With regard to the speech tasks, no difficulties were noted during phoneme repetition, but mild audible and visible groping for speech targets was noted even at the single-word/syllable level. Sentence repetition tasks were characterized by multiple false starts, revisions, articulation errors, and segmentation between words. Overall speech production (conversation and picture description) was marked by altered prosody (present in nearly every utterance), distortions upon increased speech rate and utterance complexity, syllable segmentation across words/phrases, sound substitutions, cluster reduction, vowel errors, visible and audible groping and frequently intrusive schwa (see ASRS ratings in Table 4). Mild to moderate impairment was revealed on the FDA-2 for speech intelligibility, which could be attributed to uncoordinated movement of the lips and tongue (Table 3). This patient did not demonstrate weakness or spasticity in these articulators, indicating motor execution was intact. Reduced intelligibility was secondary to the aforementioned characteristics related to AOS (particularly by distorted sound substitutions/additions and increased errors with increased utterance length, articulatory complexity, or speech rate). Thus, this participant’s performance on FDA-2 was not consistent with the presence of dysarthria.
AOS+AA1 (Participant with apraxia of speech and mild anomic aphasia #1)
AOS+AA1 presented with mild anomic aphasia (WAB-R AQ=88.2) and moderate AOS (Table 2 and Table 4). Most notably, articulation errors increased as task complexity increased. This participant demonstrated 80% accuracy on increasing word length tasks, 67% accuracy on monosyllabic word repetition (repeating a monosyllabic word three times), and 27% accuracy on multisyllabic word repetition. Errors throughout the speech tasks were primarily distorted sound substitutions, inconsistent errors with manner (e.g., /b/ for /v/ in “giving”) and place (e.g., /s/ for /θ/ in “thinks” and /b/ for /d/ in “day”), increased distortions upon increased rate/complexity, groping, difficulty with initiation, and false starts/restarts. Lip and tongue movements were mildly impaired, as was intelligibility at the word and conversation level. Once again, the below normal scores that were obtained on these subsystems were more consistent with characteristics of AOS, as movements were uncoordinated and this participant did not demonstrate difficulty with motor execution (Wertz et al., 1984).
AOS+AA2 (Participant with apraxia of speech and mild anomic aphasia #2)
AOS+AA2 presented with mild anomic aphasia (WAB-R AQ=84.4) and as the participant with the most severe AOS. Marked speech production impairments were noted across all spoken tasks. Of the 16 items on the ASRS, 11 were given a score of four, indicating the severity and pervasiveness of disturbed speech production associated with AOS. There were high error rates across speech tasks, even at the level of phoneme (61% accuracy for phoneme repetition) and monosyllabic word (47% accuracy on monosyllabic word repetition). As production difficulty increased, errors were evident in nearly all productions (e.g., repetition of increasing word length: 20% accuracy, repetition of multisyllabic words: 10% accuracy). Speech was characterized by cluster reduction, errors with manner or place, syllable segmentation across words in isolation as well as phrases and sentences, visible and auditory groping, distortions, and overall reduced intelligibility (see ASRS characteristics on Table 4). Obvious struggle with speech production was noted during picture description and conversation. Overall intelligibility was severely compromised.
It should be noted that AOS+AA2 demonstrated reduced fluency on the WAB-R picture description task; however, this performance was not due to agrammatism or non-fluent speech. Rather, due to the severity of this participant’s AOS, speech output was effortful and unintelligible, resulting in shorter utterances due to struggle with speech production. Additionally, this participant demonstrated some characteristics of mild dysarthria. Palate function was the only subsystem scored WNL on the FDA-2 (Table 3). Reflexes (e.g. drooling), respiration (during speech), lips (for speech and non-speech tasks), and laryngeal subsystems were mildly impaired. Moderate/severe impairment was noted for tongue movement (non-speech/speech; respectively) and intelligibility (conversation/words and sentences; respectively). Notably, many of the FDA-2 items use speech production tasks commonly affected in AOS (e.g., sentence repetition, AMRs and SMRs) to assess dysarthria. Due to the severity of AOS+AA2’s speech production impairments, performance on FDA-2 subtests could be confounded by AOS. Nevertheless, AOS+AA2’s impairment due to muscular involvement was mild and not sufficient to account for his severe deficits in speech production.
AA1 (Participant with anomic aphasia in the absence of apraxia of speech #1)
AA1 was classified with mild anomic aphasia (WAB-R AQ: 88.6), but did not present with errors attributable to AOS. Atypical articulation errors were not noted, and conversational speech was 100% intelligible. See Table 4 for specific ASRS ratings. Remarkable findings on the FDA-2 were slight right lip asymmetry at rest, spread, and seal and a self-report of recent onset of occasional difficulty managing pills and liquids. Reduced nasal resonance was noted, but attributed to the cleft lip and palate repair that occurred at one year and 16 years of age. All other subsystems were WNL (Table 3).
Results from neuroimaging
For each participant, brain regions with greater than 10% damage are reported. Table 5 presents lesion locations and proportion of damaged tissue for all. Areas of specific importance to this study were the AIns-L, the IFG-L, and the left motor/premotor/supplementary motor areas. The AIns-L was spared in one participant with AOS without aphasia (AOS2) and one participant with AOS and mild aphasia (AOS+AA2). Substantial AIns-L damage was found in the other two participants with AOS (AOS1; AOS+AA1), and the greatest proportion of damage to the AIns-L was seen in the participant without AOS (AA1). In terms of behavioral differences, the AIns-L was structurally spared in AOS+AA2, the participant with the most severe AOS, while nearly two-thirds was damaged in AA1, the participant without AOS.
Table 5.
Total lesion volumes and specific areas of structural damage for each participant.
| Participant | Areas Affected+ (Proportion Damaged) |
Lesion Volume |
|---|---|---|
| AOS1 | PIns (98.6%), HG (91%), ROL (69.6%), STL (44%), AIns (33%), PoCG (31.4%), putamen (14.7%) | 71.54 cm3 |
| AOS2 | SMA (31.4%), PreCG (23.3%), PCL (19.9%) | 31.23 cm3 |
| AOS+AA1 | IFGpo (95.8%), IFGpt (79.6%), ROL (69.1%), PreCG (53.9%), HG (49%), AIns (36.5%), PIns (33.6%), MFG (18.7%), PoCG (15.2%), STL (12.3%), STP (12 %) | 77.1 cm3 |
| AOS+AA2 | ROL (64.2%), IFGpo (40.9%), PreCG (33.6%), PoCG (18.1%), PIns (11.4%), HG (10.7%) | 51.7 cm3 |
| AA1 | AIns (62.6%), putamen (47.7%), pallidum (11%) | 31.23 cm3 |
All lesions were restricted to the left hemisphere
Abbreviations: AIns (anterior insula), HG (Heschl’s gyrus), IFGpo (inferior frontal gyrus – pars opercularis), IFGpt (inferior frontal gyrus – pars triangularis), PCL (paracentral lobule), PIns (posterior insula), PoCG (postcentral gyrus), PreCG (precentral gyrus), MFG (middle frontal gyrus), ROL (Rolandic operculum), SMA (supplementary motor area), STL (superior temporal lobe), STP (superior temporal pole)
Damage to the AIns-L co-occurred with damage to other areas of interest in the participants with AOS. Notably, one of the participants with AOS and mild aphasia (AOS+AA1) had damage to a large proportion of the left inferior frontal gyrus pars opercularis (IFGpo-L), inferior frontal gyrus pars triangularis (IFGpt-L), Rolandic operculum (ROL-L), and precentral gyrus (PreCG-L) in addition to the AIns-L damage. Interestingly, the other participant with the most severe AOS and mild aphasia (AOS+AA2) had substantial damage to the ROL-L, IFGpo-L, and PreCG-L, while the insula was spared. It is also interesting to note that damage to the IFG was only seen in the two participants with AOS and concomitant aphasia. The two participants with AOS without aphasia showed damage to either the ROL-L (AOS1) or the PreCG-L (AOS2). Thus, the PreCG-L was damaged in three of the four participants with AOS (AOS2, AOS+AA1, AOS+AA2). Additionally, the supplementary motor area (SMA) was damaged in one of the participants with AOS without aphasia (AOS2); the one participant with AOS that did not have any damage to the AIns-L or ROL-L. Additional lesion locations are presented in Table 5, and lesion maps for all participants are presented in Figure 2.
Discussion
Overview
Although concomitant aphasia is problematic in many previous studies of post-stroke AOS, participants with AOS were only included in this study if AOS was their only diagnosis (n=2) or primary deficit (n=2). Additionally, a rare participant with relatively isolated insula damage (n=1) provided insight into the effects of an insular lesion without damage to other cortical areas. As such, the results from this study provide an in-depth examination of individuals with patterns of brain damage that are of particular interest.
Participants with pure apraxia of speech (AOS without aphasia)
Localizing damage specific to AOS has been difficult, as the occurrence of AOS without aphasia is uncommon (McNeil et al., 1997; Graff-Radford et al., 2014). The two participants with isolated AOS (AOS1 and AOS2) provided essential data for understanding AOS. Behaviorally, these participants presented similarly, with speech characterized predominately by atypical prosody, and increasing distortions and substitutions as utterance complexity and rate increased. However, their lesion locations were markedly different. For AOS1, imaging revealed damage to parietal and sensory areas, as well as the AIns-L and ROL-L. For AOS2, damage was apparent in the primary motor (PreCG-L) and secondary motor (SMA) cortices, with parietal and posterior sensory areas intact. It is difficult to determine the specific role the AIns-L and ROL-L damage played in AOS1’s speech production deficits, as both regions have been implicated in AOS (Dronkers, 1996; Hillis et al., 2004; Richardson et al., 2012). Furthermore, the involvement of posterior regions of the Sylvan fissure and temporal/parietal areas also provokes an interesting question regarding whether damage to this region could disrupt the translation of phonological representations into motor plans. These areas are thought to be responsible for integrating sensorimotor information with speech motor information (Hickok & Poeppel, 2007; Hickok et al., 2014), and there is evidence from others studies to suggest involvement of post-central regions in AOS (see Basilakos et al., 2015; Hickok et al., 2014; Hillis et al., 2004; Odell et al., 1990).
In contrast, AOS2 had damage in the primary and secondary motor cortices, regions considered to be a part of the dorsal speech stream (Hickok & Poeppel, 2007). AOS2’s lesion location was similar to the findings of neurodegenerative cases of PAOS (Josephs et al., 2012; Whitwell et al., 2013) and support the hypothesis that relatively isolated damage to the motor areas (and secondary motor areas) is sufficient to produce the clinical presentation of stroke-induced AOS (Graff-Radford et al., 2014).
Differences in lesion location across these two participants, despite similar behavioral presentations, also suggest that AOS may be induced not only by lesions to a specific cortical area, but also by a functional disconnection from other brain regions. For instance, their profiles might represent damage to structures involved in speech production and/or feedback for error correction, but along different portions of speech processing and production streams (Hickok & Poeppel, 2007). As such, AOS1’s impairment could reflect damage to regions and/or white matter connections that provide informational input vital to the motor regions for motor speech planning.
All participants with apraxia of speech (with/without mild anomic aphasia)
The two participants with AOS and mild anomic aphasia (AOS+AA1 and AOS+AA2), also demonstrated disparity in the specific brain regions damaged. Both had substantial damage in the IFGpo-L and/or ROL-L, PreCG-L, and PoCG-L. Although the extent of brain damage to other regions varied, the one of particular interest is the AIns-L. AOS+AA1 had substantial damage to the AIns-L (37% damage), but this region was spared in AOS+AA2. When considered in conjunction with the results from AOS1 and AOS2, the commonality seen across all four participants with AOS is damage to the ROL-L (n=3; excluding AOS2) or PreCG-L (n=3; excluding AOS1). These findings provide support to previous studies that have implicated the PreCG-L in stroke-induced AOS (Graff-Radford et al., 2014). In contrast, the involvement of the IFG-L was present in both participants with AOS and concomitant aphasia, but not in the two participants with AOS in the absence of aphasia. These finding suggest that although the IFGpo-L may be a common lesion site in persons with AOS, it may more likely be related to the presentation of concomitant aphasia rather than AOS. This is a hypothesis that needs to be explored in a larger study. Although these four participants do not provide enough evidence to distinctly identify one specific region as the sole region of dysfunction, they lend support to the role of the motor regions in stroke-induced AOS. Additionally, the variability in lesioned regions across participants with AOS provokes questions regarding how various regions within the speech network might contribute to the manifestation of AOS. Further investigation is needed, not only in terms of specific cortical regions affected, but also with the consideration of connectivity to specific regions. That is, functional processes may be impaired in a region that is structurally intact due to structural damage in regions that provide input/output to that region. For example, recent studies that have investigated white matter connections to the insula (e.g., Cloutman et al., 2011, Dennis et al., 2014) suggest this is a highly connected region. Because of such connectivity, damage to surrounding areas could lead to functional impairment of insular function in the absence of structural damage to the insula.
Damage to the left anterior insula
In consideration of the role of the insula, only two participants with AOS (AOS1 and AOS+AA1) had damage to the AIns-L, while two participants presented with AOS in the absence of damage to the AIns-L (AOS2 and AOS+AA2). These data are not sufficient to rule out the possibility that AOS was a result of damage to the AIns-L in two of the participants in this study, yet it is interesting to note that AOS+AA2 (no damage to the AIns-L) presented with the most severe AOS. Additionally, AA1 (no AOS) had a large portion of the AIns-L damaged (~63%). When compared to the insular damage in AOS1 and AOS+AA1, the extent of AA1’s lesion in the AIns-L is nearly twice as large, yet did not result in AOS. A word of caution is warranted here in terms of interpreting these data. First of all, in AA1, 63% of the AIns-L was damaged, which means that 37% remained. Thus, the possibility that AOS is induced by damage specific to the remaining portions of the AIns-L cannot be ruled out. Furthermore, because both AOS1 and AOS+AA1 had damage to frontal regions as well as the insula, it is possible that damage to the AIns-L only induces AOS when there is also damage to the frontal regions and/or underlying white matter connections. The fact that AA1 was tested at 69 months post-stroke presents the possibility that more recovery of function has been able to occur in this patient. AOS may have been present in AA1 initially, but resolved over time. However, for this particular participant, this does not appear to be the case, as this participant did not report behaviors characteristic of AOS in the acute phase of stroke, nor did she present with AOS when tested in a previous study at 39 months post stroke. In addition, it should be noted that the size of the AIns-L region of interest was larger than the SPGI region described in Dronkers (1996) and Ogar et al. (2006). Thus, it is difficult to discuss the contribution of the SPGI specifically in relation to the findings of the current study. Nevertheless, despite the difficulties in generalizing from individual case studies, these findings challenge the role of the insula in motor speech planning, suggesting that involvement on the AIns-L on its own does not necessarily lead to AOS.
Lastly, due to the rarity of isolated lesions to the insula, participant AA1 provides a unique opportunity to examine the clinical implications of damage to this area. If damage to the AIns-L alone were sufficient to cause AOS, then AA1 should have presented with characteristic speech disturbances indicative of AOS. However, after undergoing a very thorough battery of speech and language evaluations, she clearly presented with mild anomic aphasia, but did not meet the diagnostic criteria for AOS. Thus, AA1’s lesion location and clinical profile strongly suggest caution in implicating the insula in motor speech planning. In terms of her language abilities, this participant’s data raise questions regarding insular involvement in aphasia and language processing. Mild aphasia following left insula damage has been reported; however, it is unclear if damage to neighboring subcortical structures was necessary for aphasia in these instances (Alexander, Naeser, & Palumbo, 1987). At least one study has suggested that insular language processes are localized in the left medial and posterior insula (cf. Ardila, 1999). When interpreting the findings of the current study, it is important to be mindful that the insula was parcellated into anterior/posterior sections, dividing the medial portion of the insula between these two regions of interest. Findings from this participant suggest that the AIns-L may be involved in linguistic abilities, but it is imperative to note that although the insula was the only cortical area damaged, the lesion also included subcortical structures of the basal ganglia (putamen and pallidum). Therefore, the extent to which these subcortical structures contributed to aphasia in this individual case cannot be determined.
Conclusion
Due to conflicting findings in the literature regarding the specific brain regions implicated in AOS, we examined the extent to which the AIns-L, IFG-L, and left primary and secondary motor cortices were affected in five participants with left hemisphere stroke in order to determine patterns of damage that are a prerequisite for AOS. These areas of interest were driven by the seminal paper of Dronkers (1996) implicating the AIns-L, more recent studies implicating the IFG-L (Hillis et al., 2004; Richardson et al., 2012), and the increasing body of evidence suggesting localized atrophy to the motor and pre-motor areas in persons with PAOS (Josephs et al., 2012; Whitwell et al., 2013). Although group analyses tend to provide more generalizable evidence than case studies, they are not without limitations and can sometimes overshadow individual variability, particularly in populations such as stroke patients. One of the strengths of this paper lies in the criteria that were utilized for participant selection. This study presents findings from a participant with relatively isolated AIns-L damage, which is rarely reported in the literature.
In addition to detailing a rare case of relatively isolated insula infarct, this study offers assessment and imaging data from two participants whose only impairment is AOS. The selection of these two participants allows for the investigation of areas involved in AOS without the confounding influence of aphasia. Finally, this study provides data from two participants with moderate and severe AOS whose structural scans did not reveal damage to the AIns-L. Overall findings suggest that damage to either the left motor areas and/or ROL-L is associated with stroke-induced AOS, while damage to the AIns-L is neither required nor sufficient to cause AOS. While the current data cannot distinguish the specific contribution of the PreCG-L and the ROL-L in the context of AOS, collectively, they provide strong evidence to suggest that the manifestations of AOS may be present following compromise to the motor system. Therefore these cases offer beneficial insight into the role of brain areas involved in speech motor control and AOS.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support of NIH-NIDCD grant (DC009571). We also thank Dr. Joe Duffy and colleagues for use of the Apraxia of Speech Rating Scale (ASRS).
This work was supported by the National Institute of Deafness and Other Communication Disorders under Grant DC009571
References
- Alexander MP, Naeser MA, & Palumbo CL (1987). Correlations of subcortical lesion sites and aphasia profiles. Brain, 110(4), 961–988. [DOI] [PubMed] [Google Scholar]
- Ardila A (1999). The role of insula in language: an unsettled question. Aphasiology, 13(1), 79–87. [Google Scholar]
- Ballard KJ, Granier JP, & Robin DA (2000). Understanding the nature of apraxia of speech: Theory, analysis, and treatment. Aphasiology, 14(10), 969–995. [Google Scholar]
- Basilakos A, Rorden C, Bonilha L, Moser D, & Fridriksson J (2015). Patterns of Poststroke Brain Damage That Predict Speech Production Errors in Apraxia of Speech and Aphasia Dissociate. Stroke. doi: 10.1161/STROKEAHA.115.009211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereda C, Ghika J, Maeder P, & Bogousslavsky J (2002). Strokes restricted to the insular cortex. Neurology, 59(12), 1950–1955. [DOI] [PubMed] [Google Scholar]
- Cloutman LL, Binney RJ, Drakesmith M, Parker GJ, & Ralph MAL (2012). The variation of function across the human insula mirrors its patterns of structural connectivity: evidence from in vivo probabilistic tractography. Neuroimage, 59(4), 3514–3521. [DOI] [PubMed] [Google Scholar]
- Darley FL (1982). Aphasia. Philadelphia: W.B. Saunders Co. [Google Scholar]
- Darley FL, Aronson AE, & Brown JR (1975). Motor speech disorders. Philadelphia: W. B. Saunders Co. [Google Scholar]
- Dennis EL, Jahanshad N, McMahon KL, Zubicaray GI, Martin NG, Hickie IB, … Thompson PM (2014). Development of insula connectivity between ages 12 and 30 revealed by high angular resolution diffusion imaging. Human Brain Mapping, 35(4), 1790–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF (1996). A new brain region for coordinating speech articulation. Nature, 384(6605), 159–161. [DOI] [PubMed] [Google Scholar]
- Duffy JR (2005). Motor Speech Disorders: Substrates, Differential Diagnosis, and Management, 2nd ed. St Louis, MI: Mosby. [Google Scholar]
- Duffy JR (2006). Apraxia of speech in degenerative neurologic disease. Aphasiology, 20(6), 511–527. [Google Scholar]
- Duffy JR, & Josephs KA (2012). The diagnosis and understanding of apraxia of speech: Why including neurodegenerative etiologies may be important. Journal of Speech, Language and Hearing Research, 55(5), S1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JR, & McNeil MR (2008). Primary progressive aphasia and apraxia of speech In Chapey R (Ed.), Language intervention strategies in adult aphasia (5th ed.). Baltimore, MD: Wolters Kluwer/Lippincott, Williams and Wilkins. [Google Scholar]
- Enderby P & Palmer R (2007). Frenchay Dysarthria Assessment (2nd ed.). Texas: Pro-Ed. [Google Scholar]
- Finley A, Saver J, Alger J, Pregenzer M, Leary M, & Ovbiagele B (2003). Diffusion weighted imaging assessment of insular vulnerability in acute middle cerebral artery infarctions. Stroke, 34, 259. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, & Frackowiak RS (1994). Statistical parametric maps in functional imaging: a general linear approach. Human brain mapping, 2(4), 189–210. [Google Scholar]
- Graff-Radford J, Jones DT, Strand EA, Rabinstein AA, Duffy JR, & Josephs KA (2014). The neuroanatomy of pure apraxia of speech in stroke. Brain and language, 129, 43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley KL, Jacks A, de Riesthal M, Abou-Khalil R, & Roth HL (2012). Toward a quantitative basis for assessment and diagnosis of apraxia of speech. Journal of Speech, Language, and Hearing Research, 55(5), S1502–S1517. [DOI] [PubMed] [Google Scholar]
- Hickok G, & Poeppel D (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8, 393–402. [DOI] [PubMed] [Google Scholar]
- Hickok G, Rogalsky C, Chen R, Herskovits EH, Townsley S, & Hillis AE (2014). Partially overlapping sensorimotor networks underlie speech praxis and verbal short-term memory: evidence from apraxia of speech following acute stroke. Front Hum Neurosci, 8, 649. doi: 10.3389/fnhum.2014.00649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G (2014). Towards an integrated psycholinguistic, neurolinguistic, sensorimotor framework for speech production. Language, Cognition and Neuroscience, 29(1), 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, & Maurer K (2004). Re-examining the brain regions crucial for orchestrating speech articulation. Brain, 127, 1479–1487. [DOI] [PubMed] [Google Scholar]
- Josephs KA, & Duffy JR (2008). Apraxia of speech and nonfluent aphasia: A new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Current Opinions in Neurology, 21, 688–692. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV,…Whitwell JL (2012). Characterizing a neurodegenerative syndrome: Primary progressive apraxia of speech. Brain, 135, 1522–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, & Parisi JE et al. (2006). Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain, 129(6), 1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A (2007). Western Aphasia Battery-Revised. San Antonio: PsychCorp. [Google Scholar]
- Marien P, Pickut BA, Engelborghs S, Martin J, & De Deyn PP (2001). Phonological agraphia following a focal anterior insulo-opercular infarction. Neuropsychologia, 39, 845–855. [DOI] [PubMed] [Google Scholar]
- McNeil MR, Doyle PJ, & Wambaugh JL (2000). Apraxia of speech: A treatable disorder of motor planning and programming In Gonzalez-Rothi L, Crosson L, & Nadeau S (Eds.) Aphasia and language: Theory to practice (pp. 221–266). New York: The Guilford Press. [Google Scholar]
- McNeil MR, Robin DA, & Schmidt RA (1997). Apraxia of speech: Definition, differentiation, and treatment In: McNeil MR (Ed.), Clinical Management of Sensorimotor Speech Disorders (pp. 311–44). New York: Thieme. [Google Scholar]
- Nagao N, Takeda K, Komori T, Isozaki E, & Hirai S (1999). Apraxia of speech associated with an infarct in the precentral gyrus of the insula. Neuroradiology, 41, 356–357. [DOI] [PubMed] [Google Scholar]
- MATLAB and Statistics Toolbox Release 2012b, The MathWorks, Inc., Natick, Massachusetts, United States. [Google Scholar]
- Mumby K, Bowen A, & Hesketh A (2007). Apraxia of speech: how reliable are speech and language therapists’ diagnoses? Clinical Rehabilitation, 21, 760–767. [DOI] [PubMed] [Google Scholar]
- Odell K, McNeil MR, Rosenbek JC, & Hunter L (1990). Perceptual characteristics of consonant production by apraxic speakers. J Speech Hear Disord, 55(2), 345–359. [DOI] [PubMed] [Google Scholar]
- Ogar J, Slama H, Dronkers N, Amici S, & Luisa Gorno-Tempini M (2005). Apraxia of speech: an overview. Neurocase, 11(6), 427–432. [DOI] [PubMed] [Google Scholar]
- Ogar J, Willock S, Baldo J, Wilkins D, Ludy C, & Dronkers N (2006). Clinical and anatomical correlates of apraxia of speech. Brain and Language, 97(3), 340–343. [DOI] [PubMed] [Google Scholar]
- Payabvash S, Souza LCS, Wang Y, Schaefer PW, & Furie KL, Halpern EF,…Lev MH (2011). Regional ischemic vulnerability of the brain to hypoperfusion: The need for location specific computed tomography perfusion thresholds in acute stroke patients. Stroke, 42, 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JD, Fillmore P, Rorden C, LaPointe LL, & Fridriksson J (2012). Re-establishing Broca’s initial findings. Brain & Language, 123, 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, & Karnath HO (2004). Using human brain lesions to infer function: A relic from a past era in the fMRI age? Nature Reviews Neuroscience, 5, 812–819. [DOI] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Fridriksson J, Bender B, & Karnath HO (2012). Age-specific CT and MRI for spatial normalization. NeuroImage, 61(4), 957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, & Brett M (2000). Stereotaxic display of brain lesions. Behavioural Neurology, 12, 191–200. [DOI] [PubMed] [Google Scholar]
- Schiff HB, Alexander MP, Naeser MA, & Galaburda AM (1983). Aphemia: Clinical-anatomic correlations. Archives of Neurology, 40(12), 720–727, [DOI] [PubMed] [Google Scholar]
- Seddoh SAK, Robin DA, Sim H-S, Hageman C, Moon JB, & Folkins JW (1996). Speech timing in apraxia of speech and conduction aphasia. Journal of Speech and Hearing Research, 39, 590–603. [DOI] [PubMed] [Google Scholar]
- Shuren K (1993). Insula and aphasia. Journal of Neurology, 240, 216–218. [DOI] [PubMed] [Google Scholar]
- Strand EA, Duffy JR, Clark HM, & Josephs K (2014). The apraxia of speech rating scale: A tool for diagnosis and description of apraxia of speech. Journal of Communication Disorders. doi: 10.1016/j.jcomdis.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupe LA, Varma DD, Gomez Y, Race D, Leigh R, & Hillis AE,…Gottesman RF (2013). Chronic apraxia of speech and Broca’s area. Stroke, 44, 740–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N,…Joliot M (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15(1), 273–289. [DOI] [PubMed] [Google Scholar]
- Wertz RT, LaPointe LL, & Rosenbek JC (1984). Apraxia of speech: The disorder and its management. New York: Grune and Stratton. [Google Scholar]
- Whitwell JL, Duffy JR, Strand EA, Machulda MM, Senjem ML, Gunter JL,…Josephs KA (2013). Neuroimaging comparison of primary progressive apraxia of speech and progressive supranuclear palsy. European Journal of Neurology, 20, 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler W, Aichert I & Staiger A (2012). Apraxia of Speech: Concepts and Controversies. Journal of Speech, Language, and Hearing Research 55: S1485–S1501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.