Abstract
A 31-year-old patient presented in the Emergency Department with a tibial fracture following a car accident that crushed the lower third of his thigh and the proximal and median part of his calf. Tibial fracture fixation with an intramedullary rod, was complicated by a Morel-Lavallée lesion. Sequential debridement procedures were performed with partially successful granulation tissue proliferation under NPWT (Negative-Pressure Wound Therapy). To further promote the already delaying granulation, plastic surgeons opted for PRP/PRF (Platelet Rich Plasma/Platelet Rich Fibrin) which allowed appropriate skin grafting. In our opinion, PRP/PRF should be considered as a viable adjuvant therapy to promote granulation.
Keywords: Tibial fracture, Platelet Rich Plasma/Platelet Rich Fibrin, skin grafting, granulation tissue
Introduction
The Morel-Lavallée lesion occurs when the skin and subcutaneous fatty tissue are traumatically and suddenly detached from the underlying fascia [1].
As the subcutaneous tissue is torn from the underlying fascia a closed degloving phenomenon occurs and a space is created, which will become filled with certain types of fluid ranging from blood to other secretions [2].
Negative-Pressure Wound Therapy-NPWT is a wound handling system that uses sub-atmospheric negative pressure, which is applied to the wound bed, aiming to remove any pathological secretions through suction, which may accumulate in the interstitial space.
Lower pressure also has the additive effect of supporting the process of angiogenesis which is crucial to the overall outcome of the healing process by promoting blood flow-a factor which plays a decisive role in granulation tissue formation [3,4,5].
PRP (Platelet Rich Plasma) is a concentrated mixture of both plasma protein and platelets obtained through a process of blood centrifugation, which aims at eliminating the red blood cells from the mixture and increasing the concentration of platelets in the plasma.
Once obtained, the overall high concentration of growth factors released from the platelets, accelerate and improve healing, which allow it to be used in a wide spectrum of applications in regenerative medicine [6,7].
PRF (Platelet rich fibrin), which is considered a second generation PRP, is made up of a resorbable fibrin membrane, rich in platelets, cytokines and growth factors which, once applied to the wound, ensures the slow and gradual release for up to 10 days, of these adjuvant factors, which promote wound healing for a prolonged period of time [8,9].
The present study aims to evaluate the potential in which PRP and PRF can increase the process of epithelization, the proliferation of granulation tissue, decrease the risk of undesirable complications, and lead to an overall improved outcome for the patient from both a functional and aesthetic perspective-two very necessary factors when aiming to improve life quality for recovering patients.
Case Report
A 30-year-old patient who was brought in the Emergency Department after a car accident in which he took part as a pedestrian.
His left lower limb suffered a tibial shaft fracture and later developed a Morel-Lavallée lesion which stretched from the distal third of his thigh to the upper-middle part of his calf. The weight of the vehicle pressing against his limb for approximatively 2-3 minutes.
Frist admitted to the Orthopaedic Department for his fractured tibia, the patient was informed of his situation and signed the informed consent form. Surgery was performed, and the tibial fracture was reduced and fixated with an intramedullary rod, which was blocked both proximally and distally with two screws.
During surgery, a prophylactic IV drip of Ceftriaxone 2g was started which was repeated after 12 hours.
During his stay at the Orthopaedic department, the patient started showing signs of local tissue suffering in the proximal third of his calf-the area most affected by the impact during the accident.
A circular pattern of soft fluctuant area with hypermobile skin developed; hypoxia and ischemia of the skin, leading to necrosis begun forming, which engulfed the epithelial tissue and underlying fat tissue of the proximal third of the calf.
It was at this point that it became evident the patient displayed clinical signs of Morel-Lavallée lesions with painful, fluctuant swelling, and surface skin necrosis.
Following the osteosynthesis surgery and due to worsening local inflammation, on the 8th day, a swab of necrotic skin tissue was harvested, and which returned positive for Klebsiella Spp.
Following the bacterial sensitivity spectrum determined via antibiogram a treatment using Ceftriaxone 2g IV every 12 hours was instated.
On the 14th day after being admitted, the patient was transferred to the Plastic Surgery Department.
A second antibiogram was obtained finding that the Klebsiella Spp. had become resistant to the initial treatment option of Ceftriaxone.
Considering available therapeutic options, it was decided to initiate a triple antibiotic therapy using Imipenem, Ciprofloxacin and Tigeciclyne.
In order to aid and promote the proliferation of granulation tissue and drain the pathological secretions, it was decided to initiate NPWT therapy; PRP and PRF applications were administrated around the exposed screws from the osteosynthesis material.
The first surgical procedure performed in the Plastic Surgery Department found a significant build-up of necrotic fat tissue, blood and lymph.
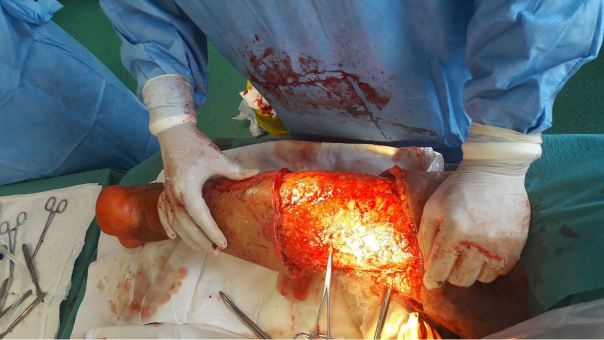
Figure 1.
Wound site after surgical debridement of necrotic tissue
Over the course of the following days, the patient underwent successive surgical debridement procedures aimed at removing the necrotic tissue, in order to assist healing and combat the spread of the local infection.
Following the surgical debridement of the necrotic tissue, the patient’s local evolution was favorable, with the gradual proliferation of granulation tissue.
Despite this, a dehiscence persisted around one of the screw implants, as well as an avulsed bone fragment, following the initial tibial fixation surgery.
After numerous unsuccessful attempts to reduce the local inflammatory process surrounding this particular area in an effort to obtain wound closure, it was decided to remove one of the screws found in its vicinity, along with the removal of the avulsed bone fragment from the fracture site, and use PRP/PRF applications to promote epithelization.
Following positive signs of overall granulation and the absence of local infection, which remitted under triple antibiotherapy, the patient underwent repeated split thickness skin graft procedures, in order to cover the skin defect on the calf and thigh.
Figure 2.
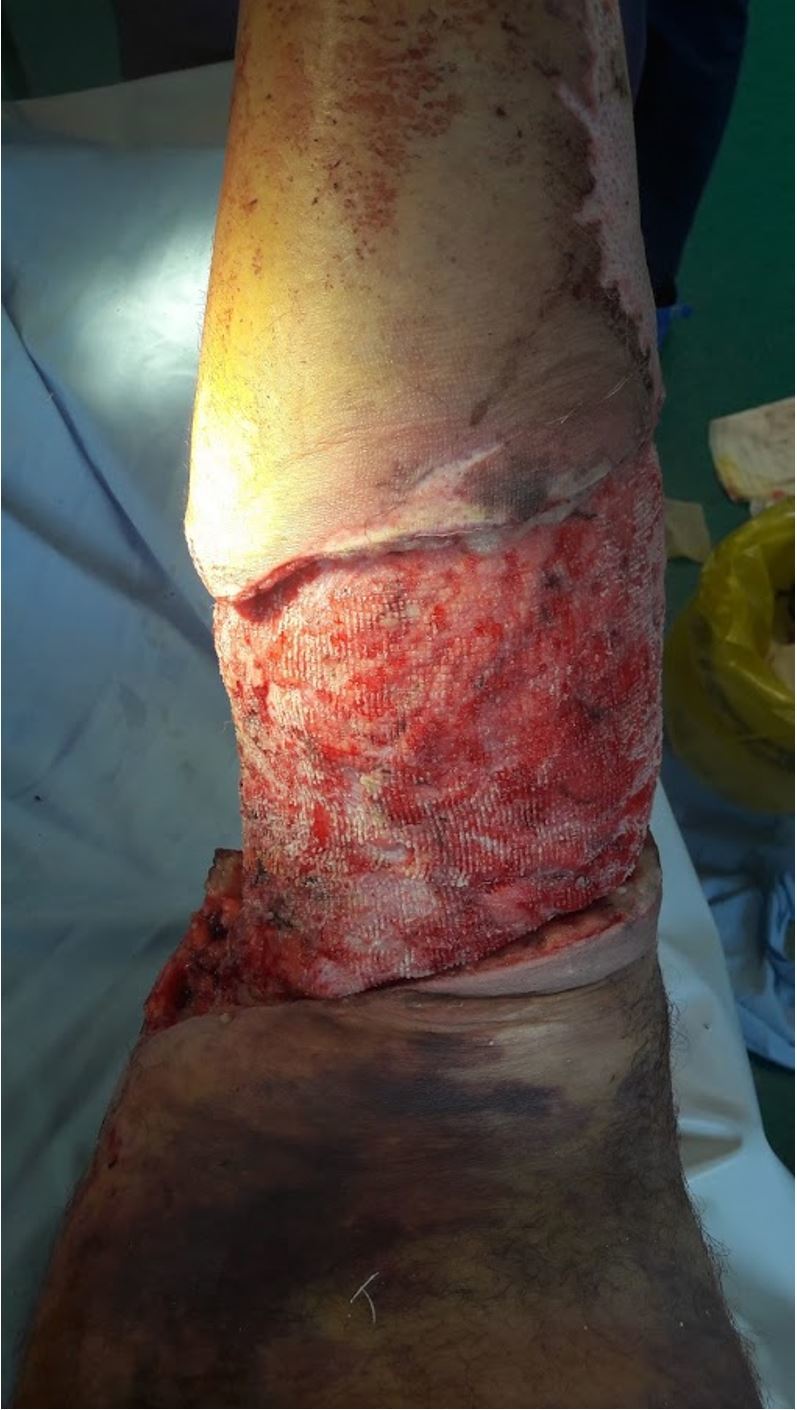
Wound site after granulation, NPWT, PRP, PRF applications
Figure 3.
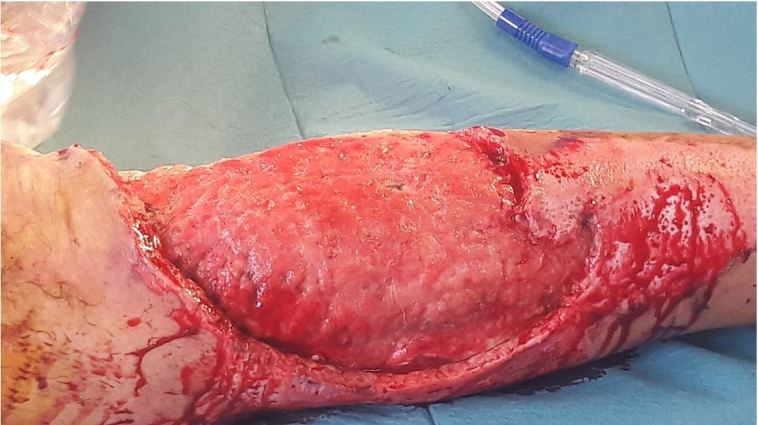
Granulation bed before Split-skin graft surgery
90 days after being admitted to the hospital through the Emergency Department, the patient’s wounds presented good integration of the skin grafts, no donor site complications, nor pathological secretions.
The result was considered adequate and the patient was released with strict indications for a close outpatient follow-up.
During the next three months, the patient had regular visits to the hospital, in order to track his progress and monitor his recovery.
At the end of the three-month period it was concluded by our team that the functional and aesthetic outcome was more than adequate with the patient returning to a normal and full life.
Discussion
From its original description in 1863 the Morel-Lavallée injury has been an ever-present challenge when considering the optimal treatment protocol. Still, given the new therapeutic options available, there is room for improvement in patient care and outcome with more studies being necessary in order to fully assess the value of modern approaches in this pathology.
PRP and PRF are known for their usability and versatility in clinical applications and thus can be considered a great aid in dealing with possibly complicated pathologies such as the Morel-Lavallée injury considered in this article. For the purposes of this paper we were unable to find any literature that outlines the advantages of using PRP and/or PRF in the management of the Morel-Lavallée injury.
During the treatment of this patient, concerns arose from using platelet concentrates together with NPWT and the possibility that the negative pressure dressing will physically remove the platelet additives, especially the liquid form Platelet Rich Plasma.
This unfavorable possibility was overturned by waiting 24 hours after applying PRP/PRF.
Given the success obtained by using the PRP and PRF for this case we consider the platelets derivates as benefiting from further investigations into more pathologies and different clinical scenarios. PRP and PRF have been cited to accelerate wound healing and have a low degree of associated morbidity, thus a better understanding of the risk/benefit ratio can be readily obtained through further studies that can further identify specific situations that may benefit from an increased intake of locally applied growth factors [10,11].
Conclusions
This report showcases the benefits of applying a mixture of modern and classical techniques in taking on one of the most challenging soft tissue lesions. The application of PRP and PRF has proven to greatly accelerate the epithelialization process and promote the proliferation of granulation tissue; the benefit in this particular case, has concluded in a good and lasting functional and aesthetic outcome for the patient.
References
- 1.Tseng S, Tornetta III. Percutaneous management of Morel-Lavallee lesions. JBJS. 2006;88(1):92–96. doi: 10.2106/JBJS.E.00021. [DOI] [PubMed] [Google Scholar]
- 2.Bonilla-Yoon I, Masih S, Patel DB, White EA, Levine BD, Chow K, eGottsegen CJ, Matcuk GR. The Morel-Lavallée lesion: pathophysiology, clinical presentation, imaging features, and treatment options. Emerg Radiol. 2014;21(1):35–43. doi: 10.1007/s10140-013-1151-7. [DOI] [PubMed] [Google Scholar]
- 3.Jones D, Neves Filho, Guimarães J, Castro D, Ferracini AM. The use of negative pressure wound therapy in the treatment of infected wounds. Case studies. Rev Bras Ortop. 2016;51(6):646–651. doi: 10.1016/j.rboe.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vikatmaa P, Juutilainen V, Kuukasjärvi P, Malmivaara A. Negative Pressure Wound Therapy: a Systematic Review on Effectiveness and Safety. Eur J Vasc Endovasc Surg. 2008;36(4):438–448. doi: 10.1016/j.ejvs.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Othman D. Negative Pressure Wound Therapy Literature Review of Efficacy, Cost Effectiveness, and Impact on Patients’ Quality of Life in Chronic Wound Management and Its Implementation in the United Kingdom. Plast Surg Int. 2012;2012:374398–374398. doi: 10.1155/2012/374398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhurat R, Sukesh M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J Cutan Aesthet Surg. 2014;7(4):189–197. doi: 10.4103/0974-2077.150734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middleton KK, Barro V, Muller B, Terada S, Fu FH. Evaluation of the Effects of Platelet-Rich Plasma (PRP) Therapy Involved in the Healing of Sports-Related Soft Tissue Injuries. Iowa Orthop J. 2012;32:150–163. [PMC free article] [PubMed] [Google Scholar]
- 8.Dohan Ehrenfest, Pinto NR, Pereda A, Jiménez P, Corso MD, Kang BS, Nally M, Lanata N, Wang HL, Quirynen M. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets. 2018;29(2):171–184. doi: 10.1080/09537104.2017.1293812. [DOI] [PubMed] [Google Scholar]
- 9.Naik B, Karunakar P, Jayadev M, Marshal VR. Role of Platelet rich fibrin in wound healing: A critical review. J Conserv Dent JCD. 2013;16(4):284–293. doi: 10.4103/0972-0707.114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masuki H, Okudera T, Watanebe T, Suzuki M, Nishiyama K, Okudera H, Nakata K, Uematsu K, Su CY, Kawase T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF) Int J Implant Dent. 2016;2(1):19–19. doi: 10.1186/s40729-016-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole BJ, Seroyer ST, Filardo G, Bajaj S, Fortier LA. Platelet-Rich Plasma. Sports Health. 2010;2(3):203–210. doi: 10.1177/1941738110366385. [DOI] [PMC free article] [PubMed] [Google Scholar]