Abstract
Introduction:
The carbohydrate antigen (CA 19-9) is a marker for pancreatic and colorectal carcinoma. In our study, we have investigated the level of CA 19-9 at 50 patients with benign and malign disease using three immunoassays.
Methods:
The COBAS e 601 (Roche) uses an ECLIA, Architect i2000 analyzer (Abbott) uses CMIA and VITROS 5600 uses integrated System Intellicheck Technology with cut off 0.0-37.0 U/mL for determination of CA 19-9. Results were with a statistical significance of p < 0.05.
Results:
Comparison of CA19-9 on COBAS with Architect show correlation coefficient R = 0.708. The results showed a regression line between immunoassay in patients with CA 19-9 treatment of y (Cobas) = 16.14 x (Architect) + 0.53. The comparison of CA19-9 on Architect and Cobas show correlation coefficient R = 0.709. The results showed regression line between immunoassay in patients with CA 19-9 treatment of y (Vitros) =–5.558 + 2.432 x (Architect) and correlation coefficient R = 0. 990. The mean concentration of CA 19-9 in the CMIA method was 41.49 U/mL, Intellicheck Technology was 103.45 U/mL and using ECLIA method was 47.25 U/mL at patients.
Conclusions:
Patients should be monitored on a single method to avoid differences in the results. The various immunoassay techniques for the detection of CA 19-9 tumor marker using different monoclonal antibodies, which leads to different results. Different antibodies recognize different parts of the molecule, and antigen heterogeneity may account in part for inter-method differences.
Keywords: CMIA, ECLIA, System Intellicheck Technology, CA19-9
1. INTRODUCTION
The carbohydrate antigen (CA 19-9) is made up of carbohydrate which constitutes 85% of this molecule in particular sialic acid, fucose, and sialyl lacto N fucopentose. The part of proteins contains proline, serine, and threonine. It was the first time described CA 19-9 in colorectal cancer cell line (SW1116) using a monoclonal antibody (1116-NS-19-9) i.e. hybridoma technology in 1979 (1-3). It is also called cancer antigen 19-9 or sialylated Lewis (a) antigen and it is a tumor marker used in the management of pancreatic cancer. It is related to the Lewis blood group antigens and only patients belonging to the Le (α-β+) or Le (α+β-) blood groups will express the CA 19-9 antigen (4). The main source of false positives results in liver disease, mainly with jaundice, pancreatitis, and cirrhosis, it is one of the reasons that some authors believe that CA 19-9 cannot be used in the diagnosis of pancreatic cancer (2, 5). The reported sensitivity and specificity of CA 19-9 for pancreas cancer are 80 and 90% retrospectively (5). The degree of elevation of CA 19-9 is associated with long-term prognosis. In patients with renal disease or benign liver disease, the CA 19-9 can be increased. It can be elevated in people with obstruction of the bile duct (5, 6). The CA 19-9 is used in the determination of gastrointestinal tumors mainly in pancreas carcinomas or colorectal carcinoma but it could be used in ovarian and bronchopulmonary tumors (adenocarcinomas and undifferentiated large cell carcinomas) (6, 7). In our study, we have investigated the level of CA 19-9 at 50 patients with benign and malign disease using three immunoassays. The tumor marker CA 19-9 is available on numerous automated platforms of autoanalyzers. Due to the high heterogeneity of polyclonal antibodies for CA 19-9, it is essential that the same assay is used for serial monitoring (8).
2. MATERIAL AND METHODS
2.1. Patients
In our study patients’ samples were collected in serum separator Vacutainer test tubes (Becton Dickinson, Rutherford, NJ 07,070 U.S.) in a volume of 3.5 mL. After collection serum samples were obtained by centrifugation at 3000 rpm using centrifuge SIGMA 3-16P (SIGMA Laborzentrifugen GmbH; Osterode am Harz; Germany) and CA 19-9 was determined. The study protocol followed the ethical guidelines given in the Declaration of Helsinki, and informed consent was obtained from all participants. In this prospective study was conducted from January 2017 to May 2017 and included 70 patient male (40) and female (30), age 65 and 75 years, hospitalized at the Oncology Clinic, University Clinical Center Sarajevo.
2.2. Serum samples
In our study CA 19-9 samples were collected, separated from the clot and stored at 2-8°C for up to 24 hours. If the serum is not tested within 24 hours, the samples were stored at -20°C. The effects of thawing were avoided by setting 50 aliquots per assay was available for all analyzers to perform correlation analyses. The samples were stored in the same manner as mentioned, to avoid variation in storage conditions (9).
2.3. Test measurement
The Architect i2000 analyzer (Abbott) uses CMIA, COBAS e 601 (Roche) uses an ECLIA and VITROS 5600 uses integrated System Intellicheck Technology with cut off 0.0-37.0 U/mL for determination of tumor marker CA 19-9 in human serum.
Chemiluminescent microparticle immunoassay - CMIA
All immunoassays require the use of the labeled material in order to measure the amount of antigen or antibody. A label is a molecule that will react as a part of the assay, so a change in signal can be measured in the blood after adding reagent solution. CMIA is noncompetitive sandwich assay technology to measure analytes. The amount of signal is directly proportional to the amount of analyte present in the sample. Architect CA 19-9 assay is two-step immunoassay to determine the presence CA 19-9 in human serum using CMIA technology. In the first step, sample, assay diluent and anti- CA 19-9 -antibody-coated paramagnetic particles are combined. CA 19-9, present in the sample, binds to the anti- CA 19-9 coated microparticles. After incubation and wash, anti- CA 19-9 -acridinium-labeled conjugate is added in the second step. Following another incubation and wash, pre-trigger and trigger solutions are then added to the reaction mixture. The pre-trigger solution (hydrogen peroxide) creates an acidic environment to prevent the early release of energy (light emission), helps to keep microparticles from clumping and splits acridinium dye off the conjugate bound to the microparticle complex (this action prepares the acridinium dye for the next step). The trigger solution (sodium hydroxide) dispenses to the reaction mixture. The acridinium undergoes an oxidative reaction when is exposed to peroxide and an alkaline solution. This reaction causes the occurrence of the chemiluminescent reaction. N-methylacridone forms and releases energy (light emission) as it returns to its ground state. The resulting chemiluminescent reaction is measured as relative light units (RLU). A direct relationship exists between the amount of CA 19-9 in the sample and RLU detected by Architect System optics.
Electrochemiluminescence-ECLIA
ECLIA is Roche’s technology for immunoassay detection of CA 19-9 in human serum. The development of immunoassays is based on the use of a ruthenium-complex and tripropylamine (TPA). The chemiluminescence reaction for the detection of the reaction complex is initiated by applying a voltage to the sample solution resulting in a precisely controlled reaction. ECLIA technology can accommodate many immunoassay principles while providing superior performance.
Integrated System–Intellicheck® Technology
The VITROS CA 19-9 test is performed using the VITROS CA 19-9 Reagent Pack and the VITROS CA 19-9. System and the VITROS 5600 Integrated System using Intellicheck® Technology. A two-step immunometric technique is used, which involves the reaction of CA 19-9 present in the sample with a biotinylated antibody (sheep polyclonal anti- CA 19-9) in the first step. The antigen-antibody complex is captured by streptavidin-coated on the well. Unbound materials are removed by washing. The second step involves the reaction of the antigen-antibody complex with a horseradish peroxidase (HRP)-labeled antibody conjugate (mouse monoclonal anti- CA 19-9). Unbound materials are removed by washing. The bound HRP conjugate is measured by a luminescent reaction. A reagent containing luminogenic substrates (a luminol derivative and a peracid salt) and an electron transfer agent, is added to the wells. The HRP in the bound conjugate catalyzes the oxidation of the luminol derivative, producing light. The electron transfer agent (a substituted acetanilide) increases the level of light produced and prolongs its emission. The light signals are read by the system. The amount of HRP conjugate bound is directly proportional to the serum concentration of CA 19-9.
2.4. Statistical analysis
Statistical analysis was performed using MedCalc software and SPSS version 16.0 software (SPSS Inc, Chicago USA). Data were analyzed using descriptive statistic average values (x), standard deviation (SDs), Pearson correlation coefficient (r) and equations of linear regression. The difference between the samples was analyzed using pair-t-test, with the statistical significance level set at p < 0.001.
3. RESULTS
3.1. Quality control testing
The three types of controls (n = 20) with low, medium and high levels of CA 19-9 were used for quality control testing. The precision of the method Cobas 601E according to the coefficient of variation of the property, since the coefficient of variation ranging from 3.0 to 4.70 % and in Architect 2000iSR was from 2.0 to 5.2 % and VITROS 5600 was from 1.03 to 5.6. The results of quality control testing for the three immunoassays are shown in Table 1.
Table 1. Quality control testing.
| Concentration spiked (U/mL) | Concentration found intra assay (mean SD, n= 20) (U/mL) | Precision intra assay (%) | Concentration found inter assay (mean SD, n= 20) (U/mL) | Reproducibility(%) |
|---|---|---|---|---|
| Architect CA 19-9 assay CMIA technology | ||||
| 26-54 | 37±1.87 | 5.0 | 41±0.21 | 5.2 |
| 102-198 | 155±6.0 | 3.9 | 145± 5.6 | 3.9 |
| 510-990 | 734±14.7 | 2.0 | 755±15.1 | 2.0 |
| Cobas CA 19-9 assay ECLIA technology | ||||
| 16.1-27.9 | 22.10±0.99 | 4.5 | 23.5±1.10 | 4.7 |
| 83.7-128 | 90.43± 2.71 | 3.0 | 95.6±3.82 | 4.0 |
| Vitros CA 19-9 assay Intellicheck® technology | ||||
| 26-44.5 | 33±1.87 | 5.6 | 36±1.21 | 3.36 |
| 40.2-74.7 | 57+.1.20 | 2.1 | 58± 0.6 | 1.03 |
| 119-178 | 149±4.7 | 3.1 | 155±5.1 | 3.29 |
3.2. Accuracy Testing
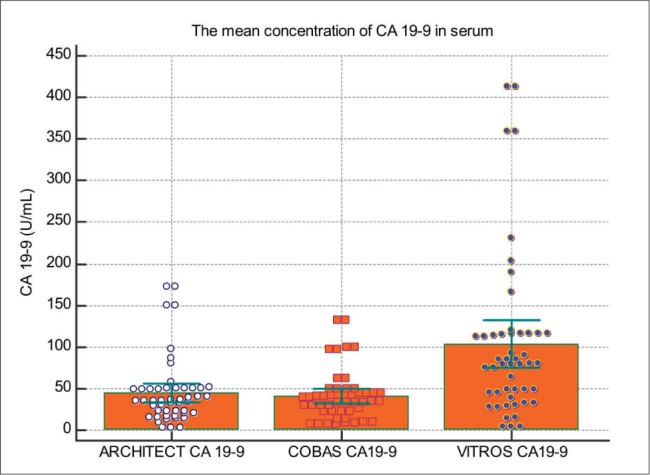
In our study, we compared CA 19-9 tumor marker measured in 50 blood sera by ARCHITECT I2000SR (CMIA) Cobas 601E (ECLIA) technology and Vitros 5600 (System Intellicheck Technology). The correlation between Architect and Roche in comparison of CA 19-9 has a coefficient of intercept = 16.148 [95 % confidence interval (Cl): 6.537 to 25.75]. The proportional difference was observed, shown by the slope = 0.078 [95 % confidence interval (Cl): 0.3823 to 0.6967] (p < 0.0001). The Cusum test of linearity was hs (p = 0.03). The regression equation for CMIA and ECLIA was y = 16.148 +0.5395x. The correlation coefficient of 0.7097 [95 % confidence interval (Cl): 0.535 to 0.8260] the results shown in Figure 1. The investigation of the correlation between ARCHITECT I2000SR (CMIA) and Vitros (System Intellicheck Technology) have a correlation coefficient of 0.994 [95 % confidence interval (Cl): 0.990 to 0.996]. The Cusum test of linearity was hs (p = 0.65). The regression equation for CMIA and System Intellicheck Technology was y = -5.558 +2.432x. The proportional difference was observed, shown by the intercept =–5.558 [95 % confidence interval (Cl):- 10.13 to – 0.985] and slope = 2.43 [95 % confidence interval (Cl): 2.35 to 2.50] and the results are shown in Figure 2. The results of our study have shown a correlation between Cobas 601E (ECLIA) and Vitros (System Intellicheck Technology) have a correlation coefficient of 0.3045 [95 % confidence interval (Cl): 0.022 to 0.541]. The Cusum test of linearity was hs (p = 0.30). We got regression equation y = 64.39 + 0.9917x. The intercept = 64.39 [95 % confidence interval (Cl):17.20 to 111.57] and slope = 0.991 [95 % confidence interval (Cl): 0.071 to 1.912] and the results are shown in Figure 3. The average mean values of CA 19-9 test concentration measured by ARCHITECT I2000SR was 41.49 +/- 39.93 U/mL, Cobas 601E was 47.25 +/- 30.40 U/mL and Vitros 5600 was 105.53 +/- 99.01 U/mL and the results are shown in Figure 4. The evaluation of method comparison analysis using Bland-Altman plot to test limits of agreement (- 1.96s to + 1.96) between ARCHITECT I2000SR (CMIA) and Cobas 601E (ECLIA) technology the mean difference (U/mL) (95 % CI) was 5.5 (-49.3 to 60.3) U/mL. The Bland-Altman plot to test limits of agreement (- 1.96s to + 1.96) between ARCHITECT I2000SR (CMIA) and Vitros (System Intellicheck Technology) the mean difference (U/mL) (95 % CI) was -58.6 (-174.2.3 to 56.9) U/mL. ). The agreement was good for the ARCHITECT I2000SR (CMIA) and Cobas 605 (ECLIA) but poor for ARCHITECT I2000SR (CMIA) and Vitros (System Intellicheck Technology).
Figure 1. Comparison of CA 19-9 concentration (U/mL) in serum measured by Architect 2000iSR CMIA (x-axis) and Cobas 601E (y-axis); y (Cobas) = 16.14 x (Architect) + 0.53; r =0.701.
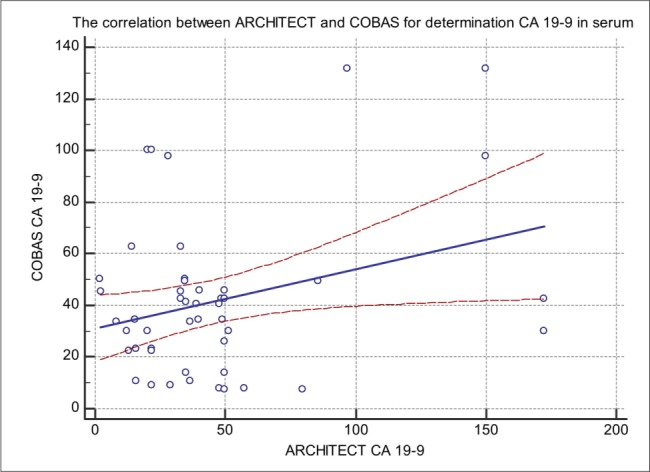
Figure 2. Comparison of CA 19-9 concentration (U/mL) in serum measured by Architect 20000iSR CMIA (x-axis) and VITROS 5600 (y-axis); y (Vitros) = 5.558 + 2432x (Architect); r = 0.994.
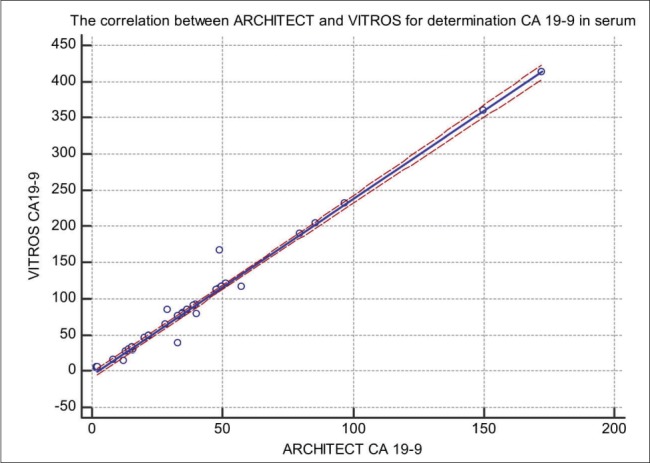
Figure 3. Comparison of CA 19-9 concentration (U/mL) in serum measured by Cobas 601E (x-axis) and VITROS 5600 (y-axis); y (Vitros) = 64.39 + 0.9917x (Cobas); r=0.304.
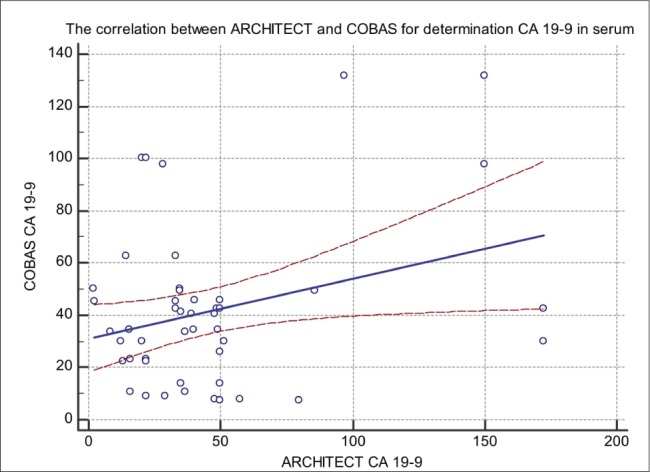
Figure 4. The CA19-9 concentration (U/mL) in patient serum using different methods.
4. DISCUSSION
The international guidelines, based on available evidence, the use of CA19-9 may be recommended only for the monitoring of pancreatic cancer (10). In other investigations, it was found that elevated serum CA 19-9 level has a sensitivity of 79-81% and a specificity of 82-90% for diagnosing pancreatic cancer in symptomatic patients (11). The correlation between CA 19-9 serum levels and pancreatic cancer resectability is not universal but undermined by the fact that 5-10% of patients with pancreatic cancer will not demonstrate elevated serum CA 19-9 serum levels given their sialyl Lewis negative state and by false positive elevations in obstructive jaundice (4). Our results have shown the precision of the method Cobas 601E according to the coefficient of variation of the property, since the coefficient of variation ranging from 3.0 to 4.70 % and in Architect 2000iSR was from 2.0 to 5.2 % and VITROS 5600 was from 1.03 to 5.6, shown in Table 1. The investigation of Stern P at all compared six routinely used immunoassays: Architect i2000 and AxSYM, Abbott Laboratories; Elecsys 2010, Roche Diagnostics; ELSA, CIS-BioInternational; Immulite 1, Diagnostic Products Corporation; and IRMA-mat, Byk-Sangtec Diagnostica coefficient of variation ranging ranged from 2.1% (Elecsys 2010) to 6.7% (ELSA) (12). The Architect CMIA method was chosen as the comparison method because it showed the best correlation with all of the other methods. In Architect CMIA, Cobas ECLIA and Vitros Intellicheck technology using Levey – Jennings report were under a range of two S.D. In our study we got good correlation between ARCHITECT i 2000SR (CMIA) and Cobas E601 (ECLIA), the correlation coefficient was 0.7097 [95 % confidence interval (Cl): 0.535 to 0.8260]. The results have show that we got proportional difference was observed, shown by the slope = 0.078 [95 % confidence interval (Cl): 0.3823 to 0.6967] (p < 0.0001) shown in Figure 1. The results of Passerini R at all. have found that regression analysis of quantitative results demonstrated a good overall agreement between ARCHITECT i2000 and Cobas 410, with a correlation coefficient of R2 = 0.86, the results that were lower than 200 U/mL, the correlation was weaker (R2 = 0.56) because of the greater effect of discordant results (13). The other investigation groups method comparison for Elecsys E170 (Roche Diagnostics, Indianapolis, IN) and ARCHITECT i2000 (Abbott) using Passing-Bablok analysis resulted in slopes ranging from 1.00 to 2.06 and correlation coefficients of 0.85 to 0.98 (14). The investigation of the correlation between ARCHITECT I2000SR (CMIA) and Vitros (System Intellicheck Technology) have got the very good coefficient of correlation 0.994 (Figure 2) and the correlation between Cobas 601E (ECLIA) and Vitros (System Intellicheck Technology) was poor ad we got correlation coefficient 0.3045 shown in Figure 3. The Architect CMIA technology had the largest analytic measurement range 2.41-172.41 U/mL followed by the VITROS Intellicheck technology 9.02-111.6 U/mL and the Cobas e601 ECLIA technology was 4.1-97.79 U/mL. The Architect CA 19-9XR assay measures lower concentrations than the other two assays mean values of CA 19-9 test concentration measured by ARCHITECT I2000SR was 41.49 +/- 39.93 U/mL, Cobas 601E was 47.25 +/- 30.40 U/mL and Vitros 5600 was 105.53 +/- 99.01 U/mL shown in Figure 4. The similar results for analytic measurement range have got other investigators too (15). The five samples of patients have a CA 19-9 concentration above 37 Um/L using Intellicheck technology and in CMIA and ECLIA technology the concentration was in the reference range. It is known from experience that repetitive sampling using different methods may show major variations in results; this fact, if not taken into account, could lead to inappropriate clinical decisions (8, 16, 17). The results of measurement of CA 19-9 in sera cannot be extrapolated from one analytical technique to another, even in cases where the same monoclonal antibody is used. One of the possible solutions is standardization of CA 19-9 measurement systems is necessary to allow the use of the results of patients in medicine.
5. CONCLUSION
In our study, we got good analytical performance on Roche and Vitros CA-19-9 assay based on 20 days precision and Abbott comparison study. The study found small differences for CA 19-9 that were obtained on ARCHITECT I 2000SR analyzer versus COBAS E601, a good correlation was observed. Then the great differences were found for CA 19-9 that were obtained on ARCHITECT I 2000SR analyzer versus VITROS 5600. Patients should be monitored on a single method to avoid differences in the results. The various immunoassay techniques for the detection of CA 19-9 tumor marker using different monoclonal antibodies, which leads to different results. Different antibodies recognize different parts of the molecule, and antigen heterogeneity may account in part for inter-method differences.
Authors’ contributions:
Designing the study, writing protocol, study conduct, data processing, writing the manuscript.
Financial support and sponsorship:
None.
Conflict of interest:
There are no conflict of interest.
REFERENCES
- 1.Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- 2.Perkins G, Slater E, Sanders G, Prichard J. Serum tumor markers. Am family Phys. 2003;68(6):1075–1082. [PubMed] [Google Scholar]
- 3.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33(3):266–270. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Kannagi R. Carbohydrate antigen sialyl Lewis a–its pathophysiological significance and induction mechanism in cancer progression. Chang Gung Med J. 2007;30:189–209. [PubMed] [Google Scholar]
- 5.European Group on Tumour Markers. Tumour markers in gastrointestinal cancers-EGTM recommendations. Anticancer Res. 1999;19(4a):2811–2815. [PubMed] [Google Scholar]
- 6.Rits RE, Nagorney DM, Jacobsen DJ, Talbot RW, Zurawski JR VR. Comparison of preoperative serum CA 19-9 levels with results of diagnostic imaging modalities in patients of preoperative serum CA9-9 levels with results of diagnostic imaging modulates in patients undergoing laparoctomy for suspected pancreatic or gallbladder disease. Pancreas. 1994;9:707–716. doi: 10.1097/00006676-199411000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Duraker N, Hot S, Polat Y, Hober N, Gencler N, Urhan N. CEA, CA 19-9 and CA 125 in the differential diagnosis of benign and malignant Pancreatic disease with or without jaundice. J Surg Oncol. 2007;95:142–147. doi: 10.1002/jso.20604. [DOI] [PubMed] [Google Scholar]
- 8.Serdarevic N, Serdarevic R, Memic A. Comparison of three immunoassays in the determination of tumor marker CA 15-3 levels in human serum. Journal of Health Sciences. 2016;6(3):154–161. [Google Scholar]
- 9.Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brunner N, Chan DW. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008;54(12):e11–79. doi: 10.1373/clinchem.2008.105601. [DOI] [PubMed] [Google Scholar]
- 10.Duffy MG, Sturgeon C, Lamerz R. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol. 2010;21:441–447. doi: 10.1093/annonc/mdp332. [DOI] [PubMed] [Google Scholar]
- 11.Ventrucci M, Pozzato P, Cipolla A. Persistent elevation of serum CA 19-9 with no evidence of malignant disease. Dig Liver Dis. 2009;4:357–363. doi: 10.1016/j.dld.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Stern P, Friedecky B, Bartos V, Bezdickova D, Vavrova J, Uhrova J, Rozprimova L, Zima T, Palicka V. Comparison of different immunoassays for CA 19-9. Clin Chem Lab Med. 2001 Dec;39(12):1278–1282. doi: 10.1515/CCLM.2001.205. [DOI] [PubMed] [Google Scholar]
- 13.Passerini R, Cassatella MC, Boveri S, Salvatici M, Radice D, Zorzino L, Galli C, Sandri MT. The pitfalls of CA19-9: routine testing and comparison of two automated immunoassays in a reference oncology center. Am J Clin Pathol. 2012 Aug;138(2):281–287. doi: 10.1309/AJCPOPNPLLCYR07H. [DOI] [PubMed] [Google Scholar]
- 14.La’ulu SL, Roberts WL. Performance characteristics of five automated CA 19-9 assays. Am J Clin Pathol. 2007;127:436–440. doi: 10.1309/H52VET3M6P7GYWG1. [DOI] [PubMed] [Google Scholar]
- 15.Hotakainen K, Tanner P, Alfthan H. Comparison of three immunoassays for CA 19-9. Clin Chim Acta. 2009;400:123–127. doi: 10.1016/j.cca.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 16.Serdarevic N, Stanciu AE. Comparison of Architect I 2000 for Determination of Scc with IMX. Clin Lab. 2013;59:1129–1133. doi: 10.7754/clin.lab.2013.121133. [DOI] [PubMed] [Google Scholar]
- 17.Serdarevic N. Comparison of Architect chemiluminescent microparticle immunoassay for determination of Troponin I in serum with AXSYM MEIA technology. Journal of Health Sciences. 2011;1(3):154–158. [Google Scholar]


