Abstract
Introduction. Carcinomas of the thyroid gland represent 3% of all malignancies, with 1.3 to 9.8% corresponding to anaplastic thyroid carcinomas (ATC). Metastases are present in 50% of patients when ATC is diagnosed. Gastrointestinal metastases are a rare finding in patients with thyroid carcinoma. Case report. A 68-year old gentleman with a history of papillary thyroid carcinoma (PTC) underwent surgery and radiopharmaceutical therapy. Restaging studies nine months later suggested wall thickening localizing to the distal stomach. Endoscopy results showed a large, infiltrative, subepithelial, and ulcerated gastric mass and biopsies revealed anaplastic thyroid carcinoma Conclusion. Incidental thickening or other findings in the stomach in a patient with ATC without gastrointestinal symptoms should be further investigated with endoscopy and biopsies to rule out gastric metastases from anaplastic thyroid carcinoma.
Keywords: Thyroid, anaplastic thyroid carcinoma, gastric metastasis, endoscopy
Introduction
Carcinomas of the thyroid gland represent 3% of all malignancies, with 1.3 to 9.8% corresponding to anaplastic thyroid carcinomas (ATC) [1]. Despite the rare incidence of ATC, it is considered one of the leading causes of thyroid cancer mortality [2].
ATC tumors develop either de-novo or in a pre-existing goiter or from a differentiated thyroid carcinoma [3].
Clinical manifestations of the disease involve local invasion with rapid progression leading to a high rate of metastases [3].
Metastases are present in 50% of patients when ATC is diagnosed, and 25% of patients will develop metastases during their disease [4].
The most common site of metastases reported arising from ATC are the lung, bone and brain [3].
Gastrointestinal metastases are a rare finding in patients with thyroid carcinoma [1].
Here, we report a case of anaplastic thyroid carcinoma with gastric metastases highlighting this rare but likely combination in a patient with an aggressive cancer like ATC and a gastric mass.
Case report
A 68-year old male patient with a long standing history of a left sided neck mass presented with a rapid increase in size of the mass associated with hoarseness.
Patient underwent ultrasound examination of the left lateral neck area which demonstrated a lobulated soft tissue density, measuring 5.05x 2x 2.65cm.
Computed tomography (CT) of the neck was performed, showing multiple enlarged cervical lymph nodes.
Left hemithyroidectomy with left radical neck dissection were performed.
Pathology report revealed 2.9cm papillary thyroid carcinoma (PTC) on the left with metastatic involvement of 14 of 55 lymph nodes.
Cancer was staged as pT3N1bMo.
Patient then received radiopharmaceutical therapy (200mCi of radioactive iodine I-131).
Restaging imaging studies nine months later noted a posterior lesion in the brain and lymphadenopathy on the right side of the neck.
A magnetic resonance imaging (MRI) of the brain was obtained and it showed 13mm enhancing lesions within the left posterior fossa and two enlarged right cervical lymph nodes.
Fine needle aspiration of the lymph nodes revealed ATC positive for BRAF V600E by immunohistochemistry (IHC).
PET/CT showed increased FDG uptake on base of tongue, right cervical, axillary and upper abdominal lymphadenopathy and left adrenal gland nodule; all findings concerning for malignant involvement.
There was also intense uptake localizing to the distal stomach, with suggestion of significant wall thickening on CT images.
Endoscopy results showed a large, infiltrative, subepithelial, and ulcerated gastric mass (Fig.1).
Figure 1.
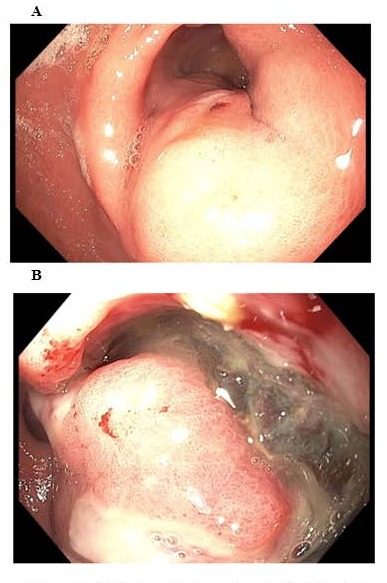
A. Endoscopic view of a subepithelial gastric mass. B. As endoscope is advanced further towards the antrum, a central ulceration is observed within the subepithelial lesion. Biopsies were taken revealing anaplastic thyroid carcinoma
Endoscopic biopsies obtained from this mass revealed poorly differentiated carcinoma which was also positive for BRAF V600E, consistent with metastatic anaplastic thyroid carcinoma (Fig.2).
Figure 2.
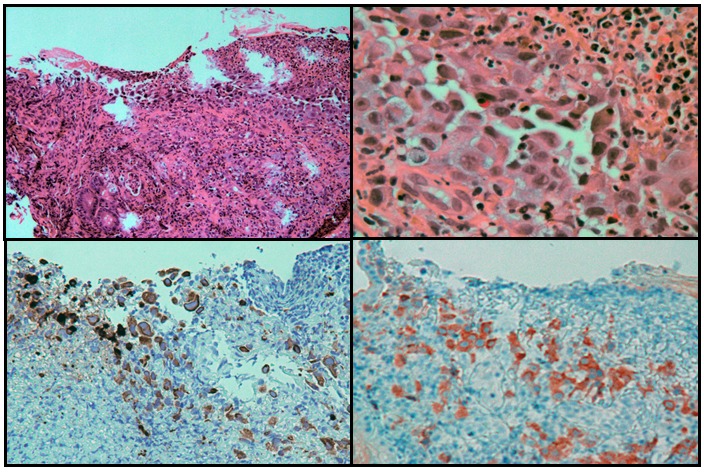
An antral ulcer (upper left, H&E stain, 100X magnification), with large epithelioid tumor cells (upper right, H&E stain, 400X magnification). The tumor cells stain for cytokeratin 7 (left lower panel, immunostaining, 200 x magnification) and for BRAF V600E mutant (right lower panel, immunostaining, 200 x magnification) by immunohistochemistry
Systemic treatment with BRAF directed targeted therapy was initiated.
Discussion
Anaplastic thyroid carcinoma is an aggressive solid malignancy with the majority of patients dying within 6 months of developing ATC [3].
Although the incidence of ATC is low compared to other thyroid malignancies, the mortality attributed to this cancer ranges between 33-50%. The median survival is 6 months, with less than 20% surviving at 1 year. Prognosis has been reported to be better in younger patients and in those without distant metastasis. Mortality rates of 70% to 95% have been reported, but a small proportion of patients experience long-term survival [5,6].
Studies showed ATC is more common in females (ratio 3:1) and the age at diagnosis is between the sixth and the seventh decade of life [7,8].
Genetic and molecular studies of ATC are encouraging for the development of new therapies [9,10].
ATC’s genotype presents chromosomal abnormalities in 85-100% of cases [11].
The most frequent genetic alterations in ATC are mutations in p53 gene (55%).
Other mutations reported are the following: RAS (22%), BRAF (45%), b-catenin (38%), PIK3CA (17%) [9,12].
Reports using array-comparative genomic hybridization (CGH) have found aberrations in regions involving EGFR, MET, BRAF, K-RAS, CCND1, FOSL1, UBE2C, CDKN2A [13,14,15].
The majority of ATC mutations are also present in patients with papillary thyroid carcinoma (PTC) (e.g. BRAF and RAS), suggesting ATC developed by dedifferentiation from preexisting PTC, gaining new mutations such as p53, catenin, beta 1, and PIK3CA [16].
There could also be mutations deriving “de novo”[17].
Our patient had a history of PTC which had transformed to ATC.
Symptoms related to ATC are the result of local symptoms resulting from compression, manifesting as hoarseness, cervical pain, dysphagia, dyspnea and stridor. Most of the patients present with a thyroid mass ranging from 3 to 20cm in size that increases in volume within 1 week [3].
Early distant metastases of ATC occur via hematogenous spread. The most common sites reported are the lungs (80%), bone (6-16%), and brain (5-13%).
A study of 121 ATC cases demonstrated 53% had metastases, 88% of them arising from the lung and 15% from bone. Besic et al. also described locations of metastatic spread in the lungs (78%), intrathoracic lymph nodes (58%), neck lymph nodes (51%), pleura (29%), adrenal glands (24%), liver (20%), brain (18%), heart (18%), and retroperitoneal lymph nodes (18%). Less common sites of distant metastases were reported to be the pericardium (13%), bones (13%), kidneys (13%), mesentery or peritoneum (13%), skin (9%), pancreas (4%), stomach (4%), diaphragm (4%), pituitary gland (2%), ovary (2%), jejunum (2%), axillary lymph nodes (2%), and gingival mucosa (2%) [18].
Metastatic disease to the stomach is a rare finding in all cancer types. It has an incidence of 0.2‑0.7% based on clinical and findings on autopsy [19,20].
The routes of gastric metastases include hematogenous or peritoneal dissemination, lymphatic infiltration or direct invasion. The most common primary tumors reported to spread to the stomach are melanoma, breast, lung and esophageal carcinoma [21,22].
The clinical presentation of metastatic tumors in the stomach is often difficult to distinguish from that of primary gastric cancer. Symptoms are variable and may include epigastric pain, nausea, vomiting, melena and anemia as a result of occult gastrointestinal blood loss. These manifestations may initially be considered as side effects of chemotherapy [23,24,25].
We could identify only one prior case report of ATC with gastric metastasis; the patient presented with gastrointestinal bleeding as a manifestation of gastric metastasis [1].
Our patient was asymptomatic from the gastric metastasis. To the best of our knowledge, this is the first case report of ATC with an asymptomatic presentation of gastric metastasis based on literature review on PubMed/Medline in the English language.
Considering the non-specific clinical manifestations, radiologic, esophagoduodenoscopy (EGD), endoscopic ultrasound (EUS) and histological evaluations are essential to distinguish between primary gastric cancer and metastatic tumors to the stomach. Imaging of gastric metastases can be presented as a thickened gastric wall or intraparietal tumor nodules on CT, MRI or EUS [23].
Our case showed gastric findings on CT similar to the ones previously reported.
On EGD, a review of case series reported metastatic gastric tumors as solitary lesions in 77.8% of cases and as multiple lesions in 22% of the patients, suggesting multiple tumors are not associated with primary malignancy [22].
Although gastric metastases may be related to the presence of abnormalities on endoscopic examination, it might be difficult to associate them with specific features due to the different morphology of the tumors [21].
Nonetheless, pathologic evaluation of the biopsies collected by EGD can confirm the diagnosis in 90-92% of cases [20,21].
Oda et al. reported the endoscopic appearance of tumors metastasizing to the stomach often resembles submucosal tumors (SMT) [21].
Endoscopic ultrasound with fine needle aspiration (EUS-FNA) is a safe and accurate technique allowing the imaging of the target lesion and the adequate sampling of it, leading to a cytological diagnosis [26].
EUS-FNA was planned in this case but cancelled once the ulceration was seen on endoscopy. Standard endoscopic biopsies in that situation were expected to lead us to the diagnosis as it happened.
Appropriate systemic treatment for metastatic tumors is the preferred treatment. Surgical resection of these tumors may be advised when there is risk of bleeding, perforation or there is a solitary metastasis [22].
Targeted therapies are showing promise in improving survival in ATC [10,27].
Systemic therapies targeting the patients’ mutations, either on a clinical trial or off label, can be considered in patients with gastric metastases form ATC. Use of target therapies or participation in clinical trials available depend on the patient and the extent of the disease. Simultaneously, palliative care should be instituted to manage symptoms of pain, nausea, dyspnea, constipation and decreased appetite.
Conclusion
ATC is an extremely aggressive malignancy with high rates of metastases. It is important to be aware of this uncommon presentation of metastasis for it to be included in the differential diagnosis of suspicious gastric lesions identified on cross sectional imaging and endoscopy.
Sometimes, incidental thickening of the gastric wall on CT may be considered as possible under distension of the stomach and not investigated in a patient with no gastrointestinal symptoms.
Our case highlights that incidental thickening or other findings in the stomach in a patient with ATC without gastrointestinal symptoms should be further investigated with endoscopy and biopsies to rule out gastric metastases from anaplastic thyroid carcinoma.
List of abbreviations
ATC: Anaplastic thyroid carcinoma
PTC: Papillary thyroid carcinoma
CT: Computed tomography
IHC: Immunohistochemistry
MRI: magnetic resonance imaging
PET/CT: Positron emission tomography-computed tomography
FDG: Fluorodeoxyglucose
CGH: Comparative genomic hybridization
EGD: Esophagoduodenoscopy
EUS: Endoscopic ultrasound
SMT: Submucosal tumors
EUS-FNA: Endoscopic ultrasound with fine needle aspiration.
References
- 1.Ayaz T, Sahin SB, Sahin OZ, Akdogan R, Gucer R. Anaplastic thyroid carcinoma presenting with gastric metastasis: a case report. Hippokratia. 2015;19(1):85–87. [PMC free article] [PubMed] [Google Scholar]
- 2.McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, van Heerden, Goellner JR. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001;130(6):1028–1034. doi: 10.1067/msy.2001.118266. [DOI] [PubMed] [Google Scholar]
- 3.Molinaro E, Romei C, Biagini A, Sabini E, Agate L, Mazzeo S, Materazzi G, Sellari-Franceschini S, Ribechini A, Torregrossa L, Basolo F, Vitti P, Elisei R. Anaplastic thyroid carcinoma: from clinicopathology to genetics and advanced therapies. Nat Rev Endocrinol. 2017;13(11):644–660. doi: 10.1038/nrendo.2017.76. [DOI] [PubMed] [Google Scholar]
- 4.Glaser SM, Mandish SF, Gill BS, Balasubramani GK, Clump DA, Beriwal S. Anaplastic thyroid cancer: Prognostic factors, patterns of care, and overall survival. Head & neck. 2016;38 Suppl 1:E2083–2090. doi: 10.1002/hed.24384. [DOI] [PubMed] [Google Scholar]
- 5.Ain KB. Anaplastic thyroid carcinoma: behavior, biology, and therapeutic approaches. Thyroid: official journal of the American Thyroid Association. 1998;8(8):715–726. doi: 10.1089/thy.1998.8.715. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson O, Lindeberg J, Zedenius J, Ekman E, Tennvall J, Blomgren H, Grimelius L, Lundell G, Wallin G. Anaplastic giant cell carcinoma of the thyroid gland: treatment and survival over a 25-year period. World journal of surgery. 1998;22(7):725–730. doi: 10.1007/s002689900460. [DOI] [PubMed] [Google Scholar]
- 7.Are C, Shaha AR. Anaplastic Thyroid Carcinoma: Biology, Pathogenesis, Prognostic Factors, and Treatment Approaches. Annals of Surgical Oncology. 2006;13(4):453–464. doi: 10.1245/ASO.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 8.Hundahl SA, Cady B, Cunningham MP, Mazzaferri E, McKee RF, Rosai J, Shah JP, Fremgen AM, Stewart AK, Holzer S. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the united states during 1996. U.S. and German Thyroid Cancer Study Group. An American College of Surgeons Commission on Cancer Patient Care Evaluation study. Cancer. 2000;89(1):202–217. doi: 10.1002/1097-0142(20000701)89:1<202::aid-cncr27>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. The Journal of clinical investigation. 2016;126(3):1052–1066. doi: 10.1172/JCI85271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME, Urbanowitz G, Mookerjee B, Wang D, Rangwala F, Keam B. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2018;36(1):7–13. doi: 10.1200/JCO.2017.73.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taccaliti A, Silvetti F, Palmonella G, Boscaro M. Anaplastic thyroid carcinoma. Frontiers in endocrinology. 2012;3:84–84. doi: 10.3389/fendo.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clinical oncology (Royal College of Radiologists (Great Britain)) 2010;22(6):486–497. doi: 10.1016/j.clon.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues R, Roque L, Krug T, Leite V. Poorly differentiated and anaplastic thyroid carcinomas: chromosomal and oligo-array profile of five new cell lines. British journal of cancer. 2007;96(8):1237–1237. doi: 10.1038/sj.bjc.6603578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J-J, Au AY, Foukakis T, Barbaro M, Kiss N, Clifton-Bligh R, Staaf J, Borg Å, Delbridge L, Robinson BG. Array-CGH identifies cyclin D1 and UBCH10 amplicons in anaplastic thyroid carcinoma. Endocrine-related cancer. 2008;15(3):801–815. doi: 10.1677/ERC-08-0018. [DOI] [PubMed] [Google Scholar]
- 15.Pozdeyev N, Gay L, Sokol ES, Hartmaier RJ, Deaver KE, Davis SN, French JD, Vanden Borre, LaBarbera DV, Tan AC, Schweppe RE, Fishbein L, Ross JS, Haugen BR, Bowles DW. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clinical cancer research: an official journal of the American Association for Cancer Research. 2018 doi: 10.1158/1078-0432.CCR-18-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nucera C, Nehs MA, Nagarkatti SS, Sadow PM, Mekel M, Fischer AH, Lin PS, Bollag GE, Lawler J, Hodin RA. Targeting BRAFV600E with PLX4720 displays potent antimigratory and anti-invasive activity in preclinical models of human thyroid cancer. The oncologist. 2011;16(3):296–309. doi: 10.1634/theoncologist.2010-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikiforov YE. Genetic alterations involved in the transition from well-differentiated to poorly differentiated and anaplastic thyroid carcinomas. Endocrine pathology. 2004;15(4):319–327. doi: 10.1385/ep:15:4:319. [DOI] [PubMed] [Google Scholar]
- 18.Besic N, Gazic B. Sites of metastases of anaplastic thyroid carcinoma: autopsy findings in 45 cases from a single institution. Thyroid: official journal of the American Thyroid Association. 2013;23(6):709–713. doi: 10.1089/thy.2012.0252. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi O, Murakami H, Yoshida T, Cho H, Yoshikawa T, Tsuburaya A, Sairenji M, Motohashi H, Sugiyama Y, Kameda Y. Clinical diagnosis of metastatic gastric tumors: clinicopathologic findings and prognosis of nine patients in a single cancer center. World journal of surgery. 2004;28(6):548–551. doi: 10.1007/s00268-004-7216-8. [DOI] [PubMed] [Google Scholar]
- 20.De Palma, Masone S, Rega M, Simeoli I, Donisi M, Addeo P, Iannone L, Pilone V, Persico G. Metastatic tumors to the stomach: clinical and endoscopic features. World journal of gastroenterology. 2006;12(45):7326–7328. doi: 10.3748/wjg.v12.i45.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oda undefined, Kondo H, Yamao T, Saito D, Ono H, Gotoda T, Yamaguchi H, Yoshida S, Shimoda T. Metastatic tumors to the stomach: analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy. 2001;33(6):507–510. doi: 10.1055/s-2001-14960. [DOI] [PubMed] [Google Scholar]
- 22.Namikawa T, Hanazaki K. Clinicopathological features and treatment outcomes of metastatic tumors in the stomach. Surgery today. 2014;44(8):1392–1399. doi: 10.1007/s00595-013-0671-9. [DOI] [PubMed] [Google Scholar]
- 23.Taal BG, Peterse H, Boot H. Clinical presentation, endoscopic features, and treatment of gastric metastases from breast carcinoma. Cancer. 2000;89(11):2214–2221. [PubMed] [Google Scholar]
- 24.Green LK. Hematogenous metastases to the stomach. A review of 67 cases. Cancer. 1990;65(7):1596–1600. doi: 10.1002/1097-0142(19900401)65:7<1596::aid-cncr2820650724>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Trouillet N, Robert B, Charfi S, Bartoli E, Joly JP, Chatelain D. Gastric metastases. An endoscopic series of ten cases. Gastroentérologie Clinique et Biologique. 2010;34(4):305–309. doi: 10.1016/j.gcb.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Tamerisa R, Irisawa A, Bhutani MS. Endoscopic ultrasound in the diagnosis, staging, and management of gastrointestinal and adjacent malignancies. The Medical clinics of North America. 2005;89(1):139–158. doi: 10.1016/j.mcna.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Cabanillas ME, Zafereo M, Gunn GB, Ferrarotto R. Anaplastic Thyroid Carcinoma: Treatment in the Age of Molecular Targeted Therapy. Journal of oncology practice. 2016;12(6):511–518. doi: 10.1200/JOP.2016.012013. [DOI] [PubMed] [Google Scholar]


