Abstract
Liver cirrhosis (LC) is the end stage of chronic liver disease characterized by the appearance of extensive fibrosis and regeneration nodes associated with hepatocyte necrosis in liver but also by the reshuffling of hepatic architecture. The triad consisting of hepatic parenchymal necrosis, regeneration and scarring is always present regardless of the type of clinical manifestation. The Child-Pugh-Turcotte classification dates back more than 30 years and has been widely used in diagnosing and assessing the severity of liver cirrhosis. This is preferred due to a low degree of complexity and a good predictive value. Prolongation of the QT interval on the electrocardiogram is common, with a prevalence exceeding 60% in patients with advanced stage of cirrhosis. In these cases, beta blockers and antiarrhythmics should be avoided or used with caution and with close QT interval monitoring. Changes in heart rate and Q-T interval are new entities in cirrhosis complications. A prolonged Q-T interval in chronic liver disease could lead to ventricular arrhythmias and sudden death. There is no report on heart rate and Q-T interval disorders in our area.
Keywords: Liver cirrhosis, Q-T interval, beta-blockers, Child-Pugh Classification
Introduction
Patients with liver cirrhosis every now and again encounter autonomic cardiovascular dysfunction showed by expanded thoughtful sensory system action and reduced heart function, which has critical implications for liver disease and survival. [1,2,3].
The baroreceptor reflex is a determinant of electrical stability in the heart. [4,5,6,7,8].
Patients with liver cirrhosis exhibit intense system activity and hyperdynamic circulation which brings a high cardiovascular output and decreased vascular resistance.
These progressions may actuate myocardial redesigning and left ventricular hypertrophy (LVH), which may lead to changes in systolic function, diastolic dysfunction and cardiomyopathy. [5,6,7].
Cardiomyopathy in patients with liver cirrhosis has been defined by a workgroup as a cardiac dysfunction characterized by the loss of stress-related contractile reaction and/or delayed-type diastolic dysfunction accompanied by electrophysiological changes in the absence of known cardiac disease. [8].
Patients with advanced cirrhosis (Child-Pugh B, Child-Pugh C) usually have rhythm disturbances (tachycardia, bradycardia). Inability to maintain increased heart rate may later contribute to reduced cardiac output, insufficient to meet the needs of systemic circulation [9,10,11].
Prolongation of the QT on the electrocardiogram is normal, with a predominance surpassing 60% in patients with end-stage liver cirrhosis.
Various studies have endeavored to build up a classification that can survey both the level of hepatic lesions and to foresee the anticipation of patients with liver cirrhosis dependent on clinical and lab parameters.
Due to the low level of many-sided quality and a decent prescient esteem, the Child-Pugh Classification (Table 1) is the most used in the staging of patients with liver cirrhosis.
Table 1.
Child-Pugh Classification
| Factor | 1 point | 2 points | 3 points |
| Total bilirubin (μmol/L) | <34 | 34-50 | >50 |
| Serum albumin (g/L) | >35 | 28-35 | <28 |
| PT INR | <1.7 | 1.7-2.30 | >2.30 |
| Ascites | None | Mild | Moderate to Severe |
| Hepatic encephalopathy | None | Grade I-II (or suppressed with medication) | Grade III-IV (or refractory) |
Total Points: 5-6 Class A, 7-9 Class B, 10-15 Class C; PT-prothrombin time, INR-international normal ratio
Hyperdinamic circulation in patients with liver cirrhosis was described 60 years ago. Experimental and clinical studies have concluded that patients with cirrhosis have a cardiac involvement known as cirrhosis of cardiomyopathy characterized by reduced cardiac contractility accompanied by diastolic dysfunction and electrophysiological abnormalities.
Material and Methods
The study included 60 patients diagnosed with liver cirrhosis irrespective of the etiology of the disease (viral, alcoholic, etc.), clinically and haemodynamically stable (not hospitalized due to cirrhosis or any related complications in the last 6 months), with chronic treatment with beta-blocker (propranolol). The patients were classified according to Child-Pugh classification (Table 1). The study was conducted at the Clinic of Internal Medicine and Cardiology Clinic of the Craiova County Emergency Clinical Hospital in 2014-2017. Detailed patient information was recorded from all patients enrolled in the study and they underwent all physical examinations, electrocardiography (ECG) and echocardiographic imaging. The informal consent forms were signed by each patient enrolled in the study, and this was approved by the Ethics Committee of the University of Medicine and Pharmacy of Craiova. Patient selection to participate in our study was based on certain inclusion and exclusion criteria.
Electrocardiographic exploration-ECG.
It is a non-invasive method used to record the electrical activity of the heart. It is done by placing 12 electrodes that collect electrical signals produced in the heart and recorded on millimeter paper. In the first phase we interpreted the electrocardiogram by measuring heart rate, establishing heart rate, electric axis, depolarization wave morphology and atrial and ventricular repolarization, atrioventricular conduction. We later focused on the QT interval, which is depolarization and ventricular repolarization, knowing that elongation is one of the electrophysiological changes present in patients with liver cirrhosis.
Variable prevalence may result from different QT interval correction methods with heart rate (QTc). Bazett's formula for calculating the QT interval is widely used, but does not completely suppress the relationship between QT and cardiac rhythm.
This is relevant in patients with liver cirrhosis, as they may typically have tachycardia. (Bazett's: QTc=QT/√RR). Subsequently, a specific "liver cirrhosis formula" was described, which is very close to that of Fridericia. Thus, the latter can be used with confidence in calculating the QT interval in patients with liver cirrhosis. (Fridericia's formula-"for liver cirrhosis": QT=QT/RR1/3.
The RR interval is expressed in seconds (RR=60/heart rate). [12]
For data processing we used Microsoft Excel (Microsoft Corp., Redmond, WA, USA) with XLSTAT suite for MS Excel (Addinsoft SARL, Paris, France) and IBM SPSS Statistics 20.0 software (IBM Corporation, Armonk, NY,USA).
Results
Based on the analysis of the distribution of patients with cirrhosis according to the Child-Pugh classes, we found that only 10% of the patients were in Class A, that in Class B there were the most patients-46.67%, only slightly less many of those staged in Class C-43.33%. (Table 2).
Table 2.
Distribution of the patients with liver cirrhosis according to severity
| Child-Pugh | Cases | % |
| Child A | 6 | 10.00% |
| Child B | 28 | 46.67% |
| Child C | 26 | 43.33% |
| Total | 60 | 100.00% |
We found that there was a significant difference between the mean age of patients in the three Child-Pugh classes (Kruskal-Wallis p <0.001), Child C patients had the highest ages (Table 3)
Table 3.
Distribution of the patients with liver cirrhosis according to severity and age
| Child-Pugh | Number of Patients | Mean Age | Standard deviation |
| Child A | 6 | 48.50 | 4.85 |
| Child B | 28 | 56.93 | 5.62 |
| Child C | 26 | 64.58 | 5.02 |
There is a significantly significant difference (p<0.001) among the three Child-Pugh classes in age distribution, patients included in Child C prevails over 60 years of age, unlike the other two classes, where the majority are subjects under the age of 60 (Table 4).
Table 4.
Distribution of patients with liver cirrhosis according to the Child-Pugh class and age category
| Child-Pugh | Child A | Child B | Child C | Total |
| Age <60 years | 6 (19.35%) | 22 (70.97%) | 3 (9.68%) | 31 (100.00%) |
| Age ≥60 years | 0 (0.00%) | 6 (20.69%) | 23 (79.31%) | 29 (100.00%) |
| Total | 6 (10.00%) | 28 (46.67%) | 26 (43.33%) | 5.00% |
Statistical analysis of the three subgroups with liver cirrhosis, according to the Child-Pugh classification, from the point of view of gender distribution (Table 5), we did not identify statistically significant differences (p Chi square=0.791>0.05).
Table 5.
Distribution of patients with liver cirrhosis according to the Child-Pugh class and gender
| Child-Pugh | Child A | Child B | Child C | Total |
| Women | 3 (50.00%) | 13 (46.43%) | 10 (38.46%) | 26 (43.33%) |
| Man | 3 (50.00%) | 15 (53.57%) | 16 (61.54%) | 34 (56.67%) |
| Total | 6 (100.00%) | 28 (100.00%) | 26 (100.00%) | 60 (100.00%) |
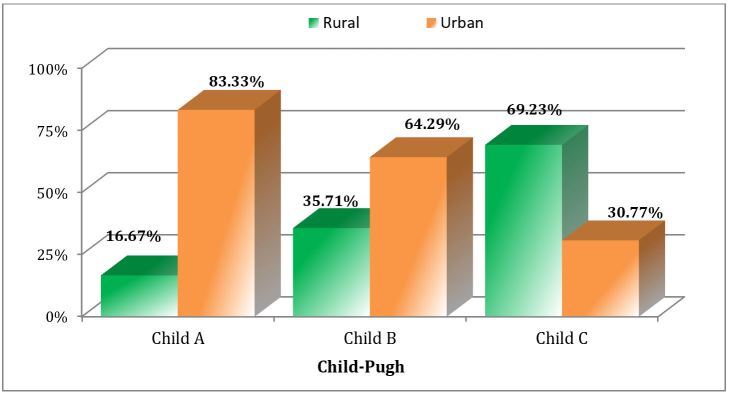
We found significant differences between the three Child-Pugh classes in the residential environment (p Chi square=0.012). We have found that most of the patients with liver cirrhosis class A and B come from urban areas-they may have a physician presentation and, by implication, faster diagnose, as highlighted in Fig.1.
Figure 1.
Distribution of patients with liver cirrhosis according to the Child-Pugh class and the environment
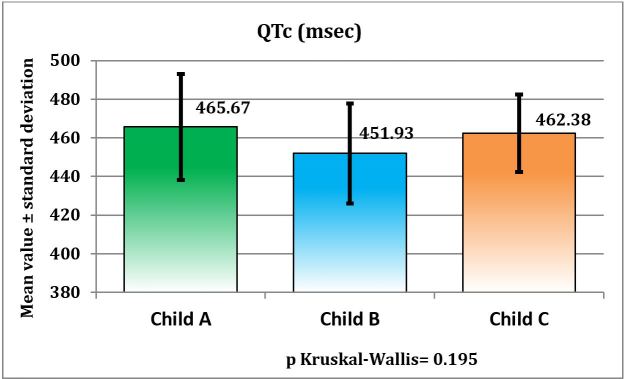
Despite the variations observed between the three Child-Pugh classes, we can state that the QTc interval does not statistically differ significantly in patients with liver cirrhosis, although it exhibited significant elongation compared to normal.
The result of the Kruskal-Wallis test was p=0.195>0.05, as evidenced by Table 6 and Figure 2 presented below.
Table 6.
Distribution of patients with liver cirrhosis according to the Child-Pugh class and QT interval
| Child-Pugh | Number of Patients | Mean value of QT interval | Standard deviation |
| Child A | 6 | 465.67 | 27.38 |
| Child B | 28 | 451.93 | 25.92 |
| Child C | 26 | 462.38 | 19.97 |
Figure 2.
Distribution of patients with liver cirrhosis according to the Child-Pugh class and QT interval
Discussions
According to data published in the literature, the mean age of patients diagnosed with liver cirrhosis is greater than 55 years, which is also evidenced in our study where the mean age was 59.4±7.34 [13]. Regarding age decades, analyzing the data from the studied group, we noticed that for patients with liver cirrhosis, more than half of them were under the age of 60 (51.67%). Due to the fact that these patients belong to the young, active population, it shows that cirrhosis is an important condition both socially and economically due to the high costs and resources it entails. A simple comparison of the decades of age of patients with hepatic cirrhosis (most of them are in the 5th and 6th decades of age) and of those with cardiovascular disease (where patients are mainly enrolled in the 7th and 8th decades) can support those previously expressed [14].
As for the gender of patients with liver cirrhosis in the studied group, we found a higher proportion of male sex (56.67%), the male/female ratio was 1.30: 1, this result can be corroborated with data present in the studies which have shown a higher prevalence of liver cirrhosis among male patients.
From the point of view of the environment, the prevalence of liver cirrhosis had slightly increased values in urban patients (51.67%), the data were not consistent with those in the literature where most patients with cirrhosis commonly come from the environment rural population due to low socio-economic status, but can be explained by the fact that the urban population is more likely to benefit from medical checks, an improvement in primary medicine and more accurate information through various media methods that contribute overall to an early diagnosis of the affection [15,16,17].
The Q-T interval showed significant changes among patients with liver cirrhosis in the study group.
The main change highlighted in our study is the significant prolongation of the Q-T interval.
A prolonged Q-T interval in chronic liver disease could lead to ventricular arrhythmias and sudden death. There is no report on heart rate and Q-T interval disorders in our area. The purpose of this study was to analyze heart rate and Q-T interval duration in patients with liver cirrhosis.
Several electrophysiological mechanisms have been suggested for cardiac contractility conduction abnormalities, including low beta-adrenoceptor density, post-receptor signal defects, abnormal excitation-contraction coupling, and molecular abnormalities [18,19].
Density and sensitivity of beta-adrenoreceptors are reduced in patients with cirrhosis, and calcium channel transport function is also affected [20,21].
This results in both the chronotropic response and the electromechanical decoupling [22]. Q-T prolongation was shown to be independent of the etiology of hepatic disease [23] and was positively associated with the severity of the disease, as shown by the Child-Pugh score [23,24] in a way that may have prognostic value. However, the cause of Q-T interval prolongation in liver disease remains obscure.
Several investigators have previously confirmed in a variety of clinical trials the presence of a prolonged QT interval in patients with cirrhosis. This abnormality had nothing to do with the etiology of cirrhosis yet was decidedly identified with the severity of the disease related to Child-Pugh score [25].
In addition, it has been shown that gender (male/female) influences Q-T probably due to hormonal differences. Some of these factors may be present in a cirrhotic patient, but the fact that Q-T interval prolongation was also detected in early-stage cirrhosis (Child-Pugh A) is only partly offset by the changes listed above.
Our study confirms the Q-T prolongation in patients with liver cirrhosis, regardless of their classification according to the Child-Pugh classification (class A, class B or class C).
Q-T prolongation from cirrhosis represents a modify that gets from the pathophysiology of the disease itself and it is not reflected by an essential anomaly identified with specific reasons for cirrhosis or its etiology.
Because cirrhotic cardiomyopathy evolves for a long time in its subclinical stage, prolongation of the QT interval is difficult to establish, this is explained by the different results of recent Q-Tc prolongation studies in cirrhotic patients from 20% in B to 60% in Child-Pugh class C severity [26,27].
The mean Q-T interval duration for prolonged Q-T in patients with liver cirrhosis was 0.457 s. Depending on the Child-Pugh severity class, the vast majority of patients were in Class B and C. By comparing the mean QT interval values between the three classes, we found that there were no statistically significant differences (the result of the Kruskal-Wallis was p=0.195>0.05.), The mean QT interval for patients in all three Child-Pugh classes had elevated values. Class B had intermediate values that did not differ significantly in either the Child A grade or the Child C grade. Interestingly, the differences were minimal in the B or C group of patients under Child-Pugh classification. A possible explanation for these results is that the prolongation of the Q-T interval may depend and may be influenced by absent factors in the Child-Pugh classification that may occur at various stages of liver cirrhosis that can significantly contribute to electrophysiological disturbances [29].
However, a recent study did not demonstrate a direct dependence between Q-T interval prolongation and autonomic function of the heart [30].
Conclusions
The highest incidence of hepatic cirrhosis in the studied group was represented by the decade 50-59 years, more than half of the patients belonging to the young active population, resulting in important social and economic consequences. There is a significant difference between the mean age of patients in the three Child-Pugh classes, the patients in the Child C class had the highest ages.
The results of our study, particularly with regard to electrocardiographic changes that occurred in patients with liver cirrhosis, demonstrated the presence of QT interval prolongation, its mean value was much higher compared to normal (0.457s).
Although most patients with liver cirrhosis had Q-T prolongation, we did not notice a significant difference between the three Child-Pugh severity classes.
However, a prospective study is needed on a larger group of patients to confirm our findings and to assess the value of the diagnosis for prolonging the QT interval in patients with cirrhosis in the prediction of cardiovascular events.
References
- 1.Valeriano V, Funaro S, Lionetti R, Riggio O, Pulcinelli G, Fiore P, Masini A, De Castro, Merli M. Modification of cardiac function in cirrhotic patients with and without ascites. Am J Gastroenterol. 2000;95(11):3200–3205. doi: 10.1111/j.1572-0241.2000.03252.x. [DOI] [PubMed] [Google Scholar]
- 2.Ates F, Topal E, Kosar F, Karincaoglu M, Yildirim B, Aksoy Y, Aladag M, Harputluoglu MM, Demirel U, Alan H, et al. The relationship of heart rate variability with severity and prognosis of cirrhosis. Dig Dis Sci. 2006;51(9):1614–1618. doi: 10.1007/s10620-006-9073-9. [DOI] [PubMed] [Google Scholar]
- 3.Hendrickse MT, Thuluvath PJ, Triger DR. Natural history of autonomic neuropathy in chronic liver disease. Lancet. 1992;339(8807):1462–1464. doi: 10.1016/0140-6736(92)92042-e. [DOI] [PubMed] [Google Scholar]
- 4.Milan A, Caserta MA, Del Colle, Dematteis A, Morello F, Rabbia F, Mulatero P, Pandian NG, Veglio F. Baroreflex sensitivity correlates with left ventricular morphology and diastolic function in essential hypertension. J Hypertens. 2007;25(8):1655–1664. doi: 10.1097/HJH.0b013e3281ddb0a0. [DOI] [PubMed] [Google Scholar]
- 5.Lantelme P, Khettab F, Custaud MA, Rial MO, Joanny C, Gharib C, Milon H. Spontaneous baroreflex sensitivity: toward an ideal index of cardiovascular risk in hypertension. J Hypertens. 2002;20(5):935–944. doi: 10.1097/00004872-200205000-00029. [DOI] [PubMed] [Google Scholar]
- 6.Okada N, Takahashi N, Yufu K, Murozono Y, Wakisaka O, Shinohara T, Anan F, Nakagawa M, Hara M, Saikawa T, Yoshimatsu H. Baroreflex sensitivity predicts cardiovascular events in patients with type 2 diabetes mellitus without structural heart disease. Circ J. 2010;74(7):1379–1383. doi: 10.1253/circj.cj-09-0960. [DOI] [PubMed] [Google Scholar]
- 7.Yufu K, Takahashi N, Okada N, Wakisaka O, Shinohara T, Nakagawa M, Hara M, Yoshimatsu H, Saikawa T. Gender difference in baroreflex sensitivity to predict cardiac and cerebrovascular events in type 2 diabetic patients. Circ J. 2011;75(6):1418–1423. doi: 10.1253/circj.cj-10-1122. [DOI] [PubMed] [Google Scholar]
- 8.Mircoli L, Rivera R, Bonforte G, Fedele L, Genovesi S, Surian M, Ferrari AU. Influence of left ventricular mass, uremia and hypertension on vagal tachycardic reserve. J Hypertens. 2003;21(8):1547–1553. doi: 10.1097/00004872-200308000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Møller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87(1):9–15. doi: 10.1136/heart.87.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braverman AC, Steiner MA, Picus D, White H. High-output congestive heart failure following transjugular intrahepatic portal-systemic shunting. Chest. 1995;107(5):1467–1469. doi: 10.1378/chest.107.5.1467. [DOI] [PubMed] [Google Scholar]
- 11.Møller S, Henriksen JH. Cardiovascular complications of cirrhosis. Gut. 2008;57(2):268–278. doi: 10.1136/gut.2006.112177. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz-del-Arbol L, Monescillo A, Jimenéz W, Garcia-Plaza A, Arroyo V, Rodés J. Paracentesis-induced circulatory dysfunction: mechanism and effect on hepatic hemodynamics in cirrhosis. Gastroenterolog. 1997;113(2):579–586. doi: 10.1053/gast.1997.v113.pm9247479. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-del-Arbol L, Urman J, Fernández J, González M, Navasa M, Monescillo A, Albillos A, Jiménez W, Arroyo V. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38(5):1210–1218. doi: 10.1053/jhep.2003.50447. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz-del-Arbol L, Monescillo A, Arocena C, Valer P, Ginès P, Moreira V, Milicua JM, Jiménez W, Arroyo V. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42(2):439–447. doi: 10.1002/hep.20766. [DOI] [PubMed] [Google Scholar]
- 15.Bernardi M, Maggioli C, Dibra V, Zaccherini G. QT interval prolongation in liver cirrhosis: innocent bystander or serious threat. Expert Rev Gastroenterol Hepatol. 2012;6(1):57–66. doi: 10.1586/egh.11.86. [DOI] [PubMed] [Google Scholar]
- 16.Moller S, Henriksen JH. Cardiovascular dysfunction in cirrhosis. Pathophysiological evidence of a cirrhotic cardiomyopathy. Scand J Gastroenterol. 2001;36(8):785–94. doi: 10.1080/003655201750313289. [DOI] [PubMed] [Google Scholar]
- 17.Puthumana L, Chaudhry V, Thuluvath PJ. Prolonged QTc interval and its relationship to autonomic cardiovascular reflexes in patients with cirrhosis. J Hepatol. 2001;35(6):733–738. doi: 10.1016/s0168-8278(01)00217-3. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Song D, Lee SS. Cirrhotic cardiomyopathy. Gastroenterol Clin Biol. 2002;26(10):842–847. [PubMed] [Google Scholar]
- 19.Zavecz JH, Bueno O, Maloney RE, O'Donnell JM, Roerig SC, Battarbee HD. Cardiac excitation-contraction coupling in the portal hypertensive rat. Am J Physiol Gastrointest Liver Physiol. 2000;279(1):G28–39. doi: 10.1152/ajpgi.2000.279.1.G28. [DOI] [PubMed] [Google Scholar]
- 20.Henriksen JH, Bendtsen F, Hansen EF, Moller S. Acute non-selective beta-adrenergic blockade reduces prolonged frequency-adjusted Q-T interval (QTc) in patients with cirrhosis. J Hepatol. 2004;40(2):239–46. doi: 10.1016/j.jhep.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Grose RD, Nolan J, Dillon JF, Errington M, Hannan WJ, BouchierI A, et al. Exercise-induced left ventricular dysfunction in alcoholic and non-alcoholic cirrhosis. J Hepatol. 1995;22(3):326–32. doi: 10.1016/0168-8278(95)80286-x. [DOI] [PubMed] [Google Scholar]
- 22.Bernard M, Calandra S, Colantoni A, Trevisani F, Rai- monto, Sica G, Schepis F, Mandini M, Simoni P, Contin M, Raimondo G. Q-T interval prolongation in cirrhosis: prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology. 1998;27(1):28–34. doi: 10.1002/hep.510270106. [DOI] [PubMed] [Google Scholar]
- 23.Puthumana L, Chaudhry V, Thuluvath PJ. Prolonged QTc interval and its relationship to autonomie cardiovascular reflexes in patients with cirrhosis. J Hepatol. 2001;35(6):733– 738. doi: 10.1016/s0168-8278(01)00217-3. [DOI] [PubMed] [Google Scholar]
- 24.Genovesi S, Prata Pizzala, Pozzi M, Ratti L, Milanese M, Pieruzzi F, Vincenti A, Stella A, Mancia G, Stramba-Badiale M. QT interval prolongation and decreased heart rate variability in cirrhotic patients: relevance of hepatic venous pressure gradient and serum calcium. Clinical Science. 2009;116(12):851–859. doi: 10.1042/CS20080325. [DOI] [PubMed] [Google Scholar]
- 25.Gaskari SA, Honar H, Lee SS. Therapy insight: Cirrhotic cardiomyopathy. Nat Clin Pract Gastroenterol Hepatol. 2006;3(6):329–337. doi: 10.1038/ncpgasthep0498. [DOI] [PubMed] [Google Scholar]
- 26.Møller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87(1):9–15. doi: 10.1136/heart.87.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Møller S, Henriksen JH. Cardiovascular complications of cirrhosis. Postgrad Med J. 2009;85(999):44–54. doi: 10.1136/gut.2006.112177. [DOI] [PubMed] [Google Scholar]
- 28.Trevisani F, Merli M, Savelli F, Valeriano V, Zambruni A, Riggio O, Caraceni P, Domenicali M, Bernardi M. QT interval in patients with non-cirrhotic portal hypertension and in cirrhotic patients treated with transjugular intrahepatic porto-systemic shunt. J Hepatol. 2003;38(4):461–467. doi: 10.1016/s0168-8278(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 29.Trevisani F, Di Micoli, Zambruni A, Biselli M, Santi V, Erroi V, Lenzi B, Caraceni P, Domenicali M, Cavazza M, Bernardi M. QT interval prolongation by acute gastrointestinal bleeding in patients with cirrhosis. Liver Int. 2012;32(10):1510–1515. doi: 10.1111/j.1478-3231.2012.02847.x. [DOI] [PubMed] [Google Scholar]
- 30.Zambruni A, Trevisani F, Di Micoli, Savelli F, Berzigotti A, Bracci E, Caraceni P, Domenicali M, Felline P, Zoli M, Bernardi M. Effect of chronic beta-blockade on QT interval in patients with liver cirrhosis. J Hepatol. 2008;48(3):415–421. doi: 10.1016/j.jhep.2007.11.012. [DOI] [PubMed] [Google Scholar]