In-depth study of the biology of any cell lineage is best performed while the cells reside in their natural morphological and physiological microenvironment. This task has been difficult to achieve for melanocytes because they make up only about one percent of the cellular milieu of the mammalian skin. The most effective strategy to ‘label’ specific cell types is to express ‘molecular beacons’ under the control of cell lineage-specific gene promoters in transgenic mice. One such transgenic mouse model is Dct-LacZ, which expresses the beta-galactosidase gene under the control of the melanocytespecific promoter of the dopachrome tautomerase (Dct) gene (Mackenzie et al., 1997). Beta-galactosidase cleaves its chromogenic substrate, X-gal, ‘labeling’ the transgene-expressing cells with blue color in visible light, which allows individual melanocytes to be visualized within the skin. The Dct-LacZ transgenic mouse model has been instrumental in studying the biology of melanocytes; for example, in helping define the existence and location of melanocyte stem cells. However, there are certain desirable features that this model lacks: (i) conditionally inducible labeling and, (ii) fluorescent label, so that it can be used to isolate melanocytes by fluorescence-activated cell sorting (FACS) following in vivo manipulation.
The year 2010 saw publication of three mouse models that expressed fluorescent labels in melanocyte-specific manner. Mort et al. (2010) reported mice that express yellow fluorescent protein (YFP) in conjunction with Tyrosinase-driven Cre recombinase. Monahan et al. (2010) published a similar strategy to express green fluorescent protein (GFP) in melanocytes. However, in both of these models, fluorescence is conditional but not inducible.
One of the most effective methods for targeted and inducible gene expression is the expression of the reverse tetracycline-controlled transactivator gene (rtTA) under the control of a tissue or cell-type-specific promoter. rtTA activates any transgene under the control of a tetracycline-responsive element (TRE) in the presence of tetracycline or its analog doxycycline. Expressing rtTA under the control of the Dct promoter would allow inducible expression of virtually any gene in a melanocyte-specific manner. Woods and Bishop (2010) reported the first such mouse last year. They were able to target GFP expression to the melanocytic compartment by crossing their Dct-rtTA mice with the TRE-H2BGFP mice (Tumbar et al., 2004). However, detailed characterization of the inducibility, specificity, and embryonic expression pattern of GFP was not presented.
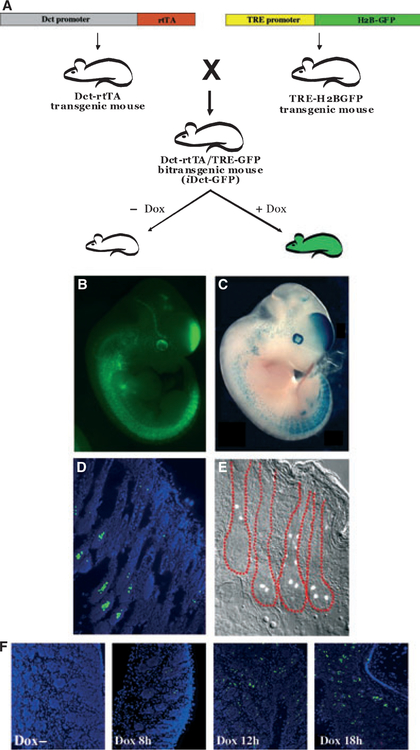
We have now generated a similar mouse model that expresses rtTA under the control of the Dct promoter (Zaidi et al., 2011). We have crossed this mouse model with the TREH2BGFP mice (Figure 1A) to generate mice (iDCT-GFP) that target GFP expression to the melanocytic compartment at all embryonic, neonatal, and adult stages (Figure 1B–E). This allows the study of melanocytes not only at the cellular level but, because the fluorescent cells can also be isolated by FACS, at the molecular level as well; such analyses are otherwise extremely challenging due to the paucity of melanocytes in the skin. The iDct-GFP mouse model is a novel tool that can be used to study melanocytes and diseases originating from them, including melanocyte development and differentiation, melanocyte and melanoma stem cells, and initiation, progression and metastasis of melanoma.
Figure 1.
(A) Making of iDct- green fluorescent protein (GFP) mice. Dct-rtTA transgenic mice were crossed with the TRE-H2BGFP mice to get the iDct-GFP bi-transgenic mice, which show tightly regulated doxycycline-inducible GFP expression. (B) An E11.5 iDct-GFP embryo. (C) A Dct-LacZ embryo (courtesy, Dr. Ian Jackson). (D) GFP expression in the skin of a 7 days old pup is detectable in the bulb and bulge regions of the hair follicles. Blue, DAPI. (E) Skin section from an adult mouse, showing GFP+ cells in the hair follicles (demarcated in red). (F) Induction of GFP expression by a single intraperitoneal injection of doxycycline. Images have been modified from Zaidi et al.
In generating the Dct-rtTA transgenic mouse, we have used the rtTA2s-M2 version of the transactivator (Urlinger et al., 2000), which functions at a much lower concentration of doxycycline than the original rtTA, is more stable in eukaryotic cells, and does not cause leaky background expression without doxycycline. The iDct-GFP mice show expression of GFP in cells located in the bulb, the bulge, and the outer root sheath areas of the hair follicles, where melanocytes and melanoblasts reside. We have immunostained these skin sections with anti-Dct (PEP8h) antibody and observed an excellent overlap of GFP and Dct positive signals, indicating that GFP is being exclusively expressed in Dct positive cells (Zaidi et al., 2011). The expression of GFP is both geno-type and doxycycline dependent, as there is no leaky background expression in the absence of doxycycline. Furthermore, GFP expression quickly responds, within 12–18 h, to a single intraperitoneal injection of doxycycline (Figure 1F). It should be noted that the TRE-H2BGFP transgenic mice have anomalous expression of H2BGFP in the hematopoietic stem cells of the bone marrow. Although this is not seen in the skin, appropriate controls should be performed when analyzing H2BGFP expression in other anatomic locations (Challen and Goodell, 2008). In order to make breeding simpler, we have established an iDct-GFP double homo-zygoustransgenic mouse line.
The following mouse lines are currently available:
- Dct-rtTA Transgenic mice (line B8):
- Genetic background: FVB ⁄ N
- Heterozygous and homozygous genotypes
- iDct-GFP Transgenic mice (DctrtTA ⁄ TRE-GFP bi-transgenic):
- Genetic background: FVB ⁄ N
- Double heterozygous and double homozygous genotypes
Genotyping
The following primer sets can be used to genotype the mice using PCR:
Dct-rtTA (line B8): Forward 5′-ACTAAGTAAGGATCAATTCAG-3′ Reverse 5′- TGTACTAGGCAGACTGTG-3′
Amplicon: 370 bp
TRE-H2BGFP: Forward 5′-GCCA
CAAGTTCAGCGTGTCC-3′ Reverse 5′-GATGCCCTTCAGCTCGATGC-3′
Amplicon: 314 bp
For both primer sets, standard PCR conditions with 57 °C annealing temperature can be used.
Activation of GFP expression
The melanocytic expression of GFP can be activated by feeding the mice doxycycline-supplemented chow diet or water. Such administration to pregnant females would activate GFP expression in embryonic stages starting at about E10.5, and would continue into neonates. Alternatively, a single intraperitoneal injection of doxycycline (80 μg ⁄ g body weight) administered to neonates or adults activates GFP expression within 12–18 h.
Fluorescence imaging
Activated GFP expression in iDct-GFP embryos and neonates is readily visible with hand-held blue flashlight and GFP filter goggles (NightSea, Bedford, MA, USA), which can also be used as a genotyping method. Adult mice can be analysed with Xenogen or CRi Maestro optical in vivo imaging systems (Caliper Life Sciences, Hopkinton, MA, USA).
References
- Challen GA, and Goodell MA (2008). Promiscuous expression of H2B-GFP transgene in hematopoietic stem cells. PLoS One 3, e2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie MA, Jordan SA, Budd PS, and Jackson IJ (1997). Activation of the receptor tyrosine kinase kit is required for the proliferation of melanoblasts in the mouse embryo. Dev. Biol 192, 99–107. [DOI] [PubMed] [Google Scholar]
- Monahan KB, Rozenberg GI, Krishnamurthy J, Johnson SM, Liu W, Bradford MK, Horner J, Depinho RA, and Sharpless NE (2010). Somatic p16(INK4a) loss accelerates melanomagenesis. Oncogene 29, 5809–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort RL, Hay L, and Jackson IJ (2010). Ex vivo live imaging of melanoblast migration in embryonic mouse skin. Pigment Cell Melanoma Res. 23, 299–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, and Fuchs E (2004). Defining the epithelial stem cell niche in skin. Science 303, 359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, and Hillen W (2000). Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc. Natl Acad. Sci. USA 97, 7963–7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SL, and Bishop JM (2010). A new transgenic mouse line for tetracycline inducible transgene expression in mature melanocytes and the melanocyte stem cells using the Dopachrome tautomerase promoter. Transgenic Res. DOI 10.1007/s11248-010-9421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi MR, Davis S, Noonan FP et al. (2011). Interferon-gamma links ultraviolet radiation to melanomagenesis in mice. Nature 469, 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]