Abstract
Over the last decade, considerable effort has been directed at developing drugs that target the spliceosome. The resulting synthetic and natural product splice modulators have opened new avenues for the interrogation of disease-associated splicing events. In this Minireview, an overview is provided of the recent advances in addressing the chemistry and chemical biology of the spliceosome and modulating its action with small molecules.
Keywords: Drug Discovery, chemical biology, drug discovery, inhibitors, RNA splicing, spliceosome
Since its discovery in 1977, the study of alternative RNA splicing has revealed a plethora of mechanisms that had never before been documented in nature. Understanding these transitions and their outcome at the level of the cell and organism has become one of the great frontiers of modern chemical biology. Until 2007, this field remained in the hands of RNA biologists. However, the recent identification of natural product and synthetic modulators of RNA splicing has opened new access to this field, allowing for the first time a chemical-based interrogation of RNA splicing processes. Simultaneously, we have begun to understand the vital importance of splicing in disease, which offers a new platform for molecular discovery and therapy. As with many natural systems, gaining clear mechanistic detail at the molecular level is key towards understanding the operation of any biological machine. This minireview presents recent lessons learned in this emerging field of RNA splicing chemistry and chemical biology.
1. Introduction
Life operates through the orchestrated translation of the four-digit genetic code into a 20 amino acid code to give proteins. During this process, DNA, the storage oligonucleotide, passes its information on through conversion into RNA, which in turn serves as an intermediary for the translation of a gene into a protein. To match the needs of longer-lived eukaryotes, this process has an additional stage of information processing. Here, the transcribed gene product mRNA undergoes splicing, a reorganization process that allows the cell and organism to rapidly alter the gene product in response to temporal or environmental challenges. Beginning in the 1970s,[1] understanding the complex mechanisms of RNA splicing has become a vital new avenue for chemical biological studies. While the bulk of splicing studies have been conducted in genetics and RNA biology laboratories, the discovery of spliceosome-targeting natural products in 2007[2] opened the door to chemists.
2. The Chemistry of Splice Modulation
Access to splice modulators (SPLMs) has evolved from the early stages of natural product isolation[3––5] to the current state of medicinal chemistry optimization and high-fidelity total synthesis.[6––17] To date, the most established set of SPLMs share a common mode of action (MOA), targeting the SF3b multiprotein component within the U2 small nuclear ribonucleoprotein (snRNP) of the spliceosome.[2] This molecular class is broken down into three families that share a common motif comprised of two functional moieties united through a central diene. Over the last decade, total synthesis has played a key role in structural determination, material access, medicinal chemistry optimization, and the facilitation of clinical trials.
2.1. Structures of Splice Modulators
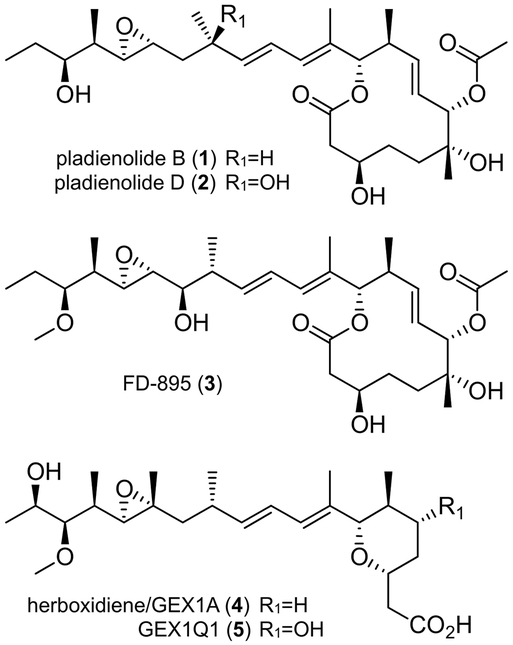
To date, the most established SPLMs arise from three distinct families of polyketide natural products. The first family is comprised of 12-membered macrolides 1−−3 (Figure 1).[3] The second family contains a pyran ring linked to a comparable side chain, as illustrated by 4−−5 (Figure 1).[4]
Figure 1.
Structures of selected examples of the 12-membered macrolide and 6-membered cyclic ether families of polyketide SPLMs.
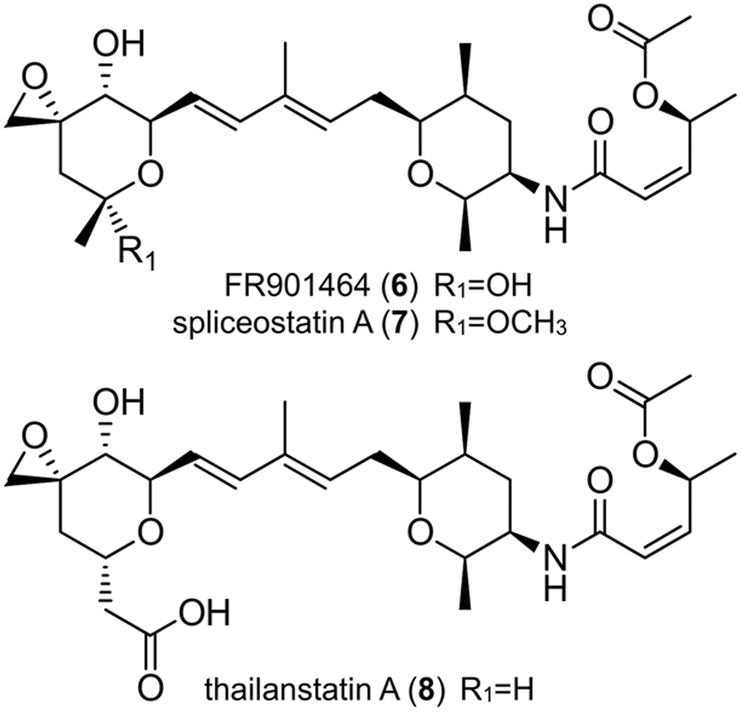
The third family is composed of four discrete chiral fragments: two pyran rings joined by a diene moiety and an acyclic side chain linked to the central pyran via an α,β-unsaturated amide bond. Examples of this family include 6−−8 (Figure 2).[5] Overall, each of polyketides 1−−8 contains 2−−3 rings, 9−−11 chiral centers, and various reactive groups (epoxide and/or α,β-unsaturated amide), to which their potent activity has in part been attributed.
Figure 2.
Structures of members of a third family of polyketide SPLMs.
2.2. Synthetic Challenges and Solutions
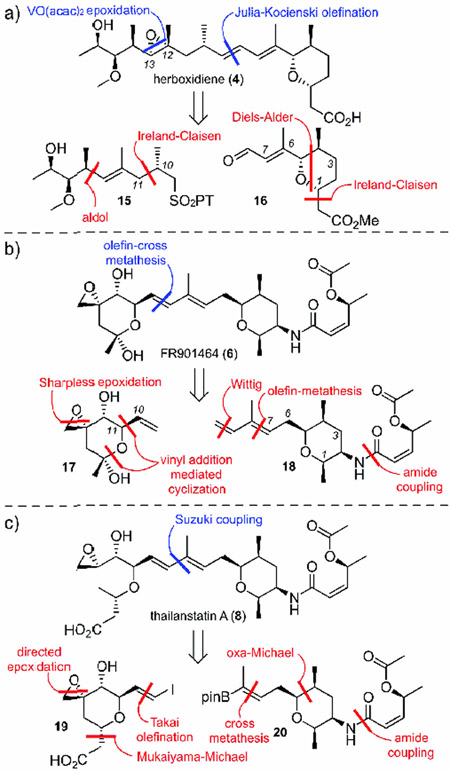
Synthetic strategies toward these three families of SPLMs primarily dissect the molecule into 2−−3 components. In the 12-membered macrolide class (Figure 3), the routes developed by Kotake and co-workers from Eisai Co.[6] and the Burkart,[7] Maier,[8] Ghosh,[9] and Chandrasekhar groups,[10] derive the macrolide core using ring-closing metathesis. The core is then attached to the respective side chains using either a Julia--Kocienski olefination (blue, (Figure 3 a,c) or Stille coupling (blue, (Figure 3 b).
Figure 3.
Synthetic disconnections implemented in the total syntheses of a--b) pladienolide B (1) or c) FD-895 (3). Bond disconnections for component coupling steps (blue) and key steps in component syntheses (red) are shown.
In the Eisai Co. synthesis of pladienolide B (1), core 10 was assembled by using a SmII-type Reformatsky reaction to create the C(3) carbinol and a Paterson aldol reaction to install the C(10)<C->C(11) stereodiad (Figure 3 a).[6] The C(6)<C->C(7) centers were created early on through Sharpless dihydroxylation. The synthesis of side chain 9 was conducted using an Evans aldol to install the C(20)<C->C(21) stereodiad followed by Shi epoxidation at C(18)<C->C(19).
In the Ghosh route to 1 (Figure 3 b), C(20)<C->C(21) in component 11 was set using a Crimmins aldol.[9] This was followed by the implementation of a Shi epoxidation to set C(18)<C->C(19). Core 12 was assembled by an epoxide ring opening to afford the C(4)<C->C(5) diad and a subsequent asymmetric crotylation to install the C(10)<C->C(11) stereocenters. Esterification, followed by ring-closing metathesis, completed the synthesis of 12.
In our studies on 3 (Figure 3 c),[7] the C(3) center was installed in core 14 using a Sammakia aldol on material that contained the C(6)<C->C(7) stereodiad. This diad was prepared by a Brown allylboration followed by 2-methoxyethoxymethyl (MEM) ether directed installation of the methyl group at C(6). The C(10)<C->C(11) centers were prepared using a Brown crotylboration. In this route (Figure 3 c), component 13 was constructed using a Crimmins aldol to install the C(20)<C->C(21) stereodiad, a Sharpless epoxidation to install C(18)<C->C(19) and a Marshall allenyl addition to create the C(16)<C->C(17) diad. The use of Marshall allenyl stannane chemistry was advantageous, since it allowed access to all four of the C(16)<C->C(17) diastereomers, a feature for which the structure--activity relationship (SAR) was not previously investigated.
A number of groups, including the Koide,[11] Webb,[12] Ghosh-Jurica,[13] Hoveyda,[14] Alvarez-Valcárcel,[15] and Nicolaou[17] groups, as well as Koehn and co-workers,[16] have explored the second and third families (Figure 4). In the first example, Webb used a Julia--Kocienski olefination strategy to complete the backbone of 4 ((Figure 4 a, blue), with the C(12)<C->C(13) epoxide being added in the last step.[12] Alternatively, the Koide group’s synthesis of 6 illustrated the use of olefin cross-metathesis to bring together both fragments ((Figure 4 b, blue).[11] The third approach was demonstrated by the Nicolaou group’s synthesis of 8, which involved the use of a Suzuki coupling to unite the two olefins of the internal diene.[17]
Figure 4.
Synthetic disconnections implemented in the total syntheses of a) herboxidiene (4), b) FR901464 (6), and c) thailanstatin A (8). Bond disconnections for component coupling steps (blue) and key steps in component syntheses (red) are shown.
A detailed evaluation of the three synthetic approaches in Figure 4 illustrates several of the key transformations required to complete the respective component syntheses. In the first example Figure 4 a),[12] core 16 was prepared through an Ireland--Claisen rearrangement to functionalize C(1) with an ester. Next, a PCC-mediated allylic alcohol transposition followed by oxidation gave the distal aldehyde in 16. Side chain 15 was assembled through the use of an aldol reaction to produce the C(13)<C->C(14) connection. Next, an Ireland--Claisen rearrangement was used to orient all of the carbon atoms correctly, and this fragment was completed by using a Mitsunobu reaction. While the routes developed to date are viable for small-scale syntheses, future efforts in the application of new carbon--carbon bond forming reactions are needed to provide effective routes to enable gram-scale access to these materials, as demonstrated by the Hoveyda laboratory.[14]
In the Koide group’s synthesis of 6 ((Figure 4 b,[11] component 17 was prepared through a vinyl addition-mediated cyclization of an epoxy aldehyde. The synthesis of the second component 18 began with an amide coupling at C(2), which was followed by a Wittig olefination. The components were then coupled through olefin-cross metathesis at C(9)<C->C(10). The diene was achieved through use of a Wittig olefination.
In the final example (Figure 4 c), Nicolaou and co-workers prepared the pyran of 20 through an oxa-Michael reaction, followed by an amide coupling and cross-metathesis.[17] Fragment 19 is prepared by a Mukaiyama--Michael addition that adds the acyl group. Next, a Takai olefination yields a vinyl iodide, which is completed by an epoxidation mediated by VO(acac)2.
2.3. Medicinal Chemistry Optimization
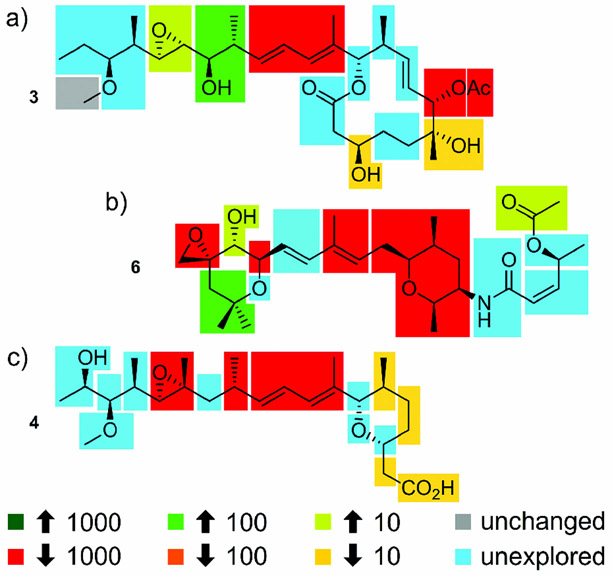
To date there is limited structural information regarding the binding site of SPLMs on SF3b, with suggestions of a tentative binding region obtained only through structure comparisons from mutational studies.[18] Despite the lack of structural data, detailed SARs of the three major families of SPLMs have been determined.[9] Of particular note is work in which four isomers of FD-895 were synthesized. Upon screening in HCT-116 cells, improved activity and stability was shown for 17S-FD-895 (23).[7] Along with ongoing synthetic modification, these efforts have provided a detailed SAR map of the 12-membered macrolide family (Figure 5 a).
Figure 5.
Structure--activity relationships (SARs) identified through synthetic and semisynthetic studies. These maps were developed using data published up to January 2017 and represent findings from in vitro cytotoxicity assays, not direct comparisons of the effect on RNA splicing. Data has been presented to show the optimal analogues for each position, as given by fold increase (up arrow) or decrease (down arrow) in activity. Unchanged denotes substitutions that have been shown to have little effect, while unexplored represents regions that lack sufficient data for assignment.
Furthermore, comparable SAR maps are also available for the second and third families, as shown for 6 (Figure 5 b) and 4 Figure 5 c), respectively.[19] One of the problems with the development of accurate SAR maps arises from the use of different cytotoxicity assays, cell lines, and culture conditions (therein making it difficult to compare the current data). Moreover, many of the activity reports do not provide splicing-modulation data using techniques such as RNAseq, reverse transcriptase polymerase chain reaction (RT-PCR), or quantitative reverse transcriptase polymer chain reaction (qRT-PCR). The lack of this data has further complicated the establishment of detailed SAR studies. That aside, the SAR maps in Figure 5 reveal that few, if any, structural modifications within these natural products lead to analogues with increased activity.
2.4. Ongoing Translational Efforts
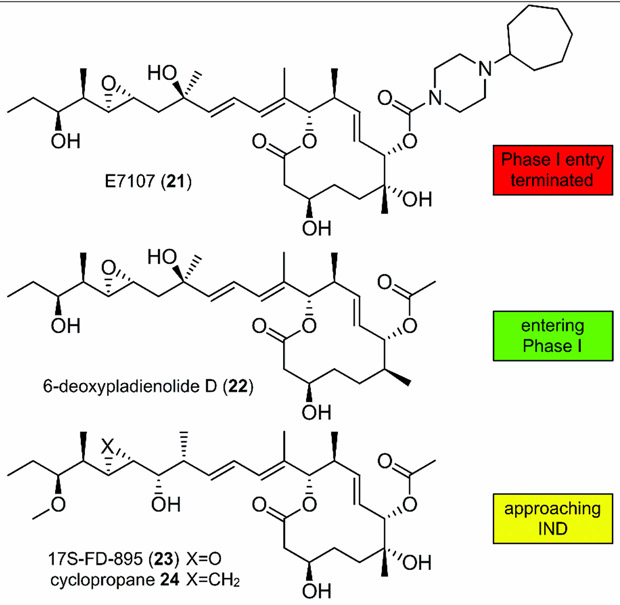
Compounds targeting SF3b have entered clinical trials with various degrees of success (Figure 6). E7107 (21), a derivative of 1, successfully altered RNA splicing in solid tumors in phase I clinical trials; however, the trials were halted due to severe toxicity.[20] Studies from H3 Biomedicine have provided detailed evaluation of 6-deoxypladienolide B (22).[21] More recently this team has translated a new candidate into Phase I clinical trials. Other compounds showing promise include 17S-FD-895 (23) and its corresponding cyclopropane 24,[22] which have recently demonstrated viable in vivo efficacy for acute myeloid leukemia (AML) by mediating stem cell maintenance.[23]
Figure 6.
Structure of the first clinical entry, E7107 (21), which entered Phase 1 clinical trials for patients with solid tumors. The next-generation analogues 6-deoxypladienolide D (H3B-8800, 22), 17S-FD-895 (23), and cyclopropane 24 are currently being examined for clinical translation for hematologic malignancies. IND=investigational new drug application.
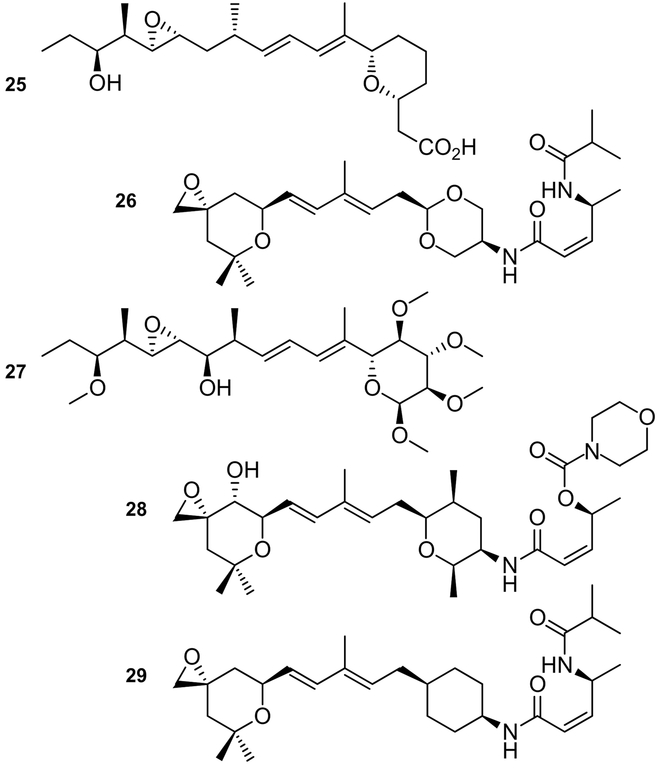
In addition to derivatives of natural products, a new generation of synthetic derivatives are currently under investigation. One of the key observations in this field was the identification of two consensus motifs within these families (Figures 1,2).[24] This was validated by the preparation of the pladienolide--herboxidiene hybrid 25 Figure 7). While 25 displayed reduced activity, it demonstrated that structural simplification could facilitate SAR studies.
Figure 7.
Exemplary structures of synthetic analogues of natural products developed from the SAR profiles. These include analogues that offer increased stability (27 or 29), provide improved synthetic access (25−−29), or serve as fusions between the pladienolide and herboxadiene families (25).
Recent studies have continued to search for a consensus motif that contains the minimum atoms necessary to elicit activity (Figure 7). Subsequent efforts in the Webb laboratory have led to the development of 26,[25] an analogue that demonstrated viable activity in cell and animal models. Further explorations have also led to complete replacements of core units, including the development of carbohydrate-based analogues such as 27,[26] meayamycin B (28),[27] or cyclohexyl-derived analogue 29.[15] While the activity of these analogues may not be comparable to their respective natural products, the improved stability in these materials, as demonstrated by 27 and 29, provide a solid foundation for next-generation advances (Figure 7).
3. Selectivity in Splice Modulation
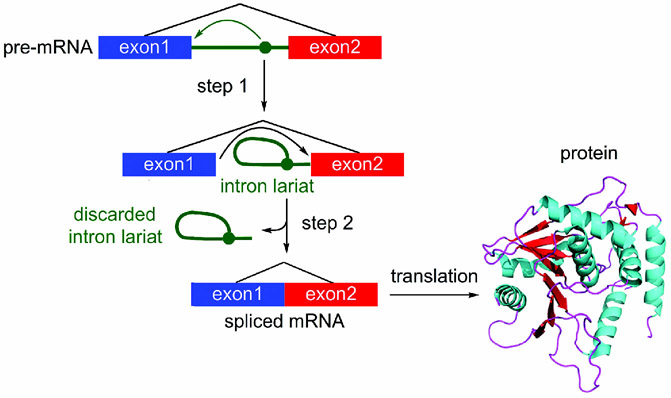
RNA splicing is a complex multistep process that is carried out by a large macromolecular machine called the spliceosome,[28] a ribonucleoprotein (RNP) particle containing five RNAs and more than 100 associated proteins.[29] During its action, the spliceosome forms a series of complexes, each of which serves to conduct a discrete step required to excise an intron, a nucleotide sequence within a gene that is removed during RNA splicing, from the pre-mRNA.[30] As illustrated in Figure 8, the process proceeds through the transesterification of an intron to form a lariat (step 1), followed by cleavage of the lariat and release (step 2) to provide the fully intact mRNA for translation into protein (Figure 8). Unsurprisingly, modulation of this process by a small molecule is extremely complex, and hence prediction of selectivity becomes increasingly complicated. Recent studies have identified some of the variables that need to be considered when evaluating a SPLM.
Figure 8.
Overview of the splicing process, depicting the conversion of pre-mRNA into spliced mRNA followed by translation into a functional protein.
3.1. Cell Selectivity
RNA splicing has been targeted in solid tumors and hematologic malignancies by using SPLMs designed to modulate the action of the SF3b complex in the spliceosome. Among the 12-membered macrolide family, 1 has been the most explored.[31] Screening in the NCI-60 cell line indicated potent activity in a unique set of cells, including NCI-H522 (non-small-cell lung cancer) and NCI-H460 (large-cell lung carcinoma) cells.[2b] Pladienolide B (1) and 6 induced in vitro cytotoxicity in A549 (lung adenocarcinoma) cells and showed in vivo efficacy in tumor xenograft models. In addition, meayamycin B (28) and 1 inhibited tumor growth in xenografts derived from HCT-116 (colon carcinoma) cells.[32] While NCI screening has been used to calibrate the activity of these compounds in tumor cell lines, early studies have indicated that many of these materials demonstrate remarkable efficacy in tumor cells over normal cells, with selectivity indices of 3−−4 orders of magnitude often observed, thus providing a wide therapeutic window for treatment.[33]
In addition to altering the normal splicing process, SPLMs also modulate splicing to deliver a gene product with differential functions to cells. One clear example involves the MCL1 and BCL-x family of genes, which in cancerous cells typically modulate alternative splicing from anti-apoptotic MCL1L (long) to pro-apoptotic MCL1S (short) upon SPLM treatment.[34, 35] In addition, SPLMs can modulate post-translational modifications, as demonstrated by recent studies illustrating that pladienolide B (1) can modulate the level of phosphorylation of SF3B1 at Thr 313.[35]
3.2. Mechanistic Selectivity
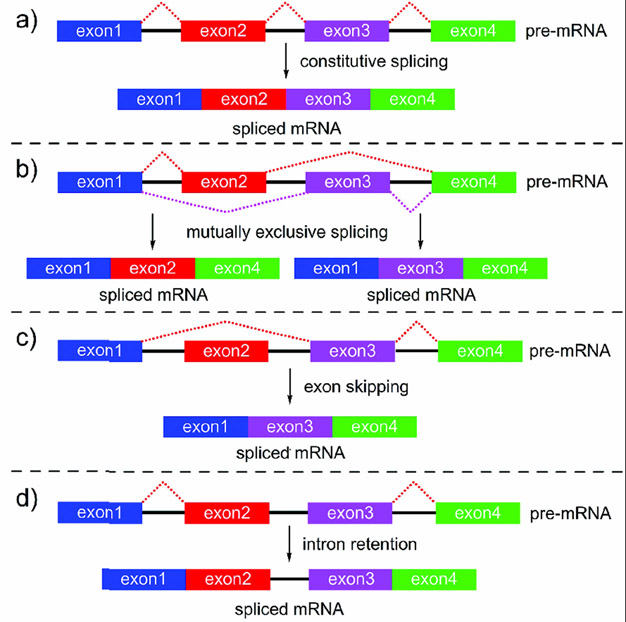
SPLMs produce different types of alternative splicing (AS) events when compared to normal constitutive splicing (Figure 9).[36] Exon skipping (ES)[37] and intron retention (IR)[38] are the most common AS events observed in SPLM-treated cells both in vitro and in vivo. Several of the best studied compounds, including pladienolide B (1), FD-895 (3), meayamycin B (28), FR901464 (6), and spliceostatin (7), have been characterized according to their ability to induce IR events by using a combination of RNAseq and RT/qRT-PCR analyses.[34, 39] Other efforts have identified detailed maps of ES events.[12, 40] While both approaches are viable, there is a need to move toward a detailed characterization of both IR and ES events in combination with other less common forms of AS, such as mutually-exclusive splicing (Figure 9).[41]
Figure 9.
Different modes of RNA splicing. a) Constitutive splicing is most common, where, as part of the normal processing of transcription, the spliceosome removes intronic (non-coding) portions of pre-mRNA. b) In diseased or abnormal cells, other pathways, such as aberrant splicing machinery could, lead to mutually exclusive splicing. c) Exon skipping or d) intron retention can also occur as part of normal splicing or in malignant cells treated with SPLMs.
3.3. Gene Selectivity
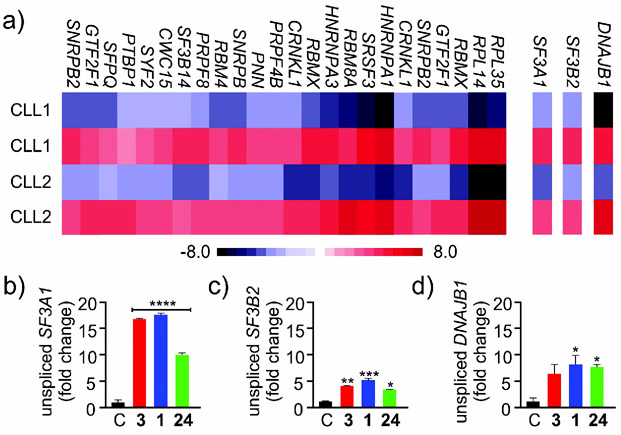
To date, RNAseq data (Figure 10 a) has provided a wealth of information about gene selectivity. The use of these data, along with that from qRT-PCR and RT-PCR analysis, has revealed two key findings.
Figure 10.
a) Examples of gene selectivity identified by RNAseq analysis. b) Examples of gene selectivity identified by qRT-PCR analysis. Three selected genes (SF3A1, SF3B2, and DNAJB1) are shown as representative examples. The level of splicing in these genes was not identical for FD-895 (3), pladienolide B (1), and cyclopropane 24, as shown in (b--d), respectively.
First, current data suggest that the different SF3b-targeting SPLMs do not necessarily target the same genes.[35] As shown in Figure 10 a, the levels of IR in CLL-B cells treated with 3 clearly differ for each gene. While this in part reflects the level of gene expression and splicing ongoing for each gene, the relative levels of the splicing in untreated cells (blue) versus treated cells (red) do not correlate. This indicates a complicated interplay between the splicing of specific genes and the efficacy of 3 in inducing IR within a specific gene.
Second, early evidence indicates that different structural features within a SPLM can alter its gene selectivity. As shown in Figure 10 b--d, the relative levels of splicing are different for SF3A1, SF3B2, and DNAJB1.[35] While complete analyses of all AS events upon drug treatment has yet to be reported for any SPLM, current studies are now providing compelling evidence that trends arise within genes from similar families, thus suggesting that gene- or gene-family-selective SPLM development could be possible.[41]
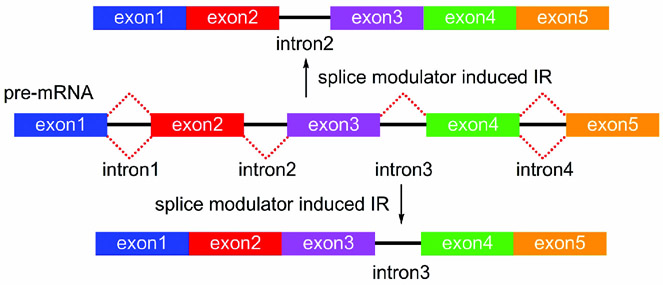
3.4. Intron/Exon Selectivity
The complexity of SPLM selectivity is not limited to the mode of splicing (Section 3.2) or gene specificity (Section 3.3), but is also reflected at the level of specific intron/exon pairs within a gene transcript. While not fully charted, SPLM activity is not required to have comparable efficacy for the removal of each intron within a gene. As shown in Figure 11, it is possible that a given SPLM may have a different rate of activity for inducing IR of one intron (intron2) versus that for another (intron3). While efforts are underway to explore this level of selectivity, the fact that multiple mechanisms exist at each intron further complicates this analysis. Each human gene contains on average 7.8 introns,[42] which adds further complexity to this problem. Recent evidence indicates that small and GC-rich introns are more prone to undergo IR/ES events.[43] Understanding the rules that guide this selectivity is not only fundamental to gauge the activity of a given SPLM, but also essential for understanding the underlying nature of the selectivity.
Figure 11.
A schematic representation of intron/exon selectivity. In this example, two different IR products bearing either intron2 (top) or intron3 (bottom) can arise from the same pre-mRNA.
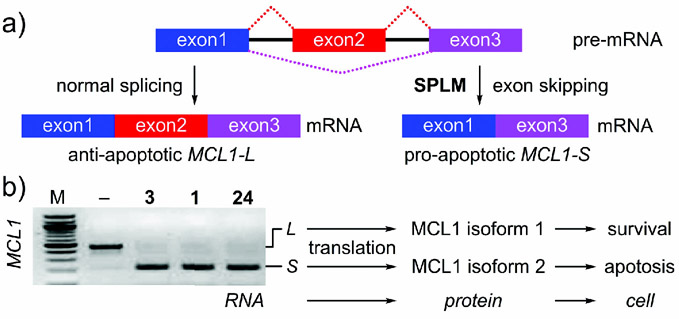
3.5. Downstream Protein Selectivity
While there is a good understanding of how to mark and identify SPLMs at the mRNA level, as well as a conceptual understanding of how these events terminate or alter protein synthesis, following these events at an analytical level will require significant additional study. Our current knowledge arises predominantly from the study of genes that are regulated by AS, such as commonly observed in MCL1.[34, 35] As shown in Figure 12, the SPLMs 1, 3, and 24 result in a shift from the long mRNA and translation of the MCL1 longer isoform 1, which inhibits apoptosis, to the short mRNA and corresponding MCL1 shorter isoform 2, which induces apoptosis.
Figure 12.
Example of splicing selectivity at the protein level. a) Mechanism of splicing modulation in MCL1. b) MCL1 splicing in mantel cell lymphoma (MCL-B) cells after treatment with control (<M->) or 100 nm 1, 3, or 24 for 4 h. The levels of spliced (S) and unspliced (L) transcripts were evaluated by RT-PCR analysis. Without splicing modulation, MCL1 undergoes normal splicing leaving the longer form. Treatment with 1, 3, or 24 results in exon skipping as noted by the formation of the shorter form.
While these effects can corroborate the potent antitumor activity of SPLMs, new tools are needed to analytically evaluate SPLM activity on genes that are prone to alternative splicing, such as MCL-1 described above. Furthermore, there is a vital need to understand the regulatory systems that lie between the formation of a spliced mRNA and its ultimately translated protein product.
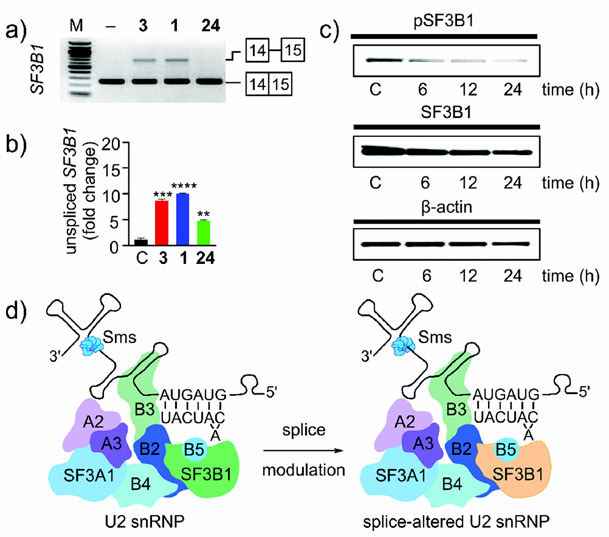
3.6. Attenuation through Feedback
The elegance and complexity of splicing modulation, however, does not end there. Recent data have indicated that mRNAs encoding the protein components of the spliceosome are among the most common families of genes to undergo splicing modulation during the action of a SPLM.[35] Remarkably, RNAseq analysis along with RT-PCR Figure 13 a) and qRT-PCR (Figure 13 b) indicate that SF3B1, the gene associated with the protein target of 1, 3, and 24, is one of the most commonly observed splicing-modified genes in cells treated with 1, 3, and 24 (Figure 13 a,b). This in turn results in a reduction in the levels of both SF3B1 and phosphorylated SF3B1 (pSF3B1; (Figure 13 c).[35] Overall, the feedback between mis-splicing of SF3B1 RNA through IR and loss of protein leads to even more compromised spliceosomes through the formation of a splice-altered U2 snRNP (Figure 13 d). While not yet established, it is likely that this splice-altered U2 snRNP also induces further modulation of splicing events.
Figure 13.
Feedback in splicing modulation. a) RT-PCR and b) qRT-PCR analysis of MCL-B cells treated with 100 nm 1, 3, or 24, or a DMSO control for 4 h. c) Pladienolide B (1) regulates the level of SF3B1 phosphorylation. JeKo-1 cells were treated with 100 nm 1 for 6 h, 12 h or 24 h. Untreated cells grown for 24 h were used as a control (C). d) Schematic representation of the feedback modulation of SF3B1. Inhibition of SF3B1 (green) results in IR in SF3B1 and leads to a reduction in the amount of SF3B1 protein (orange) within the U2 snRNP. The net effect is a reduction in SF3B1 levels and the formation of a compromised splice-altered U2 snRNP.
4. Chemical Biology of Splice Modulation
In addition to the issues of selectivity, understanding the fine details of SPLM activity must be addressed by chemical biology. Our discussion will highlight two critical features relating to the application of SPLMs, namely, the unique effects of timing and dose.
4.1. Timing in Splice Modulation
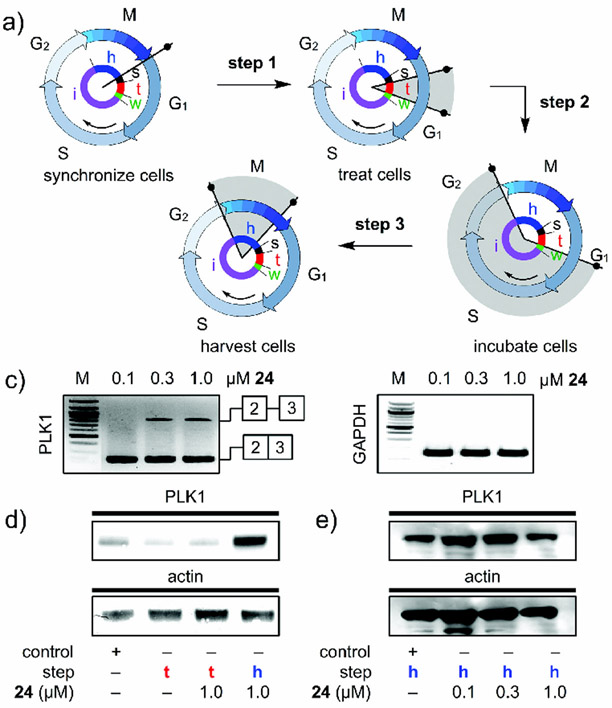
Until recently, the bulk of splicing studies were conducted using unsynchronized cells. Here, the observed effects at both the RNA and protein levels represent an average over different states of the cell cycle. Recent evidence, however, indicates that synchronization is key to providing a detailed link between splicing modulations at the RNA and protein levels and their effects on the cell. In one study, the presentation of 24 during a brief window in the G1 phase of the cell cycle (Figure 14 a) induced an effect that resulted in IR in PLK1 (Figure 14 b). While little PLK1 protein was expressed during G1 (Figure 14 d), the levels of PLK1 prior to mitosis were reduced in cells treated briefly with 24 several hours before passing from G2 to M. In this study, the direct interrogations of the temporal properties of splicing modulation were evaluated by using PLK1 as a marker of mitotic entry.
Figure 14.
A study on timing in splicing modulation. a) Clock diagrams denote the experimental timing as given by: Step 1: synchronized JeKo-1 cells were treated 1 h after release from starvation (start, s) with 24. Step 2: after incubation (treatment, t, red), the media was removed, the cells were washed with media lacking 24 and the cells were cultured (incubation, i, purple) for an additional 12 h without 24. Step 3: the cells were collected (harvest, h, blue) and evaluated. b) RT-PCR analysis was used to evaluate the levels of PLK1 in JeKo-1 cells treated (t) with 24, washed, and harvested (h). IR was observed for PLK1 after treatment with 24. c) Western blot analyses of lysates from cells treated with 24 and collected either after treatment (t) or at harvest (h). PLK1 expression arises as cells enter the G2/M transition during harvest and not at G1 during treatment. This blot confirms the increase in protein at the state of harvest (h), thus indicating that the cells were at G2/M. d) Western blot analyses of cells treated with 24 and collected at harvest (h). This blot confirms a dose-dependent reduction in the levels of PLK1 protein in cells exposed to 24 relative to controls. See Ref. [15] for further details.
4.2. Mechanistic Action of Splice Modulation
In 2007, it was shown that the molecular probes 1, 2 (Figure 1) and(7 (Figure 2) target the SF3b complex within the U2 subunit of the spliceosome.[2] This data was soon validated by the observation that pladienolide-resistant clones contained a mutation at Arg 1074 (R1074H) in SF3B1, thus suggesting that this residue is critical for activity.[44] While ongoing studies suggest that all three families of natural products (1−−8; Figures 1, 2) share a common binding site within SF3b, a lack of kinetic and structural data currently prevents this validation.[18]
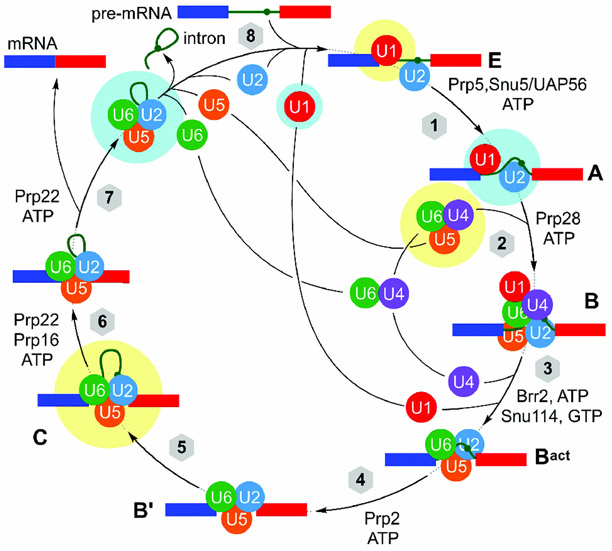
In addition to the SF3b complex, there is a vast array of different proteins, protein--protein complexes, and protein--RNA complexes in the spliceosome that could be targeted. Like many macromolecular machines, the spliceosome undergoes a complex, timed, mechanical process that serves to loop an intron from within two exons and then clip it.[45] The current understanding of this process has been highlighted schematically in Figure 15.
Figure 15.
The mechanism of splicing, depicting complexes A, B, Bact, B’, C, and E. A detailed structural understanding of each of the eight steps in this process is slowly being revealed using a combination of cryo-EM and X-ray crystallography with recent human or yeast structures, as noted by highlighting in yellow (human) and cyan (yeast).
4.3. Structural Understanding of Splicing
As shown in Figure 15, splicing begins with the formation of complexes E and A, which are composed of the U1 and U2 snRNP and mRNA. Action of the U4/U5/U6 tri-snRNP results in the formation of the precatalytic spliceosome, complex B, which in turn is activated to complex Bact through loss of the U1 and U4 snRNPs. The catalytically activated complex B’ then performs the first splicing step, cutting the intron, and the resulting complex C completes the process by fusing the two exons and removing the intron lariat. SPLMs may interrupt many of these steps.
One of the most impressive recent advances has been the development of detailed structures of specific spliceosome complexes using a combination of cryo-EM[46] and X-ray crystallography.[47] Cryo-EM has proven particularly useful to tease out the organization of the snRNPs within each complex and illustrate the structural rearrangements within each snRNP. While outside the scope of this review, structures of spliceosome complexes that include the U1 snRNP, [48] complex A, [49] the U4/U5/U6 complex, [50] complex C,[51] and the post-spliceosome complex[52] are beginning to reveal the mechanistic motions within this machine.
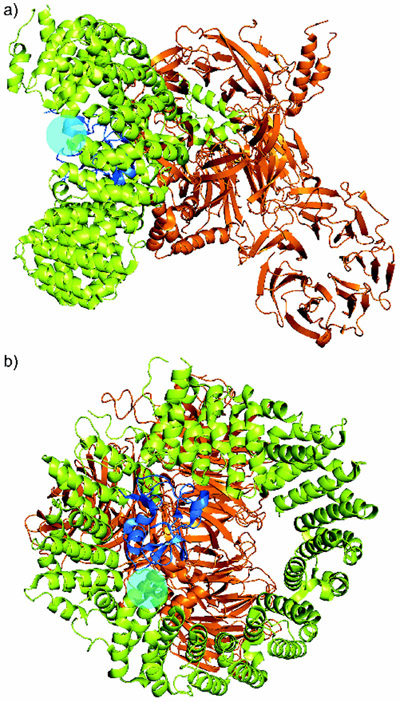
Recently teams led by Pena and Srinivasan elucidated the structure of the SF3b complex.[18] Using protein--protein crosslinking, they were able not only to elucidate the structure of the HEAT superhelix but also to determine its contacts with SF3b130, SF3b10, and SF3b14B and its proximity to p14 and U2AF6. Using this data, they utilized established pladienolide-resistance mutations to gain a first glimpse of the possible binding pocket for SPLMs (cyan circle, (Figure 16).
Figure 16.
Structure of the human SF3b core complex with a cyan sphere showing the position of the R1074 mutation found in cells resistant to pladienolide B (1). Two views are provided: a) front view, b) rotation 90° into the page.
4.4. Alternate Avenues for Modulation through Chemical Biology
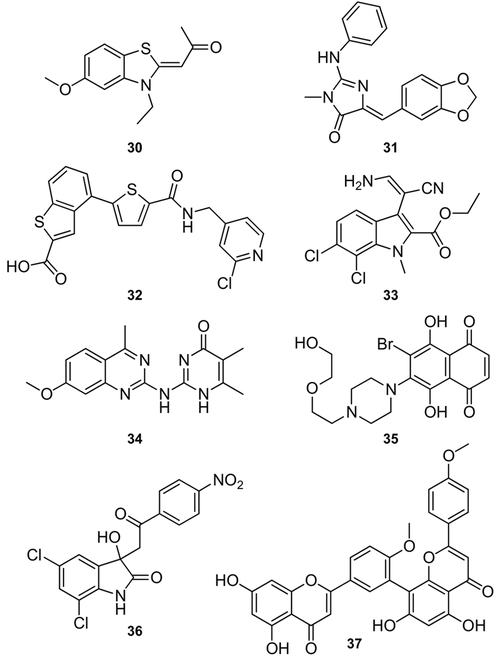
While this review has focused predominantly on SF3b-targeting inhibitors, efforts are now underway to discover new motifs that target different complexes and their associated snRNPs, as well as post-translational events associated with splicing. These materials include a variety of natural products, their derivatives, as well as synthetic leads (Figure 17). One of these leads is the CDC2-like kinase (CLK) inhibitor 30, which has been suggested to target the splicing factor SRSF4.[53] Additional studies have also identified SPLM activity in a variety of kinase inhibitors, such as the CLK1-targeting compound 31,[54] the PRP4 inhibitor 32,[55] and the potent CLK1-binding compound 33.[56] In addition, there are number of other leads for which the detailed modes of splicing modulation have not fully been established, including the inhibitor of spliceosome assembly 34,[57] 35,[58] 36,[58] and N-palmitoyl-l-leucine.[59] Other leads such as isoginkgetin (37), which inhibits precatalytic spliceosome complex B formation by blocking the binding of U4/U5/U6 tri-snRNP,[60] are already demonstrating that a variety of steps of the splicing process can be effectively modulated. Further examples, including the use of SRPK1 inhibitors, have shown remarkable in vivo utility for regulating neovascularization in tumor tissues by modulating VEGF splicing.[61]
Figure 17.
Structures of SPLMs that do not target SF3b. These include TG003 (30), leucettine L41 (31), the PRP4 inhibitor 32, KH-CB20 (33), madrasin (34), NSC659999 (35), NSC635326 (36), and isoginkgetin (37).
5. Conclusion
The advance of SPLMs brings tremendous therapeutic potential. First, it offers a new set of tools for the clinic, with immediate applications to cancer therapy. Recent evidence also supports the use of SPLMs to regulate the formation of stem cells, thus suggesting that they may also serve as future tools to guide tissue reprogramming. Second, SPLMs provide a new set of tools for basic biological studies.
Two main hurdles remain in the development of SPLM-based drugs. The first focuses on properly adapting the SF3b-targeting SPLMs for clinical use. Second, there is a need to develop a uniform standard (using defined cell lines with full RNA-seq characterization) to accurately evaluate SPLM activity and RNA splicing to provide a detailed map of molecular features that lead to distinct splicing patterns. While not always carefully identified in the literature, many of the natural product SPLMs and associated analogues used lack sufficient pharmacological stability and associated pharmacological properties for clinical applications. While initial evidence indicates that this may have contributed to problems with the clinical application of E7107 (21), further studies are needed to effectively evaluate activities in patients and develop SPLMs with reduced potential for side or off-target effects. Although a series of studies have produced materials with increased stability,[22, 53] often these compounds are significantly less effective at modulating RNA splicing.[24––28] As illustrated in the current SAR maps (Figure 5),[19] only a small number of analogues, such as 23 (Figure 6), have been identified that offer enhanced activity over their natural product counterparts.
While first discovered as natural products, it has become increasingly clear that synthetic chemistry will play an integral part in translating splicing modulators to clinical applications. This, along with studies that advance a more detailed mechanistic understanding of the splicing process[35, 62] at both the single-gene and genome-wide levels, are key to advancing SPLMs into the clinic.[63]
Acknowledgements
This work was supported by financial support from the Lymphoma Research Foundation Grant 285871, the NIH PO1-CA081534, and the Bennett Family Foundation. B. L. was supported by an NIGMS/NIH Award K12 GM068524.
Biographies
Brian León received his B.S. in Chemistry from UC Irvine under the mentorship of Prof. Larry E. Overman. He received a Ph.D. in Organic Chemistry from UC Santa Cruz under the supervison of Prof. Roger Linington. In September 2015, he began his Postdoctoral studies in the Burkart laboratory at UC San Diego.
Manoj K. Kashyap received his B.S. in Biological Sciences and M.S. in Biotechnology from Chaudhary Charan Singh University. He received a Ph.D. in Molecular Biology and Bioinformatics from the John Hopkins School of Medicine, Baltimore Institute of Bioinformatics, Bangalore, India. Currently, he is a Postdoctoral Fellow at the Moores Cancer Center, where he is actively involved in RNA splicing research.
Warren C. Chan obtained his B.S. degree from Washington University in St. Louis under the guidance of Prof. Timothy A. Wencewicz. He is currently a Ph.D. candidate at UC San Diego in the Burkart laboratory. His current research activities focus on the development of second-generation splicing modulators.
Kelsey A. Krug graduated from UC San Diego, where she received her B.S. in Biochemistry and conducted chemical biological research under the guidance of Prof. Kamil Godula. She then joined the Chemistry Ph.D. program at UC San Diego in the Burkart laboratory in 2016. Her current work focuses on the design and synthesis of splicing modulators.
Januario E. Castro obtained a M.D. from Universidad Industrial de Santander Medical School, Colombia. He is currently Professor of Clinical Medicine at UC San Diego’s Moores Cancer Center. His area of expertise is translational research and early therapy development for leukemia and lymphoma, including the application of splicing modulators as next-generation therapeutics for leukemia.
James J. La Clair received his Ph.D. degree in Chemistry from SUNY Buffalo under the tutelage of Prof. Peter T. Lansbury. His studies continued through a postdoctoral fellowship with Prof. Gilbert Stork at Columbia University. He then took up a position at the Scripps Research Institute. In 1999, he embarked on an independent research career that has included positions at UC San Diego and the Salk Institute, among other collaborative endeavors.
Michael D. Burkart received a B.A. in Chemistry from Rice University. He obtained a Ph.D. degree in Organic Chemistry from the Scripps Research Institute in 1999 under the mentorship of Prof. Chi-Huey Wong, after which time he pursued postdoctoral studies at Harvard Medical School with Prof. Christopher Walsh. Since 2002, he has been a professor at UC San Diego. His research interests include natural product synthesis, biosynthesis, and metabolic engineering.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1a.Matera AG, Wang Z, Nat. Rev. Mol. Cell Biol. 2014, 15, 108.; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Papasaikas P, Valcárcel J, Trends Biochem. Sci. 2016, 41, 33. [DOI] [PubMed] [Google Scholar]
- 2a.Kaida D.,Motoyoshi H, Tashiro E, Nojima T, Hagiwara M, Ishigami K, Watanabe H, Kitahara T, Yoshida T, Nakajima H, Tani T, Horinouchi S, Yoshida M, Nat. Chem. Biol. 2007, 3, 576.; [DOI] [PubMed] [Google Scholar]; b Kotake Y., Sagane K, Owa T, Mimori-Kiyosue Y, Shimizu H, Uesugi M, Ishihama Y, Iwata M, Mizui Y, Nat. Chem. Biol. 2007, 3, 570. [DOI] [PubMed] [Google Scholar]
- 3a.Sakai T., Sameshima T, Matsufuji M, Kawamura N, Dobashi K, Mizui Y, J. Antibiot. 2004, 57, 173.; [DOI] [PubMed] [Google Scholar]; b Seki-Asano M., Okazaki T, Yamagishi M, Sakai N, Takayama Y, Hanada K, Morimoto S, Takatsuki A, Mizoue K, J. Antibiot. 1994, 47, 1395. [DOI] [PubMed] [Google Scholar]
- 4a.Isaac BG., Ayer SW, Elliott RC, Stonard RJ, J. Org. Chem. 1992, 57, 7220.; [Google Scholar]; b Sakai Y, Yoshida T, Ochiai K, Uosaki Y, Saitoh Y, Tanaka F, Akiyama T, Akinaga S, Mizukami T, J. Antibiot. 2002, 55, 855. [DOI] [PubMed] [Google Scholar]
- 5a.Nakajima H, Sato B, Fujita T, Takase S, Terano H, Okuhara M, J. Antibiot. 1996, 49, 1196.; [DOI] [PubMed] [Google Scholar]; b Liu X, Biswas S, Berg MG, Antapli CM, Xie F, Wang Q, Tang MC, Tang GL, Zhang L, Dreyfuss G, Cheng YQ, J. Nat. Prod. 2013, 76, 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanada RM, Itoh D, Nagai M, Niijima J, Asai N, Mizui Y, Abe S, Kotake Y, Angew. Chem. Int. Ed. 2007, 46, 4350; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2007, 119, 4428. [Google Scholar]
- 7.Villa R, Mandel AL, Jones BD, La Clair JJ, Burkart MD, Org. Lett. 2012, 14, 5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller S, Mayer T, Sasse F, Maier M, Org. Lett. 2011, 13, 3940. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh AK, Anderson DD, Org. Lett. 2012, 14, 4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar V, Chandrasekhar S, Org. Lett. 2013, 15, 3610. [DOI] [PubMed] [Google Scholar]
- 11.Pham D, Koide K, Nat. Prod. Rep. 2016, 33, 637. [DOI] [PubMed] [Google Scholar]
- 12.Lagisetti C, Yermolina MV, Sharma LK, Palacios G, Prigaro BJ, Webb TR, ACS Chem. Biol. 2014, 9, 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Ghosh AK, Ma N, Effenberger KA, Jurica MS, Org. Lett. 2014, 16, 3154−−3157.; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ghosh AK, Li J, Org. Lett. 2011, 13, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng F, McGrath KP, Hoveyda AH, Nature 2014, 513, 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makowski K, Vigevani L, Alberico F, Valcárcel J, Álvarez M, ACS Chem. Biol. 2017, 12, 163. [DOI] [PubMed] [Google Scholar]
- 16.Eustáquio AS, Chang LP, Steele GL, O’Donnell CJ, Koehn FE, Metab. Eng. 2016, 33, 67. [DOI] [PubMed] [Google Scholar]
- 17.Nicolaou KC, Rhoades D, Lamani M, Pattanayak MR, Kumar SM, J. Am. Chem. Soc. 2016, 138, 7532. [DOI] [PubMed] [Google Scholar]
- 18a.Cretu C, Schmitzová J, Ponce-Salvatierra A, Dybkov O, De Laurentiis EI, Sharma K, Will CL, Urlaub H, Lührmann R, Pena V, Mol. Cell 2016, 64, 307.; [DOI] [PubMed] [Google Scholar]; b Rakesh R, Joseph AP, Bhaskara RM, Srinivasan N, RNA Biol. 2016, 13, 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Effenberger KA, Urabe VK, Jurica MS, Wiley Interdiscip. Rev. RNA 2017, 8, e1381.; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Effenberger KA, Urabe VK, Prichard BE, Ghosh AK, Jurica MS, RNA 2016, 22, 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Folco EG, Coil KE, Reed R, Genes Dev. 2011, 25, 440.; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Eskens FALM, Ramos FJ, Burger H, O’Brien JP, Piera A, De Jonge MJA, Mizui Y, Wiemer EAC, Carreras MJ, Baselga J, Taberno J, Clin. Cancer Res. 2013, 19, 6296. [DOI] [PubMed] [Google Scholar]
- 21.Arai K, Buonamici S, Chan B, Corson L, Endo A, Gerard B, Hao MH, Karr C, Kira K, Lee L, Liu X, Lowe JT, Luo T, Marcaurelle LA, Mizui Y, Nevalainen M, O’Shea MW, Park ES, Perino SA, Prajapati S, Shan M, Smith PG, Tivitmahaisoon P, Wang JY, Warmuth M, Wu KM, Yu L, Zhang H, Zheng GZ, Keaney GF, Org. Lett. 2014, 16, 5560. [DOI] [PubMed] [Google Scholar]
- 22.Villa R, Kashyap MK, Kumar D, Kipps TJ, Castro JE, La Clair JJ, Burkart MD, J. Med. Chem. 2013, 56, 6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crews LA, Balaian L, Delos Santos NP, Leu HS, Court AC, Lazzari E, Sadarangani A, Zipeto MA, La Clair JJ, Villa R, Kulidjian A, Storb R, Morris SR, Ball ED, Burkart MD, Jamieson CH, Cell Stem Cell 2016, 19, 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagisetti C, Pourpak A, Jiang Q, Cui X, Goronga T, Morris SW, Webb TR, J. Med. Chem. 2008, 51, 6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagisetti C, Palacios G, Goronga T, Freeman B, Caufield W, Webb TR, J. Med. Chem. 2013, 56, 10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhar S, La Clair JJ, León B, Hammons JC, Yu Z, Kashyap MK, Castro JE, Burkart MD, J. Am. Chem. Soc. 2016, 138, 5063. [DOI] [PubMed] [Google Scholar]
- 27.Albert BJ, Sivaramakrishnan A, Naka T, Czaicki NL, Koide K, J. Am. Chem. Soc. 2007, 129, 2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahebi M, Hanafi MM, van Wijnen AJ, Azizi P, Abiri R, Ashkani S, Taheri S, Gene 2016, 587, 107. [DOI] [PubMed] [Google Scholar]
- 29a.Lee Y., Rio DC, Annu. Rev. Biochem. 2015, 84, 291.; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wahl MC, Will CL, Lührmann R, Cell 2009, 136, 701. [DOI] [PubMed] [Google Scholar]
- 30a.Van Der Feltz C, Anthony K, Brilot A, Pomeranz Krummel DA, Biochemistry 2012, 51, 3321.; [DOI] [PubMed] [Google Scholar]; b Will CL, Lührmann R, Cold Spring Harbor Perspect. Biol 2011, 3, a003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotake Y, Kaida D, Mizui Y, Yoshida M, Tanpakushitsu Kakusan Koso 2008, 53, 28. [PubMed] [Google Scholar]
- 32.Albert BJ, McPherson PA, O’Brien K, Czaicki NL, Destefino V, Osman S, Li M, Day BW, Grabowski PJ, Moore MJ, Vogt A, Koide K, Mol. Cancer Ther. 2009, 8, 2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.Lagisetti C, Pourpak A, Goronga T, Jiang Q, Cui X, Hyle J, Lahti JM, Morris SW, Webb TR, J. Med. Chem. 2009, 52, 6979.; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kashyap MK, Kumar D, Villa R, La Clair JJ, Benner C, Sasik R, Jones H, Ghia EM, Rassenti LZ, Kipps TJ, Burkart MD, Castro JE, Haematologica 2015, 100, 945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34a.Bae J, Leo CP, Hsu SY, Hsueh AJW, J. Biol. Chem. 2000, 275, 25255.; [DOI] [PubMed] [Google Scholar]; b Kim JH, Sim SH, Ha HJ, Ko JJ, Lee K, Bae J, FEBS Lett. 2009, 583, 2758.; [DOI] [PubMed] [Google Scholar]; cGao Y, Koide K, ACS Chem. Biol. 2013, 8, 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar D, Kashyap MK, La Clair JJ, Villa R, Spaanderman I, Chien S, Rassenti LZ, Kipps TJ, Burkart MD, Castro JE, ACS Chem. Biol. 2016, 11, 2716. [DOI] [PubMed] [Google Scholar]
- 36a.Breitbart RE, Andreadis A, Nadal-Ginard B, Annu. Rev. Biochem. 1987, 56, 467.; [DOI] [PubMed] [Google Scholar]; b Chen M, Manley JL, Nat. Rev. Mol. Cell Biol. 2009, 10, 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watakabe A, Tanaka K, Shimura Y, Genes Dev. 1993, 7, 407. [DOI] [PubMed] [Google Scholar]
- 38.Kozak M, Cell Biol J. 1988, 107, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corrionero A, Miñana B, Valcárcel J, Genes Dev. 2011, 25, 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasegawa M, Miura T, Kuzuya K, Inoue A, Won Ki S, Horinouchi S, Yoshida T, Kunoh T, Koseki K, Mino K, Sasaki R, Yoshida M, Mizukami T, ACS Chem. Biol. 2011, 6, 229. [DOI] [PubMed] [Google Scholar]
- 41.Convertini P, Shen M, Potter PM, Palacios G, Lagisetti C, de la Grange P, Horbinski C, Fondufe-Mittendorf YN, Webb TR, Stamm S, Nucleic Acids Res. 2014, 42, 4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakharkar MK, Chow VTK, Kangueane P, In Silico Biol. 2004, 4, 387. [PubMed] [Google Scholar]
- 43.Galante PAF, Sakabe NJ, Kirschbaum-Slager N, de Souza SJ, RNA 2004, 10, 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yokoi A, Kotake Y, Takahashi K, Kadowaki T, Matsumoto Y, Minoshima Y, Sugi NH, Sagane K, Hamaguchi M, Iwata M, Mizui Y, FEBS J 2011, 278, 4870. [DOI] [PubMed] [Google Scholar]
- 45a.Patel AA, Steitz JA, Nat. Rev. Mol. Cell Biol. 2003, 4, 960.; [DOI] [PubMed] [Google Scholar]; b Noren CJ, Wang J, Perler FB, Angew. Chem. Int. Ed. 2000, 39, 450; [PubMed] [Google Scholar]; Angew. Chem 2000, 112, 458.; [Google Scholar]; b Paulus H, Annu. Rev. Biochem. 2000, 69, 447. [DOI] [PubMed] [Google Scholar]
- 46a.Stark H, Lührmann R, Annu. Rev. Biophys. Biomol. Struct. 2006, 35, 435.; [DOI] [PubMed] [Google Scholar]; b Valadkhan S, Jaladat Y, Proteomics 2010, 10, 4128.; [DOI] [PMC free article] [PubMed] [Google Scholar]; c Fica SM, Oubridge C, Galej WP, Wilkinson ME, Bai XC, Newman AJ, Nagai K, Nature 2017, 524, 337.; [DOI] [PMC free article] [PubMed] [Google Scholar]; d Bertram K, Agafonov DE, Liu WT, Dybkov O, Will CL, Hartmuth K, Urlaub H, Kastner B, Stark H, Lührmann R, Nature 2017, 524, 318.; [DOI] [PubMed] [Google Scholar]; e Galej WP, Wilkinson ME, Fica SM, Oubridge C, Newman AJ, Nagai K, Nature 2016, 537, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47a.Sperling J, Azubel M, Sperling R, Structure 2008, 16, 1605.; [DOI] [PubMed] [Google Scholar]; b Korneta I, Magnus M, Bujnicki JM, Nucleic Acids Res. 2012, 40, 7046.; [DOI] [PMC free article] [PubMed] [Google Scholar]; c van Roon AM, Oubridge C, Obayashi E, Sposito B, Newman AJ, Séraphin B, Nagai K, RNA 2017, 23, 968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48a.Kondo Y, Oubridge C, van Roon A-MM, Nagai K, eLife 2015, 4, 4986.; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Pomeranz Krummel DA, Oubridge C, Leung AKW, Li J, Nagai K, Nature 2009, 458, 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Behzadnia N, Golas MM, Hartmuth K, Sander B, Kastner B, Deckert J, Dube P, Will CL, Urlaub H, Stark H, Lührmann H, EMBO J. 2007, 26, 1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen THD, Galej WP, Bai X, Savva CG, Newman AJ, Scheres SHW, Nagai K, Nature 2015, 523, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Golas MM, Sander B, Bessonov S, Grote M, Wolf E, Kastner B, Stark H, Lührmann R, Mol. Cell 2010, 40, 927. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen THD, Galej WP, Fica SM, Lin P-C, Newman AJ, Nagai K, Curr. Opin. Struct. Biol. 2016, 36, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishida A, Kataoka N, Takeshima Y, Yagi M, Awano H, Ota M, Itoh K, Hagiwara M, Matsuo M, Nat. Commun. 2011, 2, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tahtouh T, Elkins JM, Filippakopoulos P, Soundararajan M, Burgy G, Durieu E, Cochet C, Schmid RS, Lo DC, Delhommel F, Oberholzer AE, Pearl LH, Carreaux F, Bazureau JP, Knapp S, Meijer L, J. Med. Chem. 2012, 55, 9312. [DOI] [PubMed] [Google Scholar]
- 55.Gao Q, Mechin I, Kothari N, Guo Z, Deng G, Haas K, McManus J, Hoffmann D, Wang A, Wiederschain D, Rocnik J, Czechtizky W, Chen X, McLean L, Arlt H, Harper D, Liu F, Majid T, Patel V, Lengauer C, Garcia-Echeverria C, Zhang B, Cheng H, Dorsch M, Huang SM, J. Biol. Chem. 2013, 288, 30125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fedorov O, Huber K, Eisenreich A, Filippakopoulos P, King O, Bullock AN, Szklarczyk D, Jensen LJ, Fabbro D, Trappe J, Rauch U, Bracher F, Knapp S, Chem. Biol. 2011, 18, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pawellek A, McElroy S, Samatov T, Mitchell L, Woodland A, Ryder U, Gray D, Lührmann R, Lamond AI, J. Biol. Chem. 2014, 289, 34683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Effenberger KA, Perriman RJ, Bray WM, Lokey RS, Ares M, Jurica MS, Biomol J. Screening 2013, 18, 1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Effenberger KA, James RC, Urabe VK, Dickey BJ, Linington RG, Jurica MS, J. Biol. Chem. 2015, 290, 27524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Brien K, Matlin AJ, Lowell AM, Moore MJ, J. Biol. Chem. 2008, 283, 33147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nowak DG, Amin EM, Rennel ES, Hoareau-Aveilla C, Gammons M, Damodoran G, Hagiwara M, Harper SJ, Woolard J, Ladomery MR, Bates DO, J. Biol. Chem. 2010, 285, 5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62a.Boswell SA, Snavely A, Landry HM, Churchman LS, Gray JM, Springer M, Nat. Chem. Biol. 2017, 501.; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Carrocci TJ, Zoerner DM, Paulson JC, Hoskins AA, Nucleic Acids Res. 2017, 45, 4837; [DOI] [PMC free article] [PubMed] [Google Scholar]; c Liu B, Abdel-Wahab O, eLife 2017, 6, e25996; [DOI] [PMC free article] [PubMed] [Google Scholar]; d Jenkins JL, Kielkopf CL, Trends Genet. 2017, 33, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Since the submission of this manuscript, two new SPLMs have been described as noted in the following publications: a Sidarovich A, Will CL, Anokhina MM, Ceballos J, Sievers S, Agafonov DE, Samatov T, Bao P, Kastner B, Urlaub H, Waldmann H, Lührmann R, eLife 2017, e23533; [DOI] [PMC free article] [PubMed] [Google Scholar]; bChung FF, Tan PF, Raja VJ, Tan BS, Lim KH, Kam TS, Hii LW, Tan SH, See SJ, Tan YF, Wong LZ, Yam WK, Mai CW, Bradshaw TD, Leong CO, Sci. Rep 2017, 7, 42504. [DOI] [PMC free article] [PubMed] [Google Scholar]