Abstract
Endothelial dysfunction is a form of subclinical cardiovascular disease that may be involved in preterm birth and small-for-gestational-age deliveries. However, concentrations of biomarkers of endothelial dysfunction before pregnancy have rarely been measured. We hypothesized that higher levels of biomarkers of endothelial dysfunction (cellular adhesion molecules and selectins) would be associated with odds of preterm birth and/or small-for-gestational-age deliveries. We included 235 women from the Coronary Artery Risk Development in Young Adults (CARDIA) study who were nulliparous at Y7, reported ≥1 live birth through Y25, and had ≥1 biomarker measured at Y7. We tested for associations between individual biomarkers and an averaged z-score representing total endothelial dysfunction with preterm birth and/or small-for-gestational-age deliveries using Poisson regression, adjusted for demographic and clinical characteristics at the exam immediately preceding index birth. At Y7, total endothelial dysfunction was similar in women who did (n=59) and did not have (n=176) preterm birth and/or small-for-gestational-age deliveries. There was no association between biomarkers of endothelial dysfunction (either individual biomarker or total score) with odds of preterm birth and/or small-for-gestational-age deliveries after adjustment: IRR = 1.01, 95% CI: 0.74, 1.39, p=0.93 for total endothelial dysfunction score. Associations were not modified by race. We conclude that biomarkers of endothelial dysfunction in nulliparous women, measured ~3 years before pregnancy, did not identify women at risk for preterm birth and/or small-for-gestational-age deliveries. This suggests that the maternal endothelial dysfunction that is believed to contribute to these birth outcomes may not be detectable before pregnancy.
Keywords: Pregnancy, vascular dysfunction, preterm birth, small for gestational age
Introduction
Women who have had a preterm birth (PTB; <37 weeks gestation) or small for gestational age (SGA; ≤ 10th percentile for birthweight) delivery have higher risk for subclinical and overt cardiovascular disease (CVD) later in life(1, 2). Endothelial dysfunction, a key feature of the atherosclerotic CVD process, is also thought to be involved in the pathogenesis of PTB and SGA(3–5). Prior studies show that pregnant women who later had PTB had endothelial dysfunction in the first trimester, as evidenced by elevated levels of intercellular adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM), compared to women who had a term delivery(6). Women who were diagnosed with intrauterine growth restriction, the condition underlying SGA, had impaired endothelial function, measured with flow-mediated dilation 6–24 months post-partum, compared to women with late-onset preeclampsia and uncomplicated pregnancies, even when blood pressure (BP) had returned to normal levels (7). However, most pregnancy studies have used a retrospective design and have not been able to investigate whether endothelial dysfunction predates pregnancy or if evidence of endothelial dysfunction in the years preceding pregnancy is also associated with incident PTB and SGA.
The purpose of this study was to examine the association between evidence of pre-pregnancy endothelial dysfunction and incident PTB and SGA. We hypothesized that women who had higher levels of circulating endothelial adhesion molecules in the years preceding pregnancy would be more likely to experience PTB or SGA. CVD risk and PTB incidence are higher in black compared to white women(8), and endothelial function is more impaired in blacks compared to whites(9). Black women also had different levels of biomarkers of endothelial activation (lower ICAM, higher e-selectin) versus white women in a large, population sample(10). Therefore we further hypothesized that the association between endothelial biomarkers and PTB/SGA would be modified by race.
Methods
The CARDIA Study.
The Coronary Artery Risk Development in Young Adults (CARDIA) Study is a population‐based, longitudinal, observational, and multi‐center study investigating determinants of coronary heart disease and its risk factors in African-American and Caucasian women and men. At baseline (1985‐1986), 5115 subjects (53% women, 52% African-American) aged 18 to 30 years were recruited from 4 metropolitan areas in the United States: Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. Validated markers of endothelial activation (11) were collected at year 7 (Y7) after baseline (1992–1993) as part of the Young Adult Longitudinal Trends in Antioxidants (YALTA) ancillary study. The study was approved by Institutional review boards at each center, and all participants signed written, informed consent.
Of the 2787 women enrolled in the study, we excluded women who reported a birth before Y7 (n=1514), those in whom no endothelial biomarkers were measured (n=85), women who did not report any live births after Y7 (n=889), and those in whom information regarding SGA was missing (n=68). Our final sample included 235 women. For characteristics of our sample at Y7, please see ST1.
Circulating Endothelial Adhesion Molecules.
We used serum and plasma p-selectin, e-selectin, ICAM and VCAM were measured at the Y7 CARDIA exam as part of the YALTA ancillary study. Blood samples were obtained via venipuncture following an overnight fast, processed, and frozen within 90 minutes. They were stored at −70°C and shipped in dry ice to a single laboratory for analysis. Participants were asked to refrain from smoking and heavy physical activity for at least 2 hours prior to the examination. Biomarkers were analyzed at the University of Minnesota’s Molecular Epidemiology and Biomarker Research Laboratory using sandwich ELISA assays (R & D Systems, Minneapolis, MN). Serum e-selectin and plasma p-selectin samples were diluted 10- and 6-fold, respectively. The within plus between day coefficients of variation (CVs) were 7.7% for e-selectin and 10.5% for p-selectin. The e-selectin assays were performed over several months, with no assay drift detected during this time. Serum ICAM samples were diluted 10-fold and plasma VCAM samples were diluted 21-fold. The within and the between day CVs were < 10% for ICAM and 9.0% for VCAM. VCAM analyses were also performed over a few months in 2010, with no assay drift detected for these analyses.
The biomarkers were then combined into a score representing total endothelial activation. This total score was calculated by taking the sum of the z-score for each biomarker and then dividing by the total number of biomarkers measured in each woman.
A higher score for an individual biomarker and total score indicates greater endothelial dysfunction.
Preterm Birth and SGA.
Information regarding PTB and SGA were obtained via self-report questionnaire and defined as a birth that occurred at < 37+0 weeks gestational age and birthweight less than the 10th percentile for gestational age, respectively (12). We only included the first birth that occurred after Y7 in our analyses as this birth was most proximal to our exposure measurement; for women who reported more than one PTB and/or SGA, we included only the event that occurred in the first birth.
Blood Pressure.
BP was measured in triplicate by a trained technician at every exam in the right arm using random zero mercury manometer with the participant seated after a 5-minute rest. Cuff size was determined after measuring the arm at a level midway between the acromion process and olecranon. There was a 1-minute break in between measurements, and the final two measurements were averaged for analysis. Self-report of hypertension diagnosis and treatment were noted at each exam using standardized surveys. Hypertension was defined as a BP reading of systolic BP (SBP) ≥140, diastolic BP (DBP) ≥90, self-report of treatment for hypertension, or use of antihypertensive medication.
Co-variable Measurements.
We included the covariates measured at the exam immediately preceding the index pregnancy. Structured interviews or self-administered questionnaires were used to obtain sociodemographic information: sex, race, birth date, education level, and smoking. Education level was determined as the maximum number of years of school completed. Smoking was classified as either never, former, or current, based on self-report. Height was recorded to the nearest 0.5 cm and weight to the nearest 0.2 kg at each examination. Body mass index (BMI) was calculated as kg/m2.
Statistical Analyses.
We compared the sociodemographic and clinical characteristics of women by quartile of endothelial dysfunction score using Kruskal-Wallis or chi-squared tests. We compared the sociodemographic and clinical characteristics of women with and without PTB and/or SGA using t-tests, Mann-Whitney U tests, or chi-squared tests.
Differences between groups for concentrations of biomarkers: ICAM, VCAM, p-selectin, and e-selectin, were evaluated individually as continuous variables using a Mann-Whitney U test before and after adjustment for covariates at the exam preceding index birth.
Poisson regression models were utilized to determine the association of the individual endothelial adhesion molecules and the combined endothelial activation score with PTB and SGA. We tested for associations for each outcome individually and pooled because vascular dysfunction can contribute to both outcomes. Our findings were similar in individual and pooled analyses, so we reported our findings for the pooled PTB/SGA condition. We adjusted for demographic and clinical characteristics: age, race, education at baseline, study center, BMI, current and former smoking, diabetes, and hypertension, at the exam immediately preceding index birth.
These associations may be affected by pre-existing hypertension or diabetes and gestational diabetes or hypertensive disorders of pregnancy. We conducted initial sensitivity analyses by excluding women who developed hypertension (n=6) or diabetes (n=2) prior to index birth and by excluding women with gestational diabetes in the index pregnancy. We conducted a second sensitivity analysis with the same exposures and outcomes in women without self-reported hypertension in pregnancy (n=195); this analysis was classified as sensitivity because the positive predictive value of self-reported hypertension in pregnancy is low and the negative predictive value is high in CARDIA (8).
We tested for effect modification by race using multiplicative interaction terms between race and the endothelial function score. Analyses were performed using STATA version 14.0 (College Station, TX).
Results
Participants.
There was no difference in age, education, BMI, proportion of women with gestational diabetes, systolic blood pressure (SBP), diastolic BP (DBP), or lipids at baseline by quartiles of endothelial dysfunction score. Women in the highest quartile were more likely to have hypertension (Table 1). Women with PTB and/or SGA (n=59) had less formal education and were more likely to be black compared to women who did not experience PTB/SGA (n=176). At the exam immediately preceding the index birth, women who reported PTB/SGA were more likely to be current smokers compared to women who did not report PTB/SGA (Table 2).
Table 1.
Demographic and clinical characteristics of women by quartile of endothelial dysfunction score at Y7 (prior to pregnancy).
| Q1 n=56 |
Q2 n=61 |
Q3 n=58 |
Q4 n=60 |
P-value | |
|---|---|---|---|---|---|
| Age (yrs) | 30 ± 1 | 30 ± 1 | 30 ± 1 | 30 ± 1 | 0.60 |
| Black Race (n, %) | 20, 36 | 23, 38 | 20, 34 | 21, 35 | 0.94 |
| Education (yrs) | 16 ± 1 | 16 ± 1 | 15 ± 1 | 16 ± 1 | 0.10 |
| Former | 8, 14 | 14, 23 | 15, 26 | 11, 18 | 0.43 |
| BMI (kg/m2) | 24.1 ± 0.8 | 24.6 ± 0.8 | 25.1 ± 0.7 | 26.5 ± 1.2 | 0.36 |
| Hypertension (n, %)* | 0, 0 | 0, 0 | 1, 1 | 5, 8 | 0.01 |
| Diabetes (n, %) | 0, 0 | 2, 1 | 0, 0 | 0, 0 | 0.12 |
| HTN in Pregnancy (n,%) | 7, 13 | 10, 16 | 9, 16 | 14, 23 | 0.46 |
| Gestational Diabetes (n,%) | 1, 2 | 2, 3 | 3, 5 | 3, 5 | 0.76 |
| SBP (mmHg) | 105 ± 1 | 102 ± 1 | 105 ± 1 | 106 ± 1 | 0.44 |
| DBP (mmHg) | 66 ± 1 | 63 ± 1 | 65 ± 1 | 69 ± 1 | 0.14 |
| LDL-C (mg/dL) | 97 ± 3 | 100 ± 3 | 106 ± 4 | 109 ± 5 | 0.59 |
| Triglycerides (mg/dL) | 62 ± 4 | 66 ± 4 | 60 ± 5 | 65 ± 5 | 0.17 |
BMI: body mass index, Gestational Diabetes: gestational diabetes in index birth, HTN in Pregnancy: hypertensive disorder of pregnancy in index birth, BMI: body mass index, SBP: systolic BP, DBP: diastolic BP, TC: total cholesterol, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol.
Table 2.
Characteristics of women with and without PTB/SGA at the exam immediately preceding index birth.
| PTB/SGA n=59 |
No PTB/SGA n=176 |
P-value | |
|---|---|---|---|
| Age (yrs) | 33 ± 1 | 32 ± 1 | 0.21 |
| Time to 1st Birth (yrs) | 2.6 ± 0.4 | 2.5 ± 0.2 | 0.98 |
| Black Race (n, %)* | 30, 50 | 55, 31 | 0.01 |
| Former | 7, 12 | 28, 16 | 0.45 |
| BMI (kg/m2) | 24.9 ± 0.4 | 25.0 ± 0.8 | 0.94 |
| Hypertension (n, %) | 3, 5 | 3, 2 | 0.15 |
| Diabetes (n, %) | 0, 0 | 2, 1 | 0.41 |
| HTN in Pregnancy (n,%) | 14, 24 | 26, 15 | 0.11 |
| Gestational Diabetes (n,%) | 3, 5 | 6, 3 | 0.56 |
| SBP (mmHg) | 106 ± 1 | 103 ± 1 | 0.13 |
| DBP (mmHg) | 68 ± 1 | 67 ± 1 | 0.83 |
| LDL-C (mg/dL) | 108 ± 6 | 104 ± 2 | 0.98 |
| Triglycerides (mg/dL) | 73 ± 6 | 73 ± 4 | 0.81 |
Time to 1st Birth: time in years from the measurement of endothelial biomarkers until the index birth, BMI: body mass index, Gestational Diabetes: gestational diabetes in index birth, HTN in Pregnancy: hypertensive disorder of pregnancy in index birth, SBP: systolic BP, DBP: diastolic BP, TC: total cholesterol, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol.
p< 0.05 for difference between women by PTB/SGA group.
Circulating Adhesion Molecules.
Women who had PTB/SGA had lower VCAM compared to women without PTB/SGA. There were no differences in unadjusted levels of other individual biomarkers or total endothelial dysfunction score in women with versus without PTB/SGA (Figure 1). There was no difference in adjusted VCAM, ICAM, e-selectin, p-selectin, or total endothelial dysfunction score by PTB/SGA status (Table 3).
Figure 1A-E.
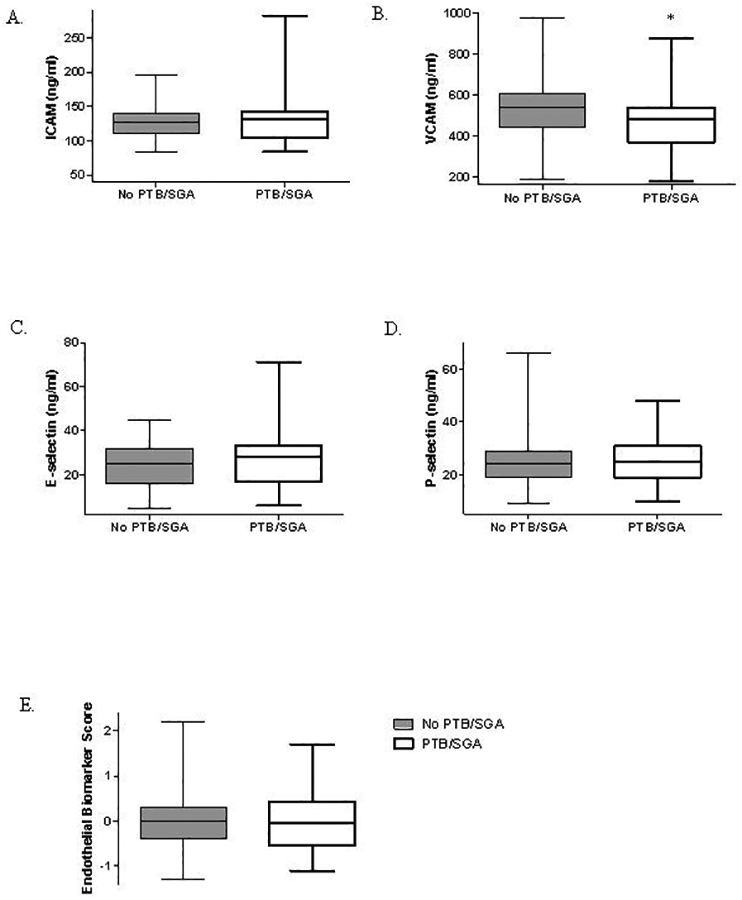
Box and whiskers plot of unadjusted levels of biomarkers of endothelial activation by PTB/SGA status.
Table 3.
Adjusted levels of biomarkers of endothelial dysfunction by PTB/SGA status.
| PTB/SGA n=59 Adjusted |
No PTB/SGA n=176 Adjusted |
P-value | |
|---|---|---|---|
| ICAM (ng/ml) | 130 (117, 141) | 126 (116, 138) | 0.57 |
| VCAM (ng/ml)* | 507(459, 568) | 535(491, 576) | 0.72 |
| E-selectin (ng/ml) | 25 (23, 28) | 25 (21, 28) | 0.15 |
| P-selectin (ng/ml) | 24 (23, 25) | 23 (21, 25) | 0.99 |
| Endothelial Dysfunction Score | −0.04(−0.22, 0.11) | −0.06(−0.24, 0.12) | 0.47 |
Levels of individual biomarkers of endothelial dysfunction and total endothelial biomarker score in women with and without PTB/SGA. Adjusted for age, race, education, study center, BMI, current and former smoking, hypertension, and diabetes at the exam immediately preceding index birth. Data are means and interquartile range.
Association of Circulating Adhesion Molecules with PTB/SGA.
Prior to modeling, we tested for interaction and observed that race did not modify the association between endothelial dysfunction score and PTB/SGA (p=0.29) and so we proceeded with pooled modeling. There were no associations between individual biomarkers (ICAM, VCAM, e-selectin, or p-selectin) or combined endothelial dysfunction score with PTB/SGA in adjusted models (Table 4).
Table 4.
Association of pre-pregnancy endothelial dysfunction with PTB/SGA in adjusted model.
| IRR | 95% CI | |
|---|---|---|
| VCAM | 0.94 | 0.68, 1.30 |
| ICAM | 0.91 | 0.64, 1.28 |
| P-selectin | 1.00 | 0.73, 1.38 |
| E-selectin | 1.19 | 0.94, 1.53 |
| Endothelial dysfunction score | 1.01 | 0.74, 1.39 |
IRR: incident rate ratio, 95% CI: 95% confidence interval. The model includes age, race, education, study center, BMI, hypertension or diabetes, and current and former smoking at the exam immediately preceding the index birth. Unit=1 SD.
The combined endothelial activation measurement was not associated with PTB/SGA in un-adjusted (IRR=1.06, 95% CI: 0.76, 1.48) or adjusted analyses (Table 4).
When we examined associations of quartiles of biomarker scores, rather the continuously measured biomarker levels, with PTB/SGA, there were also no significant associations.
Sensitivity Analysis.
Results were similar in the subset of women who did not develop hypertension or diabetes before the index birth (n=227; IRR=1.03, 95% CI: 0.75, 1.42) for combined endothelial dysfunction score), similar in women without gestational diabetes in the index pregnancy (n=226; IRR=1.02, 95% CI: 0.62, 1.68), and similar in women who did not report hypertension in the index pregnancy (n=193; for complete results please see ST2).
Discussion
We observed that circulating markers of endothelial dysfunction in the years preceding pregnancy were not associated with PTB/SGA in this prospective cohort. These findings were similar in black and white women. Because of the absence of an association of PTB/SGA with these biomarkers, other possible mechanisms need to be considered.
Although the increased CVD risk following PTB or SGA has been well-documented, it has remained unclear whether complications of pregnancy led to increased maternal CVD risk or whether the physiological stress of pregnancy uncovered pre-existing maternal predisposition to CVD (2, 8, 13, 14). This question has been difficult to address since pregnancy studies tend to have small numbers of participants, use retrospective designs, or only include women once they are already pregnant. The longitudinal design and large participant pool of the Coronary Artery Risk Development in Young Adults (CARDIA) study provided a unique opportunity to conduct a prospective study in a child-bearing cohort with a larger number of participants.
Inflammation and vascular complications of pregnancy are pathways known to lead to PTB and SGA. Two-thirds of all PTB are spontaneous and believed to be related to inflammation or infection earlier in pregnancy that interrupt adequate placentation and cause vascular disease (5, 15, 16). This inflammation can trigger vascular dysfunction and expression of endothelial adhesion molecules (ICAM, VCAM, e-selectin, p-selectin), as well as stimulate uterine contraction and PTB(5, 17). The remaining one-third of all PTB are medically indicated and believed to be caused primarily by maternal-placental vascular dysfunction (preeclampsia or intrauterine growth restriction), and maternal vascular disease or diabetes is associated with a five-fold increase in risk of SGA (18–20).
Earlier data do suggest that women who experience PTB and SGA have higher baseline CVD risk, indicating a role for pre-existing maternal factors in these birth outcomes (21, 22). ICAM and VCAM were shown to be higher early in pregnancy (around week 16 of gestation) in women who go on to have PTB, but the increased inflammation and oxidative stress associated with the pregnancy itself may have caused endothelial activation and may not be representative of maternal endothelial activation prior to the onset of pregnancy (6). Antiangiogenic factor fms-like tyrosine kinase-1 (Flt-1) is increased in the maternal circulation in response to placental hypoxia and can also cause maternal endothelial dysfunction (23). However, although circulating Flt-1 infusion was used to recapitulate the clinical features of preeclampsia (hypertension, proteinuria) during pregnancy in an animal model of the human condition, Flt-1 infusion did not induce hypertension in non-pregnant mice or have long term effects on BP following parturition (24). These and our findings indicate that the up-regulation or effects of proteins associated with vascular damage during pregnancy and longer-term CVD risk may not occur until pregnancy begins.
PTB has been shown to have a genetic component, as women who were themselves born preterm had a higher risk of delivering a preterm infant (25). This suggests that pre-existing genetic predisposition may interact with circulating or inflammatory factors in pregnancy to contribute PTB and SGA and that the vascular damage associated with these birth outcomes may expose or exacerbate subsequent CVD risk in vulnerable women. Alternatively, biomarkers other than the ones we measured in our study may better reflect pre-pregnancy endothelial function as it relates to risk of PTB and SGA.
Years after pregnancy, there is evidence of vascular impairment in women with prior PTB and SGA (2, 8). Catov et al, showed that women who had had PTB had higher BP and atherogenic lipids compared to women with only term labor (2). Yinon, et al, reported endothelial dysfunction (measured with brachial artery flow-mediated dilation) six month- two years postpartum in women with intrauterine growth restriction (7). A recent review showed that women who had pregnancies complicated by hypertension also had evidence of vascular dysfunction (higher Flt-1, central arterial stiffness, carotid intima-media thickness, and augmentation index) years after birth, though the differences between women with and without hypertensive disorders of pregnancy were greatest closer to the incident pregnancy (26). Our prospective study adds to this body of work by showing that, although women who experienced PTB and SGA have increased CVD risk postpartum (2), there was no difference in levels of circulating markers of endothelial dysfunction in the ~3 years prior to pregnancy. In fact, unadjusted VCAM was lower in women who went on to have PTB/SGA in our cohort.
When we excluded women who developed overt hypertension prior to the index pregnancy, our results were similar. There was also no association of markers of endothelial dysfunction with PTB/SGA in the women who did not report hypertension in the index pregnancy. Only two women developed diabetes prior to index pregnancy, and we did not see an effect of diabetes on our associations. Similarly, few women developed gestational diabetes in the index pregnancy, and we did not see an effect of gestational diabetes on our results. Strategies to improve endothelial function and avoid PTB and SGA, such as eating a healthy diet, exercising, maintaining a healthy weight, and managing pregnancy weight gain and glucose levels, may be more efficacious immediately preceding or during pregnancy rather than years before pregnancy onset (27) (28).
A limitation of our study is the indirect measurement of endothelial function at a single time point. However, circulating levels of endothelial adhesion molecules have been shown to correlate to endothelial cell surface expression, indicating that circulating levels of adhesion molecules reflect the activation state of the endothelial cells themselves (11). Our sample size, though larger than some other pregnancy studies, was likely too small to detect modest associations. We were also unable to distinguish between spontaneous and indicated PTB and constitutionally small infants versus those who were affected by vascular-mediated intrauterine growth restriction in our sample, which may have different etiologies (16). Our study’s strengths include the prospective design, measurement of multiple well-characterized endothelial biomarkers, and inclusion of both black and white women in different geographic regions in the United States.
We conclude that we found no evidence of an association of circulating markers of maternal endothelial dysfunction ~3 before pregnancy with incident PTB and SGA. Furthermore, biomarkers of endothelial activation were not higher in the women who went on to have PTB and/or SGA. This suggests that the endothelial dysfunction that is believed to contribute to these birth outcomes may not pre-date pregnancy. Future work should determine whether endothelial dysfunction closer to the onset of pregnancy is associated with PTB/SGA and may represent a target for intervention to avoid these birth outcomes.
Supplementary Material
Acknowledgments
Sources of Funding
CARDIA is supported by the National Heart, Lung, and Blood Institute (NHLBI) HHSN 268201300025C, HHSN 268201300026C, HHSN 268201300027C, HHSN 268201300028C, HHSN 268201300029C, HHSN 268200900041C, and partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). Cellular adhesion molecules were measured using funds from R01 HL 053560 and R01 HL093077 (Jacobs and Gross, PIs). The analyses were supported by grants from R01 DK106201 (Gunderson, PI), R01 DK090047 (Gunderson, PI) and K01 DK059944 (Gunderson, PI) from the National Institute of Diabetes, Digestive and Kidney Diseases. ALC is supported by an American Heart Association Strategically Focused Research Network Grant 14SFRN20480260 (Greenland, PI). This manuscript was reviewed by CARDIA for scientific content.
Footnotes
Disclosures
The authors of the study have no conflicts of interest to report.
Supplementary Material is available at Hypertension Research’s website
References
- 1.Bonamy AK, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth characteristics and subsequent risks of maternal cardiovascular disease: effects of gestational age and fetal growth. Circulation. 2011;124(25):2839–2846. [DOI] [PubMed] [Google Scholar]
- 2.Catov JM, Dodge R, Barinas-Mitchell E, et al. Prior preterm birth and maternal subclinical cardiovascular disease 4 to 12 years after pregnancy. Journal of women’s health. 2013;22(10):835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. British journal of obstetrics and gynaecology. 1986;93(10):1049–1059. [DOI] [PubMed] [Google Scholar]
- 4.Powers RW, Catov JM, Bodnar LM, Gallaher MJ, Lain KY, Roberts JM. Evidence of endothelial dysfunction in preeclampsia and risk of adverse pregnancy outcome. Reproductive sciences. 2008;15(4):374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF 3rd, Petraglia F. Inflammation and pregnancy. Reproductive sciences. 2009;16(2):206–215. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Scholl TO. Maternal biomarkers of endothelial dysfunction and preterm delivery. PloS one. 2014;9(1):e85716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yinon Y, Kingdom JC, Odutayo A, et al. Vascular dysfunction in women with a history of preeclampsia and intrauterine growth restriction: insights into future vascular risk. Circulation. 2010;122(18):1846–1853. [DOI] [PubMed] [Google Scholar]
- 8.Catov JM, Lewis CE, Lee M, Wellons MF, Gunderson EP. Preterm birth and future maternal blood pressure, inflammation, and intimal-medial thickness: the CARDIA study. Hypertension. 2013;61(3):641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahn DF, Duffy SJ, Tomasian D, et al. Effects of black race on forearm resistance vessel function. Hypertension. 2002;40(2):195–201. [DOI] [PubMed] [Google Scholar]
- 10.Lutsey PL, Cushman M, Steffen LM, et al. Plasma hemostatic factors and endothelial markers in four racial/ethnic groups: the MESA study. Journal of thrombosis and haemostasis : JTH. 2006;4(12):2629–2635. [DOI] [PubMed] [Google Scholar]
- 11.Kjaergaard AG, Dige A, Krog J, Tonnesen E, Wogensen L. Soluble adhesion molecules correlate with surface expression in an in vitro model of endothelial activation. Basic & clinical pharmacology & toxicology. 2013;113(4):273–279. [DOI] [PubMed] [Google Scholar]
- 12.Duryea EL, Hawkins JS, McIntire DD, Casey BM, Leveno KJ. A revised birth weight reference for the United States. Obstetrics and gynecology. 2014;124(1):16–22. [DOI] [PubMed] [Google Scholar]
- 13.Kaaja RJ, Greer IA. Manifestations of chronic disease during pregnancy. Jama. 2005;294(21):2751–2757. [DOI] [PubMed] [Google Scholar]
- 14.Tomimatsu T, Mimura K, Endo M, Kumasawa K, Kimura T. Pathophysiology of preeclampsia: an angiogenic imbalance and long-lasting systemic vascular dysfunction. Hypertension research : official journal of the Japanese Society of Hypertension. 2017;40(4):305–310. [DOI] [PubMed] [Google Scholar]
- 15.Catov JM, Bodnar LM, Ness RB, Barron SJ, Roberts JM. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. American journal of epidemiology. 2007;166(11):1312–1319. [DOI] [PubMed] [Google Scholar]
- 16.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sima AV, Stancu CS, Simionescu M. Vascular endothelium in atherosclerosis. Cell and tissue research. 2009;335(1):191–203. [DOI] [PubMed] [Google Scholar]
- 18.Kim YM, Bujold E, Chaiworapongsa T, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. American journal of obstetrics and gynecology. 2003;189(4):1063–1069. [DOI] [PubMed] [Google Scholar]
- 19.Gaudineau A [Prevalence, risk factors, maternal and fetal morbidity and mortality of intrauterine growth restriction and small-for-gestational age]. Journal de gynecologie, obstetrique et biologie de la reproduction. 2013;42(8):895–910. [DOI] [PubMed] [Google Scholar]
- 20.Hendler I, Goldenberg RL, Mercer BM, et al. The Preterm Prediction Study: association between maternal body mass index and spontaneous and indicated preterm birth. American journal of obstetrics and gynecology. 2005;192(3):882–886. [DOI] [PubMed] [Google Scholar]
- 21.Catov JM, Nohr EA, Olsen J, Ness RB. Chronic hypertension related to risk for preterm and term small for gestational age births. Obstetrics and gynecology. 2008;112(2 Pt 1):290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catov JM, Ness RB, Wellons MF, Jacobs DR, Roberts JM, Gunderson EP. Prepregnancy lipids related to preterm birth risk: the coronary artery risk development in young adults study. The Journal of clinical endocrinology and metabolism. 2010;95(8):3711–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. The Journal of clinical investigation. 2003;111(5):649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bytautiene E, Lu F, Tamayo EH, et al. Long-term maternal cardiovascular function in a mouse model of sFlt-1-induced preeclampsia. American journal of physiology. Heart and circulatory physiology. 2010;298(1):H189–193. [DOI] [PubMed] [Google Scholar]
- 25.Porter TF, Fraser AM, Hunter CY, Ward RH, Varner MW. The risk of preterm birth across generations. Obstetrics and gynecology. 1997;90(1):63–67. [DOI] [PubMed] [Google Scholar]
- 26.Grand’Maison S, Pilote L, Okano M, Landry T, Dayan N. Markers of Vascular Dysfunction After Hypertensive Disorders of Pregnancy: A Systematic Review and Meta-Analysis. Hypertension. 2016;68(6):1447–1458. [DOI] [PubMed] [Google Scholar]
- 27.Weissgerber TL, Davies GA, Roberts JM. Modification of angiogenic factors by regular and acute exercise during pregnancy. Journal of applied physiology. 2010;108(5):1217–1223. [DOI] [PubMed] [Google Scholar]
- 28.Tinius RA, Cahill AG, Strand EA, Cade WT. Maternal inflammation during late pregnancy is lower in physically active compared with inactive obese women. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2016;41(2):191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


