Abstract
Clinical symptoms of hemoptysis, chest pain, dyspnea, night sweats and weight loss seen in a patient known for 14 years with pulmonary tuberculosis with sequelae lesions, will always guide the diagnose to a reactivated pulmonary tuberculosis. Yet, in this case, the latest pulmonary radiography revealed newly emerging bilateral lesions with the appearance of a macronodular opacity of medium intensity, discreetly non-homogeneous, located apical-sub-clavicular on the right side, but also with an apical-sub-clavicular cavity lesion on the left side, well defined, with uniform opaque content, and clear-cut outline. Complementary examinations, computed tomography and biopsy bronchoscopy, confirmed the diagnosis of upper right lobe pulmonary tumor with suspicion of aspergilloma in the upper left lobe.
Keywords: Tuberculosis, tumor, squamous cell carcinoma
Introduction
Lung cancer (LC) is the most common cause of death in both sexes with one of the largest mortality rates [1].
Tuberculosis is yet another major cause of morbidity and mortality, especially in developing countries [2].
Both tuberculosis and lung cancer represent major health problems and have a large impact on public health.
The potential relationship between pulmonary tuberculosis as an infectious disease, and other diseases and health problems like cancer and diabetes mellitus have aroused the interest of scientist for years and is still an active topic today.
It has been suggested that the genetic alterations resulting from inflammation and pulmonary fibrosis caused by tuberculosis can increase the risk for lung cancer [3,4,5].
Today, we know that pulmonary tuberculosis increases the risk of developing lung cancer [6,7,8,9], although it is not unanimously accepted that having pulmonary tuberculosis can be considered a prognostic factor in this case [10,11].
Although the evolution of pulmonary tuberculosis is oscillating, with periods of aggravation and remission, sequelaes should be followed in time due to the risk of either reactivation or new lesions, both malignant and overlapping [8].
For squamous cell carcinoma (SCC), the growth is rapid with nonspecific symptomatology that can easily be confused fora known patient with a history of tuberculosis with the symptoms of reactivated disease [7], so the clinical examination should be accompanied by imaging investigations and bronchoscopic examination.
In this study we will refer to the occurrence of pulmonary cancer superimposed on tuberculosis pre-existent lung lesions, after a period of 14 years from the initial diagnostic of tuberculosis.
Imaging studies play a particularly important role both in guiding the diagnosis of pulmonary tuberculosis or its complications and in following the sometimes-unpredictable evolution of these lesions.
Case Presentation
We present here the case of a 52-year-old rural patient with a pluri-allergic status including towards penicillin, known for 14-years with bilateral pulmonary tuberculosis, who presented in the Department of Pneumophtisiology of the Emergency County Hospital of Slatina for effort dyspnea, hemoptysis, chest pain, night sweats and weight loss.
The Ethics Committee of the Emergency County Hospital of Slatina, Romania, approved this study and a written informed consent was obtained from the patient regarding the publication of the data.
At the first admission, 12 years ago, the radiographic examination revealed nodular lesions with bilaterally apical-subclavicular sequelae, medium-intensity reticulonodular lesions, discrete non-homogeneous, and with low intensity calcifications in the para-and infrahilar left regions. Moreover, a subclavicular left cavity of approximately 5cm in diameter, with a fine, opaque, 3mm thick wall, with a clear internal contour, with air content, without showing the broncho-pulmonary or solid component inside. It is also noted apical right pachypleuritis (Fig.1).
Figure 1.
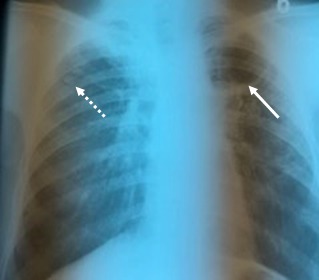
Nodular lesions with a right sub-clavicular sequela (dotted line), and a left sub-clavicular cavity lesion with a net contour without a solid component inside (continuous line). Apical right pachypleuritis shaped as a semi-uniform opacity of medium intensity, relatively homogeneous
In a new radiographic assessment after 10 years, when he presented with nonspecific symptoms, the nodular lesions were maintained apically-subclavicular right, having the same aspect, without any evolution in dynamics. Moreover, the radiographic assessment showed the appearance on the apical left side of a medium intensity opacity, discretely irregular, having rather, a sequelae aspect that could be interpreted in the context of a pachypleuritis, with the disappearance of the left cavity lesion. There were also observed lesions of bilateral pulmonary fibrosis (Fig.2).
Figure 2.
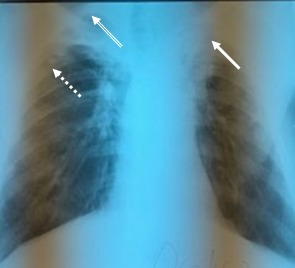
The right sub-clavicular sequela nodal lesions (dotted line) are maintained, with the presence on the apical left side of a medium intensity opacity, discrete non-homogeneous, with a suggestive aspect of pachypleuritis (continuous line). Also, on the right apical side, pachypleuritis lesions are maintained (double line) with the same appearance and characteristics as with the radiological examination 10 years ago
Surprisingly, over the next two years, when the patient presented for symptomatology that could guide the diagnosis to reactivated pulmonary tuberculosis, the radiological aspect was completely different from the previous presented features. The patient was admitted in the Pneumophtisology Department of Emergency County Hospital of Slatina for effort dyspnea, haemoptysis, chest pain, night sweats and weight loss. This was in line with a previous study by Cha SI, which showed that the clinical symptoms of pulmonary tuberculosis in patients with lung cancer does not differ significantly from those of non-lung cancer patients [12].
Laboratory tests showed mild anemia with slight decrease in average Hb concentration, mild eosinophilia (Eo=5.2%), thrombocytosis (811×103/µl count), but also the presence of an inflammatory syndrome: erythrocyte sedimentation rate (ESR)=85mm at 1 hour, fibrinogen=527mg/dl. Moreover, a mild leukocytosis at admission (WBC total count of 11.8×103/µl) was in agreement to the hypothesis raised by other studies that show that inflammation associated with infections can contribute to carcinogenesis [13]. There was no cholestasis syndrome or hepatic cytolysis syndrome, but the cholesterol value was of 265mg/dl.
Functional respiratory tests indicated severe obstructive ventilator dysfunction, with a 61% decrease of the maximum expiratory volume per second (MEVS). Bacteriological sputum examination was negative for Mycobacterium tuberculosis in both microscopy and culture, although clinical symptomatology could have guided the diagnosis for TB reactivation. The last radiography was characterized by the visualization on the right apical-subclavicular side of a macronodular opacity of medium intensity, relatively well delimited, non-homogeneous, coexisting with the pachypleuritis sequelae lesion, but also a well-defined cavity with a diameter of 4cm, on the left apical-subclavicular side, with a 3cm uniform opaque content with net outline, accompanied by medium-intensity reticulo-nodular infiltration around it (Fig.3).
Figure 3.
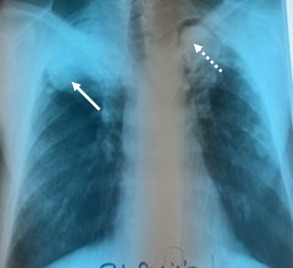
New lesions are observed-medium intensity opacity, discreetly non-homogeneous, located apically-sub-clavicular on the right side, well contoured (continuous line), but also a left apical-sub-clavicular cavity lesion, with opaque, homogeneous content (dotted line)
Suspicion is raised to the diagnosis of upper right lobe bronchopulmonary cancer and the investigation continues, knowing that there are published studies that mention the possibility of lung cancer grafting on pulmonary sequelae lesions [13].
In dynamic observation, at weekly intervals, we noticed increased levels of leukocytes to 14.8×103/µl, monocytes to 15.3%, and eosinophils to 6.4%, with a slight decrease in platelet counts compared to the latter. Biochemical analyzes showed in addition to the last biological evaluation, a slight increase in blood glucose-107mg/dl.
Also, the ESR is continuously increasing, at 105mm (1-10mm reference value) in accordance with the hypothesis of other studies showing that inflammation associated with infections can contribute to carcinogenesis [14].
The biological values compared to the last hospitalization are presented in Table 1.
Table 1.
Dynamics of biological values
| Value | Admission values | Last investigation values | References |
| Total white blood cells | 11,8 | 14,8 | 4-11/*103/µl |
| Mononucleates | 8% | 15,3% | 2-10% |
| Neutrophils | 7,1 | 8,11 | 2-8/*103/µl |
| Eosinophils | 5,2% | 6,4% | 1-5% |
| Hemoglobin | 12,9 | 12,9 | 13,5-17,5g/dl |
| Mean corpuscular hemoglobin | 31,5 | 32,95 | 32-37g/dl |
| Platelets | 811 | 751 | 150-400/*103/µl |
| Erythrocyte sedimentation rate | 85 | 105 | 1-10/mm |
| Cholesterol | 255 | 255 | 150-200mg/dl |
| Glycaemia | 101 | 107 | 74-106mg/dl |
| Microscopy for BK | NEGATIVE | NEGATIVE | +/- |
During hospitalization, the condition of the patient progressively worsens with the installation of a right brachial plexus syndrome, with hemoptoic sputum.
It is decided to perform a computer tomography (CT) examination (in which no contrast substance could be administered due to the multiple allergic background), the CT examination having, as showed in the literature, an important role in the diagnosis of pulmonary lesions that were inconclusive for the pulmonary radiography [15].
Computed tomography highlights a macronodular tissue formation within the upper right lobe (URL) with a non-homogeneous structure within its interior with hypodense areas alternating with isodense ones, with increased tissue densities (40-50UH), and irregular border, partially embracing the upper branch of the upper right lobar bronchus, accompanied by calcified nodular sequelae, features also highlighted on previous X-ray investigations (Fig.4).
Figure 4.
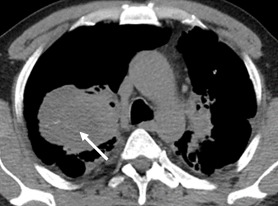
Tumor formation within the upper right lobe (URL) with a non-homogeneous structure within its interior with hypodense areas alternating with isodense ones, with increased tissue densities (40-50 UH), and irregularly contour, partially embracing the upper branch of the upper right lobar bronchus
In addition, there was a right apical thickening of the pleurae, with the emphasis on fluid in between (Fig.5), but also apical and left-thoracic pleural thickening, as well as multiple bilaterally pleuro-pulmonary sequelae, better outlined in the pulmonary window sequence (Fig.6).
Figure 5.
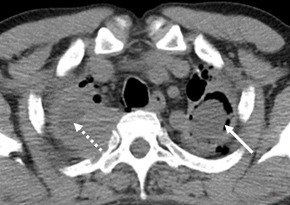
Left upper lobe cavity lesion with solid content (continuous line); Right apical thickening of the pleurae, with highlighting interior fluid (dotted line)
Figure 6.
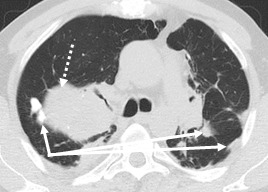
Pulmonary sequelae lesions (continuous line) complicated with new tumor tissue lesion (dotted line)
At the upper left lobe (ULL) level, the appearance of a solid cavity with relatively homogeneous content, having a suggestive aspect of aspergilloma (Fig.5).
Bronchoscopy with biopsy was performed and showed intense and diffuse congestion of the bronchial mucosa of the left bronchial tree, abundant mucopurulent secretions at the ULL and ILL level.
Basal segmental bronchi were hardly viewable due to extrinsic compression, without proliferative lesions seen at this level.
At the level of the right bronchus tree, a full luminal obstruction was highlighted before the emergence of segmental bronchi due to the presence of a voluminous, bosselated formation from which a biopsy was sampled.
A histopathological examination is performed on the bronchoscopic biopsy sample (3 small tissue fragments).
The biopsy material was processed by the classic histopathological method, that is fixation in 10% formaldehyde for 24 hours, paraffin embedding, and cutting 4-5µm sections followed by hematoxylin-eosin staining (Bioptica kit). Histopathological interpretation was done according to international protocols, and was performed in the Laboratory of Pathology from the Emergency County Hospital of Slatina.
The tumor profiling showed a malignant cell proliferation with the morphology of a non-keratinized squamous cell carcinoma.
The microscopic examination revealed on all the examined fragments non-keratinized squamous cell islets, dissociated from lymphocyte infiltrate within the desmoplastic stroma (Fig.7-8).
Figure 7.
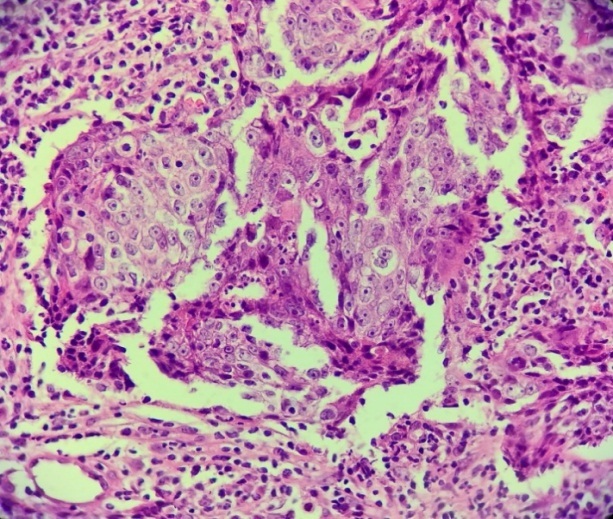
Non-keratinized squamous cell carcinoma. Hematoxylin-Eosin staining×10
Figure 8.
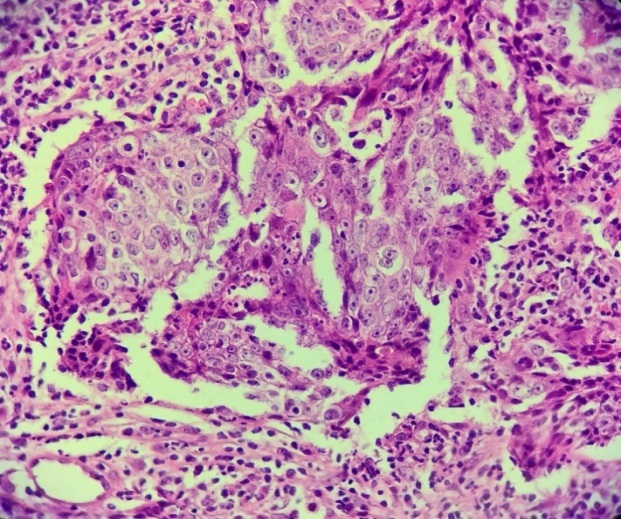
Detail of Fig.7. Non-keratinized squamous carcinoma, non-keratinized squamous cell islets, dissociated from the lymphocytic infiltrated desmoplastic stroma. Hematoxylin-Eosin staining×40
Discussions
Numerous studies addressed to the link between inflammatory and fibrotic lesions in pulmonary tuberculosis with the pathogenesis of lung cancer [5,9,16,17], although not all studies agree with the direct correlation. Luo et al. suggested that for patients with sequelae of pulmonary tuberculosis or adenocarcinoma grafted on sequelae lesions there is an increased probability for EGFR gene mutations [18].
The occurrence of overlapped lung cancer with lesions of sequelae pulmonary tuberculosis has been reported both in groups of patients and in many case presentations [6,8,19,20,21,22].
Heuvers et al indicate in a recent study conducted on a Caucasian population that the association between sequelae lesions of pulmonary tuberculosis may have a negative prognosis in lung cancer survival [10].
The association between the two entities is important because of the impact that each of them has on the population, although they may occur simultaneously or sequentially.
Recent studies reveal that tuberculous lesions are associated with a 1.7 increased risk for lung cancer development, as evidenced by Hui Ying Liang et al. meta-analysis, notably with adenocarcinoma and less with squamous cell carcinoma, although no clear causal relationship has been demonstrated [23].
There is also experimental evidence that chronic inflammation in pulmonary tuberculosis can induce cellular dysplasia and squamous cell carcinoma (SCC) [24].
First, squamous cell aggregates have consistently appeared in the pulmonary tissue associated with chronic pulmonary tuberculosis lesions and, in some cases, resembled SCC.
From the Zhou Y et al. study, the presence of pulmonary tuberculosis is a negative prognostic factor for SCC lung cancer in Chinese patients [25].
In recent years, modern imaging techniques have undergone year-to-year improvements with lower irradiating doses for the patients These improvements may also be applicable to patients with known sequelae of pulmonary tuberculosis, namely that a dose of thoracic irradiation through native CT scan, is comparable to an X-ray and can be within a certain period of time with the risk-benefit ratio being favorable.
The masks of pulmonary tuberculosis evolution can complicate the diagnosis and therefore its treatment, with direct implications for the patient. Possible overlapping lesions over tuberculosis sequelae imply a careful follow-up in clinical-imaging dynamics.
The delay in diagnosis can be determined by the patient's ignorance of delaying a doctor's appointment, limiting lung radiography investigations with misinterpretation of some suspected pulmonary tuberculosis lesions and treating them in the absence of additional investigations such as biopsy or computed tomography.
Conclusion
Pulmonary radiography plays an important role in the diagnosis of pulmonary tuberculosis, both active and sequelae, yet modern imaging by computed tomography provides more complete data, focusing faster on diagnosis, thus speeding the treatment, regardless of the disease progression.
The purpose of this case study is to raise awareness among physicians regarding the radio-imaging evolution of pulmonary tuberculosis sequelae, knowing their evolutionary possibilities, from grafting of neoplastic lesions upon preexisting sequelae lesions, to bacterial or fungal overlapping infections.
Acknowledgment
Danciu Anca, Bondari Simona and Munteanu Mihaela contributed equally to this study.
References
- 1. David F , Jacques F . The global and regional burden of cancer . In: McGuire S , editor. World cancer report 2014, World Health Organization, International agency for research on cancer . Geneva : WHO Press ; 2015 . pp. 16 – 53 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Katherine F , Philippe G , Charalambos S . The burden of disease caused by TB . In: Katherine F , Mario R , editors. Global tuberculosis report 2014, World Health Organization, International agency for research on cancer . Geneva : WHO Press ; 2014 . pp. 7 – 31 . [Google Scholar]
- 3.Ballaz S, Mulshine JL. The potential contributions of chronic inflammation to lung carcinogenesis. Clin Lung Cancer. 2003;5(1):46–62. doi: 10.3816/CLC.2003.n.021. [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert Rev Anticancer Ther. 2008;8(4):605–615. doi: 10.1586/14737140.8.4.605. [DOI] [PubMed] [Google Scholar]
- 6.Shiels MS, Albanes D, Virtamo J, Engels EA. Increased risk of lung cancer in men with tuberculosis in the alpha-tocopherol, beta-carotene cancer prevention study. Cancer Epidemiol Biomarkers Prev. 2011;20(4):672–678. doi: 10.1158/1055-9965.EPI-10-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SK, Cho LY, Yang JJ, Park B, Chang SH, Lee KS, Kim H, Yoo KY, Lee CT;, Korean Academy. Lung cancer risk and cigarette smoking, lung tuberculosis according to histologic type and gender in a population based case-control study. Lung Cancer. 2010;68(1):20–26. doi: 10.1016/j.lungcan.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Yu YH, Liao CC, Hsu WH, Chen HJ, Liao WC, Muo CH, Sung FC, Chen CY. Increased lung cancer risk among patients with pulmonary tuberculosis: a population cohort study. J Thorac Oncol. 2011;6(1):32–37. doi: 10.1097/JTO.0b013e3181fb4fcc. [DOI] [PubMed] [Google Scholar]
- 9.Engels EA, Shen M, Chapman RS, Pfeiffer RM, Yu YY, He X, Lan Q. Tuberculosis and subsequent risk of lung cancer in Xuanwei, China. Int J Cancer. 2009;124(5):1183–1187. doi: 10.1002/ijc.24042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heuvers ME, Aerts JG, Hegmans JP, Veltman JD, Uitterlinden AG, Ruiter R, Rodenburg EM, Hofman A, Bakker M, Hoogsteden HC, Stricker BH, van Klaveren. History of tuberculosis as an independent prognostic factor for lung cancer survival. Lung Cancer. 2012;76(3):452–456. doi: 10.1016/j.lungcan.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Kuo CH, Lo CY, Chung FT, Lee KY, Lin SM, Wang CH, Heh CC, Chen HC, Kuo HP. Concomitant active tuberculosis prolongs survival in non-small cell lung cancer: a study in a tuberculosis-endemic country. PLoS One. 2012;7(3):e33226–e33226. doi: 10.1371/journal.pone.0033226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cha SI, Shin K, Lee JW, Lee SY, Kim CH, Park JY, Jung TH. The clinical course of respiratory tuberculosis in lung cancer patients. Int J Tuberc Lung Dis. 2009;13(8):1002−1007–1002−1007. [PubMed] [Google Scholar]
- 13.Dacosta NA, Krinare SG. Association of lung carcinoma and tuberculosis. J Postgrad Med. 1991;37(4):185–189. [PubMed] [Google Scholar]
- 14.Coussens LM, Werb Z. Inflamation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KS, Im JG. CT in adults with tuberculosis of the chest: characteristic findings and role in management. Am J Roentgenol. 1995;164(6):1361–1367. doi: 10.2214/ajr.164.6.7754873. [DOI] [PubMed] [Google Scholar]
- 16.Nalbandian A, Yan BS, Pichugin A, Bronson RT, Kramnik I. Lung carcinogenesis induced by chronic tuberculosis infection: the experimental model and genetic control. Oncogene. 2009;28(17):1928–1938. doi: 10.1038/onc.2009.32. [DOI] [PubMed] [Google Scholar]
- 17.Yu YY, Pinsky PF, Caporaso NE, Chatterjee N, Baumgarten M, Langenberg P, Furuno JP, Lan Q, Engels EA. Lung cancer risk following detection of pulmonary scarring by chest radiography in the prostate, lung, colorectal, and ovarian cancer screening trial. Arch Intern Med. 2008;168(21):2326–2332. doi: 10.1001/archinte.168.21.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo YH, Wu CH, Wu WS, Huang CY, Su WJ, Tsai CM, Lee YC, Perng RP, Chen YM. Association between tumor epidermal growth factor receptor mutation and pulmonary tuberculosis in patients with adenocarcinoma of the lungs. J Thorac Oncol. 2012;7(2):299–305. doi: 10.1097/JTO.0b013e31823c588d. [DOI] [PubMed] [Google Scholar]
- 19.Brenner AV, Wang Z, Kleinerman RA, Wang L, Zhang S, Metayer C, Chen K, Lei S, Cui H, Lubin JH. Previous pulmonary diseases and risk of lung cancer in Gansu Province, China. Int J Epidemiol. 2001;30(1):118–124. doi: 10.1093/ije/30.1.118. [DOI] [PubMed] [Google Scholar]
- 20.Dacosta NA, Kinare SG. Association of lung carcinoma and tuberculosis. J Postgrad Med. 1991;37(4):185–189. [PubMed] [Google Scholar]
- 21.Liang HY, Li XL, Yu XS, Guan P, Yin ZH, He QC, Zhou BS. Facts and fiction of the relationship between preexisting tuberculosis and lung cancer risk: a systematic review. Int J Cancer. 2009;125(12):2936–2944. doi: 10.1002/ijc.24636. [DOI] [PubMed] [Google Scholar]
- 22.Wu CY, Hu HY, Pu CY, Huang N, Shen HC, Li CP, Chou YJ. Pulmonary tuberculosis increases the risk of lung cancer: a population-based cohort study. Cancer. 2011;117(3):618–624. doi: 10.1002/cncr.25616. [DOI] [PubMed] [Google Scholar]
- 23.Liang HY, Li XL, Yu XS, Guan P, Yin ZH, He QC, Zhou BS. Facts and fiction of the relationship between preexisting tuberculosis and lung cancer risk: a systematic review. Int J Cancer. 2009;125(12):2936–2944. doi: 10.1002/ijc.24636. [DOI] [PubMed] [Google Scholar]
- 24.Nalbandian A, Yan BS, Pichugin A, Bronson RT, Kramnik I. Lung carcinogenesis induced by chronic tuberculosis infection: the experimental model and genetic control. Oncogene. 2009;28(17):1928–1938. doi: 10.1038/onc.2009.32. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Cui Z, Zhou X, Chen C, Jiang S, Hu Z, Jiang G. The presence of old pulmonary tuberculosis is an independent prognostic factor for squ-amous cell lung cancer survival. J Cardiothorac Surg. 2013;8:123–123. doi: 10.1186/1749-8090-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]


