Abstract
Objectives:
We sought to determine the spectrum and prevalence of “background genetic noise” in the arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC) genetic test and to determine genetic associations that can guide interpretation of a positive test.
Background:
ARVC is a potentially lethal genetic cardiovascular disorder characterized by myocyte loss and fibro-fatty tissue replacement of the right ventricle. Genetic variation among the ARVC-susceptibility genes have not been systematically examined and little is known about the “background noise” associated with the ARVC genetic test.
Methods:
Using direct DNA sequencing, the coding exons/splice junctions of PKP2, DSP, DSG2, DSC2, and TMEM43 were genotyped for 93 probands diagnosed with ARVC from The Netherlands and 427 ostensibly healthy controls of various ethnicities. 82 additional ARVC cases were obtained from the literature and additional mutations were included from the ARVC Genetic Variants Database.
Results:
The overall yield of mutations among ARVC cases was 58% versus 16% in controls. 0.5% of control individuals versus 43% of ARVC cases hosted radical mutations, while 16% of controls hosted missense mutations versus a similar 21% of ARVC cases. Relative to controls, mutations in cases occurred more frequently in non-Caucasians, localized to the N-terminal regions of DSP and DSG2, and localized to highly conserved residues within PKP2 and DSG2.
Conclusions:
This study is the first to comprehensively evaluate genetic variation in healthy controls for the ARVC-susceptibility genes. Radical mutations are high probability ARVC-associated mutations whereas rare missense mutations should be interpreted in the context of race/ethnicity, mutation location, and sequence conservation.
Keywords: Arrhythmogenic right ventricular cardiomyopathy, arrhythmogenic right ventricular dysplasia, genetic testing, mutation, diagnosis
INTRODUCTION
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC) is a heritable cardiac disease characterized by fibro-fatty replacement of the right ventricle with a prevalence of 1 in 1000 to 5000 individuals1,2. It is a notable cause of sudden cardiac death (SCD) secondary to fatal ventricular arrhythmias and sudden death is a common first manifestation of disease3. Despite its name, ARVC can involve the left ventricle, in addition to the right, and is not necessarily arrhythmogenic4. This variable clinical course may include: i) a sub-clinical phase with concealed structural abnormalities during which the affected individual may present with SCD as their sentinel manifestation of the disease, ii) an overt electrical disorder with palpitations and syncope due to tachyarrhythmias stemming from the right ventricle, and iii) severe right ventricular or biventricular failure requiring cardiac transplantation or resulting in death5.
Roughly half of all ARVC is recognized as familial with an autosomal dominant inheritance with incomplete penetrance and variable expressivity. To date, mutations in several ARVC-susceptibility genes that encode essential desmosomal proteins have been identified: PKP2-encoding plakophilin 2, DSP-encoding desmoplakin, DSG2-encoding desmoglein 2, DSC2-encoding desmocollin 2, JUP-encoding plakoglobin, and TMEM43-encoding transmembrane protein 434. Desmosomes, also known as macula adherens, are macromolecular cellular structures comprised of plasma membrane proteins involved in linking intracellular intermediate filaments into a cell-spanning network allowing for effective transmission of force across cells. It is believed that functional deficits in these proteins imparted by genetic mutations result in cardiocyte detachment and cell death while subsequent inflammation results in fibro-fatty remodeling. In addition, it has been proposed that altered expression of ARVC-susceptibility genes, specifically DSP, might lead to altered Wnt/b-catenin and Tcf/Lef1 signaling causing the fibro-fatty remodeling and SCD-predisposition of ARVC6.
While non-desmosomal genes have been identified as rare causes of ARVC, the three desmosomal genes PKP2, DSP, and DSG2 harbor the majority of identified mutations accounting for approximately 40% of ARVC cases in studies from The Netherlands7,8, Italy9, and the United States10. In particular, PKP2 represents the most common gene mutated in ARVC, with a prevalence ranging from 40% (identified as the sole cause of ARVC in the cohort3,7) to approximately 10%9. The variable phenotypic expression of this disease, and the proclivity for inherited sudden cardiac death risk, make the identification of pathogenic disease biomarkers critical.
Recently, understanding of the genetic causes of ARVC has matured from the research bench to a clinically available commercial genetic test with an estimated yield of approximately 50% in patients with a likely diagnosis of ARVC11. As with other clinical tests, a true understanding of the extent and spectrum of genetic variation found in a healthy population is necessary for correct interpretation. While significant research has been dedicated to identifying pathogenic mutations, little is known about the background genetic variation in genes comprising the clinically available ARVC genetic tests. Understanding this signal-to-noise ratio is critical to determining whether an identified variant of undetermined/uncertain significance (VUS) might be the biomarker responsible for disease in the proband or whether it is a rare genetic variant with no relevance to the disease state. To this end, we sought to determine the spectrum and prevalence of background genetic variation which would meet the criteria of a “mutation” on the ARVC genetic test and to utilize this information to guide interpretation of a positive genetic test.
METHODS
Study Cohorts
From 1997 to 2007, ARVC patients were referred to the Academic Medical Center in Amsterdam, the University Medical Center Groningen in Groningen, and the University Medical Center Utrecht in Utrecht, The Netherlands, for comprehensive ARVC genetic testing following receipt of written, informed consent. For each proband, a history and physical examination, 12-lead electrocardiogram, 24-hour Holter monitoring, exercise testing, and 2-dimensional transthoracic echocardiography were conducted as described previously7,8.
To maximize the pre-genetic test possibility that a case-derived mutation is pathogenic, 93 “clinically definite,” unrelated cases were utilized for this study. ARVC diagnosis was based on the 2010 update12 of the 1994 diagnostic criteria of the Task Force of the European Society of Cardiology/International Society and Federation of Cardiology13. Similarly, 427 unrelated, ostensibly healthy individuals from various racial and ethnic backgrounds were subjected to ARVC genetic testing by PGxHealth in New Haven, Connecticut, United States. These control individuals were volunteers recruited and genotyped by PGxHealth as part of the clinical ARVC genetic test validation process. At the time of genotyping, ethnicity, sex, and age at diagnosis or genotyping, as appropriate, were recorded for each case and control subject.
To expand the spectrum of ARVC case mutations, 82 ARVC index cases genotyped in a recent United States ARVD/C Registry (Baltimore, Maryland) study by den Haan et al10 were included, yielding a combined ARVC case cohort of 175 probands from The Netherlands and the United States. In addition, for analysis of linear topology and primary sequence species conservation, mutations from the ARVD/C Genetic Variants Database14 (University Medical Center Groningen, Groningen, The Netherlands; accessed December 08, 2009) were incorporated.
Genetic Analysis
The genomic DNA of all 427 controls and 93 Dutch cases was analyzed for mutations in the translated exons and the splice site regions of the major ARVC-susceptibility genes: PKP2-encoding plakophilin 2, DSP-encoding desmoplakin, DSG2-encoding desmoglein 2, DSC2-encoding desmocollin 2, and TMEM43-encoding transmembrane protein 43. ARVC cases were additionally genotyped for JUP-encoding junction plakoglobin (also known as PKGB); however, as this gene was not genotyped for control individuals, it was excluded from statistical comparison. Among the 93 Dutch cases, identified mutations were absent in 400 reference alleles derived from unrelated, ostensibly healthy ethnically-matched Dutch volunteers. ARVC cases from the United States ARVD/C Registry were genotyped for all genes above with the exception of TMEM43 and all mutations were absent in 400 reference alleles derived from unrelated, ostensibly healthy ethnically-matched volunteers (Coriell Institute for Medical Research, Camden, New Jersey). Mutation analyses were performed using polymerase chain reaction followed by automated DNA sequencing.
Genetic Variant/Mutation Interpretation
Genetic variants predicted to alter the coding protein, through missense alteration of a codon, alteration of the canonical splice site, both in-frame and frame-shift insertion/deletion mutations, or nonsense mutations resulting in a premature truncation, were identified for this study. Any such variant observed in only ARVC cases and absent in both the ethnically-matched controls as well as the 427 control cohort, or a variant uniquely identified in one control cohort individual, were annotated as a “mutation”. Importantly, the designation of “mutation” herein is not meant to imply pathogenicity or even functional relevance to the respective protein. Rather, these are variants which, had they been discovered during the course of a clinical ARVC genetic test, would be considered a possible pathogenic variant. Mutations were classified using standard nomenclature. In-frame and frame-shift insertions and deletions, splice junction, and nonsense mutations were designated as “radical” mutations. As with the designation of “mutation,” the designation of “radical” is not meant to imply disease pathogenicity but rather serve as a descriptor for analysis of a class of mutations.
Primary Sequence and Species Conservation Analysis
Identified mutations were overlaid on the linear protein topology of each of the ARVC-associated gene products. The linear topology was annotated with known binding or functional domains, motifs, and predicted secondary structure regions from the literature15,16 as well as the UniProtKB/Swiss-Prot databank17. Sequence conservation analysis was similarly conducted utilizing primary sequences from the UCSC Genome Browser18. To calculate degree of conservation of individual residues across species, primary sequences from 44 species including primates, other placental mammals, and non-mammalian vertebrates were aligned. Degree of non-identity was determined by calculating the number of primary sequences harboring an amino acid not identical to the human residue at that location (substitution).
Statistical Analysis
Statistical analysis utilized Fisher’s exact test and two-sided t-tests, where appropriate, with a threshold of significance set to P < 0.05. Variance was expressed as mean ± standard deviation. Sequencing conservation scores were analyzed using Wilcoxon non-parametric 2-sample tests. Due to a lack of race/ethnicity matched ARVC cases, statistical comparisons between ARVC cases and non-Caucasian controls were not conducted.
RESULTS
Study Cohorts
Among the patients referred to the Dutch University Medical Centers, 93 index cases met the criteria for a “definite” diagnosis of ARVC according to the 2010 ARVC Task Force Criteria12,13,19. This Dutch Caucasian cohort was 29% female with an average age at diagnosis of 37 ± 14 years. These cases were combined with the 82 ARVC cases of North American Caucasian individuals from the United States ARVD/C Registry study cohort10 (42% female, diagnosed at 34 ± 13 years) to yield a combined ARVC case cohort of 175 probands, 35% female, with an average age at diagnosis of 35 ± 14 years.
The control cohort consisted of 427 unrelated, ostensibly healthy individuals from various racial and ethnic backgrounds including 103 (24.1%) Caucasian American, 124 (29.0%) Asian, 110 (25.8%) African, 42 (9.8%) Hispanic/Latino, 4 (0.94%) American Indian, and 12 multi-racial (2.8%) individuals. There were 32 (7.5%) individuals from a variety of other racial/ethnic backgrounds. To be included in a specific racial/ethnic group, each subject must have reported four grandparents from the same group, while multi-racial individuals had one or more grandparents of different racial/ethnic origin. This cohort was 50.1% female with an average age at genotyping of 41 ± 15 years. The cohort demographics for this study are summarized in Table 1.
Table 1 –
Study Cohort Demographics
| ARVC | Controls | |||
|---|---|---|---|---|
| Demographic | Dutch | den Haan10 | Combined | PGxHealth |
| Total | 93 | 82 | 175 | 427 |
| Caucasian | 93 | 82 | 175 | 103 |
| Asian | 0 | 0 | 0 | 124 |
| African | 0 | 0 | 0 | 110 |
| Hispanic/Latino | 0 | 0 | 0 | 42 |
| American Indian | 0 | 0 | 0 | 4 |
| Multi-Racial | 0 | 0 | 0 | 12 |
| Other | 0 | 0 | 0 | 32 |
| % Female | 29.00% | 41.98% | 35.06% | 50.11% |
| Age (years) | 37 ± 14 | 34 ± 13 | 35 ± 14 | 41 ± 15 |
Mutation Spectrum and Prevalence by Cohort
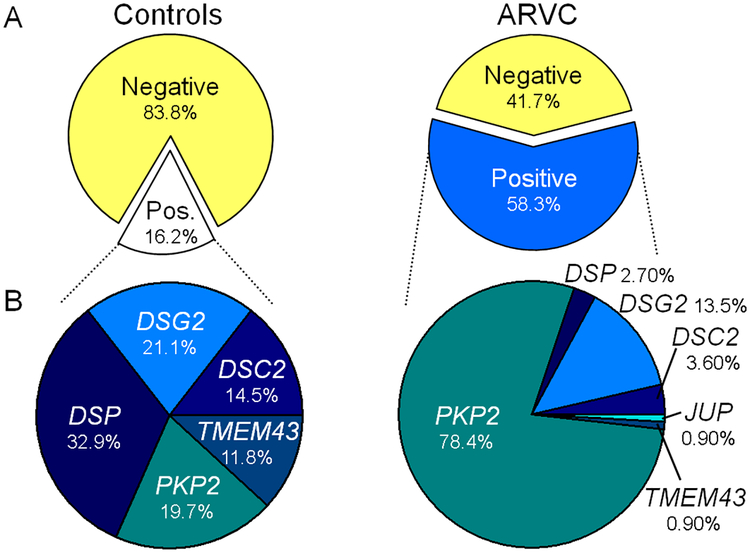
Comprehensive genetic interrogation of the control and ARVC case cohorts identified mutations in both cohorts. As demonstrated in Figure 1, among the ostensibly healthy, cosmopolitan control cohort, 69/427 (16.2%) of individuals hosted a genetic variant which met our criteria to be considered a rare “mutation” creating what would have been annotated as a possible “positive” ARVC genetic test had the subject been an ARVC case. While not meant to imply pathogenicity, we utilized this label for the purposes of further analysis of background genetic variation. 19.7% of these control cohort mutations localized to PKP2, 32.9% in DSP, 21.1% in DSG, 14.5% in DSC2, and 11.8% in TMEM43.
Figure 1 – Prevalence of mutations in control and ARVC cohorts.
A) Pie charts of the percentage of mutation-positive individuals within the control (white) and ARVC cohorts (blue) separated from mutation-negative individuals (yellow) of each respective cohort. B) Pie chart of the percentage of mutations found in each of the ARVC-associated genes among mutation-positive cases in the control (left) and ARVC (right) cohort. JUP was not genotyped in control cases and TMEM43 was not genotyped in the United States ARVD/C Registry component of the ARVC case cohort.
To test the hypothesis the frequency of mutations found in control individuals reflected random genetic variability, we compared the frequency of mutations with the relative size of the coding DNA (cDNA) for each of the genes. The 4439 nucleotides which comprise the cDNA of PKP2 represent approximately 15.7% of the total cDNA for the ARVC-associated genes, and approximately 19.7% of the mutations identified in controls localized to PKP2. Likewise, frequency of control mutations in DSP, DSG2, DSC2, and TMEM2 were similar to their respective relative cDNA size (Table 2).
Table 2 –
Relative Coding DNA Length versus Mutation Frequency
| Gene | cDNA Size (nts) | % Total cDNA | % Control Mutations | % ARVC Mutations |
|---|---|---|---|---|
| PKP2 | 4439 | 15.7% | 19.7% | 78.4% |
| DSP | 9796 | 34.6% | 32.9% | 2.7% |
| DSG2 | 5652 | 19.9% | 21.1% | 13.5% |
| DSC2 | 5124 | 18.1% | 14.5% | 3.6% |
| TMEM43 | 3341 | 11.8% | 11.8% | 0.9% |
In contrast, ARVC cases had an overall yield of 58.3% with 102/175 individuals hosting a mutation absent in at least 400 reference alleles. Among the mutation-positive ARVC cases, 78.4% of the mutations localized to PKP2, 2.7% to DSP, 13.5% to DSG, 3.6% to DSC2, 0.9% to JUP, and 0.9% TMEM43. A summary of the genetic variants identified in the ARVC and control cohort are listed in Supplemental Tables 1 and 2.
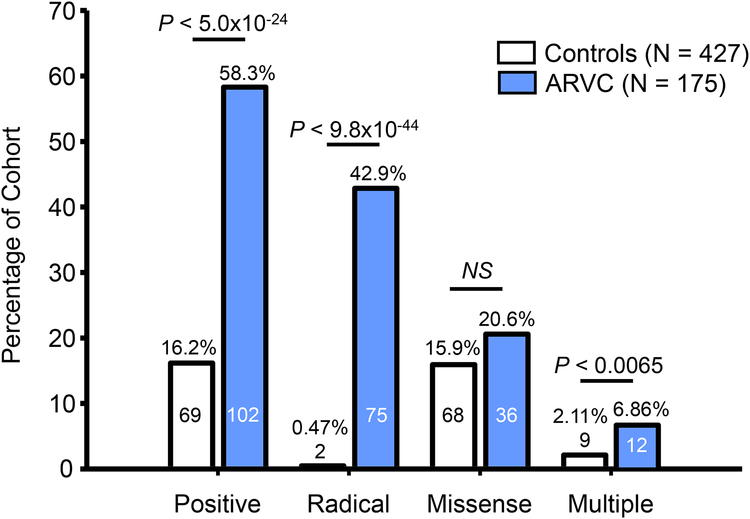
As shown in Figure 2, while there was an approximately 3.5-fold higher genetic test yield for ARVC cases than controls (58.3% vs. 16.2%, P < 5.0×10−24), approximately 1 in 6 healthy individuals genotyped met criteria for a mutation and a positive ARVC genetic test. This so-called background genetic variation was largely missense mutations, as only 2/427 (0.47%) control individuals hosted radical mutations versus 75/175 (42.9%) ARVC cases (P < 9.8×10−44) while 68/427 (15.9%) controls hosted missense mutations versus 36/175 ARVC cases (20.6%, P = ns). The two radical mutations found in two control individuals were PKP2-IVS5–1G>A and DSP-S2843-R2846del. A small percentage of control individuals hosted two or more mutations (9/427, 2.11%) compared to a higher percentage of ARVC cases (12/175, 6.86%; P < 0.0065). Among these individuals, 2/175 (1.1 %) ARVC cases hosted three mutations while no individuals in the control cohort had more than two mutations.
Figure 2 – Prevalence of radical, missense, and multiple mutations.
Bar graph depicting the number and percentage of the control (white) and ARVC case (blue) cohorts hosting mutations. The overall yield of the genetic test (positive) was divided into radical mutations, missense mutations, and individuals hosting more than one mutation (multiple). Numbers within bars denote number of individuals positive for the respective mutation type in each cohort.
Mutation Spectrum and Prevalence by Race/Ethnicity
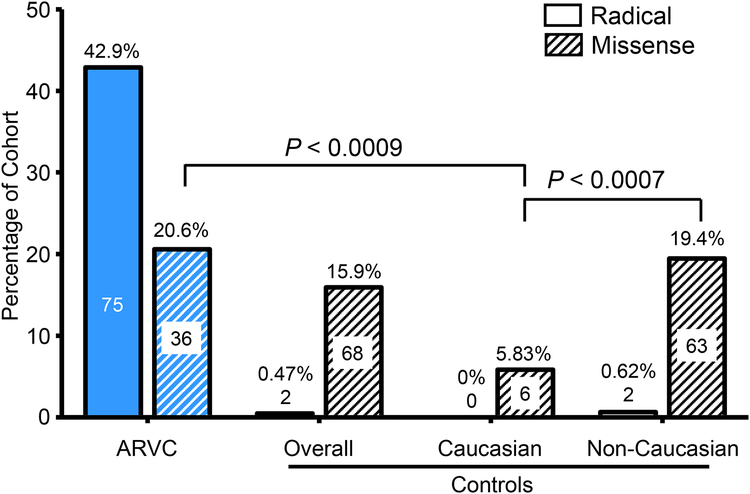
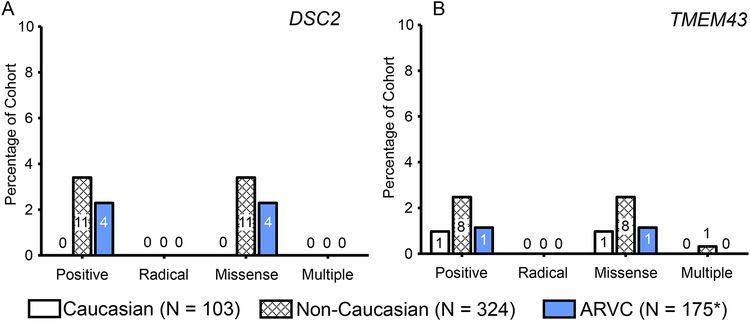
To determine whether there was race- or ethnicity-specific differences in mutation type or prevalence, particularly among missense mutations, we determined the frequency of mutations among Caucasian and non-Caucasian control subpopulations. While the yield of mutations in control individuals was 16.2% overall, mutations in non-Caucasian individuals occurred at a higher frequency than Caucasian control individuals (19.44% vs. 5.83%, respectively; P < 0.003). Specifically, as shown in Figure 3, ostensibly healthy Caucasian individuals had a much lower rate of missense mutations (6/103, 5.83%) than non-Caucasian control individuals (63/324, 19.4%; P < 0.0007). This was also lower than the overall prevalence of missense mutations among the Dutch and North American Caucasian ARVC cases (36/175, 20.6%; P < 0.0009). The prevalence of radical mutations across Caucasian and non-Caucasian subsets of the control cohort were extremely rare (0% and 0.62%, respectively).
Figure 3 – Prevalence of mutations in Caucasian and non-Caucasian individuals.
Bar graph of the number and percentage of the ARVC (blue background) or control (white background) individuals hosting radical (no lines) or missense (diagonal lines) mutations. Mutations identified in controls were subdivided into Caucasian and non-Caucasian ostensibly healthy individuals. Numbers within bars denote number of individuals positive for the respective mutation type in each cohort and cohort subset.
Mutation Spectrum and Prevalence by Gene
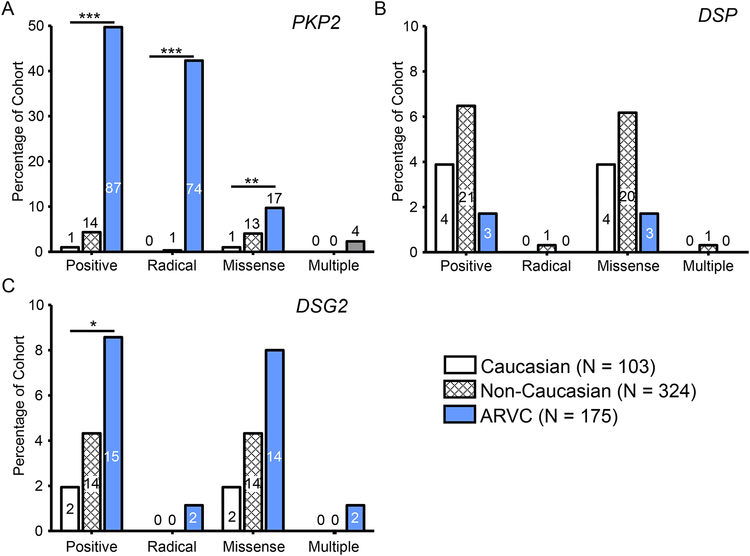
To determine whether the high background rate of genetic variation might be gene-specific, as well as race- or ethnicity-specific, we next calculated the percentage of mutation positive individuals for each gene within the control cohort, both Caucasian and non-Caucasian subsets, and the ARVC cohort (Figure 4 and 5). As demonstrated in Figure 4A, there was a higher percentage of individuals in the ARVC cohort (49.7%) with mutations in PKP2 than in Caucasian controls (0.97%, P < 2.7×10−21). While 4.32% of non-Caucasian controls hosted mutations in PKP2, statistical comparison with the ARVC cases could not be conducted due to a lack of non-Caucasian ARVC cases available in this study. This difference in mutation frequency largely reflects a higher percentage of radical mutations in the ARVC cases (42.3%) than among either the Caucasian controls (0%, P < 8.1×10−19) or the non-Caucasian (0.31%) controls. There was also a significantly higher percentage of PKP2 missense mutations in ARVC cases (9.71%) than Caucasian controls (0.97%, P < 0.005) while 4.01% of non-Caucasian controls hosted PKP2 missense mutations.
Figure 4 – Prevalence of mutations in PKP2, DSP, and DSG2.
Bar graph depicting the number and percentage of the Caucasian control (white), non-Caucasian control (white with hash) and ARVC case (blue) cohorts hosting mutations in PKP2 (A), DSP (B), and DSG2 (C). The overall yield of the genetic test (positive) was divided into radical mutations, missense mutations, and individuals hosting more than one mutation (multiple). Numbers within bars denote number of individuals positive for a mutation in the respective gene in each cohort. *, P < 0.05; **, P < 0.01; ***, P < 0.0001.
Figure 5 – Prevalence of mutations in DSC2 and TMEM43.
Bar graph depicting the number and percentage of the Caucasian control (white), non-Caucasian control (white with hash) and ARVC case (blue) cohorts hosting mutations in DSC2 (A) and TMEM43 (B). The overall yield of the genetic test (positive) was divided into radical mutations, missense mutations, and individuals hosting more than one mutation (multiple). Numbers within bars denote number of individuals positive for a mutation in the respective gene in each cohort. *, TMEM43 was not genotyped in the United States ARVD/C Registry component of the ARVC case cohort (N = 93).
Whereas mutations localizing to PKP2 were more prevalent in ARVC cases than Caucasian controls, the prevalence of mutations localizing to DSP demonstrated no statistical difference between ARVC cases and Caucasian control individuals (6.17% vs. 1.71%, respectively; Figure 4B). The prevalence of mutations in healthy non-Caucasian control individuals was 6.48%. As shown in Figure 4C, a higher percentage of ARVC case individuals hosted mutations in DSG2 compared to Caucasian controls (8.57% vs. 1.94%, P < 0.05) while there was no difference between the background genetic variation rate of non-Caucasians and ARVC cases. This difference was due to an over-representation of missense mutations among cases (8.00%) than Caucasians (1.94%, P < 0.05).
There was no discernible difference in the percentage of patients with DSC2 (Figure 5A) or TMEM43 (Figure 5B) mutations between the ARVC and both control sub-cohorts, although the overall prevalence of mutations in these genes was low.
Founder Mutations
While the ARVC case cohort analyzed is comprised of unrelated probands, we noted several mutations which were found in multiple ARVC cases which were absent in control individuals. To determine whether these potential founder mutations impacted the overall yield of genetic testing among these ARVC cases, we identified and re-analyzed the cohort yield excluding these potential founder mutations: PKP2-IVS10–1G>C which was identified in 13 ARVC cases (four Dutch), PKP2-C796R mutation in 9 Dutch cases (all Dutch), and the PKP2-R79X nonsense mutation in 8 cases (six Dutch). When each of these potential founder mutations was removed from the two ARVC cohorts, the overall yield of mutations in the ARVC cases dropped from 58.3% to 49.7% (63.4% to 43.0% in the Dutch and 52.4% to 39.0% in the North American ARVC cohort). As each of these potential founder mutations localized to PKP2, exclusion of these mutations reduced the PKP2-specific yield from 49.7% to 32.6% (53.8% to 33.3% in the Dutch and 45.1% to 31.7% in the North American ARVC cohort) while not altering other ARVC-gene frequencies.
Mutation “Hot Spots” in Desmoplakin and Desmoglein 2
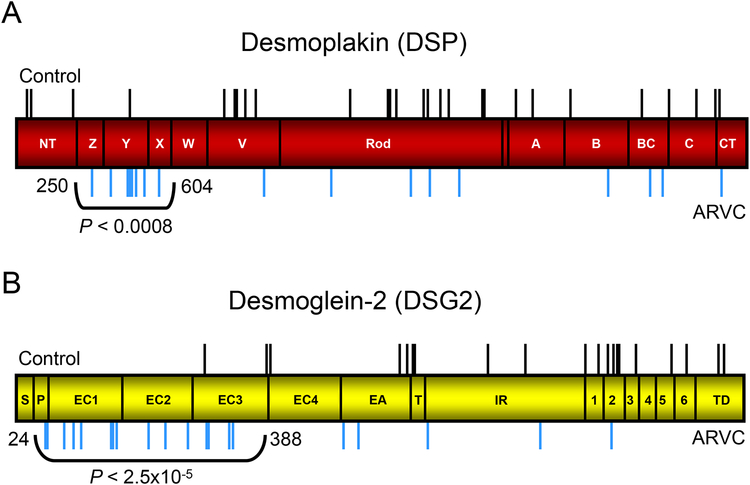
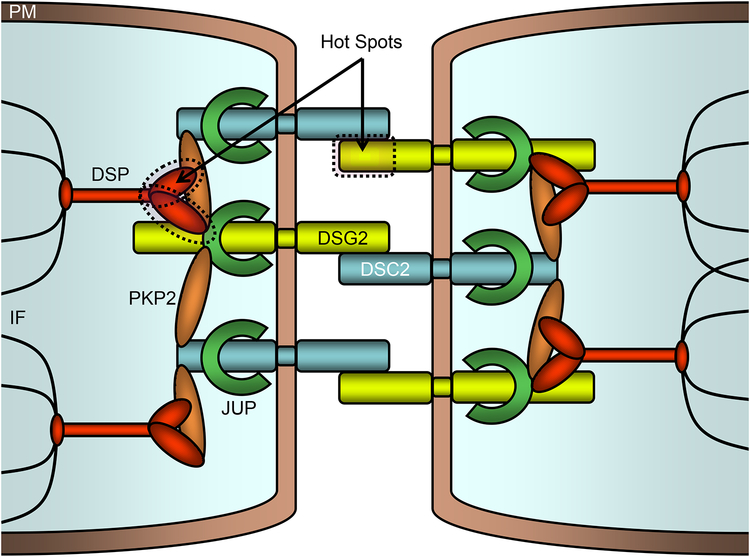
Based on the identification of key binding and functional domains on the proteins comprising the desmosome15,16, we next investigated whether missense mutations identified in ARVC cases might localize preferentially to critical protein domains compared to control mutations. As depicted in Figure 6, we identified regions on the linear topology of DSP and DSG2 which were apparent mutation “hot spots” in which a significantly larger number of missense case mutations were identified compared to controls. Specifically, an amino-terminal region of DSP encompassing three of the predicted alpha-helical bundles Z, Y, and X (residues 250 through 604) contained 8/17 (47%) of the DSP missense mutations found in ARVC cases compared to 1/28 (3.6%) of the DSP missense mutations found in controls (P < 0.0008, Figure 6A). Likewise, the amino-terminal propeptide and cadherin repeats I-III domains (residues 24 through 388) of DSG2 demonstrated an over-representation of missense mutations compared to controls (16/21 vs. 2/20, P < 2.5×10−5, Figure 6B). These mutation hot spots localize to key regions of the desmosomal macromolecular complex as demonstrated in Figure 7. Specifically, ARVC-case mutations are more prevalent in a region of DSP and DSG which include, or are closely proximal to, respective protein binding domains critical to formation of the desmosome.
Figure 6 – DSP and DSG mutation “hot spots”.
Linear topology of DSP and DSG2 with the location of all control missense mutations (black line, above topology) and ARVC case mutations (blur lines, below topology) identified. A) ARVC mutations were more frequent between residues 250 and 604 of DSP (red topology) than in control individuals. NT, Z, Y, X, W, V, predicted alpha-helical bundles; A, B, BC, C, CT, intermediate filament binding domains. B) ARVC mutations were more frequent between residues 24 and 388 of DSG2 (yellow topology) than in control individuals. S, signal peptide; P, propeptide; EC1, 2, 3, 4, cadherin repeat domains I-IV; EA, extracellular anchor; TM, transmembrane domain; IA, intracellular anchor; ICS, intracellular catenin-binding domain; IPL, proline-rich linker; RUD, repeating unit domain; DTD, desmoglein terminal domain.
Figure 7 – Mutation “hot spots” within the cardiocyte desmosome.
Schematic representation of the desmosome which links neighboring cellular intermediate filaments through a complex of interacting proteins. Two mutation hotspots (dashed outline) on DSP and DSG2 are noted. PM, plasma membrane; DSP, desmoplakin; DSG2, desmoglein 2; DSC2, desmocolin 2; PKP2, plakophilin; JUP, plakoglobin 2; IF, intermediate filaments.
Mutations and Primary Sequence Conservation in Plakophilin 2 and Desmoglein 2
In an attempt to more clearly define differences in ARVC case and healthy control missense mutations, we explored possible associations between the location of missense mutations and the conservation of the primary sequence across species for each of the ARVC-susceptibility gene products. As demonstrated in Table 3, across the five ARVC-associated genes analyzed, mutations identified in control individuals were more likely to localized to residues that were more highly substituted across species (mean of 6.71 substitutions) than ARVC cases (mean 2.85, P < 0.0001, Z-score −5.61).
Table 3 –
Sequence Conservation and Mutations in ARVC Genes
| No. of Species with Non-Conserved Residues | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort | N | 0 | 1 | 2 | 3 | 4 | 5 | >5 | Mean |
| Control | 133 | 11 | 19 | 8 | 10 | 9 | 15 | 61 | 6.71 |
| ARVC | 81 | 35 | 11 | 6 | 5 | 2 | 5 | 17 | 2.85 |
N, the total number of mutations localizing to the five ARVC associated genes analyzed; mean, the mean number of species which have a different residue at the location of the given variant for the cohort. P < 0.0001 (Z-Score −5.61).
Specifically, ARVC case-based missense mutations in PKP2 and DSG2 altered residues that were significantly more conserved across species than those missense mutations derived from controls. As shown in Table 4, among the 19 PKP2 missense mutations identified in control individuals, 13 localized to residues which were substituted in three or more species queried (mean 4.13). In contrast, among the 23 ARVC case mutations, 11 mutations localized to residues that were not substituted for other residues across species, yielding a mean degree of non-identity of 1.65 (P < 0.0001, Z-score −4.33). Similarly, 18/33 control missense mutations in DSG2 localized to residues with substitutions in more than five species (mean 9.15) while 15/27 ARVC mutations localized to non-substituted residues (mean 1.96, P < 0.0001, Z-score −4.66, Table 5). For DSP, DSC2, and TMEM43, we found no relationship, or we were underpowered to determine an association, between the degree of species conservation and whether a residue was altered in an ARVC case or control individual.
Table 4 –
Sequence Conservation and Mutations in PKP2
| No. of Species with Non-Conserved Residues | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort | N | 0 | 1 | 2 | 3 | 4 | 5 | >5 | Mean |
| Control | 19 | 1 | 5 | 0 | 3 | 1 | 2 | 7 | 5.74 |
| ARVC | 23 | 11 | 5 | 1 | 2 | 1 | 0 | 3 | 1.83 |
N, the total number of mutations localizing to PKP2; mean, the mean number of species which have a different residue at the location of the given variant for the cohort. P < 0.0001 (Z-Score −4.33).
Table 5 –
Sequence Conservation and Mutations in DSG2
| No. of Species with Non-Conserved Residues | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort | N | 0 | 1 | 2 | 3 | 4 | 5 | >5 | Mean |
| Control | 33 | 1 | 2 | 4 | 2 | 2 | 4 | 18 | 9.15 |
| ARVC | 27 | 15 | 2 | 3 | 1 | 1 | 2 | 3 | 1.96 |
N, the total number of mutations localizing to DSG2; mean, the mean number of species which have a different residue at the location of the given variant for the cohort. P < 0.0001 (Z-Score −4.66).
DISCUSSION
ARVC Genetic Test Interpretation
Understanding the background rate of genetic variability is critical to interpreting the results of a genetic test. Recently, the spectrum of background genetic variation in the genes encoding proteins implicated in the pathogenesis of another potentially lethal arrhythmia syndrome, long QT syndrome (LQTS), has been elucidated20. For LQTS, the background mutation rate for its 3 canonical genes: KCNQ1, KCNH2, and SCN5A, was ~5%. Here, we report an initial background mutation rate, unadjusted for ancestry, in the analyzed ARVC-susceptibility genes to be approximately 16%, or three-fold higher than the genetic noise prevalence seen in LQTS. This poses several challenges to interpreting the ARVC genetic test as 1 in 6 healthy individuals would meet current criteria for a so-called positive ARVC genetic test even with proper qualification of these rare “mutations” with the clinically ambiguous designation as a “variant of uncertain significance” or VUS. Herein, we hope to provide some initial genetic associations to guide the physician in determining an estimated probability of pathogenicity following the identification of a rare variant in an ARVC-susceptibility gene.
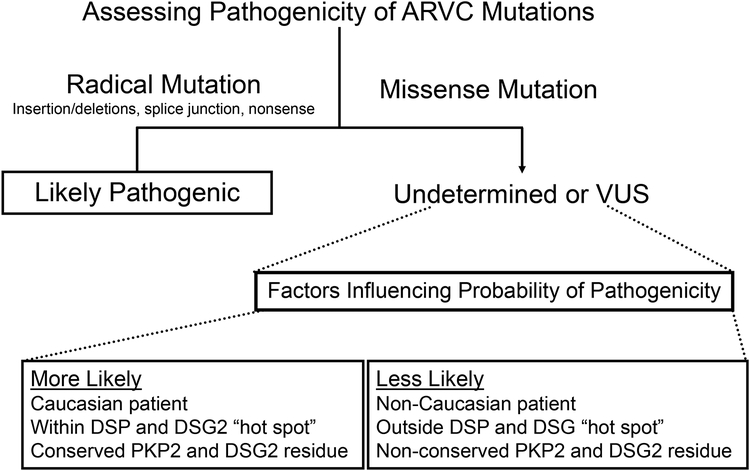
Given the background rate of mutations in the control cohort compared to ARVC cases as well as the demonstrated differences in this signal-to-noise ratio from mutation type (radical versus missense) and race/ethnicity, we created a summary of factors which we believe alter the likelihood that a given variant is pathogenic (Figure 8). First, we observe that radical mutations (insertions/deletions, splice junction mutations, and nonsense mutations) are significantly more prevalent in ARVC cases compared to controls (49.9% vs. 0.47%, respectively; P < 9.8×10−44), thus an ARVC genetic test result indicating the presence of this type of genetic variant has a high likelihood of being associated with ARVC pathogenicity. Notably, such radical mutations constitute the majority (75/102, 73.5%) of genetic alterations identified in mutation-positive ARVC cases.
Figure 8 – Assessing likelihood of pathogenicity of a positive ARVC genetic test.
Flow chart of factors influencing likelihood that a given ARVC genetic test-identified mutation is pathogenic based on comparison of mutations identified in ostensibly healthy controls versus definite ARVC cases.
If the ARVC genetic test indicates a missense mutation, we identified three associations which might strengthen or weaken the possibility of ARVC pathogenicity for the variant in question. Based on our observation that ostensibly healthy Caucasian individuals had a significantly lower rate of missense mutations than ARVC cases (5.83% vs. 20.6%, respectively; P < 0.0009), therefore, a rare missense mutation identified in a Caucasian patient is more likely pathogenic than one identified in a non-Caucasian patient. Further, specific amino-terminal regions of DSP and DSG2 may contain mutation “hot spots” as each was found to host significantly more missense mutations in ARVC cases than controls (P < 0.0008 and P < 2.5×10−5, respectively). Finally, there were significantly more ARVC missense mutations involving residues within PKP2 and DSG2 that were highly conserved across species. Given the observation that ARVC cases missense mutations demonstrate a rate of substitution of 1.65 and 1.19 for PKP2 and DSG2, respectively, compared to 4.13 (P < 0.0001) and 9.95 (P < 0.0001) for control individuals, respectively, identification of a missense mutation that involves a highly conserved residue is more likely pathogenic.
When properly interpreted, ARVC genetic testing ultimately affords the possibility of early detection of preclinical disease, identification of at-risk family members, and genetic counseling for this sudden death-predisposing disease. However, due to the potential clinical impact of such a test, and multiple factors that might influence how and when a genetic abnormality might manifest as disease, ARVC genetic testing should not be viewed as a perfectly binary (yes/no, positive/negative) test. The presence of a genetic mutation alone cannot override clinical judgment regarding the diagnosis of ARVC, nor should the absence of a mutation in the setting of compelling clinical evidence call the diagnosis into question. Regardless of the strength of statistical assessment that a mutation is pathogenic, genetic test results should be viewed as probabilistic and as one component of an overall clinical assessment.
Background Genetic Noise and Occult Disease
While it is possible that occult, undiagnosed ARVC is present in a small number of the ostensibly healthy volunteers genotyped in the control cohort, it is highly unlikely that this accounts for the significant genetic variability found in our control population. While most estimates place the prevalence of ARVC at 1 in 5000 among the general population2, a conservative estimate of 1 in 1000 would suggest a 50% chance that only a single individual within the 427 control cohort has occult ARVC1. Even if the frequency of clinically silent ARVC in our cohort were 10-fold higher, this conservative estimate would constitute approximate 1% of the entire control cohort ultimately resulting in a decrease of genetic noise from a 16 to 15% prevalence. In addition, the relative gene-specific frequency of mutations in ostensibly healthy controls supports the hypothesis that these variants are randomly occurring background genetic variation. While the majority (78%) of ARVC case mutations localized to PKP2, control mutations distributed amongst the ARVC genes in a manner reflective of the relative size of the gene.
It is also possible that the apparently high rate of mutations in healthy individuals might be somewhat inflated for this initial control cohort relative to future studies. One would expect that genetic interrogation of additional control individuals would re-encounter some of these unique genetic variants while fewer new variants would be discovered. As per our criteria for mutation designation, a variant identified in more than one healthy individual was not considered a mutation, thus genotyping additional control individuals could meaningfully reduce the estimated background mutation rate. One reason the apparent background rate is lower in the Caucasian subset of controls may be that, relative to other ancestries, more Caucasian controls have been interrogated genetically previously.
Compound and Digenic Heterozygosity
A study by Xu and colleagues genetically interrogated 198 United States and Italian ARVC cases and identified mutations localizing to PKP2 in roughly 19% of the probands with 42% of these PKP2-positive individuals, or 8% of the cohort overall, hosting at least one additional mutation in one of the ARVC-susceptibility genes21. This yield of PKP2 mutations is similar to a Danish ARVC cohort which demonstrated 8/53 (15%) individuals with PKP2 mutations22. Further, Xu and colleagues presented a number of pedigrees of kindred hosting two ARVC mutations in trans, wherein coinheritance of both mutations results in a more severe clinical phenotype compared to patients hosting only one21. In their analysis, compound and digenic heterozygosity accounts for the variable penetrance observed in their pedigrees and led authors to conclude that a single PKP2 mutation maybe insufficient to potentiate clinical disease.
While we observed a similar prevalence of multiple mutations in ARVC cases overall (7%), this was in the context of a higher percentage of PKP2-positive individuals (49.7%, or 39.9% when excluding three potential founder mutations). Thus, among the subset of genotype-positive ARVC seen in this study, 93% had a single ARVC-associated mutation. This is similar to a recent study by Fressart and colleagues which identified multiple mutations in 4% of ARVC cases23. Furthermore, we identified nine individuals (2%) in our ostensibly healthy control cohort which similarly hosted multiple mutations. Interestingly, a study by Bhuiyan and investigators recently found that mutations in DSC2 and DSG2, while less prevalent than mutations in PKP2, also occurred in the setting of another mutation in an ARVC-associated gene perhaps indicating that compound/digenic heterozygosity might play a role outside of just PKP2 mutations8.
While compound/digenic heterozygosity clearly plays a large role in explaining some of the incomplete penetrance and variable expressivity of the disease in ARVC families, we believe that the type and location of the mutation might be similarly important. We find that the frequency of radical mutations in our control cohort to be 0.5% in comparison to the ARVC case frequency of 43%. This difference alone provides significant statistical evidence that a radical mutation reported on an ARVC genetic test is likely pathogenic. Another possibility is that compound/digenic heterozygosity may play a modulatory role which is most salient in families with variable ARVC expression and may not play as meaningful a role in dictating disease penetrance in our cohorts.
LIMITATIONS
While this study is the first and largest systematic examination of the spectrum and prevalence of ARVC-associated background genetic variation, small sample sizes of both the ARVC cases and control cohorts still represent a limitation, especially considering individual ethno-geographic strata. The observed statistical associations were based on a relative small sample size compared to other similar studies in channelopathic disease and might be susceptible to sway by addition of a few outlier individuals and founder mutation20. Despite this, the observed statistical associations appear robust, were based on the largest set of controls and one of the largest case collections examined to date, and testable in future replication studies. Future studies incorporating additional phenotypically-robust ARVC cohorts and thoroughly-examined ostensibly healthy individuals genotyped for the equivalent of the ARVC genetic-test panel will further validate and extend the generalizability of these observations.
As previously discussed, a normal 12-lead electrocardiogram, Holter monitoring, and echocardiographic analysis were not a prerequisite for the individuals to be included in the control cohort. Thus, it is possible that a small number of the 69 “mutation-positive” controls may have clinically silent ARVC and a mutation identified in that individual could be falsely considered part of the background genetic variation. As mentioned earlier, statistically there should be only one, at most two, of the 427 control subjects who nevertheless have ARVC. Among these 69 mutation positive controls, two are indeed suspicious for ARVC pathogenicity: the control hosting the splice junction IVS5–1G>A mutation and the one hosting the in-frame DSP-S2843-R2846del and a concomitant PKP2-V587I missense mutation. While we acknowledge the possibility that some mutations amongst control individuals may represent incompletely penetrant mutations that may be pathogenic in another patient, for all variants to be pathogenic the average penetrance of these variants must be extremely low as the most conservative estimate of ARVC in the healthy population is 1 in 1000. Notably, we cannot exclude the possibility that ARVC cases, particularly those hosting only one ARVC-associated mutation, do not carry a compound mutation in a yet unknown ARVC-susceptibility gene.
CONCLUSIONS
In the first comprehensive study of genetic variation in ostensibly healthy individuals for ARVC-susceptibility genes, we demonstrate that radical mutations are high probability ARVC-associated mutations whereas rare missense mutations should be interpreted with great caution. A compilation of factors including mutation localization, the conservation of the primary sequence across species, and the race/ethnicity of the patient influence the probability of missense mutations truly being the pathogenic biomarker of disease.
Supplementary Material
Abbreviations:
- ARVC
arrhythmogenic right ventricular cardiomyopathy/dysplasia
- del
deletion
- DSC2
desmocollin 2
- DSG2
desmoglein 2
- DSP
desmoplakin
- IVS
intervening sequence
- JUP
plakoglobin
- LQTS
long QT syndrome
- PKP2
plakophilin 2
- SCD
sudden cardiac death
- TMEM43
transmembrane protein 43
Footnotes
DISCLOSURES
BAS, TEC, and GDP are employees of PGxHealth. AAW serves on PGxHealth’s Scientific Advisory Board. MJA is a consultant for Medtronic, St. Jude Medical, Inc., Boston Scientific, and PGxHealth and serves on PGxHealth’s Scientific Advisory Board.
REFERENCES
- 1.Peters S, Trümmel M, Meyners W. Prevalence of right ventricular dysplasia-cardiomyopathy in a non-referral hospital. Int. J. Cardiol 2004; 97:499–501. [DOI] [PubMed] [Google Scholar]
- 2.Gemayel C, Pelliccia A, Thompson PD. Arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2001; 38:1773–1781. [DOI] [PubMed] [Google Scholar]
- 3.Dalal D, Molin LH, Piccini J, Tichnell C, James C, Bomma C, Prakasa K, Towbin JA, Marcus FI, Spevak PJ, Bluemke DA, Abraham T, Russell SD, Calkins H, Judge DP. Clinical Features of Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Associated With Mutations in Plakophilin-2. Circulation. 2006; 113:1641–1649. [DOI] [PubMed] [Google Scholar]
- 4.Sen-Chowdhry S, Syrris P, McKenna WJ. Role of Genetic Analysis in the Management of Patients With Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. J Am Coll Cardiol. 2007; 50:1813–1821. [DOI] [PubMed] [Google Scholar]
- 5.Thiene G, Corrado D, Basso C. Arrhythmogenic right ventricular cardiomyopathy/dysplasia. Orphanet J Rare Dis. 2007; 2:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Gras E, Lombardi R, Giocondo MJ, Willerson JT, Schneider MD, Khoury DS, Marian AJ. Suppression of canonical Wnt/Î2-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J. Clin. Invest 2006; 116:2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Tintelen JP, Entius MM, Bhuiyan ZA, Jongbloed R, Wiesfeld ACP, Wilde AAM, van der Smagt J, Boven LG, Mannens MMAM, van Langen IM, Hofstra RMW, Otterspoor LC, Doevendans PAFM, Rodriguez L-M, van Gelder IC, Hauer RNW. Plakophilin-2 Mutations Are the Major Determinant of Familial Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Circulation. 2006; 113:1650–1658. [DOI] [PubMed] [Google Scholar]
- 8.Bhuiyan ZA, Jongbloed JDH, van der Smagt J, Lombardi PM, Wiesfeld ACP, Nelen M, Schouten M, Jongbloed R, Cox MGPJ, van Wolferen M, Rodriguez LM, van Gelder IC, Bikker H, Suurmeijer AJH, van den Berg MP, Mannens MMAM, Hauer RNW, Wilde AAM, van Tintelen JP. Desmoglein-2 and Desmocollin-2 Mutations in Dutch Arrhythmogenic Right Ventricular Dysplasia/Cardiomypathy Patients: Results From a Multicenter Study. Circ Cardiovasc Genet. 2009; 2:418–427. [DOI] [PubMed] [Google Scholar]
- 9.Pilichou K, Nava A, Basso C, Beffagna G, Bauce B, Lorenzon A, Frigo G, Vettori A, Valente M, Towbin J, Thiene G, Danieli GA, Rampazzo A. Mutations in Desmoglein-2 Gene Are Associated With Arrhythmogenic Right Ventricular Cardiomyopathy. Circulation. 2006; 113:1171–1179. [DOI] [PubMed] [Google Scholar]
- 10.den Haan AD, Tan BY, Zikusoka MN, Llado LI, Jain R, Daly A, Tichnell C, James C, Amat-Alarcon N, Abraham T, Russell SD, Bluemke DA, Calkins H, Dalal D, Judge DP. Comprehensive Desmosome Mutation Analysis in North Americans With Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Circ Cardiovasc Genet. 2009; 2:428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awad MM, Calkins H, Judge DP. Mechanisms of Disease: molecular genetics of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Nat Clin Pract Cardiovasc Med. 2008; 5:258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MGPJ, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DMY, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: Proposed Modification of the Task Force Criteria. Circulation. 2010; 121:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKenna W, Thiene G, Nava A, Fontaliran F, Blomstrom-Lundqvist C, Fontaine G, Camerini F. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br. Heart J 1994; 71:215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Zwaag P, Jongbloed J, van den Berg M, van der Smagt J, Jongbloed R, Bikker H, Hofstra R, van Tintelen J. A genetic variants database for arrhythmogenic right ventricular dysplasia/cardiomyopathy. Hum Mutat. 2009; 30:1278–1283. [DOI] [PubMed] [Google Scholar]
- 15.Virata M, Wagner R, Parry D, Green K. Molecular structure of the human desmoplakin I and II amino terminus. Proc Natl Acad Sci U S A. 1992; 89:544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green KJ, Gaudry CA. Are desmosomes more than tethers for intermediate filaments? Nat Rev Mol Cell Biol. 2000; 1:208–216. [DOI] [PubMed] [Google Scholar]
- 17.Junker VL, Apweiler R, Bairoch A. Representation of functional information in the SWISS-PROT Data Bank. Bioinformatics. 1999; 15:1066–1067. [DOI] [PubMed] [Google Scholar]
- 18.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler aD. The Human Genome Browser at UCSC. Genome Res. 2002; 12:996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox MGPJ, vanderSmagt JJ, Noorman M, Wiesfeld AC, Volders PGA, vanLangen IM, Atsma DE, Dooijes D, Houweling AC, Loh P, Jordaens L, Arens Y, Cramer MJ, Doevendans PA, vanTintelen JP, Wilde AAM, Hauer RNW. ARVD/C Diagnosis: Impact of New Task Force Criteria. Circ Arrhythm Electrophysiol. 2010; 3:126–133. [DOI] [PubMed] [Google Scholar]
- 20.Kapa S, Tester DJ, Salisbury BA, Harris-Kerr C, Pungliya MS, Alders M, Wilde AAM, Ackerman MJ. Genetic Testing for Long-QT Syndrome: Distinguishing Pathogenic Mutations From Benign Variants. Circulation. 2009; 120:1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu T, Yang Z, Vatta M, Rampazzo A, Beffagna G, Pillichou K, Scherer SE, Saffitz J, Kravitz J, Zareba W, Danieli GA, Lorenzon A, Nava A, Bauce B, Thiene G, Basso C, Calkins H, Gear K, Marcus F, Towbin JA. Compound and Digenic Heterozygosity Contributes to Arrhythmogenic Right Ventricular Cardiomyopathy. J Am Coll Cardiol. 2010; 55:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen AH, Benn M, Tybjærg-Hansen A, Haunso S, Svendsen JH. Missense Variants in Plakophilin-2 in Arrhythmogenic Right Ventricular Cardiomyopathy Patients - Disease-Causing or Innocent Bystanders? Cardiology. 2010; 115:148–154. [DOI] [PubMed] [Google Scholar]
- 23.Fressart V, Duthoit G, Donal E, Probst V, Deharo J-C, Chevalier P, Klug D, Dubourg O, Delacretaz E, Cosnay P, Scanu P, Extramiana F, Keller D, Hidden-Lucet Fo, Simon Fo, Bessirard V, Roux-Buisson N, Hebert J-L, Azarine A, Casset-Senon D, Rouzet Fo, Lecarpentier Y, Fontaine G, Coirault C, Frank R, Hainque B, Charron P. Desmosomal gene analysis in arrhythmogenic right ventricular dysplasia/cardiomyopathy: spectrum of mutations and clinical impact in practice. Europace. 2010; 12:861–868. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.