Abstract
It is generally believed that there is correlation between cancer prognosis and pretreatment PLR and NLR. However, there are limited data about their role in diffuse large B cell lymphoma (DLBCL). This study aims to determine the prognostic value of pretreatment PLR and NLR for patients who have DLBCL. The associations between clinical characteristics and NLR and PLR were evaluated among 182 DLBCL patients from January 2005 to June 2016. The optimal cutoff values for high PLR (⩾150) and NLR (⩾2.32) in prognosis prediction were determined. The effect of NLR and PLR on survival was evaluated through multivariate Cox regression analysis, univariate analysis, and log-rank test. According to the evaluation results, patients with high NLR and PLR had significantly shorter OS (P=0.026 and P=0.035) and PFS (P=0.024 and P=0.022) compared with those who have low PLR and NLR. On multivariate analyses, IPI>2, elevated LDH, and PLR⩾2.32 were prognostic factors for OS and PFS in DLBCL patients. Therefore, we demonstrated that high PLR and NLR predicted adverse prognostic factors in DLBCL patients.
1. Introduction
With a prevalence of about 25% non-Hodgkin's lymphomas (NHL) [1], diffuse large B-cell lymphoma (DLBCL) is a common lymphomas group. This malignancy is a heterogeneous entity, including variable clinical behaviors, morphologic variants, various biologic abnormalities, and different responses to treatment [2]. Some prognostic factors, such as gene expression profiling (GEP) [3], early interim analysis with positron emission tomography after chemotherapy [4, 5], and International Prognostic Index (IPI) [6], have been studied. However, the treatment for some patients who have favorable prognostic factors fails and vice versa.
In recent years, increasing attention has been attached to the correlation between cancer and inflammation. There is antitumor activity in inflammation and activation of immune system, which promotes tumor growth, human cancer progression, and carcinogenesis [7]. Systemic inflammation is an independent risk factor for treatment response, overall survival (OS), and event-free survival in patients with DLBCL [8–10].
Platelet/lymphocyte ratio (PLR) and pretreatment neutrophil/lymphocyte ratio (NLR) are common clinical parameters of inflammation and blood system, and they are related to the adverse prognosis in several malignancies [11–14]. However, the use of PLR and NLR as a prognostic marker for DLBCL is seldom reported [15–17]. Therefore, this study aims to explore the potential prognostic function of NLR and PLR in DLBCL by comparing the pretreatment complete blood count (CBC) data of patients with other risks of mortality.
2. Materials and Methods
2.1. Patients and Methods
The inclusion criteria are diagnosed de novo DLBCL, treated with R-CHOP (prednisone, hydroxydaunorubicin, vincristine, cyclophosphamide, and rituximab) or R-chop-like chemotherapy for a minimum of 4 cycles in which the clinical data are complete, and follow-up at the First Affiliated Hospital of Wenzhou Medical University between January 2005 and June 2016. The patients with HIV associated DLBCL, transformed NHL, or inflammatory conditions, including collagen diseases or infections, or other diseases of the hematological system, pretreatment with induction radiotherapy or chemotherapy, as well as a previous malignancy or non-cancer-associated death were excluded. From January 2005 to June 2016, 182 DLBCL patients were enrolled in the study, which was performed according to the principles of the Declaration of Helsinki, which had obtained the approval of the Institutional Review Board of our hospital.
2.2. Clinical Data
The clinicopathologic characteristics and demographic data of patients and were collected from medical records, which include International Prognostic Index (IPI), LDH, pathology type, ECOG-PS, and the presence of B symptoms.
Lymphocyte, neutrophil, and platelet counts were obtained from standard CBC data before the initiation of any treatment (pretreatment). NLR was calculated using the formula absolute neutrophil count which was divided through absolute lymphocyte count. PLR was calculated as the absolute platelet count which was divided through absolute lymphocyte count. ROC curves were used to determine the best threshold values for sensitivity and specificity.
Cheson BD et al. defined the relapse and response criteria [18]. PFS was defined as the time from diagnosis to relapse. And OS was from diagnosis to death.
2.3. Statistical Analysis
IBM SPSS Statistic v. 21.0 (SPSS Inc., Chicago, IL, USA) was used to perform statistical analysis. The categorical variables were demonstrated according to the frequency (%). The categorical variables were analyzed through Chi-square/Fisher's exact test. Prognostic factors of OS and PFS were determined through univariate and multivariate Cox regression analyses. Variables with P<0.05 in univariate analysis were introduced to a multivariate analysis. Two-tailed P-values were reported. P<0.05 was considered of statistical significance.
3. Results
The enrollment was carried out among 182 patients. The median follow-up time was 24 months (6-120 months). 59 (range 18-80) years was the median age. Table 1 shows other clinical and laboratory characteristics of the patients who had been dead by the time of the last follow-up. The median time of death was 22 (7-89) months. Their death resulted from lymphoma relapse by the time of the last follow-up.
Table 1.
Peripheral NLR/PLR and clinical characteristics of DLBCL patients.
| Characteristics | Total (n=182) | PLR | NLR | ||||
|---|---|---|---|---|---|---|---|
| ⩾150(n=90) | <150(n=92) | P-value | ⩾2.32(n=96) | <2.32(n=86) | P-value | ||
| Gender | 0.659 | 0.883 | |||||
| Male, n (%) | 96 | 49 | 47 | 50 | 46 | ||
| Age | 0.883 | 0.235 | |||||
| >60 | 90 | 44 | 46 | 43 | 47 | ||
| ⩽60 | 92 | 46 | 46 | 53 | 39 | ||
| Ann Arbor stage, n (%) | 0.431 | 0.001 | |||||
| I | 43 | 21 | 22 | 13 | 30 | ||
| II | 61 | 26 | 35 | 28 | 33 | ||
| III | 19 | 9 | 10 | 12 | 7 | ||
| IV | 59 | 34 | 25 | 43 | 16 | ||
| B symptoms, n (%) | 0.039 | 0.005 | |||||
| Yes | 35 | 23 | 12 | 26 | 9 | ||
| No | 147 | 67 | 80 | 70 | 77 | ||
| ECOG PS, n (%) | 0.032 | 0.048 | |||||
| <2 | 142 | 64 | 78 | 69 | 73 | ||
| ⩾2 | 40 | 26 | 14 | 27 | 13 | ||
| Extranodal sites of disease, n (%) | 0.285 | 0.002 | |||||
| >1 | 40 | 23 | 17 | 30 | 10 | ||
| ⩽1 | 142 | 67 | 75 | 66 | 76 | ||
| IPI, n (%) | 0.053 | 0.001 | |||||
| 0 | 37 | 16 | 21 | 16 | 21 | ||
| 1 | 55 | 20 | 35 | 18 | 37 | ||
| 2 | 38 | 21 | 17 | 24 | 14 | ||
| 3 | 30 | 18 | 12 | 23 | 7 | ||
| 4 | 14 | 11 | 3 | 10 | 4 | ||
| 5 | 8 | 4 | 4 | 5 | 3 | ||
| LDH, n (%) | 0.006 | 0.006 | |||||
| ⩽1 × ULN | 112 | 46 | 66 | 50 | 62 | ||
| >1 × ULN | 70 | 44 | 26 | 46 | 24 | ||
| Bone marrow involvement, n (%) | 0.567 | 1.000 | |||||
| YES | 13 | 5 | 8 | 7 | 6 | ||
| NO | 169 | 85 | 84 | 89 | 80 | ||
| Pathology type | 0.728 | 0.728 | |||||
| GCB subtype | 43 | 20 | 23 | 24 | 19 | ||
| Non-GCB subtype | 139 | 70 | 69 | 72 | 67 | ||
∗ Serum LDH level >ULN (upper limit of normal), the normal range of LDH in our center is 0-250U/L.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status; IPI, International Prognostic Index; LDH, lactate dehydrogenase; GCB, germinal center B cell.
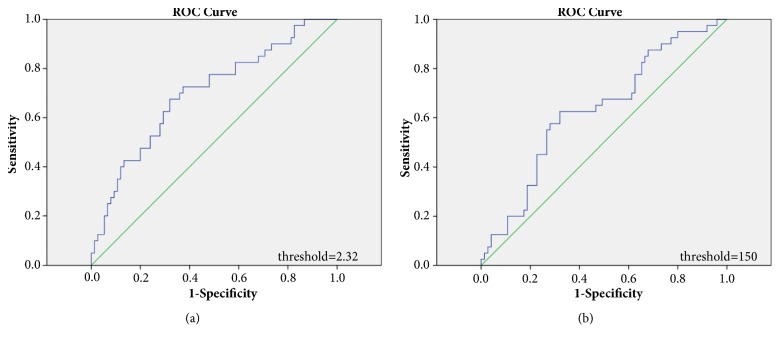
The optimal cutoff values of pretreatment PLR and NLR were determined through ROC analysis. The area under the ROC curves for NLR [Figure 1(a)] and PLR [Figure 1(b)] was 0.698 (95% CI: 0.596–0.799, P <0.001) and 0.631 (95% CI: 0.524–0.737, P=0.021), respectively (Figure 1). According to the ROC curve, the optimal cutoff levels were 2.32 for NLR and 150 for PLR.
Figure 1.
ROC curves analysis for NLR (a) and PLR (b).
There were 96 patients with NLR⩾2.32 and 86 patients with NLR<2.32. Table 1 shows the correlation between NLR and the clinical factors. The patients who have high NLR showed a higher disease stage (P=0.001) more frequently, with B symptoms (P=0.005), and had significantly lower ECOG-PS (P=0.048), higher LDH (P=0.006), more extranodal sites of disease (P=0.002), and higher IPI (P=0.001) at diagnosis. NLR is not significantly related to clinical factors, such as gender, age, bone marrow infiltration, and pathology type (Table 1).
There were 92 patients with PLR<150 and 90 patients with PLR⩾150. The associations between PLR and the clinical factors are shown in Table 1. The patients with high PLR have lower ECOG-PS (P=0.032), with B symptoms (P=0.039), and had a higher LDH (P=0.006) at diagnosis. PLR was not related to other clinical features, including disease stage, age, IPI, gender, pathology type, extranodal sites of disease, and bone marrow infiltration (Table 1).
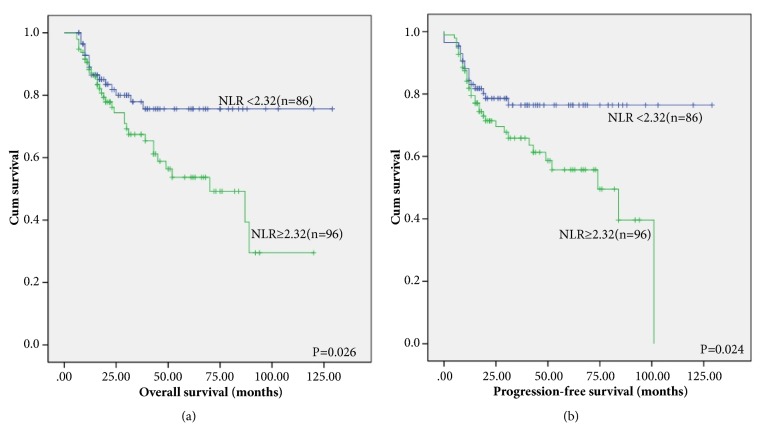
Through Kaplan-Meier analysis, whether NLR and PLR were associated with PFS and OS was valued. The group with a NLR<2.32 has shorter OS and PFS compared to those in the group with a NLR≥2.32 [Figure 2(a), P=0.026; Figure 2(b), P=0.024]. In the NLR<2.32 group, the 2-year OS rates were 83.7, but in the NLR≥2.32 group, they were 78.1 [Figure 2(a)]. Correspondingly, the 2-year PFS rates separately were 80.0 in the NLR<2.32 group. However, the PFS rate of the NLR≥2.32 group was 74.0 [Figure 2(b)].
Figure 2.
Kaplan–Meier survival analysis of NLR. OS (a) and PFS(b) according to NLR in DLBCL patients.
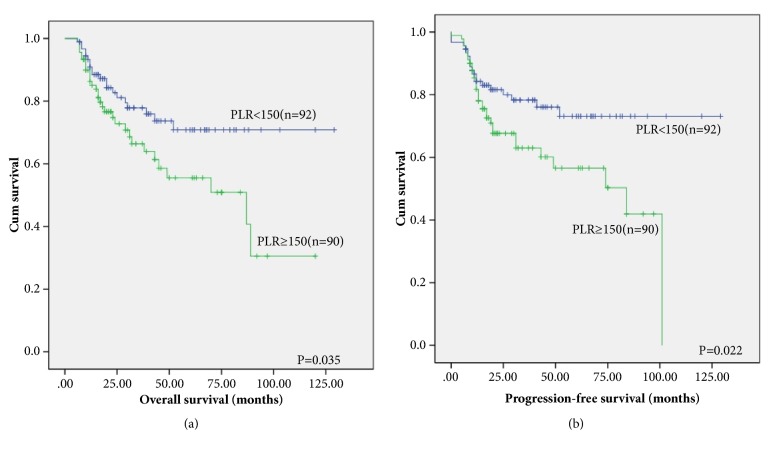
According to Kaplan-Meier curves, the patients who have low PLR experienced superior PFS [Figure 3(b), P=0.022] and OS [Figure 3(a), P=0.035] than the patients with a high PLR. The two-year OS rates were 84.8% in the PLR<150 group and 76.7% in the PLR≥150 group [Figure 3(a)]. Correspondingly, the two-year PFS rates were 82.6% in the PLR<150 group and 71.1% in the PLR≥150 group [Figure 3(b)].
Figure 3.
Kaplan–Meier survival analysis of PLR. OS (a) and PFS (b) according to PLR in DLBCL patients.
Tables 2 and 3 summarized the univariate and multivariate analysis results of clinical factors that influence PFS and OS in DLBCL patients.
Table 2.
Univariate analysis of clinical factors for PFS and OS in 182 patients.
| Characteristics | OS | PFS | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (>60) | 0.862 | 0.497-1.496 | 0.597 | 0.840 | 0.489-1.443 | 0.528 |
| Gender | 0.923 | 0.531-1.602 | 0.775 | 0.918 | 0.534-1.576 | 0.755 |
| B symptoms | 0.504 | 0.279-0.911 | 0.023 | 0.500 | 0.278-0.900 | 0.021 |
| ECOG PS (⩾2) | 0.427 | 0.231-0.788 | 0.007 | 0.497 | 0.271-0.912 | 0.024 |
| LDH (>ULN) | 0.405 | 0.232-0.707 | 0.001 | 0.385 | 0.222-0.667 | 0.001 |
| Stage (III and IV) | 0.527 | 0.301-0.921 | 0.025 | 0.574 | 0.331-0.993 | 0.047 |
| Bone marrow involvement | 2.227 | 0.945-5.252 | 0.067 | 0.506 | 0.215-1.191 | 0.119 |
| IPI (>2) | 0.379 | 0.216-0.665 | 0.001 | 0.420 | 0.241-0.732 | 0.002 |
| Extranodal sites of disease (>1) | 0.451 | 0.247-0.824 | 0.010 | 0.507 | 0.280-0.919 | 0.025 |
| Pathology type | 0.895 | 0.447-1.789 | 0.753 | 0.830 | 0.416-1.656 | 0.598 |
| NLR⩾150 | 1.909 | 1.066-3.418 | 0.030 | 0.527 | 0.298-0.932 | 0.028 |
| PLR⩾2.32 | 0.551 | 0.314-0.969 | 0.038 | 0.528 | 0.302-0.924 | 0.025 |
Abbreviations: NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio.
Table 3.
Multivariate analysis of clinical factors for PFS and OS according to PLR.
| Characteristics | OS | PFS | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | |
| B symptoms | 1.270 | 0.649-2.483 | 0.485 | 1.471 | 0.758-2.855 | 0.254 |
| ECOG PS (⩾2) | 0.753 | 0.234-2.423 | 0.635 | 0.711 | 0.222-2.277 | 0.566 |
| LDH (>ULN) | 1.704 | 0.719-4.036 | 0.046 | 1.895 | 0.813-4.419 | 0.039 |
| Stage (III and IV) | 0.823 | 0.350-1.934 | 0.655 | 0.801 | 0.339-1.895 | 0.614 |
| IPI (>2) | 1.252 | 0.707-2.217 | 0.041 | 1.168 | 0.659-2.070 | 0.044 |
| Extranodal sites of disease (>1) | 1.411 | 0.526-3.781 | 0.494 | 1.412 | 0.524-3.806 | 0.495 |
| PLR⩾2.32 | 1.549 | 0.868-2.763 | 0.038 | 1.458 | 0.818-2.597 | 0.041 |
By carrying out univariate Cox regression analysis, it can be found that independent predictors of OS were a higher clinical stage (P=0.025), a higher ECOG-PS (P=0.007), higher IPI (P=0.001), with B symptoms (P=0.023), elevated LDH (P=0.001), more extranodal sites of disease (P=0.010), higher NLR (P=0.030), and elevated PLR (P=0.038). Independent predictors of PFS had higher clinical stage (P=0.047), higher ECOG-PS (P=0.024), more extranodal sites of disease (P=0.025), with B symptoms (P=0.021), higher IPI score (P=0.002), elevated LDH (P=0.001), high NLR (P=0.028), and elevated PLR (P=0.025) (Table 2).
Multivariate analyses were performed through the Cox proportional hazard model, including all parameters that are significant at P<0.05. Neutrophils is a type of inflammatory cells, and thrombocytes are present as a nonspecific response to inflammation induced by cancer. Due to the correlation between NLR and PLR, two multivariate analyses will be carried out separately, one with NLR and one with PLR to avoid the problem with colinearity. The results revealed that PLR⩾2.32 (P=0.038; P=0.041), LDH>ULN (P=0.046; P=0.039), and IPI>2 (P=0.041; P=0.044) were independent prognostic factors for PFS and OS in DLBCL patients in PLR model (Table 3). In NLR model, the results revealed only that LDH>ULN (P=0.046; P=0.044) and IPI>2 (P=0.039; P=0.046) were independent prognostic factors for PFS and OS in DLBCL patients (Table 4).
Table 4.
Multivariate analysis of clinical factors for PFS and OS according to NLR.
| Characteristics | OS | PFS | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | |
| B symptoms | 1.302 | 0.669-2.535 | 0.437 | 1.494 | 0.772-2.892 | 0.233 |
| ECOG PS (⩾2) | 0.690 | 0.213-2.237 | 0.537 | 0.661 | 0.204-2.146 | 0.491 |
| LDH (>ULN) | 1.701 | 0.720-4.015 | 0.046 | 1.879 | 0.807-4.377 | 0.044 |
| Stage (III and IV) | 0.700 | 0.297-1.649 | 0.415 | 0.697 | 0.295-1.648 | 0.411 |
| IPI (>2) | 1.326 | 0.743-2.366 | 0.039 | 1.223 | 0.685-2.181 | 0.046 |
| Extranodal sites of disease (>1) | 1.293 | 0.483-3.461 | 0.609 | 1.314 | 0.489-3.532 | 0.588 |
| NLR⩾150 | 1.564 | 0.853-2.867 | 0.148 | 1.527 | 0.830-2.809 | 0.173 |
4. Discussion
It is commonly recognized that inflammation plays a very significant role in the development of cancer and may affect cancer patients' survival [19]. Systemic inflammation promotes tumor metastasis and progression through the promotion of angiogenesis, apoptosis inhibition, and DNA damage [20]. It has been found that hematological indices for these systemic inflammatory conditions, such as platelet count, NLR and PLR, and leukocyte count, are independent prognostic factors in patients with various cancer types [21–23]. But whether NLR and PLR are associated with prognosis in hematological malignancy is under strong research interest. Only little research investigated pretreatment NLR's prognostic role in people suffering from DLBCL [17, 24, 25], and the utility of PLR in DLBCL patients remains unknown.
In this study, we found that the patients with higher NLR and PLR had markedly short OS and PFS compared with those with low NLR and PLR. The patients with a high NLR more frequently showed significantly lower ECOG-PS, had a higher disease stage, with B symptoms, and more extranodal sites of disease, and had a higher IPI and a higher LDH at diagnosis. The patients with a high PLR more frequently showed significantly lower ECOG-PS, with B symptoms, and had a higher LDH at diagnosis. Multivariate analyses also showed high PLR to be an independent prognostic factor for mortality in DLBCL.
Although cancer and inflammation have been strongly linked with each other, the mechanism between increased NLR and PLR and poor tumor prognosis is still unclear. Tumor-associated inflammatory responses consist of a series of inflammatory mediators and inflammatory cells. Together, these generate a tumor-related inflammatory microenvironment, which plays vital roles in tumor progression and pathogenesis [26]. Furthermore, such factors may lead to decreased sensitivity of antitumor therapy. On the other hand, tumor-related inflammatory responses can result in changes in blood components such as platelets, lymphocytes, and neutrophils [27, 28].
Lymphocytes are of importance for NHL patients' immune surveillance. A number of studies demonstrated a relationship between lymphocytopenia and poor clinical outcome in NHL of various subtypes, including DLBCL [29–31]. Inhibitory immunologic cytokines such as transforming growth factor-b and IL-10, which is associated with systemic inflammation, can lead to significant immunosuppressive effects with consequent impaired cytolytic activity of the lymphocytes [32].
Neutrophils as a type of inflammatory cells promote tumor progression by producing a series of inflammatory factors that can inhibit apoptosis, promote angiogenesis, and damage cellular DNA [33–35]. Generating neutrophil extracellular traps caused by the elevated level of peripheral blood neutrophils had been reported to contribute to cancer-related thrombosis in breast and lung cancer models [36].
The rationale of NLR is to compare inflammatory responses of host (neutrophils) with the cancer that has the immune responses of host (lymphocytes). In recent years, it has been shown that NLR at diagnosis is one prognostic factor in DLBCL patients receiving R-chemotherapy [37]. In our study, we found that the patients with an NLR<2.32 at diagnosis experienced more superior PFS and OS than those within an NLR⩾2.32 at diagnosis. Nevertheless, we did not find any statistical significance in multivariate analysis, which may be because one type-II error is secondary to comparatively modest size of sample as well as the low event rate within the two categories. To clearly determine if NLR could improve present established risk stratification systems' prognostic value, further evidence within research with one bigger sample size is required.
Inflammatory cytokines released by various cancer entities, like IL-3, IL-6, and IL-10, are capable of stimulating megakaryocytes proliferation, which can produce platelets [38, 39]. Hence, the presence of the thrombocytosis may be one nonspecific response towards cancer-related inflammation. Recent clinical and experimental research reveals that platelet activation in the circulatory system contributes to the metastasis of tumors through growth factors that the platelets activated secret and then protects tumor cells from being attacked by immune system, promotes tumor cells for arresting in endothelium, and enhances tumor motility and growth [40–42]. Moreover, it had been reported that PLR is one prognostic marker for a number of cancers [43, 44].
Consistent with this finding, we found that patients with a PLR<150 at diagnosis experienced more superior PFS and OS than those with a PLR⩾150 at diagnosis. PLR predictive ability was also confirmed in our multivariate and univariate analysis, indicating that PLR as one prognostic factor for DLBCL patients may be more superior to NLR. Our finding was similar to the previous study [15, 16]. We assumed that the findings might be because the platelets are less stable and more resistant inflammatory markers than neutrophils; thus, the findings could better reflect the real response of host towards cancer-related inflammation [45].
NLR and PLR are calculated from blood cell count, which is routinely recorded in nearly all oncology records, an easily available measure in daily clinical practice, and is inexpensive to test, which can provide useful prognostic information for the management and treatment of DLBCL. Therefore, these markers can be easily accepted by clinicians.
We acknowledge that our study has certain limitations. Firstly, this study is one single-center retrospective study. Secondly, the sample size is small. Thirdly, the treatment duration of patients is relatively long. Therefore, it may lead to many biases. Hence, a multicenter and wider retrospective study should be designed for supporting the preliminary results.
In conclusion, this study demonstrated that a high NLR and PLR were associated with shorter OS and PFS. They are inexpensive instruments which are helpful to predict the outcomes of patients with DLBCL receiving R-chemotherapy. Nevertheless, further research is still needed for investigating the role of NLR and PLR among patients based on larger sample size.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (no. 81703851).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Sabattini E., Bacci F., Sagramoso C., Pileri S. A. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: An overview. Pathologica. 2010;102(3):83–87. [PubMed] [Google Scholar]
- 2.Habermann T. M. New developments in the management of diffuse large B-cell lymphoma. International Journal of Hematology. 2012;17(1):S93–S97. doi: 10.1179/102453312X13336169156014. [DOI] [PubMed] [Google Scholar]
- 3.International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. The New England Journal of Medicine. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 4.Mikhaeel N. G. Interim fluorodeoxyglucose positron emission tomography for early response assessment in diffuse large B cell lymphoma: where are we now? Leukemia & Lymphoma. 2009;50(12):1931–1936. doi: 10.3109/10428190903275610. [DOI] [PubMed] [Google Scholar]
- 5.Dührsen U., Hüttmann A., Jöckel K.-H., Müller S. Positron emission tomography guided therapy of aggressive non-Hodgkin lymphomas the PETAL trial. Leukemia & Lymphoma. 2009;50(11):1757–1760. doi: 10.3109/10428190903308031. [DOI] [PubMed] [Google Scholar]
- 6.Rosenwald A., Wright G., Chan W. C., et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. The New England Journal of Medicine. 2002;346(25):1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 7.Dmitrieva O. S., Shilovskiy I. P., Khaitov M. R., Grivennikov S. I. Interleukins 1 and 6 as main mediators of inflammation and cancer. Biochemistry (Moscow) 2016;81(2):80–90. doi: 10.1134/S0006297916020024. [DOI] [PubMed] [Google Scholar]
- 8.Periša V., Zibar L., Knezović A., Periša I., Sinčić-Petričević J., Aurer I. Prognostic nutritional index as a predictor of prognosis in patients with diffuse large B cell lymphoma. Wiener Klinische Wochenschrift. 2017;129(11-12):411–419. doi: 10.1007/s00508-016-1077-7. [DOI] [PubMed] [Google Scholar]
- 9.Zhou S., Ma Y., Shi Y., et al. Mean platelet volume predicts prognosis in patients with diffuse large B-cell lymphoma. Hematological Oncology. 2018;36(1):104–109. doi: 10.1002/hon.2467. [DOI] [PubMed] [Google Scholar]
- 10.Zhou S., Fang F., Chen H., et al. Prognostic significance of the red blood cell distribution width in diffuse large B-cell lymphoma patients. Oncotarget . 2017;8(25):40724–40731. doi: 10.18632/oncotarget.16560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo G., Guo M., Liu Z., et al. Blood Neutrophil–Lymphocyte Ratio Predicts Survival in Patients with Advanced Pancreatic Cancer Treated with Chemotherapy. Annals of Surgical Oncology. 2014;22(2):670–676. doi: 10.1245/s10434-014-4021-y. [DOI] [PubMed] [Google Scholar]
- 12.Guthrie G. J. K., Charles K. A., Roxburgh C. S. D., Horgan P. G., McMillan D. C., Clarke S. J. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Critical Review in Oncology/Hematology. 2013;88(1):218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Feng J.-F., Liu J.-S., Huang Y. Lymphopenia predicts poor prognosis in patients with esophageal squamous cell carcinoma. Medicine (United States) 2014;93(27, article no. 257) doi: 10.1097/MD.0000000000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gungorduk K., Ertas I. E., Ozdemir A., et al. Prognostic significance of retroperitoneal lymphadenectomy, preoperative neutrophil lymphocyte ratio and platelet lymphocyte ratio in primary fallopian tube carcinoma: A multicenter study. Cancer Research and Treatment. 2015;47(3):480–488. doi: 10.4143/crt.2014.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni J., Wang Y.-Q., Zhang Y.-P., et al. Value of Neutrophil/Lymphocyte Ratio and Platelet/Lymphocyte Ratio for Prognostic Evaluation of Diffuse Large B-cell Lymphoma. Zhongguo shi yan xue ye xue za zhi / Zhongguo bing li sheng li xue hui = Journal of experimental hematology / Chinese Association of Pathophysiology. 2016;24(2):427–432. doi: 10.7534/j.issn.1009-2137.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Zhao P., Zang L., Zhang X., et al. The Lymphocyte–Monocyte Ratio and the Platelet–Lymphocyte Ratio at Diagnosis as Independent Prognostic Factors in Primary Gastrointestinal Diffuse Large B Cell Lymphoma. Indian Journal of Hematology and Blood Transfusion. 2017;33(3):333–341. doi: 10.1007/s12288-016-0720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J., Zhou M., Xu J.-Y., et al. Prognostic role of pretreatment neutrophillymphocyte ratio in patients with diffuse large B-cell lymphoma treated with RCHOP. Medicine (United States) 2016;95(38) doi: 10.1097/MD.0000000000004893.e4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheson B. D., Horning S. J., Coiffier B., et al. Report of an International Workshop to Standardize Response Criteria for Non-Hodgkin's Lymphomas. Journal of Clinical Oncology. 1999;17(4):1244–1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 19.Shalapour S., Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. The Journal of Clinical Investigation. 2015;125(9):3347–3355. doi: 10.1172/jci80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMillan D. C. Systemic inflammation, nutritional status and survival in patients with cancer. Current Opinion in Clinical Nutrition & Metabolic Care. 2009;12(3):223–226. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 21.Wu G., Yao Y., Bai C., et al. Combination of platelet to lymphocyte ratio and neutrophil to lymphocyte ratio is a useful prognostic factor in advanced non-small cell lung cancer patients. Thoracic Cancer. 2015;6(3):275–287. doi: 10.1111/1759-7714.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S., Oh S. Y., Kim S. H., et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13, article 350 doi: 10.1186/1471-2407-13-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia W., Wu J., Jia H., et al. The peripheral blood neutrophil-to-lymphocyte ratio is superior to the lymphocyte-to-monocyte ratio for predicting the long-term survival of triple-negative breast cancer patients. PLoS ONE. 2015;10(11) doi: 10.1371/journal.pone.0143061.0143061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho C.-L., Lu C.-S., Chen J.-H., Chen Y.-G., Huang T.-C., Wu Y.-Y. Neutrophil/Lymphocyte Ratio, Lymphocyte/Monocyte Ratio, and Absolute Lymphocyte Count/Absolute Monocyte Count Prognostic Score in Diffuse Large B-Cell Lymphoma: Useful Prognostic Tools in the Rituximab Era. Medicine. 2015;94(24):p. e993. doi: 10.1097/MD.0000000000000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keam B., Ha H., Kim T. M., et al. Neutrophil to lymphocyte ratio improves prognostic prediction of International Prognostic Index for patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leukemia & Lymphoma. 2015;56(7):2032–2038. doi: 10.3109/10428194.2014.982642. [DOI] [PubMed] [Google Scholar]
- 26.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 27.Mizunuma M., Yokoyama Y., Futagami M., Aoki M., Takai Y., Mizunuma H. The pretreatment neutrophil-to-lymphocyte ratio predicts therapeutic response to radiation therapy and concurrent chemoradiation therapy in uterine cervical cancer. International Journal of Clinical Oncology. 2015;20(5):989–996. doi: 10.1007/s10147-015-0807-6. [DOI] [PubMed] [Google Scholar]
- 28.Feng J. F., Huang Y., Liu J. S. Combination of neutrophil lymphocyte ratio and platelet lymphocyte ratio is a useful predictor of postoperative survival in patients with esophageal squamous cell carcinoma. OncoTargets and Therapy. 2013;6:1605–1612. doi: 10.2147/OTT.S52501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oki Y., Yamamoto K., Kato H., et al. Low absolute lymphocyte count is a poor prognostic marker in patients with diffuse large B-cell lymphoma and suggests patients' survival benefit from rituximab. European Journal of Haematology. 2008;81(6):448–453. doi: 10.1111/j.1600-0609.2008.01129.x. [DOI] [PubMed] [Google Scholar]
- 30.Kim D. H., Baek J. H., Chae Y. S., et al. Absolute lymphocyte counts predicts response to chemotherapy and survival in diffuse large B-cell lymphoma. Leukemia. 2007;21(10):2227–2230. doi: 10.1038/sj.leu.2404780. [DOI] [PubMed] [Google Scholar]
- 31.Cox M. C., Nofroni I., Ruco L., et al. Low absolute lymphocyte count is a poor prognostic factor in diffuse-large-B-cell-lymphoma. Leukemia & Lymphoma. 2008;49(9):1745–1751. doi: 10.1080/10428190802226425. [DOI] [PubMed] [Google Scholar]
- 32.Salazar-Onfray F., López M. N., Mendoza-Naranjo A. Paradoxical effects of cytokines in tumor immune surveillance and tumor immune escape. Cytokine & Growth Factor Reviews. 2007;18(1-2):171–182. doi: 10.1016/j.cytogfr.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? The Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 34.Jackson J. R., Seed M. P., Kircher C. H., Willoughby D. A., Winkler J. D. The codependence of angiogenesis and chronic inflammation. The FASEB Journal. 1997;11(6):457–465. doi: 10.1096/fasebj.11.6.9194526. [DOI] [PubMed] [Google Scholar]
- 35.Grivennikov S. I., Greten F. R., Karin M. Immunity, Inflammation, and Cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demers M., Krause D. S., Schatzberg D., et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proceedings of the National Acadamy of Sciences of the United States of America. 2012;109(32):13076–13081. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porrata L. F., Ristow K., Habermann T., Inwards D. J., Micallef I. N., Markovic S. N. Predicting survival for diffuse large B-cell lymphoma patients using baseline neutrophil/lymphocyte ratio. American Journal of Hematology. 2010;85(11):896–899. doi: 10.1002/ajh.21849. [DOI] [PubMed] [Google Scholar]
- 38.Alexandrakis M. G., Passam F. H., Moschandrea I. A., et al. Levels of serum cytokines and acute phase proteins in patients with essential and cancer-related thrombocytosis. American Journal of Clinical Oncology. 2003;26(2):135–140. doi: 10.1097/00000421-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Klinger M. H. F., Jelkmann W. Role of blood platelets in infection and inflammation. Journal of Interferon & Cytokine Research. 2002;22(9):913–922. doi: 10.1089/10799900260286623. [DOI] [PubMed] [Google Scholar]
- 40.Gay L. J., Felding-Habermann B. Contribution of platelets to tumour metastasis. Nature Reviews Cancer. 2011;11(2):123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takagi S., Sato S., Oh-hara T., et al. Platelets Promote Tumor Growth and Metastasis via Direct Interaction between Aggrus/Podoplanin and CLEC-2. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0073609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho M. S., Bottsford-Miller J., Vasquez H. G., et al. Platelets increase the proliferation of ovarian cancer cells. Blood. 2012;120(24):4869–4872. doi: 10.1182/blood-2012-06-438598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asher V., Lee J., Innamaa A., Bali A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clinical and Translational Oncology. 2011;13(7):499–503. doi: 10.1007/s12094-011-0687-9. [DOI] [PubMed] [Google Scholar]
- 44.Templeton A. J., Ace O., McNamara M. G., et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiology, Biomarkers & Prevention. 2014;23(7):1204–1212. doi: 10.1158/1055-9965.epi-14-0146. [DOI] [PubMed] [Google Scholar]
- 45.Racz J. M., Cleghorn M. C., Jimenez M. C., et al. Predictive Ability of Blood Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios in Gastrointestinal Stromal Tumors. Annals of Surgical Oncology. 2015;22(7):2343–2350. doi: 10.1245/s10434-014-4265-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.