Abstract
Objective:
To assess survival and identify predictors of survival in patients with systemic sclerosis-interstitial lung disease (SSc-ILD) who participated in the Scleroderma Lung Studies (SLS) I and II.
Methods:
SLS I randomized 158 SSc-ILD patients to 1 year of oral cyclophosphamide (CYC) versus placebo. SLS II randomized 142 patients to 1 year of oral CYC followed by 1 year of placebo versus 2 years of mycophenolate (MMF). Counting process cox proportional hazard modeling identified variables associated with long-term mortality in SLS I and II. Internal validation was performed using joint modeling.
Results:
After a median follow-up of 8 years, 42% of SLS I patients died, and when known, the cause of death was most often attributable to SSc. There was no significant difference in the time to death between treatment arms in SLS I or II. Higher baseline skin score, older age, and a decline in the forced vital capacity (FVC) and the diffusing capacity for carbon monoxide (DLCO) over 2 years were independently associated with an increased risk of mortality in SLS I. The Cox model identified the same mortality predictor variables using the SLS II data.
Conclusion:
In addition to identifying traditional mortality risk factors in SSc (skin score, age), this study demonstrated that a decline in the FVC and the DLCO over 2 years was a better predictor of mortality than the baseline FVC and DLCO. These findings suggest that short-term changes in surrogate measures of SSc-ILD progression may have important effects on long-term outcomes.
Keywords: Systemic sclerosis, interstitial lung disease, treatment, survival
INTRODUCTION
Interstitial lung disease (ILD) is the leading cause of death in systemic sclerosis (SSc)1, accounting for over one-third of SSc-related deaths in a multi-center observational study of over 5000 patients with SSc2. In addition, ILD occurs in the majority of patients with SSc,3, and is found in 79% of patients with SSc at autopsy4.
Immunosuppressant agents, such as mycophenolate (MMF) and cyclophosphamide (CYC), are currently used to treat SSc-ILD5,6,7. However, randomized controlled trials (RCTs) have demonstrated that while some patients experience improvement in lung function after treatment with MMF or CYC, other patients experience ILD progression despite treatment with immunosuppression6,7. Moreover, not all patients with ILD develop symptoms or will have progressive disease that leads to death even in the absence of treatment3,8.
The present study sought To develop a mortality prediction model using data from two RCTs for SSc-ILD (Scleroderma Lung Study [SLS] I and II)6,7. Using data from RCTs (in contrast to an observational cohort) may minimize confounding due to factors that affect survival, such as timing of treatment initiation, access to health care, socioeconomic status, as well as co-morbid conditions. From these 2 RCTs with rigorous entry criteria, close monitoring of pulmonary function every 3 months for 2 years, and standard treatment regimens, the hypothesis was that in this controlled setting of 300 participants with SSc and ILD new predictors of mortality could be discovered.
METHODS
Study participants
All participants enrolled in SLS I6 (NCT01762449; NCT00004563) and SLS II7 (NCT00883129) were eligible to participate in the SLS long-term follow up study. SLS I and II included adult patients with SSc with evidence of ILD on high-resolution computed tomography (HRCT) with a duration of disease ≤7 years from onset of the first non-Raynaud’s ymptom of SSc. Please see Supplementary Appendix for complete eligibility criteria. The Institutional Review Board of each site approved the studies; and only participants who provided informed consent were included in the present analyses.
SLS I and II study design
In SLS I, 158 participants were randomized to receive either oral CYC or matching placebo for one year, followed by an additional year of observation off-treatment as previously published6. In SLS II, 142 patients were randomized to receive either MMF for 2 years or oral CYC for 1 year followed by an additional year placebo using a double-dummy design to maintain the blinding as reported7.
SLS I and II assessment measurements
Complete details of SLS I and II assessment measurements are in the Supplementary Appendix. The FVC (primary SLS I and II endpoint) and DLCO (secondary SLS I and II endpoint) were measured every 3 months during the 24-month study period for both studies6,7. HRCT thoracic imaging was obtained at baseline and 24 months in SLS II and at baseline and 12 months in SLS I. Quantitative imaging analysis (to quantify the extent of ILD) was performed previously published and described in the Supplementary Appendix).
Long-Term follow up assessment
During both the SLS I and II study periods, when the statistical center was informed of a participant's death, clinical research associates were asked to collect source documentation to determine the cause of death. A mortality and morbidity committee adjudicated the causes of death to determine whether the cause was related to underlying SSc, medication, or another cause based on expert consensus. Following the 24-month study periods, patients or designated surrogates were contacted to assess morbidity and mortality outcomes. Please see Supplementary Appendix for further details of the long-term follow up assessment.
Statistical analysis
Baseline characteristics
Summary statistics were generated for baseline characteristics from the two cohorts. Group comparisons were performed using two-sample t-tests and chi-square tests.
Primary outcome: Survival
The primary outcome was survival. The Kaplan-Meier estimate was used to generate survival curves, and the log-rank test was used to compare survival between groups. If survival status was unknown, survival time was censored at the date when the participant was last known to be alive. Cox proportional hazard models were developed to evaluate the impact of covariates shown previously to be associated with survival, including treatment, baseline MRSS, age, sex, race, disease duration, type of SSc (limited or diffuse), serological subtype (Scl-70 antibody positive, RNA Polymerase III antibody positive), %-predicted values for FVC or DLCO, and the radiographic quantitative extent of ILD/fibrosis. Models for FVC and DLCO were first fit using the baseline measure as the covariate of interest, and then, in separate models using the longitudinal assessments over 24 months as a time-varying covariate. Final models were validated by fitting joint models for longitudinal and survival data using the SAS macro, JMFit14. See Supplementary Appendix for details on variables definitions and selection and joint modeling methods.
All tests were 2-sided. All analyses were performed using SAS 9.4 (SAS Institute; Cary, NC).
RESULTS
Participant characteristics
Baseline characteristics of participants in SLS I and SLS II were fairly similar (Table 1). SLS II participants were slightly older and had shorter disease durations compared with SLS I participants. While the FVC%-predicted did not differ between the two cohorts, SLS II participants had slightly more restrictive ventilatory impairment, as reflected by lower total lung capacity (TLC), despite less diffusion impairment.
Table 1.
Baseline characteristics of SLS I and SLS II participants.
| SLS I | SLS II | ||||
|---|---|---|---|---|---|
| Measure | N | Mean ± SD ( CYC; Placebo) |
N | Mean ± SD ( CYC; MMF) |
P-value*
(SLS I vs SLS II) |
| Age, years | 158 | 48.5 ± 12.3 (48.4±12.3; 47.7±12.5) P=0.693 |
142 | 52.3 ± 9.7 (52.0 ± 9.8; 52.6 ± 9.7) P=0.725 |
0.004 |
| Gender (F/M) | 158 | 70%/30% (76%/24%; 65%/35%) P=0.117 |
142 | 74%/26% (78%/22%; 70%/30%) P=0.248 |
0.477 |
| Race % | |||||
| White | 158 | 67% (67%; 66%) | 142 | 68% (66%; 71%) | 0.002 |
| African American | 16% (15%; 18%) | 23% (26%; 20%) | |||
| Asian | 4% (4%; 5%) | 6% (4%; 9%) | |||
| Other | 12% (14%; 10%) | 1% (3%; 0%) | |||
| Unknown | 1% (0%; 1%) P=0.776 |
0% P=0.324 |
|||
| Diffuse %/Limited % | 158 | 59%/41% (62%/38%; 57%/43%) P=0.512 |
142 | 59%/41% (55%/45%; 62%/38%) P=0.363 |
0.855 |
| Disease duration,years | 158 | 3.2 ± 2.1 (3.2±2.3; 3.1±1.8) P=0.867 |
139 | 2.6 ± 1.8 (2.5±1.8; 2.6±1.7) P=0.757 |
0.008 |
| FVC, % predicted | 158 | 68.1 ± 12.1 (67.6±11.3; 68.6±13.0) P=0.640 |
142 | 66.5 ± 9.1 (66.5±9.9; 66.5±8.3) P=1.000 |
0.194 |
| FEV1/FVC, % | 155 | 82.8 ± 8.0 (82.8±5.8; 82.8±7.4) P=0.999 |
142 | 82.6 ± 5.6 (83.3±5.6; 81.8±5.5) P=0.105 |
0.802 |
| TLC, % reference | 156 | 69.6 ± 13.1 (69.8±12.9; 69.3±13.3) P=0.845 |
142 | 65.9 ± 10.9 (65.5±12.0; 66.3±10.0) P=0.668 |
0.008 |
| DLCO, % reference | 158 | 47.2 ± 14.0 (47.1±13.7; 47.4±14.3) P=0.881 |
142 | 54.0 ±12.7 (54.1±14.1; 54.0±11.1) P=0.963 |
<.001 |
| BDI (focal score; 0-12)† | 156 | 5.7 ± 1.8 (5.6±1.7; 5.7±2.0) p=0.772 |
134 | 7.1 ± 2.2 (7.1±2.3; 7.3±2.1) P= 0.537 |
<.001 |
| HAQ-DI (score, 1-3)‡ | 157 | 0.83 ± 0.7 (1.0±0.7; 0.7±0.7) P=0.018 |
142 | 0.72 ± 0.7 (1.7±0.7; 0.7±0.6) p=0.874 |
0.176 |
| Visual Analog Scale(VAS) – breathlessness (0-100) | 156 | 28.4 ± 26.1 (27.2±24.6; 29.5±27.6) P=0.581 |
139 | 24.5 ± 28.1 (24.4±24.5; 29.5±27.4) P=0.983 |
0.220 |
| Modified Rodnan Skin Score (MRSS)(0-51) | 158 | 14.8 ± 10.9 (15.6±11.4; 14.0±10.5) P=0.361 |
142 | 14.7 ± 10.5 (14.0±10.6; 15.3±10.4) P=0.471 |
0.936 |
| Lung fibrosis(QLFib) score,whole lung (WL), % | 125 | 10.2 ± 10.4 (10.3±10.5; 10.1±10.4) P=0.904 |
137 | 8.6± 6.9 (8.9±7.0; 8.3±6.8) P=0.578 |
0.148 |
| Lung fibrosis (QLFib) score, worstzone (ZM), % | 125 | 26.5 ± 21.9 (28.2±23.4; 25.0±20.5) P=0.407 |
137 | 22.8 ± 19.6 (22.6±19.3; 23.0±20.2) P=0.903 |
0.152 |
| Quantitative ILD (QILD) score, % WL | 125 | 35.5 ± 16.9 (35.8±17.1; 35.3±16.9) P=0.855 |
137 | 29.5 ± 14.0 (31.6±14.4; 27.2±13.2) P=0.064 |
0.002 |
| Quantitative ILD (QILD) score, % ZM | 125 | 58.1 ± 21.7 (58.1±22.3; 58.0±21.3) P=0.977 |
137 | 51.2 ± 20.3 (53.2±19.9; 50.0±20.9) P=0.517 |
0.009 |
T-tests were used for all comparisons with the exception of gender and diffuse vs. limited disease (chi-square test).
High score denotes worse dyspnea
High score denotes worse function
Definition of abbreviations: FVC = forced vital capacity; FEV1 = forced expired volume in 1 sec; TLC = total lung capacity; DLCO = single-breath diffusing capacity for carbon monoxide; % pred = % predicted value; DL/VA = ratio of DLCO to the alveolar volume; BDI = baseline dyspnea index; HAQ-DI = health assessment questionnaire for scleroderma-Disability Index; VAS = visual analog score (for breathlessness); MRSS = Modified Rodnan Skin Score; QLfib WL, % = quantitative extent of lung fibrosis (reticulations) in whole lung high-resolution computed tomography (HRCT); QLfib ZM, % = quantitative extent of lung fibrosis in the zone of maximal involvement on HRCT; QILD WL, % = quantitative extent of interstitial lung disease (fibrosis + GGO + honeycombing) in whole lung on HRCT; QILD ZM = quantitative extent of interstitial lung disease (fibrosis + GGO + honeycombing) in the zone of maximal involvement on HRCT.
Participant disposition
SLS I
Twelve years after the first patient was randomized in SLS I, 66 of 158 (42%) participants had died (CYC: 38; Placebo 28). Among the 37 patients for whom the cause of death was known, 24 deaths (65%) were attributable to underlying SSc of which 16 (CYC 8; Placebo: 8) were due to respiratory failure (Table 2). Two of the deaths (1 CYC, 1 Placebo) due to “Respiratory Failure” were not attributed to underlying SSc. Survival status could not be determined in 34 participants. The median follow-up time for all patients in SLS I was 8 years.
Table 2.
Long-term morbidity and mortality outcomes of SLS I and II participants
| SLS I | SLS II | |||
|---|---|---|---|---|
| CYC (N=79) |
Placebo (N=79) |
CYC (N=73) |
MMF (N=69) |
|
| Subject Status | ||||
| Alive | 29 (37%) | 29 (37%) | 50 (68%) | 51 (74%) |
| Lost to follow-up* | 10 (13%) | 22 (28%) | 7 (10%) | 4 (6%) |
| No data available | 2 (3%) | 0 | 0 | 0 |
| Deceased | 38 (48%) | 28 (35%) | 16 (22%) | 14 (20%) |
| Death related to SSc | 12 | 12 | 7 | 8 |
| Death unrelated to SSc | 6 | 4 | 8 | 3 |
| Unknown if related to SSc | 20 | 12 | 1 | 3 |
| Cause of Death† | ||||
| Respiratory failure (ILD)‡ | 9 | 9 | 6 | 7 |
| Aspiration | 0 | 0 | 2 | 0 |
| Pulmonary hypertension | 0 | 0 | 0 | 1 |
| Myocardial infarction | 0 | 0 | 1 | 0 |
| Cancer | 4 | 3 | 2 | 1 |
| Heart failure | 4 | 6 | 2 | 1 |
| Renal failure | 2 | 0 | 0 | 0 |
| Complications from lung transplantation | 1 | 0 | 0 | 0 |
| Complica ions from hip fracture | 0 | 0 | 1 | 0 |
| Gastroint stinal tract failure | 1 | 1 | 0 | 0 |
| Sepsis | 1 | 0 | 0 | 0 |
| Seizures | 0 | 1 | 0 | 0 |
| Infection | 0 | 1 | 0 | 0 |
| Other | 1 | 0 | 0 | 0 |
| Unknown | 19 | 11 | 2 | 4 |
| Organ Failure | ||||
| No organ failure | 63 (80%) | 60 (76%) | 58 (79%) | 58 (84%) |
| Any organ failure | 14 (18%) | 19 (24%) | 8 (11%) | 7 (10%) |
| No data available | 2 (3%) | 0 | 7 (10%) | 4 (6%) |
| Type of Organ Failure†: | ||||
| Supplementary oxygen use | 12 | 17 | 5 | 6 |
| Lung transplantδ | 1 | 2 | 0 | 1 |
| Dialysis | 2 | 0 | 0 | 1 |
| Total parenteral nutrition | 0 | 0 | 1 | 1 |
| Cardiac ablation | 2 | 1 | 1 | 2 |
| Pacemaker | 0 | 1 | 1 | 0 |
| Malignancy | 7 | 6 | 0 | 1 |
| Development of pulmonary hypertension | Not collected | Not collected | 2 | 9 |
| Median follow up time (IQR),months | 97.4 (37.3,121.9) | 86.7 (32.3,120.2) | 40.78 (24.9,54.5) | 42.2 (36.4,58.7) |
These patients were not in death registries, but were unreachable.
Subjects could have more than one cause of death and type of organ failure recorded. In SLS I-CYC group, 1 person died of sepsis and respiratory failure, 1 died of heart and renal failure, 1 died of respiratory failure and renal failure, and 1 died of respiratory failure and GI failure. In SLS I-Placebo group, 1 person died of respiratory failure and heart failure, 1 died of respiratory failure and GI failure, 1 died of respiratory failure and infection, and 1 died of respiratory failure and seizures.
In the SLS1 study, 2 deaths (1 CYC, 1 Placebo) due to “Respiratory Failure” were not attributed to underlying SSc based on the case report form.
No patients received heart, kidney, liver or bone marrow transplantations during the follow up period.
Definitions of abbreviations: CYC = cyclophosphamide; MMF = mycophenolate; ILD = interstitial lung disease.
SLS II
Eight years after the first patient was randomized in SLS II, 30 of 142 (21%) participants had died (CYC: 16; MMF: 14). Among the 26 patients for whom the cause of death was known, 15 deaths (58%) were attributable to underlying SSc of which 13 (CYC: 6; MMF: 7) resulted from respiratory failure (Table 2). Survival status could not be determined in 12 participants The median follow-up time for all patients in SLS II was 3.6 years.
Cyclophosphamide does not improve long-term survival compared with placebo in SLS I
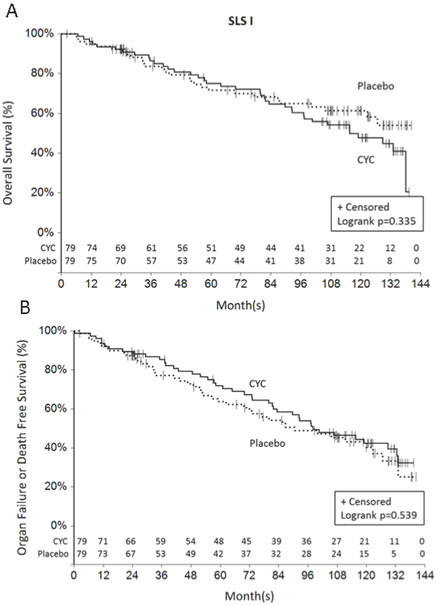
During the 24-month study period of SLS I, 6 participants randomized to CYC and 6 participants randomized to placebo expired15. During the 12-year long-term follow-up period, there was no significant difference in the time to death (p=0.335 by log-rank test; Figure 1a), nor the time to death or organ failure (p=0. 539 by log-rank test; Figure 1b) for patients randomized to CYC versus placebo in SLS I. Moreover, time to the development of organ failure did not differ between the two study arms(p=0.185 by log-rank test;Supplementary Figure S1), nor did time to the development of malignancy (p=0.701 by log-rank test; Supplementary Figure S2). Types/locations of malignancies in SLS I included anus (N=1), colon (N=2), vulvar (N=1), prostate (N=1), sarcoma (N=1) and breast (N=1) within the CYC arm, and colon (N=1), esophageal (N=1), lung (N=3) and Hodgkin’s lymphoma (N=1) within the placebo arm.
Figure 1.
Time death (Figure 1a) and time to death or organ failure (Figure 1b) from randomization in SLS I. There was no significant difference in the time to death (p=0.335 by log-rank test; Figure 1a), nor the time to death or organ failure (p=0. 539 by log-rank test; Figure 1b) for patients randomized to CYC versus placebo in SLS I. The last known date they were known to be alive was used for the survival analysis.
There is no difference in long-term survival between patients randomized to mycophenolate versus cyclophosphamide in SLS II
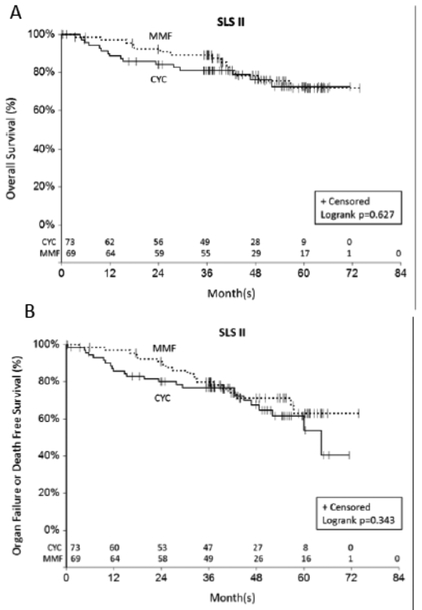
Over twice as many deaths occurred in the CYC arm (N=11) compared with the MMF arm (N=5) during the 24-month study period in SLS II (p =0.160 by log-rank test).7. During the 8-year long-term follow-up period, an additional 5 deaths occurred in the CYC arm compared with an additional 9 deaths in the MMF arm. There was no significant difference in the time to death (p =0.627 by log-rank test; Figure 2a), nor the time to death or organ failure (p=0.343 by log-rank test; Figure 2b) for patients randomized to CYC versus MMF in SLS II. However, there appeared to be a separation in the survival curves favoring MMF within the first two years (Figure 2a). There was no significant difference in the time to the development of organ failure between the two groups (p=0.692 by log-rank test Supplementary Figure S3). Two malignancies occurred during the follow-up period (MMF: N=1 thyroid cancer, N=1 papillary urothelial carcinoma; CYC: None).
Figure 2.
Time death (Figure 2a)and time to death or organ failure (Figure 2b) from randomization in SLS II. There was no significant difference in the time to death (p=0.627 by log-rank test; Figure 2a), nor the time to death or organ failure (p=0.343 by log-rank test; Figure 2b) for patients randomized to CYC versus MMF in SLS II. The last known date they were known to be alive was used for the survival analysis.
Longitudinal assessments of FVC and DLCO predict long-term survival in SLS I and II
SLS I: Cox proportional hazards model s and joint models
The basic model developed from the SLS I cohort as described in the Supplementary Methods, consisted of the following covariates: treatment arm (CYC vs. placebo), baseline extent of cutaneous sclerosis (MRSS), age at randomization (years), and sex. Among these variables, increased age and MRSS were associated with increased mortality. The following variables were independently associated with mortality in the final models: (1) Baseline FVC%-predicted; (2) Longitudinal assessment of the FVC%-predicted measured as a time-varying covariate over 24 months; and (3) longitudinal assessment of the DLCO%-predicted measured as a time-varying covariate over 24 months (Table 3). None of the quantitative lung fibrosis/ILD scores was associated with long-term survival when added to the base model. The Akaike information criterion (AIC)16, 17, 18,which estimates the quality of each model relative to each of the other models was slightly lower (better) for the models that included the longitudinally measured FVC and DLCO parameters compared with those that included the baseline FVC and DLCO parameters (Table 3). Thus, the final SLS I survival models demonstrated that decreased age, decreased extent of cutaneous sclerosis, as well as an improved course of the FVC%-predicted and the DLCO%-predicted over 24 months were associated with better survival outcomes (Table 3). As stated in the Supplementary Methods, we created separate models for the FVC and DLCO variables to avoid collinearity.
Table 3.
Final models for predicting death in SLS I
| FVC Models | |||
|---|---|---|---|
| Cox Model using Baseline FVC as covariate |
Cox Model using FVC as time- dependent covariate |
Joint model usig FVC as a time- dependent covariate |
|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Baseline MRSS | 1.03 (1.01, 1.05)* | 1.03 (1.01, 1.06)** | 1.03 (1.01, 1.06)** |
| Age (years) | 1.06 (1.04, 1.08)**** | 1.06 (1.04, 1.08)**** | 1.06 (1.03, 1.08)**** |
| FVC%-predicted | 0.97 (0.95, 0.99)** | 0.96 (0.94, 0.97)**** | 0.97 (0.96, 0.98)**** |
| AIC | 564 | 546 | 789 |
| DLCO Models | |||
|---|---|---|---|
| Cox Model using Baseline DLCO as covariate |
Cox Model using DLCO as time- dependent covariate |
Joint model usig DLCO as a time- dependent covariate |
|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Baseline MRSS | 1.04 (1.01, 1.06)** | 1.04 (1.02, 1.07)** | 1.04 (1.01, 1.06)** |
| Age (years) | 1.05 (1.03, 1.08)**** | 1.05 (1.02, 1.07)**** | 1.05 (1.03, 1.07)**** |
| DLCO%-predicted | 0.98 (0.96, 1.00) | 0.96 (0.95, 0.98)**** | 0.96 (0.94, 0.98)**** |
| AIC | 569 | 525 | 791 |
P<0.05
P<0.01
P<0.001
P<0.0001
Definitions of abbreviations: HR = hazards ratio; AIC = Akaike information criterion; FVC = forced vital capacity; DLCO = single-breath diffusing capacity for carbon monoxide; MRSS = Modified Rodnan Skin Score.
SLS I: Joint model validation analysis
Using the same basic model as above (e.g. MRSS, age), the longitudinal assessment of the FVC%-predicted was significantly associated with the outcome (Table 3). When added to the basic model, the longitudinal assessment of the DLCO%-predicted was also significantly associated with the outcome (Table 3). As noted above, the programming for the joint model does not allow for the inclusion of two longitudinally measured covariates simultaneously.
SLS I: Exploratory analyses
In an exploratory analysis, we examined whether the change from baseline in the FVC%-predicted and DLCO%-predicted at 12-months predicted survival. When added to the basic model (e.g. MRSS, age), none of the change scores was significantly associated with survival. In addition, we explored whether combined, categorical changes in the FVC%-predicted and DLCO%-predicted at 12 and 24 months predicted survival. None of the categorical declines at 12 months (e.g., FVC decline ≥ 10%; FVC decline ≥ 15%; DLCO decline ≥ 15%; FVC decline ≥ 10% and DLCO decline ≥ 15%; FVC decline ≥ 10% or DLCO decline ≥ 15%) were significantly associated with long-term survival when added to the basic model; however, these individual categorical declines at 24 months were associated with long-term survival when added to the basic model (Suplementary Table S1). Too few patients (N=1) experienced a FVC decline 5-9% and DLCO decline ≥15% to include this covariate in the model.
SLS II: Cox proportional hazards model
The basic model developed from the SLS II cohort consisted of the following covariates: treatment arm (CYC vs. MMF), baseline extent of cutaneous sclerosis (MRSS), age at randomization (years), and sex. Similar to SLS I, increased age and increased MRSS at baseline were associated with increased mortality in SLS II. Baseline FVC%-predicted and baseline DLCO%-predicted were not significantly associated with time to death when added to the basic model comprised of age and MRSS. However, the longitudinal assessment of the FVC%-predicted and the longitudinal assessment of the DLCO%-predicted were each associated with the outcome when added to the basic model(Table 4).None of the quantitative lung fibrosis/ILD scores was associated with long-term survival when added to the base model. Therefore, the final SLS II survival models demonstrated that decreased age, decreased extent of cutaneous sclerosis, as well as an improved course of the FVC%-predicted and the DLCO%-predicted over 24 months were associated with better survival outcomes (Table 4).
Table 4.
Final models for predicting death in SLS II
| FVC Models | |||
|---|---|---|---|
| Cox Model using Baseline FVC as covariate |
Cox Model using FVC as time- dependent covariate |
Joint model usig FVC as a time- dependent covariate |
|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Baseline MRSS | 1.49 (0.82, 2.56) | 1.61 (0.93, 2.77) | 1.66 (0.98, 2.83) |
| Age (years) | 1.09 (1.04, 1.14)*** | 1.09 (1.04, 1.14)*** | 1.08 (1.04, 1.13)*** |
| FVC%-predicted | 1.17 (0.64, 2.14) | 0.48 (0.28, 0.81)** | 0.51 (0.30, 0.88)* |
| AIC | 250 | 217 | 347 |
| DLCO Models | |||
|---|---|---|---|
| Cox Model using Baseline DLCO as covariate |
Cox Model using DLCO as time- dependent covariate |
Joint model usig DLCO as a time- dependent covariate |
|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Baseline MRSS | 1.56 (0.92, 2.67) | 1.86 (1.00, 3.47) | 1.85 (1.07, 3.19)* |
| Age (years) | 1.08 (1.03, 1.13)*** | 1.08 (1.02, 1.15)** | 1.07 (1.03, 1.12)** |
| DLCO%-predicted | 0.95 (0.85, 1.06) | 0.83 (0.72, 0.96)* | 0.84 (0.76, 0.94)** |
| AIC | 249 | 150 | 360 |
P<0.05
P<0.01
P<0.001
P<0.0001
Definitions of abbreviations: HR = hazards ratio; AIC = Akaike information criterion; FVC = forced vital capacity; FVC = forced vital capacity; DLCO = single-breath diffusing capacity for carbon monoxide; MRSS = Modified Rodnan Skin Score.
SLS II: Joint model validation analysis
Using the same basic model as above (e.g. MRSS, age), the longitudinal assessment of the FVC%-predicted was significantly associated with the outcome (Table 4). When added to the basic model, the longitudinal assessment of the DLCO%-predicted was also significantly associated with the outcome (Table 4).
SLS II: Exploratory analyses
In an exploratory analysis, we examined whether the change from baseline in the FVC%-predicted and DLCO%-predicted at 12-months predicted survival. When added to the basic model (e.g. MRSS, age), the change in FVC from baseline to 12 months predicted long-term survival (Estimate 0.52 [CI 0.31, 0.90]; p<0.05), but not the change in DLCO from baseline to 12 months. In addition, we explored whether combined, categorical changes in the FVC%-predicted and DLCO%-predicted at 12 months predicted survival as described above. We found that an FVC decline ≥10% at 12 months and FVC decline ≥15% at 12 months, were each associated with long-term survival when added to the base model with the covariates of MRSS and age (Supplementary Table S2). None of the patients in SLS II had a decline in DLCO ≥15% at 12 months in SLS II; therefore, we were unable to analyze any of the the composite categorical decline variables that included the DLCO.
Use of potential disease modifying agents
While no data was available regarding the use of potential disease modifying agents beyond the 24-month study period in SLS I, in SLS II these data were collected and demonstrated that the majority of patients consumed MMF following the 24-month study period. Please see Supplementary Table S3 for a list of all potential disease modifying agents used following cessation of study drug in SLS II.
DISCUSSION
The present study is the first study to examine mortality outcomes in patients who participated in two of the largest RCTs for SSc-ILD. The results presented herein demonstrate that treatment with 1-year of CYC compared with placebo does not improve long-term survival outcomes in patients with SSc-ILD. The findings also demonstrate that there is no difference in long-term survival between patients randomized to CYC versus MMF in SLS II.
Both SLS I and II demonstrated that treatment with immunosuppression led to short-term improvements in surrogate measures of SSc-ILD outcomes6,7.However, the present findings suggest that short-term treatment with CYC and MMF may not improve long-term outcomes of patients with SSc-ILD. Where known, the majority of patients in both SLS I and II died of complications related to SSc, and respiratory failure due to end-stage lung disease was the leading cause of death. These findings are consistent with recent reports of mortality outcomes in observational cohorts1,2.
Relatively few malignancies occurred in either cohort. Furthermore, although studies have demonstrated an increase in hematological malignancies in SSc19, only one case of lymphoma occurred in the placebo arm of SLS I. The paucity of malignancies observed in both cohorts may in part be due to the length of the follow up period, especially in SLS II where the median follow up was only 4 years, as well as the observation that respiratory failure was the leading cause of death.
These findings highlight a need to determine the appropriate duration of treatment for SSc-ILD. In SLS I, very few patients reported use of immunosuppression in year two of the study despite the accepted view that ILD progression generally occurs up to 5 years from disease onset in SSc. This may explain why there was no difference in long-term survival between the two study arms in SLS I. It is plausible that initiation of a maintenance therapy regimen after induction therapy may affect long-term survival outcomes. In SLS II, many patients continued on immunosuppression after the trial concluded (MMF was most common). The use of MMF in the CYC arm may in part explain why the trend for an MMF-related survival benefit observed in the first two years diminished in the subsequent follow up years.
More research is needed to determine the appropriate length of treatment for immunosuppression in SSc-ILD. A prior small retrospective study demonstrated improvement/stability in the FVC after 18 months of azathioprine (AZA) maintenance therapy in patients who first completed a 6-month induction course of intravenous CYC for SSc-ILD20. However, without a control arm, it is impossible to discern whether the observed improvement/stability in the FVC represents a true treatment response versus the natural course of ILD in SSc. An additional study demonstrated that patients who responded favorably to pulse CYC and were subsequently treated with AZA experienced a higher rate of improvement or stabilization in lung function compared with patiets who did not respond to pulse CYC21
The present analysis also revealed significant predictors of long-term mortality in SSc-ILD. In line with prior observational studies22-24, increased skin score and increased age were independently associated with increased mortality. In contrast to prior observational studies12,23,25,26, however, male gender and African American race were not associated with an increased risk of mortality. Regarding gender, the SLS I and II cohorts were comprised predominantly of women; thus, these studies may be underpowered to detect true gender differences in long-term survival. In terms of race, our findings could potentially suggest that in the context of a clinical trial, in which all patients have equal access to healthcare and uniform follow-up, race does not play a substantial role in predicting long-term survival.
Consistent with prior observational studies21,24,27,28, low baseline FVC was associated with an increased risk of mortality. However, the course of the FVC and the DLCO over 24 months appeared to be more robust predictors of long-term survival in both SLS I and II than the baseline measurements of these parameters when comparing the AIC for the models. The individual parameter estimates were similar for both the Cox model and the joint model that we used as a validation approach, suggesting that the relationship between survival and FVC (or DLCO) is not biased by non-ignorable missing data.
A recent single-center observational cohort study of patients with SSc-ILD also found that pulmonary function trends at 1- and 2-years predicted intermediate to long-term mortality29. This study demonstrated that 1-year categorical trends in the FVC and DLCO were the most accurate prognostic determinants of mortality, while at 2-years, changes in gas transfer were the most important predictors of mortality29. As this was an observational study, the authors could not adequately control for treatment effect and selection bias. We were able to replicate some, but not all of these findings in the SLS I or II cohorts. Taken together, the findings of the present study provide further evidence that trends in pulmonary function may offer more prognostic information than baseline pulmonary function measurements. This may in part be due to the fact that substantially variability exists in a single FVC and DLCO measurement. Repeated measurements of the FVC/DLCO may yield more clinically meaningful information regarding ILD progression and survival.
The findings of the present study should be interpreted in the context of specific limitations. There were subtle differences in the baseline characteristics of the SLS I and SLS II cohorts. For instance, the DLCO was lower in SLS I, and this may have been due to less scrupulousness in excluding PAH. The BDI was also lower in SLS I, although this differences could be related to using different instruments to administer the BDI in the two studies. The QILD/QLF-ZM was higher in SLS II, while the QILD-WL was higher in SLS I. However, in SLS I, non-volumetric CT scans of 1-2 mm slice thickness were acquired at 10 mm increments, while in SLS II volumetric CT scans of 1-1.5mm slice thickness were acquired contiguously. Overall, the two cohorts were strikingly similar.
A number of SLS I and, to a lesser degree, SLS II participants, were lost to follow-up during the course of this long-term follow-up study. This can introduce bias, especially in cases where early censoring occurred. The Cox-proportional hazards model was used to deal with time to event data in the presence of censoring. Moreover, while a morbidity and mortality committee adjudicated the causes of death during the 24-month study periods, less detailed information was obtained during long-term follow up periods regarding causes of death. In addition, although we successfully collected information on the use of immunosuppression following the conclusion of SLS II, the timing, duration, and dosages of immunosuppression reported by patients varied widely and precluded any kind of meaningful statistical analysis of these data (in SLS I, very few patients continued on immunosuppression during the second year; beyond this point no data was available regarding immunosuppression use).Finally,whilePH was identified as the cause of death in only one of the SLS II patients, this co-morbidity may have influenced survival rates in both cohorts.
Notable strengths of the present manuscript include the use of two relatively large, well-charactriz SSc-ILD cohorts undergoing standard treatment approaches with uniform follow up measurements over the course of 2 years. These cohorts were comprised of patients from multiple SSc Centers of Excellence across the US, augmenting the generalizability of our study findings to a US population. Furthermore, we identified the same mortality predictor variables in both cohorts, suggesting that our results are likely reproducible in other similar SSc cohorts. Finally, we used a joint model as a means of internal validation.
In summary, the findings of the present analyses demonstrate that increased baseline skin score, increased baseline age, and the course of the FVC and DLCO over 2 year, are important predictors of long-term survival in SSc-ILD. Treatment with immunosuppression may not improve long-term survival in patients with SSc-ILD, in contrast to hematopoetic stem cell transplantation30-32, which seems to offer a more sustained improvement in long-term survival and may especially help those patients who have early,rapidly progressive SSc with organ involvement. Future studies are needed to determine how the duration of immunosuppression affects long-term survival among patients with SSc-ILD. With the emergence of promising new therapies for SSc-ILD (e.g. anti-fibrotics, or combination therapy with anti-fibrotics and immunosuppression), additional studies are needed to compare how these novel approaches affect survival compared with the current standard of care for SSc-ILD.
Supplementary Material
KEY POINTS.
What is already known about this subject?
Observational studies have identified factors associated with an increased risk of mortality in SSc, including age, extent of cutaneous sclerosis and severity of ILD.
What does this study add?
In addition to identifying traditional risk factors for mortality, this study found that short-term progression of ILD was a better predictor of mortality than baseline severity of ILD.
Specifically, patients who experienced a decline in lung function and diffusing capacity over two years had a substantially increased risk of mortality even after adjusting for treatment arm assignment, as well as baseline disease severity.
How might this affect clinical practice?
Patients with SSc-ILD who experience an early decline in lung function and have an increased risk of death may benefit from receiving a more aggressive treatment approach that could include escalation of immunosuppression, addition of antifibrotic therapy, and/or evaluation for hematopoetic stem cell transplant.
SSc providers should closely monitor lung function when ILD is present to accurately identify declines in lung function and promptly intervene to improve patient outcomes.
ACKNOWLEDGEMENTS AND AFFILIATIONS
This work was supported in part by the NIH/NIAID: N01-AI05419 (KMS) and HHSN-272201100025C (KMS); the Scleroderma Foundation (ERV); NHI/NIAMS: R01 AR 070470 and K24 AR 063121 (DK); NHLBI/NIH: R01 HL089758 (DPT), R01 HL089901 (RME), U01 HL 60587 (DPT) and U01 HL 60606 (RME). Bristol—Myers Squibb supplied cyclophosphamide for use in SLS I and Hoffmann-LaRoche supplied mycophenolate mofetil for use in SLS II. We thank John Dermond and Grace Ibrahim for their assistance contacting participants in SLS I and II, respectively.
The following persons and institutions participated in the Scleroderma Lung Study 1: University of California at Los Angeles (UCLA), Los Angeles: P.J. Clements, D.P. Tashkin, R. Elashoff, J. Goldin, M. Roth, D. Furst, K. Bulpitt, D. Khanna, W.-L.J. Chung, S. Viasco, M. Sterz, L. Woolcock, X. Yan, J. Ho, S. Vasunilashorn, I. da Costa; University of Medicine and Dentistry of New Jersey, New Brunswick: J.R. Seibold, D.J. Riley, J.K. Amorosa, V.M. Hsu, D.A. McCloskey, J.E. Wilson; University of Illinois Chicago, Chicago: J. Varga, D. Schraufnagel, A. Wilbur, M. Lopata, S. Arami, P. Cole-Saffold; Boston University, Boston: R. Simms, A. Theodore, P. Clarke, J. Korn, K. Tobin, M. Nuite; Medical University of South Carolina, Charleston: R. Silver, M. Bolster, C. Strange, S. Schabel, E. Smith, J. Arnold, K. Caldwell, M. Bonner; Johns Hopkins School of Medicine, Baltimore: R. Wise, F. Wigley, B. White, L. Hummers, M. Bohlman, A. Polito, G. Leatherman, E. Forbes, M. Daniel; Georgetown University, Washington, D.C.: V. Steen, C. Read, C. Cooper, S. Wheaton, A. Carey, A. Ortiz; University of Texas at Houston, Houston: M. Mayes, E. Parsley, S. Oldham, T. Filemon, S. Jordan, M. Perry; University of California at San Francisco, San Francisco: K. Connolly, J. Golden, P. Wolters, R. Webb, J. Davis, C. Antolos, C. Maynetto; University of Alabama at Birmingham, Birmingham: B. Fessler, M. Olman, C. Sanders, L. Heck, T. Parkhill; University of Connecticut Health Center, Farmington: N. Rothfield, M. Metersky, R. Cobb, M. Aberles, F. Ingenito, E. Breen; Wayne State University, Detroit: M. Mayes, K. Mubarak, J.L. Granda, J. Silva, Z. Injic, R. Alexander; Virginia Mason Research Center, Seattle: D. Furst, S. Springmeyer, S. Kirkland, J. Molitor, R. Hinke, A. Mondt; Data Safety and Monitoring Board: Harvard Medical School, Boston — T. Thompson; Veterans Affairs Medical Center, Brown University, Providence, R.I. — S. Rounds; Cedars Sinai–UCLA, Los Angeles — M. Weinstein; Clinical Trials Surveys, Baltimore — B. Thompson; Mortality and Morbidity Review Committee: UCLA, Los Angeles — H. Paulus, S.Levy;Johns Hopkins Unversity, Baltimore — D. Martin.
The following persons and institutions participated in the Scleroderma Lung Study 2: University of Boston, Boston: A.C. Theodore, R.W. Simms, E. Kissin, F.Y. Cheong; Georgetown University, Washington, D.C.: V.D. Steen, C.A. Read Jr., C. Fridley, M. Zulmatashvili; Johns Hopkins University, Baltimore: R.A. Wise, F.M. Wigley, L. Hummers, G. Leatherman; Medical University of South Carolina, Charleston: R.M. Silver, C. Strange, F.N. Hant, J. Ham, K. Gibson, D. Rosson; University of California, Los Angeles (UCLA), Los Angeles: D.P. Tashkin, R.M. Elashoff, M.D. Roth, P.J. Clements, D. Furst, S. Kafaja, E. Kleerup, D. Elashoff, J. Goldin, E. Ariola, G. Marlis, J. Mason-Berry, P. Saffold, M. Rodriguez, L. Guzman, J. Brook; University of California, San Francisco (UCSF), San Francisco: J. Golden, M.K. Connolly, A. Eller, D. Leong, M. Lalosh, J. Obata; University of Illinois, Chicago: S. Volkov, D. Schraufnagel, S. Arami, D. Franklin; Northwestern University, Chicago: J. Varga, J. Dematte, M. Hinchcliff, C. DeLuca, H. Donnelly, C. Marlin; University of Medicine and Dentistry of New Jersey, New Brunswick: D.J. Riley, V.M. Hsu, D.A. McCloskey; University of Michigan, Ann Arbor: K. Phillips, D. Khanna, F.J. Martinez, E. Schiopu, J. Konkle; University of Texas, Houston: M. Mayes, B. Patel, S. Assassi, F. Tan; National Jewish Health, Denver: A. Fischer, J. Swigris, R. Meehan, K. Brown, T. Warren, M. Morrison; University of Utah, Salt Lake City: M. B. Scholand, T. Frecht, P. Carey, M. Villegas; University of Minnesota, Minneapolis: J. Molitor, P. Carlson.
This manuscript was based on work previously published at the following conferences: Systemic Sclerosis World Congress 2018 (Volkmann ER, Tashkin DP, Sim M, et al. The course of the forced vital capacity during treatment for systemic sclerosis-related interstitial lung disease predicts long-term survival in 2 independent cohorts. Journal of Scleroderma and Related Disorders 2018;3(15):69-101) and the American College of Rheumatolgy Annual Meeting 2017 (Volkmann ER, Tashkin DP, Sim M, et al. The course of the forced vital capacity during treatment for systemic sclerosis-related interstitial lung disease predicts long-term survival in 2 independent cohorts. Arthritis Rheumatol 2017;69(Suppl 10)).
References
- 1.Elhai M, Meune C, Avouac J, et al. Trends in mortality in patients with systemic sclerosis over 40 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxf rd) 2012;51:1017–26. [DOI] [PubMed] [Google Scholar]
- 2.Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010;69:1809–15. [DOI] [PubMed] [Google Scholar]
- 3.Wells AU. Interstitial lung disease in systemic sclerosis. Press Med 2014;43:e329–e343. [DOI] [PubMed] [Google Scholar]
- 4.D’Angelo WA, Fries JF, Masi AT, et al. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969;46:428–40. [DOI] [PubMed] [Google Scholar]
- 5.Volkmann ER, Tashkin DP. Treatment of systemic sclerosis-related interstitial lung disease: A review of existing and emerging therapies. A Am Thorac Soc 2016;13:2045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tashkin DP, Elashoff R, Clements PJ, et l. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 2006;354:2655–66. [DOI] [PubMed] [Google Scholar]
- 7.Tashkin DP, Roth MD, Clements PJ, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease: Scleroderma lung study II (SLS-II), a double-blind, parallel group, randomised controlled trial. Lancet Resp Med 2016;4:708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkmann ER, Chung A, Tashkin DP. Managing systemic sclerosis-related interstitial lung disease in the modern treatment era. J Scleroderma Relat Disord 2017;2:72–83. [Google Scholar]
- 9.Mahler DA, Weinberg DH, Wells CK, et al. The measurement of dyspnea. Contents, interobserver agreement and physiologic correlates of two new clinical indexes. Chest 1984;85:751–758. [DOI] [PubMed] [Google Scholar]
- 10.Mahler DA, Ward J, Fierro-Carrion G, et al. Development of self-administered verions of modified baseline and transition dyspnea indexes in COPD. COPD 2004;1:1–8. [DOI] [PubMed] [Google Scholar]
- 11.Clements PJ, Lachenbruch PA, Seibold JR, et al. Skin thickness score in systemic sclerosis (SSc): an assessment of inter-observer variability in three independent studies. J Rheumatol 1993;20:1892–6. [PubMed] [Google Scholar]
- 12.Goldin J, Elashoff R, Kim HJ, et al. Treatment of scleroderma-interstitial lung disease with cyclophosphamide is associated with less progressive fibrosis on serial thoracic high-resolution CT scan than placebo: findings from the scleroderma lung study. Chest 2009;136:1333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HJ, Li G, Gjertson D, et al. Classification of parenchymal abnormality in scleroderma lung using a novel approach to denoise images collected via a multicenter study. Acad Radiol 2008;15:1004–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang D, Chen MH, Ibrahim JG, e al. JMFit: A SAS macro for joint models of longitudinal and survival data. J Stat Softw 2016;71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tashkin DP, Elashoff R, Clements PJ, et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med. 2007;176:1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bozdogan H Model selection and Akaike’s information criterion (AIC): The general theory and its analytical extensions. Psychometrika 1987;52:345–70. [Google Scholar]
- 17.Snipes M, Taylor DC. Model selection and Akaike information criteria (AIC): An example from wine ratings and prices. Wine Economics and Policy 2014;3:3–9. [Google Scholar]
- 18.Symonds MRE, Moussalli A. A brief guide to model selection, multimodel inference and model averaging in behavioual ecology using Akaike’s information criterion. Behav Ecol Sociobiol 2011;65:13–21. [Google Scholar]
- 19.Zeineddine N, Khoury LE, Mosak J Systemic sclerosis and malignancy: a review of current data. Journal of Clinical Medicine Research. 2016;8:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berezne A, Ranque B, Valeyre D, et al. Therapeutic strategy combining intravenous cyclophosphamide followed by oral azathioprine to treat worsening interstitial lung disease associated with systemic sclerosis: a retrospective multicenter open-label study. J Rheumatol 2008;35(6):1064–72. [PubMed] [Google Scholar]
- 21.Iudici M, Cuomo G, Vettori S, et al. Low-dose pulse cyclophosphamide in interstitial lung disease associated with systemic sclerosis (SSc-ILD): efficacy of maintenance immunosuppression in responders and non-responders. Semin Arthritis Rheum 2015;44:437–44. [DOI] [PubMed] [Google Scholar]
- 22.Steen VD, Conte C, Owens GR, et al. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum 1994;37:1283–9. [DOI] [PubMed] [Google Scholar]
- 23.Mayes MD, Lacey JV Jr, Beebe-Dimmer J, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum 2003;48:2246–55. [DOI] [PubMed] [Google Scholar]
- 24.Nihtyanova SI, Schreiber BE, Ong VH, et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol 2014;66:1625–35. [DOI] [PubMed] [Google Scholar]
- 25.Domsic RT, Nihtyanova SI, Wisniewski SR, et al. Derivation and external validation of a prediction rule for five-year mortality in patients with early diffuse cutaneous systemic sclerosis. Arthritis Rheumatol 2016;68:993–1003. [DOI] [PubMed] [Google Scholar]
- 26.Mendoza F, Derk CT. Systemic sclerosis mortality in the United States 1999-2002: Implications for patient care. J Clin Rheumatol 2007;13:187–92. [DOI] [PubMed] [Google Scholar]
- 27.Assassi S, Sharif R, Lasky RE, et al. Predictors of interstitial lung disease in early systemic sclerosis: a prospective longitudinal study of the GENISOS cohort. Arthritis Res Ther 2010;12:R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan C, Knight C, Lunt M, et al. Predictors of end stage lung disease in a cohort of patients with scleroderma. Ann Rheum Dis 2003;62:146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goh NS, Hoyles RK, Denton CP, et al. Short-term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheum 2017;69:1670–8. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan KM, Goldmuntz EA, Keyes-Elstein L, et al. Myeloablative autologous stem-cell transplantation for severe scleroderma. NEJM 2018;378:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Laar JM, Garge D, Sont JK, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA 2014;11:2490–8. [DOI] [PubMed] [Google Scholar]
- 32.Shouval R, Furie N, Raanani P, et l. Autologous hematopoietic stem cell transplantation for systemic sclerosis: A systematic review and meta-analysis. Biol Blood Marrow Transplant 2018;24:937–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.