Summary
Transcriptional silencing and anti-silencing mechanisms modulate bacterial physiology and virulence in many human pathogens. In Shigella species, many virulence plasmid genes are silenced by the histone-like nucleoid structuring protein H-NS and anti-silenced by the virulence gene regulator VirB. Despite the key role that these regulatory proteins play in Shigella virulence, their mechanisms of transcriptional control remain poorly understood. Here, we characterize the regulatory elements and their relative spacing requirements needed for the transcriptional silencing and anti-silencing of icsP, a locus that requires remotely located regulatory elements for both types of transcriptional control. Our findings highlight the flexibility of the regulatory elements’ positions with respect to each other, and yet, a molecular roadblock docked between the VirB binding site and the upstream H-NS binding region abolishes transcriptional anti-silencing by VirB, providing insight into transcriptional anti-silencing. Our study also raises the need to re-evaluate the currently proposed VirB binding site. Models of transcriptional silencing and anti-silencing at this genetic locus are presented, and the implications for understanding these regulatory mechanisms in bacteria are discussed.
Introduction
Nucleoid structuring proteins (NSPs) mediate transcriptional silencing of bacterial genes by binding, compacting and organizing DNA, activities that frequently render promoter regions inaccessible (Dorman and Deighan, 2003; Dorman, 2007). Transcriptional anti-silencing proteins relieve this repression through poorly characterized mechanisms, although it is clear that remodeling of the NSP-DNA complex is involved (reviewed in [Stoebel et al., 2008]). Using the human bacterial pathogen Shigella flexneri as a model, our goal is to further characterize mechanisms of transcriptional silencing and anti-silencing of target genes found on the large (222 kb) virulence plasmid. At temperatures below 37°C, many of these target genes are transcriptionally silenced by the NSP H-NS (Hromockyj and Maurelli, 1989; Beloin and Dorman, 2003). Upon a switch to 37°C, VirF, the master activator of virulence genes, triggers the production of the anti-silencing protein VirB (Tobe et al., 1991), which subsequently functions to relieve H-NS-mediated silencing of virulence genes that is not relieved by the temperature increase alone (Wing et al., 2004). Despite the importance of the interplay between H-NS and VirB for Shigella virulence, mechanistic insight into transcriptional silencing and anti-silencing of virulence genes in Shigella is limited (Beloin et al., 2002; Turner and Dorman, 2007; Gao et al., 2013).
The transcriptional silencing activity of H-NS can be explained by its DNA binding preference and the resulting nucleoprotein complexes that form (reviewed in [Picker and Wing, 2016]). H-NS preferentially binds AT-rich DNA (Williams and Rimsky, 1997; Navarre et al., 2006; Bouffartigues et al., 2007), which is a common feature of bacterial promoters (Landick et al., 2015) and horizontally acquired genetic loci (Navarre et al., 2007), including virulence genes. Although a high-affinity binding site for H-NS has been proposed (Bouffartigues et al., 2007; Lang et al., 2007), AT-rich DNA tracts that produce narrow minor groove widths primarily govern the DNA binding preference of H-NS (Gordon et al., 2011). Once bound to DNA, H-NS oligomerizes along the helix into regions with lower binding affinities (Lang et al., 2007; Fang and Rimsky, 2008), leading to the formation of large H-NS-DNA complexes. Two H-NS-DNA complexes have been described: H-NS nucleoprotein filaments that coat long, contiguous stretches of DNA, and H-NS bridging complexes that bring two discrete regions together by direct H-NS-H-NS interactions (Dame et al., 2000, 2005, 2006; Arold et al., 2010). Both of these nucleoprotein complexes have been implicated in the silencing of virulence genes in Shigella (Tobe et al., 1993; Falconi et al., 1998; Prosseda et al., 2004; Turner and Dorman, 2007).
The anti-silencing protein VirB belongs to the ParB protein superfamily (Turner and Dorman, 2007; Taniya et al., 2003) that contains both chromosomal and plasmid partitioning factors, including SopB and Spo0J. Consequently, VirB appears to have been co-opted to a new role in Shigella where it serves to relieve transcriptional silencing mediated by H-NS and its family members (Turner and Dorman, 2007; Stoebel et al., 2008; Picker and Wing, 2016). VirB is a bona fide anti-silencing protein because it up-regulates transcription of its target genes in the presence of H-NS, but has little to no effect on these genes in its absence ([Wing et al., 2004; Turner and Dorman, 2007; Basta et al., 2013] and reviewed in [Stoebel et al., 2008]). The DNA binding activity of VirB is necessary for its role as a transcriptional regulator (Beloin et al., 2002; Gao et al., 2013). Even though a DNA recognition site for VirB, 5ʹ-RWG(G)AAAT-3ʹ, has been proposed (Taniya et al., 2003), this site is only loosely supported by in vitro assays (Taniya et al., 2003), making the validity of the proposed site uncertain.
To date, the best studied example of a transcriptionally silenced and anti-silenced virulence gene locus in Shigella is the icsB promoter, which controls the ipa operon located within the invasion locus on the large virulence plasmid (Taniya et al., 2003; Turner and Dorman, 2007). Here, DNA sequences required for both transcriptional silencing by H-NS and anti-silencing by VirB are promoter-proximal, located within 150 bp upstream of the icsB transcription start site (TSS; [Turner and Dorman, 2007]). The VirB binding site at this genetic locus is organized as a near-perfect inverted repeat separated by a single base pair. Interestingly, only the upstream half (known as Box 2) is reported to be necessary for VirB-dependent regulation of the icsB promoter in vivo and VirB binding in vitro (Turner and Dorman 2007). As such, this site appears to be consistent with the currently proposed VirB binding site (Taniya et al., 2003).
To improve our understanding of transcriptional silencing and anti-silencing and how this process regulates virulence gene expression in Shigella, our work has focused on characterizing another H-NS and VirB-regulated gene, icsP (Wing et al., 2004; Castellanos et al., 2009; Africa et al., 2011; Hensley et al., 2011), which encodes the IcsA-specific outer membrane protease (Wing et al., 2005; Egile et al., 1997; Shere et al., 1997; Steinhauer et al., 1999). Several features of the icsP locus make it different from the icsB locus and justify our interest in characterizing its silencing and anti-silencing. These include its regulation by two promoters (Hensley et al., 2011), its location outside of the invasion locus (100 kb away) and the unusually long, 1.5 kb intergenic region upstream of the icsP gene (Castellanos et al., 2009). Our previous studies of icsP reveal that H-NS silences the transcription of icsP at 37°C in the absence of VirB (Wing et al., 2004). Consistent with its role as an anti-silencing protein, VirB has little to no effect on icsP promoter activity in the absence of a functional hns gene (Wing et al., 2004). VirB-dependent anti-silencing of icsP relies on a DNA sequence (Castellanos et al., 2009) resembling that found at the icsB promoter: a near-perfect inverted repeat, with each half bearing similarity to the proposed VirB binding site (Taniya et al., 2003). Unlike the VirB regulatory site at the icsB promoter, however, both halves of the inverted repeat are required for VirB-dependent regulation of icsP (Castellanos et al., 2009). Strikingly, and in contrast to the icsB promoter, these sites are remotely located, centered 1137 bp upstream of the primary icsP TSS (Castellanos et al., 2009). These differences raise questions about the mechanism of transcriptional anti-silencing mediated by VirB.
Here, we focus on the detailed characterization of the remotely located regulatory elements controlling the expression of icsP and their relative spacing requirements to improve our understanding of the mechanisms of transcriptional silencing and anti-silencing of virulence gene expression in Shigella (Wing et al., 2004; Stoebel et al., 2008; Picker and Wing, 2016). We present a model of transcriptional regulation by H-NS and VirB at the icsP locus and discuss the implications of our findings for those studying mechanisms of transcriptional silencing and anti-silencing in other bacteria.
Results
Remote DNA sequences located between –900 and –436 are required for H-NS-mediated silencing of the icsP promoter
Previous studies revealed that sequences located between –1232 (full-length icsP intergenic region) and –351 relative to the primary TSS (+1) of icsP were required for full silencing of the icsP promoter, with partial silencing observed when sequences up to 2665 were present (Castellanos et al., 2009). However, it was unclear if this silencing was mediated by H-NS, and the sequences involved needed to be more precisely mapped. To address this, a 5ʹ truncation series of the icsP intergenic region was constructed, extending as far as –92 (Egile et al., 1997; Hensley et al., 2011). Subsequently, each DNA fragment was introduced into our PicsP-lacZ transcriptional reporter pAFW04 (Basta et al., 2013), and the resulting constructs were assayed for β-galactosidase activity in the Escherichia coli strain MC4100 and an isogenic mutant lacking a functional hns allele (MC4100 hns::Knr). These strains have been routinely used to investigate the role of H-NS in the regulation of Shigella promoters (Beloin and Dorman, 2003; Wing et al., 2004; Basta et al., 2013; Picker and Wing, 2016) because they avoid the genetic instability routinely exhibited by Shigella hns mutants (Schuch and Maurelli, 1997) and eliminate interference arising from the dysregulation of virB in the absence of hns (Tobe et al., 1993; Falconi et al., 1998). Our data show that DNA sequences located between –900 and –436 are needed for H-NS-mediated silencing of the icsP promoter in vivo (Fig. 1A). In wild-type cells, 90% repression was observed when this region is present in its entirety, and 5ʹ truncations from –436 to –92 displayed repression levels similar to those observed in the hns mutant. These data raise the possibility that H-NS binds throughout this remote region to confer direct H-NS-mediated silencing of the icsP promoter.
Fig. 1. Precise mapping of DNA sequences required for H-NS-mediated silencing of PicsP are capable of binding H-NS.
A. Activities of the PicsP-lacZ and 5ʹ truncation derivatives in wild-type E. coli MC4100 and its isogenic hns::Knr mutant. β-Galactosidase activities are expressed as a percentage of repression exhibited by the full-length PicsP-lacZ in wild-type MC4100. Upstream boundary of constructs relative to the primary TSS (+1) (Hensley et al., 2011) are listed from left to right: –1232 (full-length), –1056 bp, –900 bp, –893 bp, –874 bp, –838 bp, –683 bp, –665 bp, –637 p, –601 bp, –550 bp, –436 bp, –351 bp, –254 bp and –92 bp. All data generated in the wild-type (MC4100) background, except those annotated ns, are statistically different from the full-length promoter (P < 0.001). All data generated in the hns::Knr mutant, except those annotated NS, are statistically different from the identical construct measured in the wild-type strain (P < 0.05).
B. Schematic of the six DNA targets (T1–T6) used in EMSAs to identify H-NS binding regions. All coordinates given are relative to the primary icsP TSS (Hensley et al., 2011). Inverted arrows represent the VirB boxes essential for VirB-dependent regulation (Castellanos et al., 2009).
C. In vitro binding of purified, recombinant H-NS-His6 to six DNA targets (T1–T6) as determined by EMSAs. Each radiolabeled target was incubated with final concentrations of 0, 10.7, 13.4 or 16.1 μM (for each panel, lanes 1–4, respectively) of H-NS-His6. DNA and the resulting nucleoprotein complexes were separated by polyacrylamide gel electrophoresis.
H-NS directly binds to two discrete regions upstream of the icsP gene
To determine if H-NS binds directly to the DNA located upstream of the icsP gene, electrophoretic mobility shift assays (EMSAs) were used. The full intergenic region upstream of icsP (1.2 kb) was divided into six nearly equal length DNA targets (Fig. 1B). Each radiolabeled target was incubated with increasing concentrations of purified, His-tagged H-NS, and the resulting DNA-protein complexes were resolved by polyacrylamide gel electrophoresis (Fig. 1C). Because H-NS oligomerizes along DNA (Lang et al., 2007), H-NS-DNA complexes do not appear as discrete shifted bands in EMSAs, but instead, appear more diffuse (Azam and Ishihama, 1999; Doyle et al., 2007). Nonetheless, we fully expected that H-NS would interact with some of the six DNA fragments at lower concentrations than others.
H-NS caused pronounced shifts of T4 and T5 at the lowest concentration used (10.7 μM; Fig. 1C), although moderate shifts of T1, T3 and T6 were also observed with this concentration. The only targets, however, to solely exhibit discrete shifts at the highest concentration of H-NS (16.1 μM) were T1, T4 and T5 (T3 showed a mixture of discrete and non-discrete shifts). In contrast, T2 failed to shift even in the presence of the highest H-NS concentration used (16.1 μM). Taken together, these data are consistent with our 5’ truncation analysis because the sequences bound by H-NS in vitro (Fig. 1B and C; T3–T5) overlap with those required for H-NS-mediated silencing in vivo (Fig. 1A; –900 to –436), thus supporting our hypothesis that H-NS directly binds to the icsP intergenic region to silence the icsP promoter. Strikingly, these data also reveal that T1 displays high affinity for H-NS in vitro, a region demonstrated not to be sufficient for H-NS mediated silencing of icsP (Fig. 1A). Notably, this DNA target contains icsP promoter elements and has the highest AT content of all six targets used in our EMSAs (70% AT-rich), which likely contributes to its affinity for H-NS (Williams and Rimsky, 1997; Navarre et al., 2006; Bouffartigues et al., 2007).
Spacing and helical phasing requirements between the two discrete regions displaying high affinity for H-NS in vitro
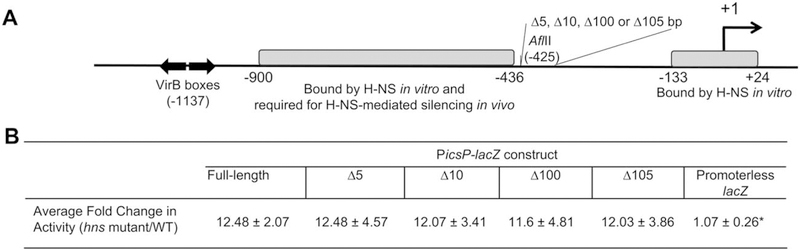
The presence of two discrete H-NS binding regions (T5–T3 and T1) identified by our EMSAs suggests that H-NS silences the icsP promoter by bridging these two regions of DNA, a demonstrated activity of H-NS (Dame et al., 2005). A similar arrangement of H-NS binding regions at the virF promoter is crucial for the bridging of two regions by H-NS, resulting in transcriptional silencing (Falconi et al., 1998; Prosseda et al., 2004). At this locus, bridging can be disrupted by changing the helical phasing of the two H-NS binding regions with respect to each other (Prosseda et al., 2004). To determine if H-NS-mediated silencing of the icsP promoter is also affected by the spacing between and/or helical orientation of the regions bound by H-NS, four derivatives of the PicsP-lacZ construct (pAFW04) were created. Each construct, containing either a change in a half helical turn (5 or 100 bp deletion) or full helical turn (10 or 105 bp deletion) immediately downstream of the AflII site at position –425 (Fig. 2A), was introduced into MC4100 and the isogenic MC4100 hns mutant strain, and β-galactosidase activities were measured.
Fig. 2. Helical phasing and spacing requirements between H-NS binding regions at the icsP promoter.
A. Schematic showing the size and location of deletions made in the icsP promoter region relative to key regulatory elements and regions (not drawn to scale).
B. Effect of deletions between the two regions binding H-NS in vitro. Fold change in promoter activity of the PicsP-lacZ reporter and deletion derivatives when compared in the hns::Knr mutant and wild-type backgrounds. The asterisk represents data that are statistically different from the fold change observed with full-length PicsP-lacZ, (P < 0.05).
Regardless of the deletion tested, the average fold change in β-galactosidase activity between the hns mutant strain and wild-type remained approximately 12-fold (Fig. 2B), demonstrating that none of these deletions affected H-NS-mediated silencing of the icsP promoter. Thus, we conclude that, unlike the virF promoter, the spacing and helical phasing of the icsP regulatory elements with respect to each other can be altered without affecting H-NS-mediated silencing.
Seven sites matching the proposed VirB binding site do not significantly contribute to VirB-dependent regulation in vivo
Having mapped the DNA sequences required for H-NS-mediated silencing of the icsP promoter, we next turned our attention to the sequences required for VirB-dependent regulation. Two putative VirB binding sites (known as boxes 1 and 2), each matching the proposed recognition site (Taniya et al., 2003; Turner and Dorman, 2007), are required for the regulation of the icsP promoter by VirB in vivo (Castellanos et al., 2009). Notably, these boxes are organized as an inverted repeat and are positioned over 1 kb upstream of the primary icsP TSS (Egile et al., 1997; Hensley et al., 2011). Interestingly, seven other putative binding sites with at least a 6/7 match to the proposed recognition site were also identified in the intergenic region immediately upstream of the icsP gene ([Castellanos et al., 2009]; Fig. 3A), but their contribution to VirB-dependent regulation had not been tested.
Fig. 3. Investigating the role of seven sites that closely match the proposed VirB binding site in vivo.
A. Schematic showing the location of seven sites displaying at least a 6/7 or 7/8 match to the proposed VirB binding site 5ʹ-RWG(G)AAAT-3ʹ (Taniya et al., 2003). The central base pair of each site is indicated.
B. Activities of PicsP-lacZ reporter and derivatives carrying transversion mutations made in each of the seven sites, all seven sites and the previously characterized box 1, box 2 or both. Average β-galactosidase activities are expressed as percentage activity relative to the full-length PicsP-lacZ in wild-type S. flexneri (2457T). Average fold changes between S. flexneri wild-type and isogenic virB mutant are also provided. The asterisk represents data that are statistically different from the average relative activity observed in wild-type S. flexneri carrying PicsP-lacZ. ^, represents data that are statistically different from the fold change observed with full-length PicsP-lacZ, (P < 0.05).
To assess the involvement of these seven sites in VirB-dependent regulation of the icsP promoter, each site was subjected to site-directed mutagenesis using transversion mutations. The eight resulting promoter fragments carrying mutations of each of the seven putative binding sites, or a combination of all seven mutated sites, were cloned upstream of the lacZ gene in our transcriptional reporter, pHJW20 (Supporting Information Table S1). The resulting reporter constructs were then introduced into wild-type S. flexneri strain 2457T or a virB mutant derivative, and β-galactosidase activities were measured.
Mutagenesis of these putative binding sites alone or in combination did not significantly alter the VirB-dependent regulation of the icsP promoter (Fig. 3B). In contrast, mutagenesis of either or both of the required boxes 1 and 2 completely abolished VirB-dependent regulation (Fig. 3B), as demonstrated previously (Castellanos et al., 2009). Based on these data, we conclude that the seven sites displaying at least a 6/7 match to the proposed VirB-binding site do not contribute significantly to VirB-dependent regulation of the icsP promoter. Interestingly, none of these seven sites were organized as an inverted repeat separated by a single base pair.
VirB binds DNA sites required for VirB-dependent regulation
Next, we chose to study the direct interaction of VirB with the remotely located VirB boxes. To do this, a combination of in vitro electrophoretic mobility shift assays and DNase I protection assays were used to test binding of purified His-tagged VirB to radiolabeled DNA products of the icsP promoter containing either wild-type or mutated boxes 1 and 2. A 54 bp DNA fragment containing wild-type boxes was retarded when VirB was added at the lowest concentration (0.94 μM), while retardation of an identically sized DNA fragment containing mutated boxes began at the second lowest concentration (1.88 μM) (Fig. 4A). With increasing concentrations of VirB, the formation of discrete VirB-DNA complexes in the middle of the gel, as well as higher order complexes in the gel wells, are evident on the DNA bearing wild-type boxes (Fig. 4A and B panel i). In contrast, only higher order VirB-DNA complexes form with the DNA fragment containing mutated boxes (Fig. 4A and B, panel ii). These data suggest that the wild-type boxes are required for the formation of specific, discrete VirB-DNA complexes and that higher order complexes form through non-specific interactions. We therefore conclude that VirB displays higher binding affinity and specificity for the DNA fragment containing the wild-type boxes than the mutated boxes.
Fig. 4. In vitro binding of VirB to DNA sites required for VirB-dependent regulation.
A. EMSA of recombinant VirB-His6 with 54 bp DNA targets carrying either centrally located wild-type boxes or mutated boxes. Increasing amounts of purified recombinant VirB-His6 at final concentrations of 0, 0.94, 1.88, 3.75 and 5.00 μM (from left to right) were incubated with radiolabeled DNA.
B. Densitometric trace analysis of each lane in (A). Panels i and ii show traces of DNA targets containing wild-type and mutated boxes respectively. Concentrations of recombinant VirB-His6 are indicated.
C. DNase I protection analysis of VirB-His6 bound to a 250 bp DNA fragment carrying either wild-type boxes or mutated boxes (coding strand radiolabeled). Panels i and ii show sequencing gels of DNA products after DNase I cleavage of complexes formed with increasing final concentrations of VirB-His6 (0, 0.31, 0.62 and 1.2 μM) on DNA bearing wild-type boxes and mutated boxes respectively. Panel iii shows a similar gel of DNA products after DNase I cleavage of complexes formed on DNA bearing wild-type boxes with increasing final concentrations of VirB-His6 (0, 0.77, 1.54, 3.08 and 4.62 μM). A Maxam-Gilbert ladder (GA) was used to identify coordinates relative to the primary icsP TSS. The solid bars indicate initial regions of protection by VirB, the open bar highlights lack of protection observed on DNA bearing mutated boxes, and the dotted line represents an extended region of protection observed.
Next, to precisely map sequences protected by VirB at the icsP promoter, DNase I protection assays were used. VirB protection was observed between positions –1154 to –1122 (relative to the primary TSS of icsP) at the two highest protein concentrations used, 0.62 and 1.2 μM (Fig. 4C, panel i and Supporting Information Fig. S1, panel i). This protection is consistent with the region that contains the VirB boxes required for VirB-dependent regulation of the icsP promoter. In contrast, VirB was unable to protect the DNA fragment containing mutated boxes at the concentrations needed to protect the wild-type VirB binding site (Fig. 4C, compare panels i and ii, and Supporting Information Fig. S1). Interestingly, with just a two-fold increase in the concentration of VirB needed to protect the wild-type boxes (0.77–1.54 μM), an extended region of protection was observed in both directions away from the initially protected site (Fig. 4C, panel iii). These findings are consistent with the oligomerization of VirB along the DNA. Cumulatively, these data indicate that VirB specifically binds to the remote boxes required for VirB-dependent regulation of the icsP promoter, and may oligomerize along DNA bidirectionally from these sites.
The remote VirB binding sites are cis-acting elements needed for VirB-dependent regulation
Since it is unusual for bacterial transcriptional regulators to modulate transcription from remotely located DNA sites (>1 kb upstream of the regulated promoter), we next chose to investigate how these remote VirB binding sites were used to modulate icsP promoter activity. To start, we sought to determine if the binding sites function as bona fide, remote cis-acting elements necessary for the transcriptional regulation of icsP or if these sites function in trans by controlling the production of a small protein or sRNA that subsequently regulates icsP promoter activity.
Two existing full-length PicsP-lacZ reporter constructs (Supporting Information Table S1) bearing either wildtype VirB binding sites (pHJW20) or mutated sites (pMIC18) (Castellanos et al., 2009) were introduced into wild-type S. flexneri and an isogenic virB mutant. Subsequently, pBR322 or derivatives carrying either DNA sequences upstream of –665 (pKLP09) or upstream of –255 (pADK05) relative to the primary TSS of the icsP promoter was also introduced. The activities of the lacZ reporters were then measured using β-galactosidase assays, and data were expressed as fold change in the VirB-dependent regulation of the lacZ reporter (Supporting Information Fig. S2). Our data show that the VirB binding sites and the downstream sequences contained in pKLP09 or pADK05 do not significantly influence VirB-dependent regulation of the PicsP-lacZ reporter bearing mutated sites when placed in trans (Supporting Information Fig. S2). Consequently, we conclude that the VirB binding sites found in the icsP intergenic region function as remote, cis-acting elements that are required for VirB-dependent regulation of icsP.
The VirB boxes function as a single cis-acting element
The finding that mutagenesis of either box 1 or box 2 resulted in a complete loss of VirB-dependent regulation ([Castellanos et al., 2009]; Fig. 3B) raised the possibility that, rather than functioning as two distinct binding sites, the two boxes actually function together as a single cis-acting element. To test this, base pair insertions (2, 3 and 4 bp) were made in between the two binding sites ordinarily organized as a near-perfect inverted repeat separated by a single base pair. Three promoter fragments bearing these insertions were then introduced into the PicsP-lacZ transcriptional reporter (pHJW20), and β-galactosidase activities were measured in wild-type S. flexneri and an isogenic virB mutant (Table 1).
Table 1.
Effect of inserting base pairs between the two halves of the VirB binding site.
| Average relative β-galactosidase activity (%) |
||
|---|---|---|
| Wild-type S. flexneri (2457T) | virB mutant (AWY3) | |
| PicsP-lacZ | 100 | 6 ± 1* |
| 2 bp insertion | 8 ± 1* | 6 ± 1* |
| 3 bp insertion | 7 ± 1* | 6 ± 1* |
| 4 bp insertion | 7 ± 1* | 7 ± 1* |
| Promoterless lacZ | 3 ± 0* | 6 ± 1* |
All data are statistically different (t-test) from the average relative activity observed in wild-type S. flexneri carrying PicsP-lacZ, (P < 0.05).
Insertion of two, three or four base pairs between the two sites abolished VirB-dependent activity of the icsP promoter (Table 1). Thus, in combination with the previous data presented in this work (Fig. 4, Supporting Information Figs S1 and S2), and in contrast to the site that regulates the icsB promoter (Turner and Dorman, 2007), we conclude that the remote VirB binding site that regulates the icsP promoter functions as a single cis-acting element comprised of a near-perfect inverted repeat separated by a single base pair. Based on these findings, it is likely that VirB binds to this site as a dimer.
Plasticity of the spacing and helical phasing of the VirB binding site with respect to the promoter-distal H-NS binding region
An earlier study revealed that a DNA fragment carrying a VirB binding site transplanted upstream of a H-NS binding region in the E. coli proU promoter allowed VirB to relieve silencing of this promoter by H-NS (Kane and Dorman, 2011). This was striking not only because VirB is not naturally produced by E. coli, but also because changes in the spacing of the VirB binding site relative to the H-NS binding region did not alter VirB-dependent regulation at this artificial proU promoter construct. To investigate these requirements at the naturally occurring icsP promoter, three deletions of increasing size (Δ5, Δ10 and Δ50 bp) were created (upstream of –1038) between the VirB binding site and downstream sequences, including the promoter-distal region required for H-NS-mediated silencing and the promoter elements (Supporting Information Fig. S3A). Each of the resulting constructs were then introduced into wild-type S. flexneri and an isogenic virB mutant, and β-galactosidase activities were measured (Supporting Information Fig. S3B). None of the deletions compromised VirB-dependent regulation of the icsP promoter (Supporting Information Fig. S3B). Furthermore, because the 5 bp deletion places the VirB binding site on the opposite face of the DNA helix with respect to the downstream H-NS binding region and promoter elements, we conclude that the helical phasing of the VirB binding site relative to these features is not critical for its role as a transcriptional anti-silencer of this locus.
A molecular roadblock, LacI, placed between the VirB binding site and the upstream region bound by H-NS blocks VirB-mediated anti-silencing
To further probe the relationship between the VirB binding site and the promoter-distal H-NS binding region, we took inspiration from a study that investigated the oligomerization along DNA of ParB, the closest homologue of VirB, at the P1 plasmid centromere (Rodionov et al., 1999). In that study, oligomerization of ParB along DNA was blocked by docking a DNA binding protein to its introduced binding site, which was positioned close to, but not overlapping, the initial ParB binding site. Like ParB, VirB is known to form oligomers in vivo (Beloin et al., 2002) and in addition, our DNase I protection assays suggest VirB oligomerizes bi-directionally from the VirB binding site (Fig. 4B, panel iii). We therefore reasoned that VirB binding to its recognition site is likely followed by its oligomerization along DNA, and that VirB oligomerization towards the promoter-distal H-NS binding region may be key for the transcriptional anti-silencing of the icsP promoter.
To test this hypothesis, two constructs were created that placed two tandem lacO sites approximately 80 bp either upstream or downstream of the cis-acting VirB binding site (Fig. 5A). The use of pQE2, a medium-copy plasmid that expresses lacIq, ensured LacI production and LacI binding to the lacO recognition sites in the absence of IPTG and its dissociation in the presence of IPTG. With these constructs, the effect of a DNA binding protein acting as a molecular roadblock, positioned either on the upstream or downstream flank of the VirB binding site, was tested in the context of our PicsP-lacZ reporter, pAFW04.
Fig. 5. A LacI molecular roadblock placed downstream, but not upstream, of the essential VirB binding site abolishes VirB-dependent antisilencing of the icsP promoter.
A. Schematic showing the inserts bearing tandem lacO sites located either 87 bp upstream or 80 bp downstream of the VirB binding site (not drawn to scale). Double forward slashes represent intergenic sequences not drawn.
B. Effect of lacO sites in the presence or absence of LacI on VirB-dependent regulation of the icsP promoter. Average β-galactosidase activities are expressed as percent activity relative to the full-length PicsP-lacZ in wild-type S. flexneri (2457T). All data, except those labeled ns, are statistically significant from the average relative activity observed in wild-type S. flexneri carrying the wild-type PicsP-lacZ reporter in the absence of IPTG (P < 0.05).
VirB-dependent regulation of the icsP promoter was not affected when the tandem lacO sites were positioned upstream of the remote VirB binding site, regardless of whether IPTG was present or absent from the growth medium (Fig. 5B). These data indicate that VirB-dependent regulation of the icsP promoter is neither impacted by the inserted lacO sites nor LacI binding to these sites. Because VirB-dependent regulation was still observed in the absence of IPTG, conditions supporting LacI binding, we conclude that the 87 bp between the LacI binding sites and the VirB binding site provides sufficient room for both proteins to simultaneously dock at their recognition sites.
In the construct where the tandem lacO sites were placed 80 bp downstream of the VirB binding site, a modest decrease (less than 1.2-fold effect) in VirB-dependent promoter activity was observed in the presence of IPTG (i.e., absence of bound LacI) when compared to the wild-type PicsP-lacZ reporter. In contrast, a dramatic decrease in VirB-dependent regulation was detected in the absence of IPTG (i.e., presence of bound LacI; 17-fold effect), reducing promoter activity to levels similar to those observed in the virB mutant (Fig. 5B). These data demonstrate that a LacI molecular roadblock located downstream of the VirB binding site significantly interferes with VirB-dependent regulation of the icsP promoter. In combination with our results from DNase I protection assays (Fig. 4B, panel iii), these data support our current hypothesis that VirB oligomerization along DNA toward, but not away from, the region required for H-NS-mediated silencing is required for VirB-dependent transcriptional anti-silencing of the icsP promoter. As such, these data provide important insight into transcriptional anti-silencing by VirB.
Discussion
The xenogeneic silencing and anti-silencing of virulence genes has had a profound effect on the evolution of many bacterial pathogens and continues to play a key role in controlling the pathogenicity of these organisms (Stoebel et al., 2008; Ali et al., 2012; Marteyn et al., 2012; Picker and Wing, 2016). Many virulence genes on the Shigella virulence plasmid are transcriptionally silenced by H-NS and anti-silenced by VirB (Porter and Dorman, 1997; Beloin and Dorman, 2003; Le Gall et al., 2005; Turner and Dorman, 2007; Picker and Wing, 2016; Weatherspoon-Griffin et al., 2016). Despite VirB being a key regulator of Shigella virulence, very little is known about how VirB functions to offset transcriptional silencing mediated by H-NS (Turner and Dorman, 2007). To help address this gap in knowledge, in this study, we thoroughly mapped the genetic elements necessary for transcriptional silencing and anti-silencing of the icsP locus located on the large Shigella virulence plasmid. Our work demonstrates the necessity of remotely located DNA binding sites while highlighting the flexibility of key regulatory elements required for transcriptional silencing and anti-silencing at this genetic locus. Moreover, our work demonstrates that a molecular roadblock placed between the key regulatory elements (i.e., the VirB binding site and the H-NS-bound region) completely blocks transcriptional anti-silencing by VirB. Finally, our findings support the need to re-evaluate the currently proposed VirB binding site (Taniya et al., 2003; Turner and Dorman, 2007). Based on our findings, a model of transcriptional silencing and anti-silencing and a summary diagram of regulatory elements identified by this work are presented in the graphical abstract and Fig. 6, respectively.
Fig. 6.
Schematic of findings presented in this study. The icsP intergenic region is depicted. The flexibility of regulatory elements is highlighted by experiments described in grey text. Precise requirements or major effects on regulation of the icsP promoter by VirB are highlighted by experiments described in black text. Black dotted line depicts the high affinity H-NS binding region. Hatched boxes depict the lower affinity H-NS binding regions. Dark grey shaded area depicts the region required for H-NS-mediated silencing of PicsP in vivo. Light grey shaded box depicts the sequences required for VirB-dependent regulation in vivo. Black inverted arrows depict the single, remote, cis-acting VirB binding site required for VirB-dependent transcriptional anti-silencing of the icsP promoter. White arrows depict sequences strongly matching the proposed VirB binding site (Taniya et al., 2003) that do not contribute to VirB-dependent regulation of the icsP promoter.
Our study reveals that a stretch of DNA located between 900 and 436 bp upstream of the primary icsP TSS (AT content 68%) is required for H-NS-mediated transcriptional silencing of icsP. This is corroborated by our EMSAs because three of the six DNA targets (targets 3, 4 and 5), each containing at least part of this region, bind H-NS in vitro with relatively high affinity. Strikingly, a 100 bp, 79% AT-rich region located in targets 4 and 5 (–844 to –744) contains a 20 bp, 95% AT-rich stretch starting at –782. Taken together, the DNA sequences identified by our in vivo 5’ truncation analysis and EMSAs are consistent with established binding characteristics of H-NS (Navarre et al., 2006; Bouffartigues et al., 2007; Lang et al., 2007). Another AT-rich region (79%; –133 to +24; found in target 1) was found to display relatively high affinity for H-NS in our EMSAs. The binding of H-NS to this region, which contains icsP promoter elements, is consistent with other findings that demonstrate H-NS commonly binds to bacterial promoter regions in vivo (Landick et al., 2015). Nonetheless, our 5’ truncation analysis of the icsP promoter region reveals that the promoter-proximal sequences alone are not sufficient for H-NS mediated silencing, bringing into question their role in this regulatory process.
Based on previous work (Basta et al., 2013) and data presented here, a repression loop involving the H-NS bound promoter-distal and promoter-proximal sites does not seem likely for three reasons. First, removal of the upstream H-NS binding region causes a complete loss of H-NS-mediated silencing (Fig. 1A). So, unless the upstream region functions to stabilize the H-NS interaction with the promoter region, a mechanism of promoter occlusion that directly involves the promoter region seems unlikely. Secondly, analysis of the ospZ promoter, which lies divergent to the icsP promoter, revealed that there was no requirement for icsP promoter-proximal sequences for H-NS-mediated silencing (Basta et al., 2013). Instead, DNA sequences overlapping the region necessary for H-NS-mediated silencing of icsP were required (Basta et al., 2013). Finally, both small and large deletions that place the two regions that bind H-NS in vitro on the same or opposite faces of the DNA helix do not impact H-NS-mediated silencing (Fig. 2B), suggesting that if a H-NS bridge complex does form, it must tolerate these spatial changes. As such, we favor a model of transcriptional silencing where H-NS forms a nucleoprotein filament along the icsP intergenic region from the upstream H-NS binding region identified by this study. Future studies aimed at fully characterizing the nature of the H-NS-DNA complex that forms at the icsP promoter, as well as identifying which step in transcription is inhibited by H-NS, are underway.
Regarding VirB-dependent regulation of the icsP promoter, our previous work had shown that remote sites located over 1 kb upstream of the icsP promoter were required for VirB-dependent regulation (Castellanos et al., 2009). This same study identified seven additional sites further downstream, each displaying at least a 6/7 match to the proposed VirB binding site (Taniya et al., 2003). However, despite being closer to the icsP gene than the required VirB binding site (Fig. 3A), none of these sites contribute to VirB-dependent regulation (Fig. 3B). This finding not only validates our previous result that VirB regulates transcription from a remote site, but also challenges our understanding of the proposed VirB binding site sequence; clearly not all sites resembling the currently proposed site are involved in VirB-dependent regulation of icsP (Taniya et al., 2003; Turner and Dorman, 2007). When we expanded our search on the large virulence plasmid for the previously proposed 7–8 bp VirB binding site (Taniya et al., 2003), ~250 perfect matches were identified, and this number increases to ~4000 if a single base pair mismatch is tolerated. As such, it seems highly unlikely that all of these sites are involved in VirB binding and VirB-dependent regulation of Shigella virulence genes. Due to the functional differences in the VirB binding sites found at the icsB (Turner and Dorman, 2007) and icsP promoters highlighted by this study, it is clear that there is a pressing need to improve our understanding of VirB-DNA interactions on the virulence plasmid in vivo. Further, it will be important to determine which of these DNA-protein interactions are needed for the anti-silencing of virulence genes in this important human pathogen.
It is unusual for bacterial transcription factors to influence promoter activity from more than 250 bp upstream of a bacterial promoter (Collado-Vides et al., 1991), and yet, the essential cis-acting VirB binding site at the icsP locus is centered at –1137 relative to the primary icsP TSS. This location places the VirB binding site only 237 bp upstream of the region required for H-NS-mediated silencing (Fig. 1B). Our data show that small or large deletions that maintain or alter the helical phasing of these regulatory elements do not impact VirB-dependent transcriptional anti-silencing of the icsP promoter (Supporting Information Fig. S3). These findings demonstrate a level of flexibility inherent to transcriptional anti-silencing complexes, a finding consistent with previous work (Kane and Dorman, 2011). Given our work reveals that VirB oligomerizes along DNA in vitro (Fig. 4C), it is possible that these extended VirB-DNA interactions facilitate this flexibility. Going forward, it would be interesting to probe the limits of this apparent spatial flexibility.
New insights into the VirB anti-silencing mechanism come from our study where a molecular roadblock was introduced on either side of the essential VirB binding site. Strikingly, we found that the LacI roadblock only interferes with transcriptional anti-silencing when placed in between the VirB binding site and the region required for H-NS-mediated silencing (Fig. 5B). While our finding is consistent with VirB oligomerizing along the DNA towards the region required for H-NS-mediated silencing, which is also supported by the extended footprints (Fig. 4C, panel iii), it remains unclear if VirB oligomerization along DNA occurs in vivo or if this activity is needed for VirB-dependent transcriptional anti-silencing. Future experiments that more thoroughly investigate the mechanism of transcriptional anti-silencing by VirB in vivo are in progress.
In summary, we have: (i) identified that a remote region is required for H-NS-mediated silencing of icsP, (ii) demonstrated that VirB binds directly to a remote, single cis-acting site arranged as a near-perfect inverted repeat, (iii) revealed the significant plasticity in the spacing requirements between the two H-NS binding regions as well as the VirB binding site and region required for H-NS-mediated silencing and (iv) determined that a protein docked immediately downstream of the VirB binding site, but upstream of the region required for H-NS-mediated silencing, completely blocks VirB-dependent regulation. More generally, our findings stress the importance of considering the involvement of DNA sequences outside of the canonical promoter region when studying transcription regulation (Collado-Vides et al., 1991; Gralla and Collado-Vides, 1996), especially when the regulation is being imparted by NSPs and their counter-silencing proteins. While our work highlights that there can be considerable flexibility in the architecture of nucleoprotein complexes controlling transcriptional silencing and anti-silencing, our work also shows the apparent ease with which transcriptional anti-silencing can be disrupted. This insight may prove useful in the design of new antibacterials that could be broadly applicable because gene regulation mediated by transcriptional silencing and anti-silencing is common in bacteria and central to numerous aspects of bacterial physiology, including virulence.
Experimental procedures
Bacterial strains, plasmids and media
The bacterial strains and plasmids used in the present study are listed in Table 2 and Supporting Information Table S1 respectively. E. coli strains were grown routinely at 37°C in Luria-Bertani (LB) broth (Miller, 1972) with aeration or on LB agar (LB broth containing 1.5% [w v–1] agar). Shigella flex-neri strains were routinely grown at 37°C in Tryptic Soy broth (TSB) with aeration or on trypticase soy agar (TSA; TSB containing 1.5% w v–1 agar). Where appropriate, antibiotics were added to achieve the following final concentrations: ampicillin, 100 μg ml–1; chloramphenicol, 25 μg ml–1 and kanamycin, 50 μg ml–1. To ensure that Shigella strains had maintained the large virulence plasmid during manipulation, Congo Red binding was tested on TSA plates containing 0.01% (w v–1) Congo Red (Sigma Chemical Co., St. Louis, Mo.).
Table 2.
Bacterial strains used in this work.
| Strain | Descriptiona | Source or reference |
|---|---|---|
| E. coli | ||
| MC4100 | E. coli strain K-12 with araD and lacZ deletion | (Pogliano and Beckwith, 1994) |
| MC4100 hns | MC4100 hns::Knr The first 37 amino acids of H-NS are expressed, resulting in a dominant-negative effect on other H-NS-like proteins in the cell. | (Yamada et al., 1991) |
| S. flexneri | ||
| 2457T | S. flexneri serotype 2a | (Formal et al., 1958) |
| AWY3 | 2457T virB::Tn5; Knr | (Wing et al., 2004) |
Knr, kanamycin resistance.
Plasmid construction
Plasmids and plasmid constructs used in this study are fully described in the supporting information (Supporting Information Table S1 and Plasmid construction). All constructs had their DNA sequences verified by Sanger sequencing. DNA sequences of oligonucleotide primers and duplexes used in this work are available upon request.
Quantification of icsP promoter activity using the PicsP-lacZ reporter and derivatives
To measure promoter activities, the PicsP-lacZ fusion plasmids described in this work were introduced into S. flexneri and E. coli strains by electroporation. Activities of the icsP promoter constructs were determined by measuring β-galactosidase activity as described previously (Wing et al., 2004), using the Miller protocol (Miller, 1972). Overnight cultures were diluted 1:100 and grown for 5–7 h in either TSB medium (S. flexneri) or LB (E. coli) at 37°C with shaking at 325 rpm (LabLine/Barnstead 4000 MaxQ), prior to cell lysis. Routinely, β-galactosidase levels were measured in early stationary phase cultures grown from three independent transformants because experiments had shown that icsP expression significantly increases under these conditions (Hensley et al., 2011). Assays routinely contained three independent biological replicates and were repeated three times. For statistical analyses, a Student’s t-test assuming equal variance was routinely used.
To assess the effect of LacI binding to lacO recognition sites engineered upstream or downstream of the essential VirB binding sites in pAFW04, wild-type S. flexneri (2457T) and the isogenic virB mutant (AWY3) were simultaneously transformed with pAFW04 or a derivative (pHS27, pDRG01 or MAP07) and the lacIq-expressing plasmid, pQE2. Overnight cultures bearing pQE2 and a pAFW04 derivative were back-diluted 1:100 and grown for 2 h at 37°C. Cell cultures were either induced with a final concentration of 250 μM IPTG or not induced and then all cultures were grown for an additional 3 h. Cells were then harvested by centrifugation and resuspended in an equal volume of PBS before lysis. β-galactosidase activities were determined using the Miller protocol (Miller, 1972).
Purification of His-tagged H-NS and VirB proteins
For the purification of C-terminally tagged H-NS-His6 protein, the pQE60 derivative pCTH01 was used. H-NS-His6 was purified as described previously with a few exceptions (Deighan et al., 2003). Briefly, the protein was produced in the E. coli strain M15 carrying the plasmid pREP4. The expression of the C-terminally His-tagged proteins were induced in 500 ml cultures growing exponentially with 1 mM IPTG (isopropyl-β-thiogalactopyranoside). After a 2 h induction, the cells were harvested and frozen at –80°C overnight. The cell pellet was then thawed on ice and resuspended in lysis buffer (60 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl [pH 8.0]). Cells were lysed by sonication, and cellular debris was pelleted by centrifugation at 10,000 × g at 4°C. Cell lysates were applied to Ni-NTA columns (Qiagen) pre-equilibrated with lysis buffer. The columns were then washed with 10 bed volumes of wash buffer (100 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl [pH 8.0]). Proteins were eluted by the addition of 2.5 bed volumes of elution buffer (equilibration buffer with 500 mM imidazole). The H-NS-His6 fractions were collected and analyzed on SDS-PAGE followed by Coomassie staining. The H-NS fractions were combined and dialyzed against a storage buffer (350 mM NaCl, 50 mM Tris-HCl [pH 8.0], 1 mM EDTA, 0.5 mM DTT, 0.1 mM PMSF, 30% glycerol). Protein concentration was determined using a Bradford assay. Previous studies using an identical His-tag H-NS fusion protein, produced in a manner similar to that described above, was shown to retain normal function of H-NS in assays (Williams and Rimsky, 1997).
The C-terminally VirB-His6 protein was produced from pAJH03 and purified by Monserate Biotech. The hexa-his tag was not found to interfere with VirB expression or activity because His-tagged VirB was observed to restore icsP expression to wild-type levels in a strain lacking virB in vivo (data not shown). SDS-PAGE and western blots of purified proteins used in this work are shown in Supporting Information Fig. S4.
Electrophoretic mobility shift assays
To test H-NS binding to the six DNA fragments taken from the icsP intergenic region in vitro, 0.25 pmol of 32P-labeled PicsP DNA (PCR amplified from pHJW20 with the following primers: W63 and W64 target 1, W65 and W66 target 2, W67 and W68 target 3, W69 and W70 target 4, W71 and W148 target 5, W73 and W74 target 6 and gel purified by electroelution) was incubated at 37°C for 30 min with 0, 107, 134 or 161 pmol of purified His-tagged H-NS protein in a 10 μl reaction containing 50 mM Tris-HCl (pH 8.0), 20 mM KCl, 10% glycerol, 100 μg ml–1 BSA and 25 μg ml–1 poly (dI-dC). DNA loading dye solution was added to the reaction and directly subjected to 5% polyacrylamide gel electrophoresis (PAGE) in 0.5X TBE running buffer. Radioactive signals were detected using a Typhoon 9410 (Amersham) variable mode imager.
To test VirB binding in vitro, two 54 bp icsP promoter fragments containing either wild-type or mutated VirB sites were used. To create each target, primer pairs W391/W392 and W393/W394, respectively, were denatured at 95°C for 5 min and subsequently annealed using a cycle that decreased by 1°C every minute until a final temperature of 25°C. Each sample was then gel purified and electroeluted into the surrounding buffer, and the resulting DNA was phenol-chloroform extracted and ethanol precipitated. For radiolabeling of the non-coding strand, 4.8 pmol of each target was single-end labeled using T4 polynucleotide kinase (Promega Cat. No. M4101) according to manufacturer directions using [γ32P] ATP (specific activity, 3000 Ci mmol–1; Perkin Elmer). Unincorporated radionucleotides were removed using Illustra ProbeQuant G-50 Micro Columns according to manufacturer directions (GE Healthcare).
Increasing concentrations of purified VirB-His6 (Monserate Biotech) were incubated with ~0.02 pmol of each icsP promoter target at 37°C for 20 min in 1x binding buffer (10 mM potassium phosphate, pH 7.5, 25 mM NaCl, 0.5 mM β-mercaptoethanol, 0.5 mM EDTA, 50% glycerol, 25 ng μl–1 herring sperm DNA) in a total reaction volume of 20 μl. The reactions were then resolved on a 6% native polyacrylamide gel in 1x TBE at 120 v for 40 min. The gel was transferred to Whatman paper, covered with plastic wrap, exposed to a phosphor-imaging screen and then scanned with the Typhoon 9410 (Amersham) Variable Mode Imager. For densitometric lane trace analysis, ImageJ software (http://imagej.nih.gov) was used.
DNase I protection assays
DNase I protection assays to identify VirB bound regions of the icsP promoter were carried out using PCR amplified DNA fragments. Primers W515 and W516 were used to amplify a 250 bp fragment containing wild-type VirB boxes (amplified using pHJW20 as a template) or mutated VirB boxes (amplified using pMIC18 as a template). Prior to the PCR, one of the primers W515 or W516 was labeled with T4 polynucleotide kinase and [γ−32P]-ATP (32P-labeled W515 allowed detection of the coding strand; 32P-labeled W516 allowed detection of the non-coding strand). To detect the initial binding site of VirB, approximately 0.25 pmol of labeled DNA and 0, 6.2, 12.3 or 24.6 pmol of the purified His-tagged VirB were incubated at 37°C for 20 minutes in a 20 μl reaction containing 50 mM Tris-HCl (pH 8.0), 20 mM KCl, 10% glycerol, 100 μg ml–1 BSA and 25 μg ml–1 poly (dI-dC). To investigate the potential for VirB oligomerization along DNA, the same conditions were used, but the following amounts of VirB were added; 0, 15.4, 30.8, 61.6 or 92.4 pmol. Samples were treated with 0.06 U of DNase I (New England Biolabs) for 30 seconds followed by phenol-chloroform DNA extraction and ethanol precipitation. DNA was resuspended in a gel loading buffer (40% deionized formamide, 5 M urea, 5 mM NaOH, 1 mM EDTA, 0.025% bromophenol blue and 0.025% xylene cyanol) and analyzed by 6% denaturing PAGE by comparing to an appropriate DNA sequence ladder generated by the Maxam and Gilbert A + G reaction (Maxam and Gilbert, 1986). Radiolabeled DNA fragments were detected using a Typhoon 9410 (Amersham) variable mode imager.
Supplementary Material
Acknowledgements
We thank David Fujimoto of the Monserate Biotechnology Group for purifying the recombinant VirB protein used in this study and R. Martin Roop, II for guidance with preliminary EMSAs. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R15AI090573 and by NIH grant P20 RR-016464 from INBRE Program of the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. MAP was a recipient of a Higher Education Graduate Research Opportunity Fellowship in 2015 from the Nevada Space Grant Consortium (NVSGC) National Aeronautics and Space Administration (NASA) Training Grant #NNX10AN23H and was awarded the Hermsen Fellowship in 2017. RO-D was a recipient of a National Science Foundation (NSF) Research Experience for Undergraduates (REU) scholarship DBI 1005223. PU, MMAK, JCD, MIC were partially supported by the NV INBRE undergraduate research opportunity program (National Institute of General Medical Sciences; 8 P20GM103440–11). CTH was supported in part by the Post-9/ 11 GI Bill. DJH was a Lieutenant (LT) Medical Service Corps (MSC) United States Navy (USN) microbiologist, NAVMED MPT&E when this work was undertaken. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Navy, Department of Defense, or U.S. Government.
Footnotes
The authors declare no conflicts of interest.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- Africa LA, Murphy ER, Egan NR, Wigley AF, and Wing HJ (2011) The iron-responsive Fur/RyhB regulatory cascade modulates the Shigella outer membrane protease IcsP. Infect Immun 79: 4543–4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SS, Xia B, Liu J, and Navarre WW (2012) Silencing of foreign DNA in bacteria. Curr Opin Microbiol 15: 175–181. [DOI] [PubMed] [Google Scholar]
- Arold ST, Leonard PG, Parkinson GN, and Ladbury JE (2010) H-NS forms a superhelical protein scaffold for DNA condensation. Proc Natl Acad Sci U S A 107: 15728–15732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam TA, and Ishihama A (1999) Twelve species of the nucleoid-associated protein from Escherichia coli. Sequence recognition specificity and DNA binding affinity. J Biol Chem 274: 33105–33113. [DOI] [PubMed] [Google Scholar]
- Basta DW, Pew KL, Immak JA, Park HS, Picker MA, Wigley AF, et al. (2013) Characterization of the ospZ promoter in Shigella flexneri and its regulation by VirB and H-NS. J Bacteriol 195: 2562–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloin C, and Dorman CJ (2003) An extended role for the nucleoid structuring protein H-NS in the virulence gene regulatory cascade of Shigella flexneri. Mol Microbiol 47: 825–838. [DOI] [PubMed] [Google Scholar]
- Beloin C, McKenna S, and Dorman CJ (2002) Molecular dissection of VirB, a key regulator of the virulence cascade of Shigella flexneri. J Biol Chem 277: 15333–15344. [DOI] [PubMed] [Google Scholar]
- Bouffartigues E, Buckle M, Badaut C, Travers A, and Rimsky S (2007) H-NS cooperative binding to highaffinity sites in a regulatory element results in transcriptional silencing. Nat Struct Mol Biol 14: 441–448. [DOI] [PubMed] [Google Scholar]
- Castellanos MI, Harrison DJ, Smith JM, Labahn SK, Levy KM, and Wing HJ (2009) VirB alleviates H-NS repression of the icsP promoter in Shigella flexneri from sites over 1 kb upstream of the transcription start site. J Bacteriol 191: 4047–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado-Vides J, Magasanik B, and Gralla JD (1991) Control site location and transcriptional regulation in Escherichia coli. Microbiol Rev 55: 371–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame RT, Luijsterburg MS, Krin E, Bertin PN, Wagner R, and Wuite GJ (2005) DNA bridging: a property shared among H-NS-like proteins. J Bacteriol 187: 1845–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame RT, Noom MC, and Wuite GJ (2006) Bacterial chromatin organization by H-NS protein unravelled using dual DNA manipulation. Nature 444: 387–390. [DOI] [PubMed] [Google Scholar]
- Dame RT, Wyman C, and Goosen N (2000) H-NS mediated compaction of DNA visualised by atomic force microscopy. Nucleic Acids Res 28: 3504–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deighan P, Beloin C, and Dorman CJ (2003) Three-way interactions among the Sfh, StpA and H-NS nucleoid-structuring proteins of Shigella flexneri 2a strain 2457T. Mol Microbiol 48: 1401–1416. [DOI] [PubMed] [Google Scholar]
- Dorman CJ (2007) H-NS, the genome sentinel. Nat Rev Microbiol 5: 157–161. [DOI] [PubMed] [Google Scholar]
- Dorman CJ, and Deighan P (2003) Regulation of gene expression by histone-like proteins in bacteria. Curr Opin Genet Dev 13: 179–184. [DOI] [PubMed] [Google Scholar]
- Doyle M, Fookes M, Ivens A, Mangan MW, Wain J, and Dorman CJ (2007) An H-NS-like stealth protein aids horizontal DNA transmission in bacteria. Science 315: 251–252. [DOI] [PubMed] [Google Scholar]
- Egile C, d’Hauteville H, Parsot C, and Sansonetti PJ (1997) SopA, the outer membrane protease responsible for polar localization of IcsA in Shigella flexneri. Mol Microbiol 23: 1063–1073. [DOI] [PubMed] [Google Scholar]
- Falconi M, Colonna B, Prosseda G, Micheli G, and Gualerzi CO (1998) Thermoregulation of Shigella and Escherichia coli EIEC pathogenicity. A temperature-dependent structural transition of DNA modulates accessibility of virF promoter to transcriptional repressor H-NS. Embo J 17: 7033–7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang FC, and Rimsky S (2008) New insights into transcriptional regulation by H-NS. Curr Opin Microbiol 11: 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal SB, Dammin GJ, LaBrec EH, and Schneider H (1958) Experimental Shigella infections: characteristics of a fatal infection produced in guinea pigs. J Bacteriol 75: 604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Zou T, Mu Z, Qin B, Yang J, Waltersperger S, et al. (2013) Structural insights into VirB-DNA complexes reveal mechanism of transcriptional activation of virulence genes. Nucleic Acids Res 41: 10529–10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon BR, Li Y, Cote A, Weirauch MT, Ding P, Hughes TR, et al. (2011) Structural basis for recognition of AT-rich DNA by unrelated xenogeneic silencing proteins. Proc Natl Acad Sci U S A 108: 10690–10695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla JD, and Collado-Vides J, (1996) Organization and function of transcription regulatory elements. In: E. coli and Salmonella Neidhardt F (ed). Washington, DC: ASM Press, pp. 1232–1245. [Google Scholar]
- Hensley CT, Kamneva OK, Levy KM, Labahn SK, Africa LA, and Wing HJ (2011) Two promoters and two translation start sites control the expression of the Shigella flexneri outer membrane protease IcsP. Arch Microbiol 193: 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromockyj AE, and Maurelli AT (1989) Identification of an Escherichia coli gene homologous to virR, a regulator of Shigella virulence. J Bacteriol 171: 2879–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane KA, and Dorman CJ (2011) Rational design of an artificial genetic switch: Co-option of the H-NS-repressed proU operon by the VirB virulence master regulator. J Bacteriol 193: 5950–5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landick R, Wade JT, and Grainger DC (2015) H-NS and RNA polymerase: a love-hate relationship? Curr Opin Microbiol 24: 53–59. [DOI] [PubMed] [Google Scholar]
- Lang B, Blot N, Bouffartigues E, Buckle M, Geertz M, Gualerzi CO, et al. (2007) High-affinity DNA binding sites for H-NS provide a molecular basis for selective silencing within proteobacterial genomes. Nucleic Acids Res 35: 6330–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall T, Mavris M, Martino MC, Bernardini ML, Denamur E, and Parsot C (2005) Analysis of virulence plasmid gene expression defines three classes of effectors in the type III secretion system of Shigella flexneri. Microbiology 151: 951–962. [DOI] [PubMed] [Google Scholar]
- Marteyn B, Gazi A, and Sansonetti P (2012) Shigella: a model of virulence regulation in vivo. Gut Microbes 3: 104–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam AM, and Gilbert W (1986) A method for determining DNA sequence by labeling the end of the molecule and cleaving at the base. Isolation of DNA fragments, end-labeling, cleavage, electrophoresis in polyacrylamide gel and analysis of results. Mol Biol 20: 581–638. [PubMed] [Google Scholar]
- Miller J (1972) Experiments in Molecular Genetics Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Navarre WW, McClelland M, Libby SJ, and Fang FC (2007) Silencing of xenogeneic DNA by H-NS-facilitation of lateral gene transfer in bacteria by a defense system that recognizes foreign DNA. Genes Dev 21: 1456–1471. [DOI] [PubMed] [Google Scholar]
- Navarre WW, Porwollik S, Wang Y, McClelland M, Rosen H, Libby SJ, and Fang FC (2006) Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science 313: 236–238. [DOI] [PubMed] [Google Scholar]
- Picker MA, and Wing HJ (2016) H-NS, its family members and their regulation of virulence genes in Shigella species. Genes (Basel) 7: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano JA, and Beckwith J (1994) SecD and SecF facilitate protein export in Escherichia coli. Embo J 13: 554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, and Dorman CJ (1997) Differential regulation of the plasmid-encoded genes in the Shigella flexneri virulence regulon. Mol Gen Genet 256: 93–103. [DOI] [PubMed] [Google Scholar]
- Prosseda G, Falconi M, Giangrossi M, Gualerzi CO, Micheli G, and Colonna B (2004) The virF promoter in Shigella: more than just a curved DNA stretch. Mol Microbiol 51: 523–537. [DOI] [PubMed] [Google Scholar]
- Rodionov O, Lobocka M, and Yarmolinsky M (1999) Silencing of genes flanking the P1 plasmid centromere. Science 283: 546–549. [DOI] [PubMed] [Google Scholar]
- Schuch R, and Maurelli AT (1997) Virulence plasmid instability in Shigella flexneri 2a is induced by virulence gene expression. Infect Immun 65: 3686–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shere KD, Sallustio S, Manessis A, D’aversa TG, and Goldberg MB (1997) Disruption of IcsP, the major Shigella protease that cleaves IcsA, accelerates actin-based motility. Mol Microbiol 25: 451–462. [DOI] [PubMed] [Google Scholar]
- Steinhauer J, Agha R, Pham T, Varga AW, and Goldberg MB (1999) The unipolar Shigella surface protein IcsA is targeted directly to the bacterial old pole: IcsP cleavage of IcsA occurs over the entire bacterial surface. Mol Microbiol 32: 367–377. [DOI] [PubMed] [Google Scholar]
- Stoebel DM, Free A, and Dorman CJ (2008) Anti-silencing: overcoming H-NS-mediated repression of transcription in Gram-negative enteric bacteria. Microbiology 154: 2533–2545. [DOI] [PubMed] [Google Scholar]
- Taniya T, Mitobe J, Nakayama S, Mingshan Q, Okuda K, and Watanabe H (2003) Determination of the InvE binding site required for expression of IpaB of the Shigella sonnei virulence plasmid: involvement of a ParB box A-like sequence. J Bacteriol 185: 5158–5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe T, Nagai S, Okada N, Adter B, Yoshikawa M, and Sasakawa C (1991) Temperature-regulated expression of invasion genes in Shigella flexneri is controlled through the transcriptional activation of the virB gene on the large plasmid. Mol Microbiol 5: 887–893. [DOI] [PubMed] [Google Scholar]
- Tobe T, Yoshikawa M, Mizuno T, and Sasakawa C (1993) Transcriptional control of the invasion regulatory gene virB of Shigella flexneri: activation by virF and repression by H-NS. J Bacteriol 175: 6142–6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner EC, and Dorman CJ (2007) H-NS antagonism in Shigella flexneri by VirB, a virulence gene transcription regulator that is closely related to plasmid partition factors. J Bacteriol 189: 3403–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherspoon-Griffin N, Picker MA, and Wing HJ, (2016) The genetic organization and transcriptional regulation of Shigella virulence genes. In: Shigella: Molecular and Cellular Biology Picking WD & Picking WL (eds). UK: Caister Academic Press, pp. 65–107. [Google Scholar]
- Williams RM, and Rimsky S (1997) Molecular aspects of the E. coli nucleoid protein, H-NS: a central controller of gene regulatory networks. FEMS Microbiol Lett 156: 175–185. [DOI] [PubMed] [Google Scholar]
- Wing HJ, Goldman SR, Ally S, and Goldberg MB (2005) Modulation of an outer membrane protease contributes to the virulence defect of Shigella flexneri strains carrying a mutation in the virK locus. Infect Immun 73: 1217–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing HJ, Yan AW, Goldman SR, and Goldberg MB (2004) Regulation of IcsP, the outer membrane protease of the Shigella actin tail assembly protein IcsA, by virulence plasmid regulators VirF and VirB. J Bacteriol 186: 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Yoshida T, Tanaka K, Sasakawa C, and Mizuno T (1991) Molecular analysis of the Escherichia coli hns gene encoding a DNA-binding protein, which preferentially recognizes curved DNA sequences. Mol Gen Genet 230: 332–336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.