Abstract
Background:
Absent T-cell immunity resulting in life-threatening infections provides a clear rationale for hematopoetic stem cell transplantation (HSCT) in patients with severe combined immunodeficiency (SCID). Combined immunodeficiencies (CIDs) and “atypical” SCID show reduced, not absent T-cell immunity. If associated with infections or autoimmunity, they represent profound combined immunodeficiency (P-CID), for which outcome data are insufficient for unambiguous early transplant decisions.
Objectives:
We sought to compare natural histories of severity-matched patients with/without subsequent transplantation and to determine whether immunologic and/or clinical parameters may be predictive for outcome.
Methods:
In this prospective and retrospective observational study, we recruited nontransplanted patients with P-CID aged 1 to 16 years to compare natural histories of severity-matched patients with/without subsequent transplantation and to determine whether immunologic and/or clinical parameters may be predictive for outcome.
Results:
A total of 51 patients were recruited (median age, 9.6 years). Thirteen of 51 had a genetic diagnosis of “atypical” SCID and 14 of 51 of CID. About half of the patients had less than 10% naive T cells, reduced/absent T-cell proliferation, and at least 1 significant clinical event/year, demonstrating their profound immunodeficiency. Nineteen patients (37%) underwent transplantation within 1 year of enrolment, and 5 of 51 patients died. Analysis of the HSCT decisions revealed the anticipated heterogeneity, favoring an ongoing prospective matched-pair analysis of patients with similar disease severity with or without transplantation. Importantly, so far neither the genetic diagnosis nor basic measurements of T-cell immunity were good predictors of disease evolution.
Conclusions:
The P-CID study for the first time characterizes a group of patients with nontypical SCID T-cell deficiencies from a therapeutic perspective. Because genetic and basic T-cell parameters provide limited guidance, prospective data from this study will be a helpful resource for guiding the difficult HSCT decisions in patients with P-CID.
Keywords: T-cell deficiency, combined immunodeficiency, hematopoietic stem cell transplantation, natural history
Human genetic disorders leading to deficiencies in T-cell number and/or function are a heterogeneous group of inherited diseases. They can range from absent T-cell immunity with early onset of severe clinical manifestations to significant residual T-cell immunity with late onset of milder clinical manifestations. The characteristic clinical feature of complete T-cell deficiencies is infection susceptibility, while incomplete T-cell deficiencies in addition present with manifestations of impaired immune regulation and malignancy.1–4 The key treatment to restore T-cell immunocompetence is hematopoetic stem cell transplantation (HSCT) and in some cases gene therapy or enzyme replacement therapy. There is little debate that this intensive therapy is required to prevent lethal complications in patients with absent T-cell immunity. However, the threshold of T-cell immunity required to prevent severe clinical complications is unknown. In patients with low to intermediate T-cell function, it is therefore frequently difficult to make a prognosis and to balance this prognosis against the risks of HSCT.
Currently, there is no accepted classification of T-cell deficiencies established from this therapeutic perspective (for present classifications of T-cell disorders, see Table E1 in this article’s Online Repository at www.jacionline.org). Patients with very low T-cell numbers are usually classified as severe combined immunodeficiency (SCID). Although these patients have a clear HSCT indication, this classification does not consider that there are also patients with normal T-cell numbers, but severely impaired T-cell function with the same risk for early severe complications and the same clear HSCT indication (eg, ORAI calcium release-activated calcium modulator 1 [ORAI1], caspase recruitment domain family, member 11, or IkBkB deficiency or patients with the IL-2 receptor gamma chain [IL2RG]R222C mutation). At the other end of the spectrum, common variable immunodeficiency (CVID), although predominantly an antibody deficiency,5 can be associated with significantly impaired T-cell immunity and manifestations related to this.6,7 Current definitions also do not consider a threshold of T-cell immunity below which the diagnosis of CVID is inappropriate and early HSCT is a necessary consideration. Preliminary criteria for a threshold of T-cell immunity separating CVID from more profound T-cell deficiencies, which we derived by expert consensus in the European Society for Immunodeficiencies (ESID) Registry Working Group, remain to be prospectively validated.8
TABLE E1.
Current definitions of non-SCID T-cell disorders
| Condition | Clinical criteria | T-cell criteria | Genetic criteria | Source |
|---|---|---|---|---|
| “Leaky” SCID | Treatment with HSCT | CD3 <1000/μL (<2 y), <800/μL (2–4 y), <600/μL (>4 y) AND PHA < 30% of lower limit AND absence of maternal engraftment |
None | Shearer et alE4 |
| “Leaky” SCID | Infant identified by NBS because of low TRECs | CD3 300–1500/μL AND naive T cells reduced AND T-cell proliferation reduced |
Incomplete defect in known SCID gene | Kwan et alE5 |
| “Variant” SCID | Infant identified by NBS because of low TRECs | <1500/μL, <2500/μL, or <3505/μL T cells | None | Kwan et alE5 |
| “Atypical” SCID | Not transplanted in first year of life | CD31 T-cell counts above 100/μL at diagnosis | Mutation in known SCID gene | Felgentreff et alE6 |
| P-CID | Not transplanted in first year of life AND at least 1 of the following: Major infection Major immune dysregulation Malignancy |
At least 2 of the following: CD4: <700, if <2 y; <500, if 2–4 y; <300, if >4 y; OR CD8: <350, if <2 y; <250, if 2–4 y; <150, if >4 y OR naive CD41: <30% <2 y, <25% 2–6 y, <20% >6y OR PHA or anti-CD3 <30% of lower limit OR g/δ T cells >15% of total CD3 |
None | This study |
| CID | Treatment with HSCT | CD3 >500/mL | None | Roifman et alE7 |
| CVID | Age >4 y AND at least 1 of the following Increased susceptibility to infection Autoimmune manifestations Granulomatous disease Unexplained polyclonal lymphoproliferation Affected family member with antibody deficiency |
At least 1 of the following: Marked decrease in IgG AND marked decrease in IgA Poor antibody response to vaccines OR absent isohemagglutinins AND no evidence of profound T-cell deficiency (2 of the following) CD4: 4–6 y <300/μL, 6–12 y <250/μL, >12 y <200/μL OR naive CD4: 4–6 y <25%, 6–16 y <20%, >16 y <10% OR T-cell proliferation absent |
None | ESID Registry |
TREC, T-cell receptor excision circle.
In this study, we focus on patients with disorders of T-cell immunity that can neither be classified as SCID nor as CVID, but rather as combined immunodeficiencies (CIDs). Our present article considers the following categories of CID, including “atypical SCID,” taking into consideration the previous work of Roifman et al3 and Felgentreff et al14: (1) “bona fide” CID, diseases in which mutations in affected genes cause T-cell deficiencies and the clinical problem is mainly restricted to the immune system (eg, lymphocyte-specific protein tyrosine kinase, IL2-inducible T-cell kinase [ITK], zeta-chain (TCR)-associated protein kinase 70kDa [ZAP70], macrophage stimulating 1, Coronin 1A, dedicator of cytokinesis 8, dedicator of cytokinesis 2, and MHC class I and II deficiencies).9–13 A subgroup are syndromic CID, diseases in which mutations cause additional clinical problems (eg, cartilage hair hypoplasia, severe cases of 22q11 deletion syndrome, Cernunnos, and STIM1 or purine nucleoside phosphorylase deficiencies); (2) atypical SCID,14 in which patients survive infancy despite T-cell deficiencies caused by hypomorphic mutations in SCID-associated genes (eg, recombination-activating genes [RAG] 1/2, DNA cross-link repair 1C [DCLRE1CI], IL2RG, ORAI1, or inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase beta [IKBKB]); and (3) CID of unknown cause, in which T-cell deficiency can be documented, but no disease-causing mutation is detected. We label patients from 1 of these 3 groups of patients with CID as profound combined immunodeficiency (P-CID) if their T-cell deficiency is associated with at least 1 severe infection or manifestation of impaired immune regulation.
The motivation for the P-CID study is the recurrent difficult question, if and at what time point HSCT should be performed. The study therefore targets patients in whom impaired T-cell immunity leads to significant complications, but is not severe enough for an unambiguous early transplant decision. We prospectively collect data on mortality, morbidity, quality of life (QOL), and treatment decisions on parameters of T-cell immunity and on results of genetic studies in (initially) nontransplanted children with CID. The goal of the study was to analyze these data for predictors of outcome to provide guidance on whether and when patients with CID should undergo transplantation. Here, we report interim analysis of the first 51 patients recruited, to evaluate the feasibility of the study concept and raise awareness for our project.
METHODS
Study design and objectives
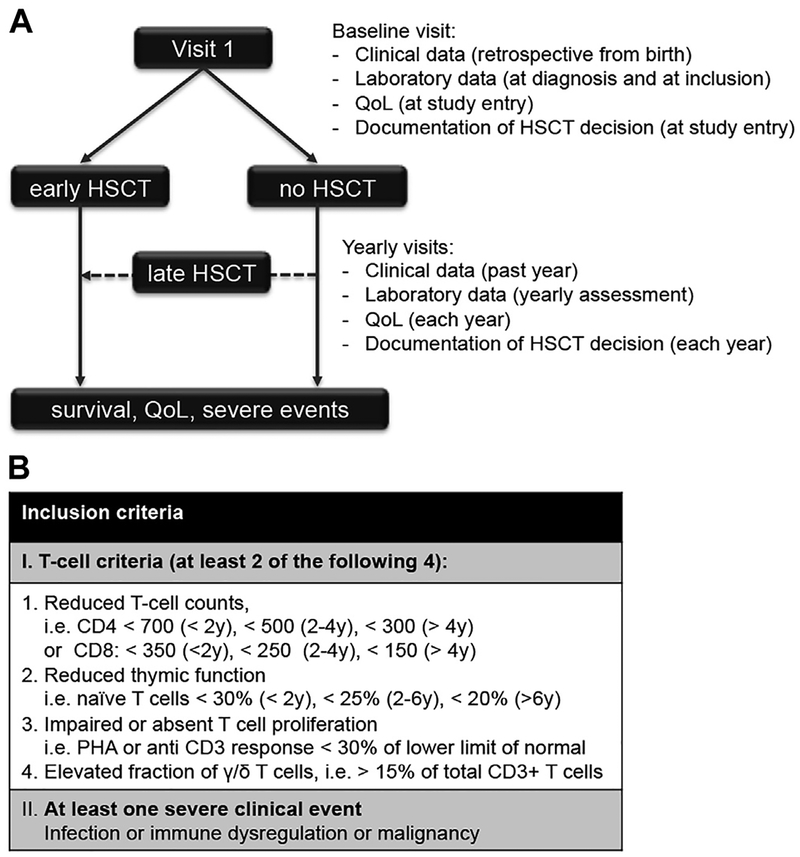
The P-CID study is a prospective international multicenter observational cohort study. The study protocol has been published.15 The primary objective was to compare data on the natural history of age and severity-matched patients with P-CID with or without transplantation after study inclusion. Secondary objectives were to determine whether and which immunologic parameters and clinical events (eg, infections, immune dysregulation, and chronic lung disease) are predictive for outcome, assessed as mortality, morbidity, and QOL. The main hypothesis is that patients with P-CID undergoing early HSCT have a better 5-year survival than patients with similar disease severity who undergo late HSCT or no HSCT. Fig 1, A, illustrates the study design. Patients are evaluated and treated according to local protocols, including the HSCT decision. Baseline and follow-up documentation of genetic, immunologic, and clinical parameters including infections and manifestations of immune dysregulation, the transplant decision, severe transplant-related events, and QOL is provided on standardized case report forms provided through the study Web site (www.pcid-study.org). At least 6 yearly study visits are scheduled.
FIG 1.
Overview of the study design and inclusion criteria. A, Each patient is seen in at least 6 study visits (1 baseline visit and 5 follow-up visits). At each visit, clinical data, laboratory data, QOL, and the local center’s decision on HSCT are documented. This decision is not influenced by the study protocol. Patients who undergo HSCT within 12 months after inclusion are followed in the “early HSCT” arm, the other patients in the “No HSCT” arm. Some patients receive HSCT during follow-up (“late HSCT”). After an observation period of at least 5 years, survival, QOL, and the frequency of severe clinical events are assessed in the whole cohort. B, Inclusion criteria. Nontransplanted HIV-negative patients, 1 to 16 years of age, with impaired T-cell immunity and at least 1 severe clinical event are included into the study.
Inclusion and exclusion criteria
Inclusion criteria are shown in Fig 1, B. We recruit nontransplanted patients with impaired T-cell immunity and at least 1 associated clinical manifestation (infection, immune dysregulation, or malignancy) who are 1 year or older and 16 years or younger at inclusion. The definition of impaired T-cell immunity was derived from a previous retrospective analysis of 73 patients with atypical SCID, where a combination of at least 2 of 4 abnormal T-cell criteria (CD4 or CD8 T-cell counts, percentage of naive CD4 T cells, T-cell proliferation, and percentage of γ/δ T cells) best characterized the cohort.14 Because of lack of standardized protocols for proliferation to anti-CD3 stimulation, we initially only considered results of PHA stimulation for the assessment of T-cell proliferation. After the first 20 patients, we realized that good coverage of our target population required the inclusion of anti-CD3 proliferation data. An amendment of the study protocol was issued and retrospective data were obtained where possible. However, for the present interim analysis, these data are still incomplete and only PHA responses were evaluated. We exclude patients with typical SCID16 phenotype, defined by (1) presentation with a SCID-defining infection (eg, pneumocystis jirovecii pneumonia, persistent cytomegalovirus, or generalized BCG infection) or Omenn syndrome in the first year of life AND (2) evidence of impaired T-cell immunity (CD3+ T-cell counts of <300/μL OR absent PHA or anti-CD3 response) AND (3) absence of evidence for a secondary immunodeficiency. The requirement for significantly impaired T-cell immunity delineates patients with P-CID from patients with mild CID and CVID.8 We exclude patients with Wiskott-Aldrich syndrome, CD40 ligand deficiency, or ataxia telangiectasia because larger cohorts have been published, offering the potential to address the P-CID study question in a disease-specific way.17,18
Patient recruitment
The first 51 patients were recruited at 15 study centers between November 2011 and March 2014. After informed consent had been obtained from the parents/legal guardians, eligibility was verified by the principal investigator on the basis of assessment of inclusion and exclusion criteria. The study was approved by local ethics committees (lead institutional review board approval by University of Freiburg, institutional review board no. 230/11) and registered in the World Health Organization study registry (www.drks.de/DRKS00000497).
Morbidity measure and disease categorization
To provide a quantification of clinical problems that have developed because of the immunodeficiency over time until study entry, a morbidity measure was calculated on the basis of the following 5 items: invasive bacterial infection (including pneumonia), viral or opportunistic infection, autoimmune cytopenia, other immune dysregulation, and chronic lung disease. For each patient, the number of clinical events related to this morbidity item (from birth up to study inclusion) was counted and divided by the patient’s age to correct for the time of exposure. The resulting 5 scores were added and used as a measure of morbidity. One limitation of the morbidity measure is the failure to differentially weigh acute versus chronic events. Each episode of an autoimmune disease requiring treatment and each acute infection requiring admission to hospital counted as a single severe event. Persistent infections (eg, EBV, HPV, or molluscum) or persistent immune dysregulation (eg, thyroiditis, chronic inflammatory bowel disease, or splenomegaly) were also counted as single events. We have used the term “morbidity measure” instead of “morbidity score” to reflect that it is a rough, preliminary tool requiring future validation. It is a goal of this study to identify the best predictors for a severe outcome and this analysis will allow adapting the morbidity measure to a proper risk score, which will then need prospective validation in an independent cohort. Because of this limitation, we also categorized the disease at study entry as either “severe” or “mild” as judged by the treating physician. This assessment represents an overall evaluation of the clinical status at study inclusion. Patients considered for HSCT because of overall disease severity or patients considered too sick for HSCTwere rated as “severe.” Patients not considered for HSCTor considered for transplantion for “prophylactic” reasons only were rated as “mild.”
Statistics
We used Kaplan-Meier plots to illustrate time-to-event data. We purposely elected not to provide any P values in this article because this is just an initial analysis. All statistical analyses were done using “R” software for Windows, version 3.2.2. (https://www.r-project.org/). Further information on the study protocol is available in this article’s Online Repository at www.jacionline.org.
RESULTS
Age distribution and genetic characteristics of the first 51 patients
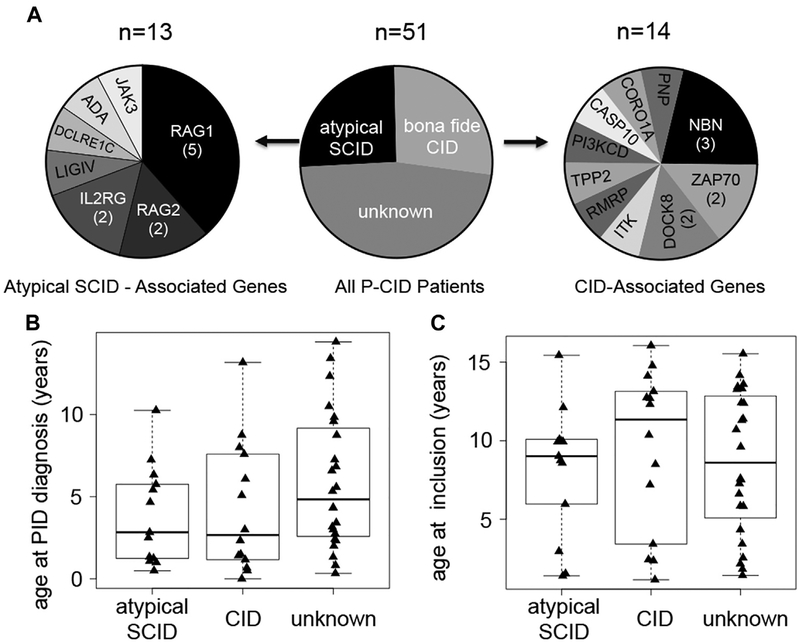
Between November 2011 and March 2014, 51 patients (29 males, 22 females) were recruited by 15 centers. A molecular diagnosis was established in 27 of 51 patients (Fig 2, A). Thirteen of these had atypical SCID (as defined in the Introduction). Their median age at clinical diagnosis was 2.7 years (Fig 2, B). Genetic diagnoses included 7 patients with RAG1 or RAG2 mutations, 2 with IL2RG mutations, and 1 each with adenosine deaminase, Janus kinase 3, DCLRE1CI, and LIG4 mutations. Fourteen had “bona fide” CID (as defined in the Introduction) with mutations in coronin-1A, dedicator of cytokinesis 8 (2), ITK, tripeptidyl-peptidase II, and ZAP70 (2), purine nucleoside phosphorylase, PIK3CD, caspase-10, and RNA component of mitochondrial RNA processing endoribonuclease, while 3 had nibrin mutations. Their median age at clinical diagnosis was 2.6 years. In the 24 patients without genetic diagnosis, the median age at diagnosis was 4.9 years (Fig 2, B). The median age at study entry was 9.6 years (1.2–16 years) without apparent differences between the 3 groups (Fig 2, C).
FIG 2.
Molecular diagnoses and age at study inclusion. A, Among 51 patients, 13 had mutations in genes that can also cause a SCID phenotype (“atypical” SCID; left pie chart). Fourteen patients had mutations in other genes associated with CID (right pie chart). Twenty-four patients currently remain without genetic diagnosis. ADA, Adenosine deaminase; CASP10, caspase-10; CORO1A, coronin-1A; DCLRE1C, DNA cross-link repair 1C; DOCK8, dedicator of cytokinesis 8; JAK3, Janus kinase 3; LIGIV, ligase IV; NBN, nibrin; PI3KCD, phosphoinositide 3-kinase; PNP, purine nucleoside phosphorylase; RAG1/2, recombination-activating genes 1 and 2; RMRP, RNA component of mitochondrial RNA processing endoribonuclease; TPP2, tripeptidyl-peptidase II. B, Age at diagnosis, that is, the time point at which the treating physician diagnosed an underlying immunodeficiency. C, Age at inclusion into the P-CID study. Data for B and C were evaluated separately for patients with atypical SCID, CID, or unknown molecular cause.
Analysis of CID-related illnesses
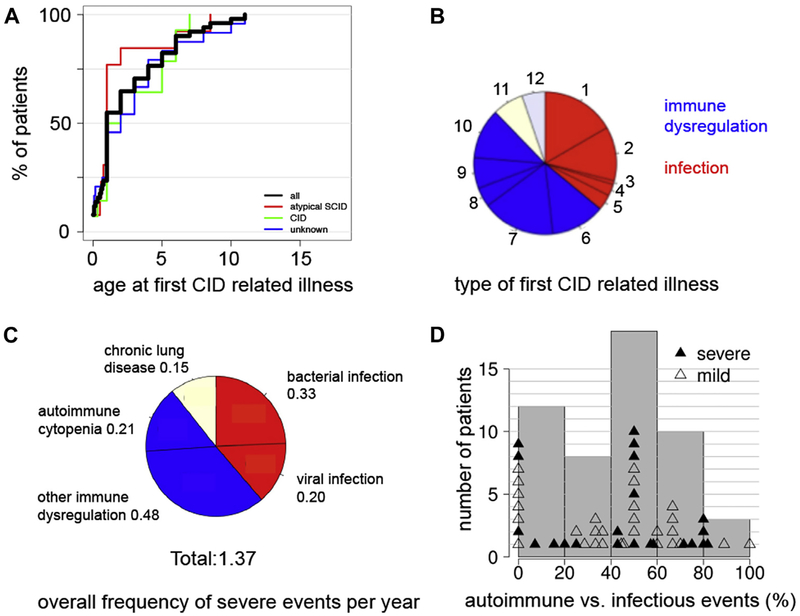
Seventy-six percent of patients had their first clinical manifestation before the age of 5 years in all 3 patients groups (Fig 3, A). Among the events observed during the first year after initial presentation, 37% were infections, mostly invasive bacterial infections (including pneumonia; 17%) and severe acute viral infections (12%). Notably, 51% of events were related to immune dysregulation, most frequently severe eczema (17%), autoimmune disease (12%), and autoimmune cytopenia (11%) followed by lymphoproliferation (7%) and inflammatory bowel disease (4%) (Fig 3, B). Other organ manifestations including chronic lung and liver disease were diagnosed in the first year of illness in 7% of patients each. Among all events that had occurred until study inclusion in all patients, 51% were manifestations of immune dysregulation, a third of which were episodes of autoimmune cytopenia, while the other half were infections or chronic lung disease (Fig 3, C). To further characterize the spectrum of clinical manifestations until study entry, we grouped the patients according to the percentage of infectious versus immune dysregulation events in their individual history. Remarkably, although most patients had both infections and immune dysregulation, patients with a predominant immune dysregulation phenotype were more frequent than those with a predominant infection phenotype (Fig 3, D). This distribution was not different in patients judged to have a mild versus severe clinical phenotype.
FIG 3.
Key clinical characteristics of the P-CID study cohort. A, Cumulative percentage of patients having suffered from their first immunodeficiency-related illness at a given age for all patients (black line) and the 3 subgroups (color code indicated in figure). B, Type of immunodeficiency-related illnesses within the first year of clinical presentation. 1, invasive bacterial infection; 2, severe acute viral infection; 3, persistent viral infection; 4, opportunistic infection; 5, other infection; 6, autoimmune disease; 7, skin disease; 8, gastrointestinal disease; 9, lymphoproliferation; 10, autoimmune cytopenia; 11, chronic lung disease; 12, other ID-related organ complication. C, Overall frequency of severe clinical events. The pie chart illustrates the relative frequency of all individual clinical events observed in all patients of the cohort. The number indicates the mean number of events per year among all patients in the study. D, Relative distribution of infections versus manifestations of immune dysregulation as defined for the morbidity measure for all individual patients (mild disease course, open triangles; severe disease course, filled triangles). “50%” indicates an equally balanced distribution of infections/immune dysregulation events. Patients between the range of 50% to 100% predominantly had manifestations of immune dysregulation, and patients between the range of 0% to 50% predominantly had infections. Gray bars summarize the individual patients in groups.
Patient morbidity, treatment, and QOL
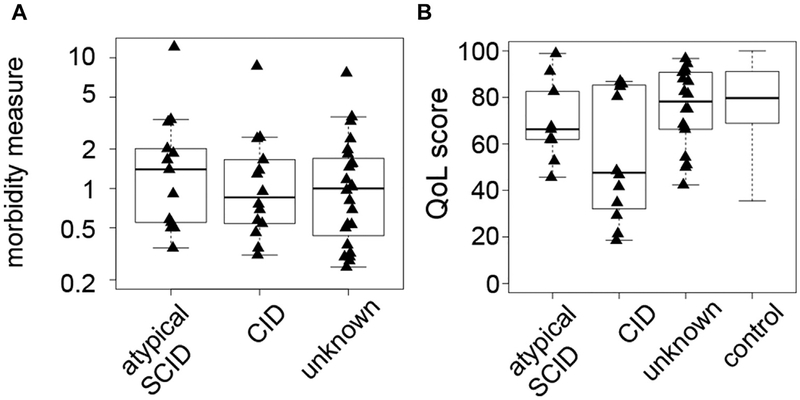
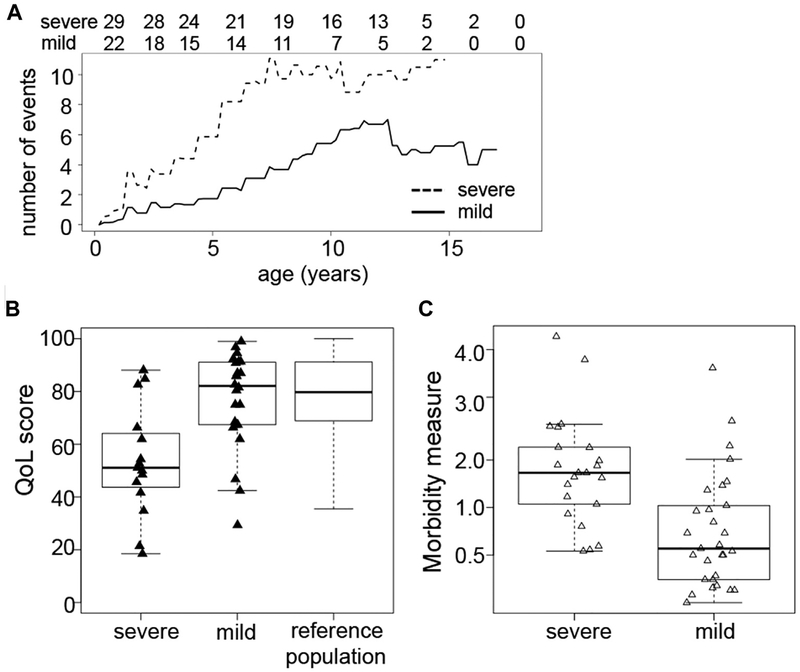
Most patients had 0.5 to 1 clinical event per year, whereas several had 2 to 4 events with no relevant differences between the patient groups (Fig 4, A). QOL was reduced compared with a healthy reference population (Fig 4, B). Overall disease severity (mild vs severe) as judged by the treating physician delineated 2 groups with different rates of accumulation of severe events (see Fig E1, A, in this article’s Online Repository at www.jacionline.org). The physician judgment correlated well with the morbidity measure and QOL (Fig E1, B and C). The severity of the disease course in this P-CID cohort was also illustrated by the treatment history. Twenty patients (39%) had received steroids, 10 patients immunosuppressive drugs, and 9 patients rituximab, while 2 patients were splenectomized. Forty patients (78%) received immunoglobulins (the 11 other patients either had normal vaccine responses or were just recently diagnosed and therefore not yet on immunoglobulin substitution) and 48 (94%) received prophylactic antimicrobials, mostly cotrimoxazole. One patient with later-diagnosed RAG1 deficiency received IgG replacement after a bacterial pneumonia at age 6 months, but none of the other patients had received prophylactic treatment in infancy.
FIG 4.
Morbidity assessment and baseline QOL of the P-CID study cohort. A, Morbidity measure in patients with atypical SCID, CID, and unknown molecular diagnosis. B, QOL at study entry as assessed by PedsQL Generic Core Scales of patients with atypical SCID, CID, and unknown molecular diagnosis vs a reference population of healthy children.E1
FIG E1.
Correlation of different parameters assessing disease severity. A, Mean cumulative number of events at a given age for patients judged to have a severe (dashed line) or a mild disease course (solid line) at study entry. The absolute number of patients at risk at a given time is indicated in the line above the figure. B, QOL scores at study entry for patients with a severe or a mild disease score. C, Morbidity measure in patients with severe and mild disease course as judged by the treating physician.
Immunologic parameters at study entry
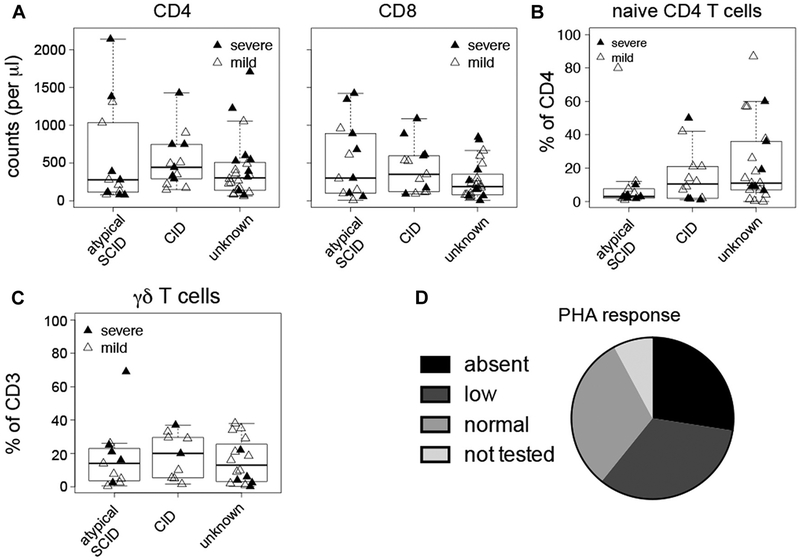
T-cell counts were variable without significant differences between the patient groups (Fig 5, A). Twenty-four patients (47%) had CD4 counts below 300/μL and 30 (59%) had CD8 T-cell counts below 300/μL. The median percentage of naive CD4+ T cells was around 10% in all patient groups (Fig 5, B), and 34 patients (74%) had below 20% naive T cells. Fourteen of 36 patients (39%) had above 20% γ/δ T cells (Fig 5, C), with a similar distribution in all groups. Surprisingly, we did not observe relevant differences in any of these T-cell parameters between patients categorized as mild or as severe by the treating physician (Fig 5, A–C). PHA responses were absent or low in 31 (61%) (Fig 5, D).
FIG 5.
Immunologic parameters at study inclusion. In A-C, data are shown separately for patients with atypical SCID, CID, and unknown molecular diagnosis. Filled triangles represent patients with a severe disease course, open triangles patients with a mild disease course. A, Counts of CD4 T cells (left panel) and CD8 T cells (right panel). B, Naive CD4 T cells determined as percentage of CD45RA+CD62L+ or CD45RA+CD31+ of CD4 T cells. C, Percentage of γ/δ TCR+ among CD3 T cells. D, T-cell proliferation response (absent, <10%; low, 10% to 30%; normal, >30% of local control) after stimulation with PHA.
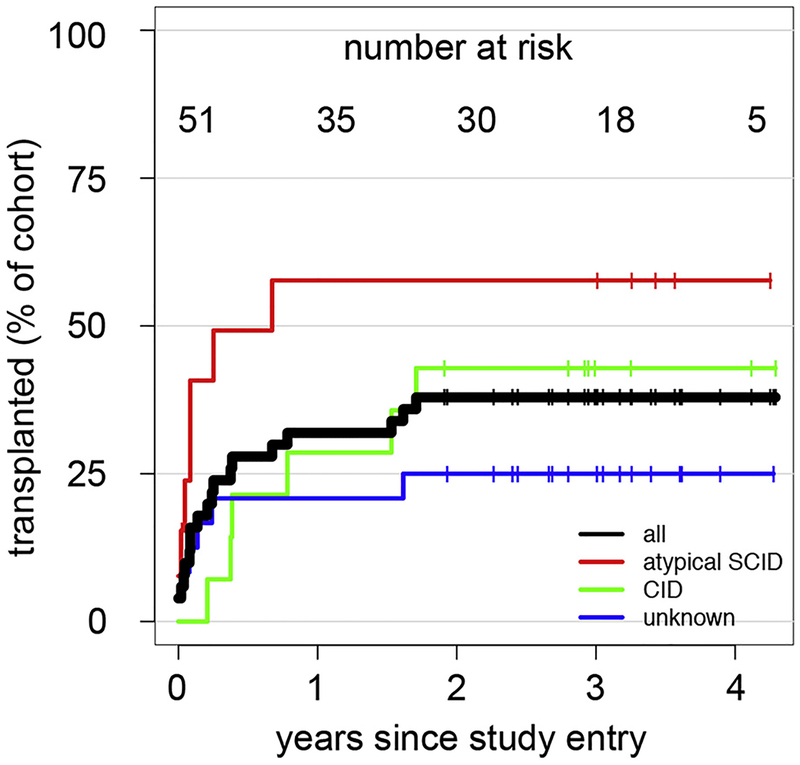
Analysis of HSCT decisions
Sixteen of 51 patients (31%) underwent transplantation within the first year after inclusion, and 3 additional patients underwent transplantation within current follow-up (Fig 6). The percentage of patients with atypical SCID (as defined in the Introduction) who underwent transplantation within the first year was twice as high as that of patients without molecular diagnosis. Interestingly, of 22 patients with severe disease as judged by the treating physician, only 13 underwent transplantation. Reasons for not transplanting included lack of donor (3), disease too severe (3), family denial (4), and death (2). However, of 29 patients with mild disease, 6 underwent transplantation for “prophylactic reasons,” that is, family history or because the genetic diagnosis (DCLEREC1, LIGIV, IL2RG, nibrin) was considered an HSCT indication. So far, 5 patients died, all from infections: 2 after HSCT (RAG1, ZAP70) and 3 without HSCT (ITK, RAG1, RAG2).
FIG 6.
Patients undergoing HSCT since study inclusion. Kaplan-Meyer curve indicating the cumulative percentage of patients who underwent transplantation at a given time after study inclusion. Results are shown for all patients (black line) and the 3 subgroups (color code indicated in figure). Vertical lines indicate the time point of censoring for individual patients. The absolute number of patients at risk at a given time is indicated at the top of the figure.
Feasibility assessment of a matched-pair analysis
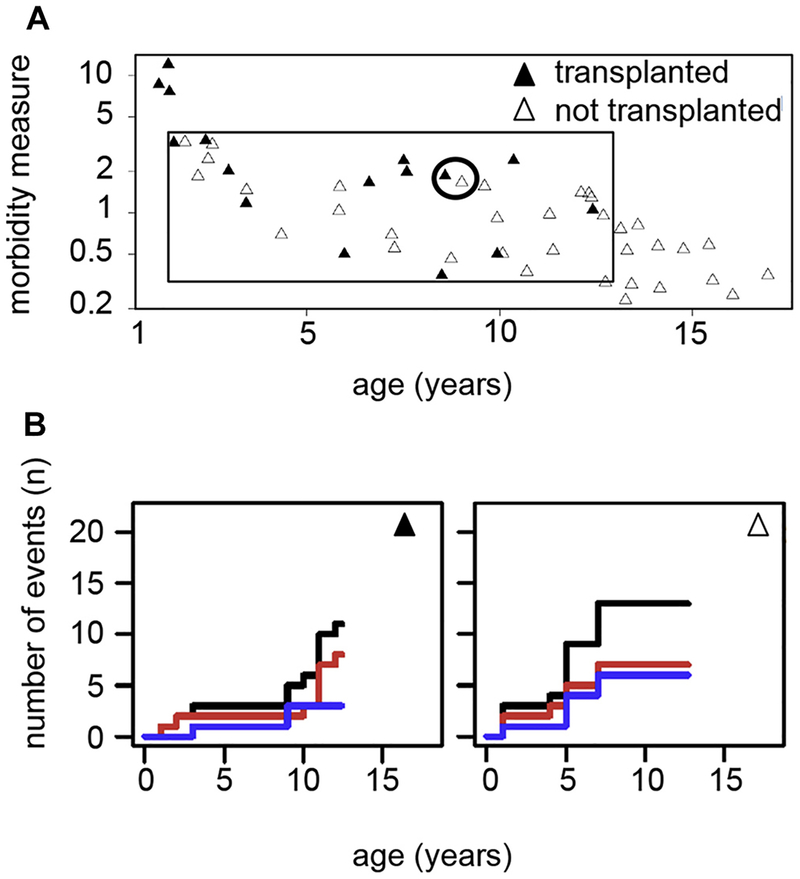
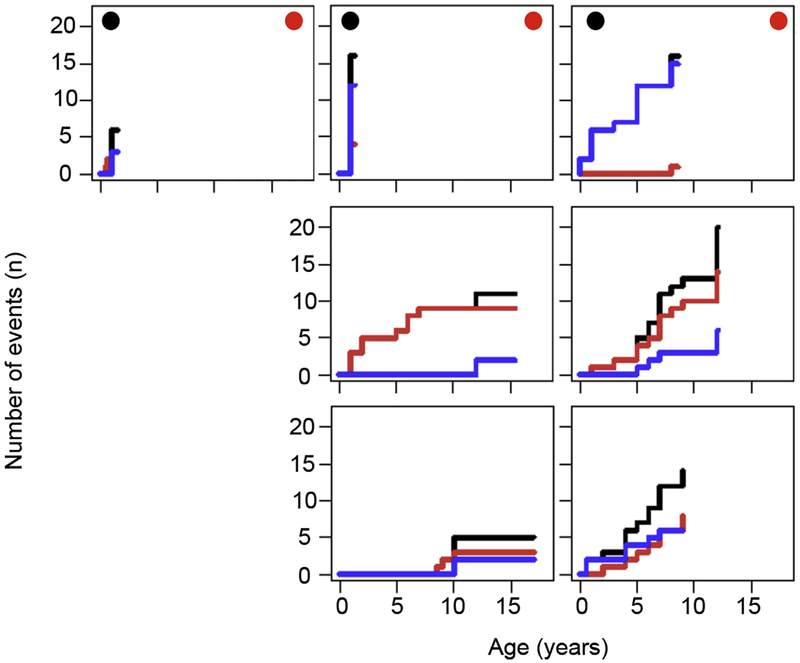
To estimate how informative the cohort will be with respect to a matched-pair outcome analysis of patients who underwent or did not undergo transplantation, we related the age-corrected morbidity measure in all patients to their current HSCT status. Three patients had an early intensive disease course and underwent transplantation within the first 2 years of life (Fig 7, A), reflecting the boundary between CID and typical SCID. In contrast, 13 patients had a mild disease course up to age 12 to 16 years, reflecting the boundary between CID and CVID. Although these patients will be informative for analysis of the predictive value of immunologic parameters, it is unlikely that they can eventually be matched with corresponding (non-) transplanted patients. However, 35 patients (69%) had moderate disease severity, where transplant decisions were variable despite similar morbidity. These patients provide opportunities for future matched-pair analysis. To visualize the disease course of such matched pairs, we composed a graphical chart reflecting the cumulative incidence of CID-related health events over time including a separate display of infections and immune dysregulation (Fig 7, B). The charts illustrate that the P-CID study will allow comparing patient pairs of similar age and similar disease profile, but divergent HSCT decisions. We also used these charts to determine whether grouping patients according to their genetic defect would be a better strategy for analysis. Fig E2 in this article’s Online Repository at www.jacionline.org illustrates the known clinical heterogeneity in the 7 RAG1/2 deficient patients, arguing against this approach.
FIG 7.
Graphical illustration documenting the feasibility of the planned matched-pair analysis. A, Assessment of morbidity measures plotted against the age at study inclusion for all patients. Patients who underwent transplantation within the first year after inclusion are represented by filled triangles, nontransplanted patients by open triangles. Inside the black-rimmed box are patients who will be included in the future matched-pair analysis. Outside the box are patients who are either very sick very early (“SCID-like,” left outside the box) or patients who are quite healthy as teenagers (“CVID-like,” right outside the box) and therefore not informative for the matched-pair analysis. The circle indicates the patient pair further visualized in B. B, Example of a patient pair with a similar morbidity measure who will quality for a matched-pair analysis. The staircase diagrams summarize the clinical course of an individual patient over time. Individual clinical events are indicated by an upward step on the y-axis. The cumulative course of all clinical events per patient is indicated by a black line. A separate analysis of infections and manifestations of immune dysregulation is given in red and blue, respectively.
FIG E2.
Summary of clinical course of RAG-deficient patients. Each staircase diagram summarizes the clinical course of an individual patient with a mutation in RAG1 or 2 over time. Individual clinical events are indicated by an upward step on the y-axis. The cumulative course of all clinical events per patient is indicated by a black line. A separate cumulative analysis of infections and manifestations of immune dysregulation is given in red and blue, respectively. Black circles in the upper left corner indicate patients who underwent HSCT less than 1 year after study inclusion. Red circles in the upper right corner indicate patients with severe course as judged by their primary physician.
DISCUSSION
The P-CID study targets patients in whom impaired T-cell immunity leads to significant complications, but is not severe enough for an unambiguous early transplant decision. For these patients, decisions on the indication and time point for HSCT must carefully weigh the risks of transplantation against risks of further disease evolution. Given the rarity and phenotypic heterogeneity of the individual genetically defined conditions, natural history studies are challenging. We hypothesize that a clinical and immunologic rather than a genetic definition of P-CID best delineates a cohort in which guidance for transplant decisions can be obtained. We therefore initiated a prospective long-term natural history study for patients with P-CID. A key feature is the parallel observation of patients with a similar clinical course as a result of their T-cell deficiency who undergo or do not undergo transplantion in the observation period including a careful analysis of the HSCT decision. This interim analysis documents the feasibility of this study approach.
Recently, profound T-cell deficiencies have been defined from different perspectives and it is important to relate the P-CID cohort to these definitions (see Table E1 in this article’s Online Repository at www.jacionline.org).3,8,14,16,19 From the “perspective of HSCToutcome,” the Primary Immune Deficiency Treatment Consortium study 6901 defines “leaky” SCID in patients with reduced, but present (>300/μL) T cells (<1000/μL if <2 years, <800/μL if 2–4 years, and <600/μL if >4 years) and reduced PHA response or a pathologic mutation in a known SCID gene.20 Similarly, “CID” has been delineated from “typical SCID” in a retrospective cohort of transplanted patients by more than 500/μL T cells.3 From the “diagnostic perspective” of T-cell receptor excision circle–based newborn screening (NBS), Kwan et al19 define “leaky” SCID in an infant identified by NBS with 300 to 1500/μL T cells, reduced naive T cells, and reduced proliferation, supported by an incomplete defect in a known SCID gene. Thirteen of the first 50 Primary Immune Deficiency Treatment Consortium 6901 patients and 10 of 52 patients identified by NBS fulfilled these definitions, all underwent transplantation early, and all 18 with a genetic diagnosis had mutations in known SCID genes.19,20 None of these patients would have qualified for our study. The NBS study also defines idiopathic T-cell lymphopenia (or variant SCID) as a condition with less than 1500/μL, less than 2500/μL, or less than 3505/μL T cells plus “lack of naive T cells” defined according to local screening authorities. Fourteen such patients have been reported by Kwan et al, all without a molecular diagnosis. Some of these patients would probably qualify for the P-CID study once they acquire clinical symptoms during follow-up. Thus, the P-CID study targets a unique cohort with minimal overlap to other ongoing protocols.
The P-CID definition was derived from an “intention-to-treat perspective.” We excluded patients with T-cell deficiency in whom the treatment decision was clear in infancy. These include patients with typical SCID and also patients with normal T-cell numbers, but severe functional defects leading to manifestations severe enough for HSCT in infancy (eg, ORAI1, IKBK, caspase recruitment domain family, member 11 deficiency, and some patients with IL2RGR222C mutations21–23). Instead, we focus on patients in whom the treatment decision is less clear. This includes some patients with atypical SCID (as defined in the Introduction) that could potentially be identified by NBS, but importantly an equal number of patients with forms of bona fide CID (as defined in the Introduction) that will most likely not be identified by NBS (eg, ZAP70 deficiency24). Patients with these moderate T-cell deficiencies are not specifically recruited by other current studies.
This interim analysis demonstrates that our study protocol indeed recruits the targeted patient population. The T-cell inclusion criteria were initially derived from a retrospective study on patients with atypical SCID,14 but in this study proved suitable to equally identify patients with bona fide CID. A third of the patients had significant lymphopenia, more than 50% had less than 10% naive T cells, and 61% had reduced/absent PHA proliferation. Detailed further phenotypic and functional immunotyping is ongoing. Overall, patients with P-CID had the targeted profound T-cell deficiency, which was also reflected by treating physician’s decision to use cotrimoxazole prophylaxis and immunoglobulins in 94% and 78%. Our inclusion criteria in addition required at least 1 severe clinical event. Immune dysregulation was frequent as first, predominant, and even only overt manifestation of CID. This led to the use of steroids and immunosuppressive drugs in more than half of the patients. Overall morbidity was significant with a median of 1 severe event per year, reduced QOL, and significant mortality. In accordance with the initial study assumptions, one-third of patients underwent transplantation within the first year after study inclusion. Genetic diagnoses were equally diverse among patients with atypical SCID and bona fide CID. For patients without molecular diagnosis, ongoing genetic studies promise novel insights into the molecular regulation of human T-cell immunity. In summary, the P-CID study succeeded in recruiting patients with impaired T-cell deficiency associated with significant morbidity, but with a spectrum of molecular diagnoses and age profile (median, 9.6 years) that is unique and differs from other prospective studies on T-cell deficiencies.
A key question to the study concept is whether the heterogeneity in genetics, disease course, and age can be sufficiently controlled to perform comparative outcome analysis with and without HSCT or to determine predictors for outcome at the cohort level. First, the molecular heterogeneity will have consequences for the immunologic and clinical variability of the patients. Some genetic defects will affect T-cell numbers more than T-cell function and some defects will in addition affect other immune cell functions. Subgrouping will help to determine which of these factors contribute to outcome. Patients in whom additional defects outside of the immune system (eg, radiosensitivity) have a significant impact on life expectancy or QOL are rare in our cohort and can be excluded for certain elements of the analysis. Second, the problem of a variable time of exposure to the risks of the T-cell deficiency can be statistically addressed to account for the delayed entry (see this article’s Methods section in the Online Repository at www.jacionline.org). Third, we expect that different center policies and experience in addition to patient-specific variables including donor availability and the will of the patient’s family will have an impact on the transplant decision. Data from this interim analysis illustrate that this variability actually benefits the study concept. The P-CID study recruits patients who undergo transplantation soon after inclusion and others with similar age and with similar disease severity who do not. We expect that among the additional 70 patients, at least 50 will be in the window of interest that will allow forming matched pairs for comparative outcome analysis. This is based on the fact that we recruit only few patients who are very sick very early (“SCID-like”) and few patients who are quite healthy as teenagers (“CVID-like”).
The study results so far confirmed our assumption that the genetic diagnosis has limited value as a predictor of disease evolution. There was no relevant difference in age at diagnosis, age at first illness, overall age-corrected morbidity, or QOL between patients with atypical SCID, bona fide CID, or genetically undefined disease. The highly variable course of the 8 patients with RAG deficiency, only 3 of whom have undergone transplantation so far, illustrates that knowledge about the affected gene does not imply a clear prognosis and treatment decision.
More unexpectedly, our results also indicate that basic measurements of T-cell immunity (ie, T-cell numbers, naive T cells, and PHA proliferation) cannot easily identify patients with P-CID with a poor prognosis. Additional patients in long-term follow-up will help extend these initial observations.
In summary, the P-CID study for the first time defines and characterizes a group of patients with non-SCID T-cell deficiencies from a therapeutic perspective. This first analysis indicates that the P-CID study will reach its study goals. Further recruitment is needed to reach 120 patients and study discipline during long-term follow-up will continue to be a collaborative challenge. However, the potential of P-CID, both as a clinical study and as a unique platform for molecular and immunologic studies by all participants, makes us confident that we will achieve our goals.
METHODS
Study protocol
Advanced genetic and immunologic studies.
All patients undergo genetic studies according to center policy. If no genetic diagnosis is reached, study consent foresees the possibility to perform advanced genetic studies including whole-exome and whole-genome sequencing. This is performed in separate research projects outside the clinical study protocol. Among members of the P-CID consortium, flow cytometry panels for extensive B- and T-cell phenotyping have been standardized. These panels are performed at each study visit in all centers offering these investigations. Study consent foresees the possibility of using frozen samples for additional phenotypic and functional studies in the context of separate research projects. Biomaterial (serum, PBMC, DNA, fibroblasts) is stored locally from all patients, and this is documented in the common database at each study visit.
Decision to transplant.
The decision to transplant is an individual decision based on the severity of clinical manifestations, the severity of the T-cell deficiency, the local center’s policy (ie, how these factors are interpreted), donor availability, and the will of the patient’s family. Reasons for transplanting/not transplanting are carefully documented at every visit. The study protocol does not issue recommendations for transplantation indication, donor selection, or conditioning regimes.
Outcome parameters.
We analyze overall and event-free survival, the incidence of severe events (infections, autoimmunity, malignancy), and the incidence of chronic lung disease and QOL at study entry and 5 years after study inclusion. Chronic lung disease is defined as the presence of bronchiectasis, interstitial lung disease, or other defined chronic lung disease (eg, granulomatous and lymphocytic interstitial lung disease). The severity is judged by the impairment of lung function as assessed by lung function testing and by the need for home oxygen. A “morbidity measure” is assessed for each patient at study entry and 5 years after study inclusion by dividing the cumulative number of clinical events (bacterial infections, viral or opportunistic infections, immune dysregulatory events, and chronic lung disease) by the patient’s age. QOL is evaluated using the PedsQL Generic Core Scales compared with a reference population.E1 Outcome parameters will be compared between patients receiving HSCT (group 1) and patients not receiving HSCT (group 2). To account for patient heterogeneity, we will also analyze patient outcome in a matched-pair analysis. Here, patient pairs with similar age and morbidity measure at study entry, but divergent decision on HSCT during follow-up, will be compared. Finally, we will determine to which extent clinical events and immunologic parameters and their changes are predictive for outcome (survival, morbidity, and QOL). For this, immunologic and clinical data are documented retrospectively at study entry and prospectively yearly thereafter.
Statistical considerations
Statistical adjustment for time of exposure.
In the P-CID study, the age at study inclusion is between 1 and 16 years and therefore the time of exposure to the risks of the T-cell deficiency before the controlled observation is variable. Left-truncationE2 is a statistical adjustment to account for this fact. It is used when individuals are not observed at the event (eg, chronic lung disease) under study but come under observation at some known later time (called the left-truncation time). In this case, the calculation of the risk of developing chronic lung disease does not include individuals whose left-truncation times exceed the given event time (ie, who already have chronic lung disease before study entry).
Predictive value of clinical and immunologic parameters.
We aim to model which variables predict whether disease course is mild versus severe using the following variables of interest: illnesses (infections, immune dysregulation, malignancy, chronic lung disease), CD4 and CD8 T-cell counts, percentage naive T cells, and PHA and anti-CD3 response. As a statistical model for severe/mild status of patients, we use a multivariable logistic regression model with an added least absolute shrinkage and selection operator penalty term that simultaneously performs modeling and variable selection for an optimal value of a parameter lambda.E3 To determine an optimal value of λ, 17-fold cross-validation is used.
Matched-pair analysis.
The primary variable for matching will be disease severity assessed by the morbidity measure at study inclusion. As a secondary variable, patients can be selected for age at study entry. Matching for disease category, that is, “atypical” SCID versus “bona fide” CID, will be performed only if further observations indicate significant clinical differences between these groups (not observed in the “first fifty” analysis). We assume that among 120 patients, at least 30 patient pairs can be formed. The pairs will be analyzed both visually and analytically. Visually, an assessment will be made by the investigators through comparing the plots of the course of clinical events within pairs (see Fig 7). For an analytical assessment, binary outcomes such as 5-year survival rates can be estimated using conditional logistic regression.
Sample size considerations.
The original study protocol specifies that we intended to recruit 120 patients by March 2015 and 200 patients by March 2017, when we wanted to close recruitment. Of these, it was estimated that 40% will receive primary HSCT, 30% secondary HSCT, and 30% no HSCT. To determine, how many patients would be needed to compare outcomes in those undergoing 1 versus 2 versus no HSCT, sample size calculations have been performed. Assuming a reference 5-year mortality of 20%, 60 patients in each of the 3 groups would allow detecting a relative risk of 2.2 with a power of 80%.
We have currently (February 2016) enrolled 93 patients and have recently initiated a number of additional centers including large centers in the United States and Canada. We are therefore confident that we can enroll at least 120 patients until March 2017. The initial assumptions with respect to HSCT were reasonable, with the reference mortality being lower than expected in the range of 10%. The basis for choosing 120 as a revised minimum recruitment goal was (1) a pragmatic consideration of the recruitment potential in the Inborn Error Working Party/ESID community, (2) a modification of the analysis concept from 3 groups to 2 groups with group 1 receiving HSCT within the 5-year follow-up and group 2 remaining without HSCT, and (3) to be able to analyze at least 30 patient pairs (transplanted/not transplanted) matched for age and disease severity.
Key messages.
P-CID defines a distinct patient group in which impaired T-cell immunity leads to significant complications, but is not severe enough for an unambiguous transplant decision.
In the first 51 patients of the P-CID study, neither the genetic diagnosis nor basic current measurements of T-cell immunity were good predictors of disease evolution.
Acknowledgments
The P-CID study is funded by the German Federal Ministry of Education and Research (BMBF grant no. 01 EO 0803) and a Chaim Roifman Scholar Award from the Canadian Immunodeficiency Society. This project has further received funding from the European Union’s Horizon 2020 Research and Innovation Programme under the ERA-NET Cofund action N°8643578.
We thank our patients and their family for participating in this study. We are grateful to the technicians from the CCI Advanced Diagnostic Unit for excellent technical assistance and our study nurse Henrike Ritterbusch for her dedicated support of this study.
Abbreviations used
- CID
Combined immunodeficiency
- CVID
Common variable immunodeficiency
- HSCT
Hematopoietic stem cell transplantation
- IL2Rg
IL-2 receptor gamma chain
- ITK
IL2-inducible T-cell kinase
- NBS
Newborn screening
- ORAI1
ORAI calcium release-activated calcium modulator 1
- P-CID
Profound combined immunodeficiency
- QOL
Quality of life
- RAG
Recombination-activating gene
- SCID
Severe combined immunodeficiency
- ZAP70
Zeta-chain (TCR)-associated protein kinase 70kDa
Footnotes
Disclosure of potential conflict of interest: C. Speckmann receives grant support from the German Ministry for Education and Research (BMBF); payments for lectures from Octapharma and CSL Behring; and travel support from Orphan Europe and CSL Behring. A. Aiuti receives grant support from the European Research Council, Fondazione Telethon Rome, Italian Ministry of Health, and GSK. M. H. Albert receives grant support from GSK; receives payment for lectures from Biotest, MSD, and Jazz; holds stock in Amen and BMS; and receives travel support from Octapharma. T. Avcin serves as a consultant for Octapharma and receives payment for lectures from Octapharma. C. Cancrini receives grant support from Ministero della Salute and European Community and serves as a consultant for UCB CellTech UK. A. Finocchi receives grant support from Telethon. H. Bobby Gaspar serves as a consultant for Orchard Therapeutics and holds stock in Orchard Therapeutis. K. Gilmour receives travel support from UKPIN. M. Hoenig receives payment for lectures from CSL Behring and Jazz Pharma and travel support from CSL Behring and Jazz Pharma. L. Notarangelo serves on the board of Novimmune and receives grant support from the National Institutes of Health and royalties from Up-to-Date. M. G. Seidel serves as a consultant for Baxalta and Novartis; received payments for lectures from Jazz Pharmaceuticals, Novartis, and CSL Behring; and received travel support from Jazz Pharmaceuticals, Octapharma, and Amgen. P. Soler-Palacin receives travel support from the P-CID study group; expert testimony from CSL Behring, Octapharma, Grifols, and Baxter; grant support from CSL Behring; and payments for lectures from Grifols. K. Warnatz serves on the board for BioTest, CSL Behring, and LFB Biomedicaments; receives grant support from BMS, CSL Behring, and BioTest; and receives payments for lecture from LFB Biomedicaments, Baxter, GSK, CSL Behring, Pfizer, BioTest, Novartis Pharma, Roche, Octapharma, and UCB Pharma. A. Worth receives research support from the National Institute of Health Research and Wellcome Trust. A. Uhlmann receives travel support from BMBF. S. Ehl receives research support from BMBF, the Canadian Immunodeficiency Society, and European Union’s Horizon 2020 Research and Innovation Programme; serves as a consultant for UCD and Novartis but not in the context of this study; and received payments from lectures for CSL Behring. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Buckley R Primary immunodeficiency diseases due to defects in lymphocytes. N Engl J Med 2000;343:1313–24. [DOI] [PubMed] [Google Scholar]
- 2.Notarangelo LD. Primary immunodeficiencies. J Allergy Clin Immunol 2010;125: S182–94. [DOI] [PubMed] [Google Scholar]
- 3.Roifman CM, Somech R, Kavadas F, Pires L, Nahum A, Dalal I, et al. Defining combined immunodeficiency. J Allergy Clin Immunol 2012;130:177–83. [DOI] [PubMed] [Google Scholar]
- 4.Al-Herz W, Bousfiha A, Casanova J-L, Chatila T, Conley ME, Cunningham-Rundles C, et al. Primary immunodeficiency diseases: an update on the classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency. Front Immunol 2014;5:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ameratunga R, Brewerton M, Slade C, Jordan A, Gillis D, Steele R, et al. Comparison of diagnostic criteria for common variable immunodeficiency disorder. Front Immunol 2014;5:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gathmann B, Mahlaoui N, Ceredih F, Gérard L, Oksenhendler E, Warnatz K, et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol 2014;134:116–26. [DOI] [PubMed] [Google Scholar]
- 7.Malphettes M, Gerard L, Carmagnat M, Mouillot G, Vince N, Boutboul D, et al. Late-onset combined immune deficiency: a subset of common variable immunodeficiency with severe T cell defect. Clin Infect Dis 2009;49:1329–38. [DOI] [PubMed] [Google Scholar]
- 8.ESID Registry – working definitions for clinical diagnosis of PID. 2015. Available at: http://esid.org/Working-Parties/Registry/Diagnosis-criteria. Accessed July 15, 2016.
- 9.Nehme NT, Pachlopnik Schmid J, Debeurme F, Andre-Schmutz I, Lim A, Nitschke P, et al. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood 2012;119:3458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Davis J, Lamborn I, Freeman A, Jing H, Favreau A, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med 2009;361: 2046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hauck F, Randriamampita C, Martin E, Gerart S, Lambert N, Lim A, et al. Primary T-cell immunodeficiency with immunodysregulation caused by autosomal recessive LCK deficiency. J Allergy Clin Immunol 2012;130:1144–52.e11. [DOI] [PubMed] [Google Scholar]
- 12.Huck K, Feyen O, Niehues T, Ruschendorf F, Hubner N, Laws HJ, et al. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J Clin Invest 2009; 119:1350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arpaia E, Shahar M, Dadi H, Cohen A, Roifman CM. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking zap-70 kinase. Cell 1994;76:947–58. [DOI] [PubMed] [Google Scholar]
- 14.Felgentreff K, Perez-Becker R, Speckmann C, Schwarz K, Kalwak K, Markelj G, et al. Clinical and immunological manifestations of patients with atypical severe combined immunodeficiency. Clin Immunol 2011;141:73–82. [DOI] [PubMed] [Google Scholar]
- 15.Speckmann C, Uhlmann A, Doerken S, Wolkewitz M, Pohl A, Ehl S. A prospective outcome study of patients with profound combined immunodeficiency (P-CID). LymphoSign J 2015;2:91–106. [Google Scholar]
- 16.Shearer WT, Dunn E, Notarangelo LD, Dvorak CC, Puck JM, Logan BR, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol 2014;133:1092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moratto D, Giliani S, Bonfim C, Mazzolari E, Fischer A, Ochs HD, et al. Long-term outcome and lineage-specific chimerism in 194 Wiskott-Aldrich syndrome patients treated by hematopoietic cell transplantation between 1980–2009: an international collaborative study. Blood 2011;118:1675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkelstein JA, Marino MC, Ochs H, Fuleihan R, Scholl PR, Geha R, et al. The X-linked hyper-IgM syndrome: clinical and immunologic features of 79 patients. Medicine (Baltimore) 2003;82:373–84. [DOI] [PubMed] [Google Scholar]
- 19.Kwan A, Abraham RS, Currier R, Brower A, Andruszewski K, Abbott JK, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA 2014;312:729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dvorak CC, Cowan MJ, Logan BR, Notarangelo LD, Griffith LM, Puck JM, et al. The natural history of children with severe combined immunodeficiency: baseline features of the first fifty patients of the Primary Immune Deficiency Treatment Consortium Prospective Study 6901. J Clin Immunol 2013;33:1156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pannicke U, Baumann B, Fuchs S, Henneke P, Rensing-Ehl A, Rizzi M, et al. Deficiency of innate and acquired immunity caused by an IKBKB mutation. N Engl J Med 2013;369:2504–14. [DOI] [PubMed] [Google Scholar]
- 22.Stepensky P, Keller B, Buchta M, Kienzler AK, Elpeleg O, Somech R, et al. Deficiency of caspase recruitment domain family, member 11 (CARD11), causes profound combined immunodeficiency in human subjects. J Allergy Clin Immunol 2013;131:477–85.e1. [DOI] [PubMed] [Google Scholar]
- 23.Fuchs S, Rensing-Ehl A, Erlacher M, Vraetz T, Hartjes L, Janda A, et al. Patients with T(+/low) NK(+) IL-2 receptor γ chain deficiency have differentially impaired cytokine signaling resulting in severe combined immunodeficiency. Eur J Immunol 2014;44:3129–40. [DOI] [PubMed] [Google Scholar]
- 24.Hauck F, Blumenthal B, Fuchs S, Lenoir C, Martin E, Speckmann C, et al. SYK expression endows human ZAP70-deficient CD8 T cells with residual TCR signaling. Clin Immunol 2015;161:103–9. [DOI] [PubMed] [Google Scholar]
- E1.Varni JW, Seid M, Kurtin PS. PedsQL™ 4.0: reliability and validity of the Pediatric Quality of Life Inventory™ Version 4.0 Generic Core Scales in healthy and patient populations. Med Care 2001;39:800–12. [DOI] [PubMed] [Google Scholar]
- E2.Wolkewitz M, Allignol A, Schumacher M, Beyersmann J. Two pitfalls in survival analyses of time-dependent exposure: a case study in a cohort of oscar nominees. Am Stat 2010;64:205–11. [Google Scholar]
- E3.Tibshirani R. Regression shrinkage and selection via the lasso. J Royal Stat Soc Series B (Methodological) 1996;58:267–88. [Google Scholar]
- E4.Shearer WT, Dunn E, Notarangelo LD, Dvorak CC, Puck JM, Logan BR, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol 2014;133:1092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Kwan A, Abraham RS, Currier R, Brower A, Andruszewski K, Abbott JK, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA 2014;312:729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Felgentreff K, Perez-Becker R, Speckmann C, Schwarz K, Kalwak K, Markelj G, et al. Clinical and immunological manifestations of patients with atypical severe combined immunodeficiency. Clin Immunol 2011;141:73–82. [DOI] [PubMed] [Google Scholar]
- E7.Roifman CM, Somech R, Kavadas F, Pires L, Nahum A, Dalal I, et al. Defining combined immunodeficiency. J Allergy Clin Immunol 2012;130:177–83. [DOI] [PubMed] [Google Scholar]