Abstract
Obesity is a major contributor to the greater prevalence of chronic disease morbidity and mortality observed in rural versus nonrural areas of the U.S. Nonetheless, little research attention has been given to modifying this important driver of rural/urban disparities in health outcomes. Although lifestyle treatments produce weight reductions of sufficient magnitude to improve health, the existing research is limited with respect to the long-term maintenance of treatment effects and the dissemination of services to underserved populations. Recent studies have demonstrated the feasibility of delivering lifestyle programs through the infrastructure of the U.S. Cooperative Extension Service (CES), which has more than 2,900 offices nationwide and whose mission includes nutrition education and health promotion. In addition, several randomized trials have shown that supplementing lifestyle treatment with extended-care programs consisting of either face-to-face sessions or individual telephone counseling can improve the maintenance of weight loss. However, both options entail relatively high costs that inhibit adoption in rural communities. The delivery of extended care via group-based telephone intervention may represent a promising, cost-effective alternative that is well suited to rural residents who tend to be isolated, have heightened concerns about privacy, and report lower quality of life. The Rural Lifestyle Eating and Activity Program (Rural LEAP) is a randomized trial, conducted via CES offices in rural communities, targeted to adults with obesity (n=528), and designed to evaluate the effectiveness and cost-effectiveness of extended-care programs delivered via group or individual telephone counseling compared to an education control condition on long-term changes in body weight.
Keywords: Obesity, obesity management, weight loss, rural health services, cost-benefit analysis
Clinical trial number
1. Introduction
Obesity disproportionately affects Americans living in rural areas and contributes to higher prevalences of chronic diseases and all-cause mortality observed in rural versus nonrural communities [1–6]. In rural areas, the convergent effects of poverty, low educational attainment, limited access to preventive health services, and cultural factors regarding diet and exercise contribute to higher rates of obesity and associated diseases [7–13].
Weight loss can reverse many of the adverse consequences linked to obesity [14]. According to national guidelines [15] and literature reviews [16–20], the most effective means of achieving weight reductions at the individual level is comprehensive lifestyle treatment. Participants in lifestyle interventions are taught behavioral strategies (e.g., goal setting and selfmonitoring) to modify their eating and physical activity patterns so as to produce a negative energy balance and weight loss. Comprehensive lifestyle treatment routinely results in weight losses of 5–10% [16–20]. Reductions of this magnitude can produce beneficial effects on hypertension, glucose intolerance, and hyperlipidemia, and can prevent the onset of type 2 diabetes [15,21–23].
The limited availability of obesity treatment options in rural communities prevents many residents from engaging in weight-control efforts [24]. With a nationwide network of more than 2,900 offices and a mission that includes nutrition education and health promotion, the Cooperative Extension Service (CES) [25,26] has the potential to play an important role in providing treatment for obesity in rural communities.
While lifestyle interventions can produce clinically meaningful weight reductions, the long-term maintenance of those losses remains a challenge. Without follow-up care, participants commonly regain one-third to one-half of their initial weight loss within one year [18,27–31]. Providing extended care via face-to-face sessions improves long-term outcomes [28,32], but is time- and cost-intensive [29,33,34], thereby limiting adoption in low-resources settings. Delivering extended care via telephone counseling may decrease the burdens associated with face-to-face treatment while producing equivalent weight-loss outcomes [35].
Most studies supporting phone-based interventions (e.g., [31,35]) have utilized individual telephone counseling, which requires a significant amount of provider time and consequently poses a major barrier to widespread implementation. Group-based phone counseling may overcome this challenge, but evidence of its effectiveness is limited. Befort and colleagues [36] found that group phone counseling produced weight losses equivalent to individual phone counseling, but at lower costs, and in a subsequent trial [37] showed that extended care delivered via group phone counseling improved weight-loss maintenance compared with a newsletter control group. In an efficacy study conducted largely with urban participants, Donnelly et al. [38] found equivalent outcomes for lifestyle treatment delivered via face-to-face sessions versus group conference call counseling. However, the impact of extended-care interventions delivered via group- versus individual telephone counseling in less resourced, rural areas remains unknown.
The Rural Lifestyle Eating and Activity Program (Rural LEAP) is a randomized trial designed to evaluate the comparative effectiveness and cost efficiency of three methods of delivering extended-care counseling for weight-loss maintenance in rural communities. The intervention is delivered through the infrastructure of the CES, and the primary outcome is weight change over the course of 18 months following initial treatment.
2. Methods
2.1. Overview
The study consists of three distinct phases: (1) Phase I, a non-randomized 4-month weight-loss phase (Months 1 through 4) during which all participants received the same face-to-face group lifestyle intervention for weight loss; (2) Phase II, a 12-month extended-care phase (Months 5 through 16) during which participants receive additional treatment according to randomized assignment to group-based (GRP) phone counseling, individual-based (IND) phone counseling, or an education control (CTRL) group; and (3) Phase III, a 6-month no-contact follow-up period (Months 17 through 22).
2.2. Participants
The study was approved by the Institutional Review Board of the University of Florida. Participants included 528 adults, 21–75 years of age, with body mass indices (BMI) of 30–45 kg/m2. Eligible participants were free of uncontrolled diabetes and hypertension and had no active manifestations of cardiovascular, cerebrovascular, renal, or hepatic disease. Exclusion criteria included the use of medications known to affect body weight, musculoskeletal conditions that precluded walking for 30 minutes, and weight loss > 4.5 kg in the preceding 6 months. Psychosocial contraindications included clinically significant depression and substance abuse.
2.3. Recruitment and Screening
Study announcements were mailed to residential addresses within 14 rural counties in northern Florida [39]. All 14 counties were designated in whole or in part as “Health Professional Shortage Areas” [40]. In response to the mailings and other outreach activities (e.g., presentations at churches and community events), 851 adults completed a preliminary telephone screening and met basic eligibility criteria for study participation. An in-person screening visit was then conducted at a local CES site during which the study was described and informed consent was obtained. At the screening visit, a medical history was collected, and a study nurse measured the potential participant’s height, weight, and resting blood pressure, and a fasting blood sample was drawn and analyzed for metabolic and lipid profiles. Following review of the screening visit results, 220 individuals were excluded, 103 individuals declined to participate, and the remaining 528 were enrolled into Phase I of the study (see Figure 1).
Fig. 1.
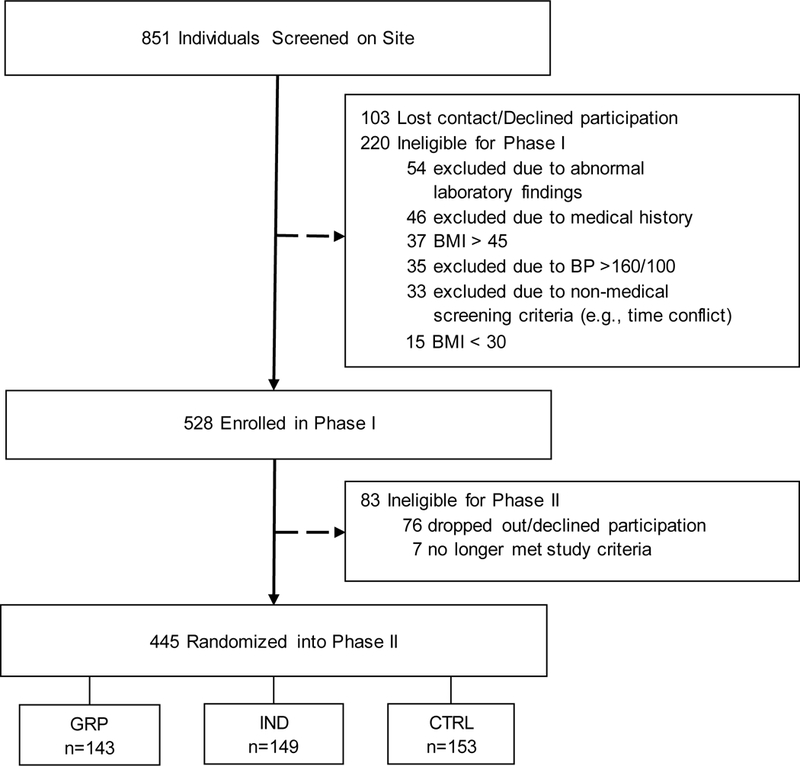
Participant flow during screening, enrollment, and randomization.
Baseline characteristics of Phase I participants, those not randomized to Phase II, and participants randomized to Phase II are shown in Table 1. The study sample was composed largely of middle-aged, White, non-Hispanic women, with a high school diploma or GED as their highest level of education, and a mean BMI in the Class II obesity range [41]. Approximately half of participants had an annual household income less than $50,000. Chi-square tests were conducted for differences in the categorical demographic variables between participants not randomized to Phase II and those randomized to Phase II. The only variable that was significant was Obesity Class,X2 (2, n = 528) = 6.49, p = .04 (unadjusted for multiple comparisons), with a larger of percentage of individuals with Class III obesity among those not randomized to Phase II.
Table 1.
Baseline characteristics for participants enrolled inPhase I (n = 528), those not randomized (n = 83), and those randomized to Phase II (n = 445).
| Phase I participants M (SD) or n (%) |
Participants not randomized to Phase II M (SD) or n (%) |
Participants randomized to Phase II M (SD) or n (%) |
|
|---|---|---|---|
| Age (years) | 54.5 (10.7) | 49.9 (11.8) | 55.4 (10.3) |
| Body mass index (BMI, kg/m2) | 36.6 (3.8) | 37.3 (4.3) | 36.4 (3.7) |
| Obesity Class | |||
| Class I (BMI = 30.0 to 34.9) | 200 (37.9%) | 28 (33.7%) | 172 (38.6%) |
| Class II (BMI = 35.0 to 39.9) | 223 (42.2%) | 30 (36.1%) | 193 (43.4%) |
| Class III (BMI = 40.0 to 45.0) | 105 (19.9%) | 25 (30.1%) | 80 (18.0%) |
| Gender (female) | 434 (82%) | 66 (79.5%) | 368 (83%) |
| Education, highest level completed | |||
| < High school | 12 (2%) | 1 (1.2%) | 11 (2%) |
| High school or GED | 280 (53%) | 53 (63.9%) | 227 (51%) |
| Associate’s degree | 66 (13%) | 12 (14.5%) | 54 (12%) |
| Bachelor’s degree | 117 (22%) | 14 (16.9%) | 103 (23%) |
| Advanced degree | 53 (10%) | 3 (3.6%) | 50 (11%) |
| Race/ethnicity | |||
| White, Non-Hispanic/Latino | 391 (74%) | 63 (75.9%) | 328 (74%) |
| Black, Non-Hispanic/Latino | 100 (19%) | 18 (21.7%) | 82 (18%) |
| Other, Non-Hispanic/Latino | 16 (3%) | 0 (0.0%) | 16 (4%) |
| White, Hispanic/Latino | 16 (3%) | 2 (2.4%) | 14 (3%) |
| Black, Hispanic/Latino | 1 (0%) | 0 (0.0%) | 1 (0%) |
| Other, Hispanic/Latino | 4 (1%) | 0 (0.0%) | 4 (1%) |
| Annual household income | |||
| < $20,000 | 67 (13%) | 18 (21.7%) | 49 (11%) |
| $20,000-$34,999 | 93 (18%) | 11 (13.3%) | 82 (18%) |
| $35,000-$49,999 | 93 (18%) | 14 (16.9%) | 79 (18%) |
| $50,000-$74,000 | 123 (23%) | 16 (19.3%) | 107 (24%) |
| > $75,000 | 120 (23%) | 20 (24.1%) | 100 (22%) |
| Unknown/refused | 32 (6%) | 4 (4.8%) | 28 (6%) |
2.4. CES Offices and Interventionists
The initial weight-loss intervention in the current trial is delivered on site via CES sites in 14 rural counties in northern Florida. All 14 counties have a centrally-located site available for the intervention. Interventionists are CES Family and Consumer Sciences Agents or individuals with a bachelor’s or master’s degree in nutrition, exercise science, or psychology. Fifty percent of the counties employed local CES Agents with the remaining sites employing other qualified individuals to conduct the intervention. All interventionists were provided with training in lifestyle treatment and nutrition education that included two 8-hour workshops held semi-annually, plus case management reviews with a clinical health psychologist (MGP) or registered dietitian (MNS), each with extensive experience overseeing behavioral interventions for obesity. The case management reviews are conducted weekly during Months 1 through 4, biweekly during Months 5 through 10, and monthly during Months 11 through 16. Interventions sessions are audio-taped and random reviews are carried out to ensure treatment fidelity. Corrective feedback is provided during training, case management reviews, or individually with the interventionists.
2.5. Phase I Lifestyle Intervention
Phase I consisted of 16 weekly face-to-face group sessions conducted at local CES offices. Each group consisted of 4–16 participants, and each session lasted approximately 90 minutes. Intervention content was derived from the Diabetes Prevention Program (DPP) [42,43]. Modifications to the DPP approach included group rather than individual counseling [44] and home-based rather than center-based physical activity [45]. The DPP intervention was further modified to address weight-management challenges experienced by individuals living in rural areas (i.e., lack of places to exercise, traditions of high-fat Southern cooking, absence of social support for weight loss, etc.). Similar to the DPP, the Rural LEAP intervention was guided by social-cognitive and self-regulation theories [46–48] and incorporated key behavioral modification strategies including goal setting, self-monitoring of dietary intake and physical activity, stimulus control, cognitive restructuring, and problem solving [33]. Utilization of problem-solving skills, in particular, was emphasized as a way to address the specific challenges experienced by adults living in rural communities [49,50]. Topics and objectives for the 16 Phase I and 18 Phase II weight-loss treatment modules are presented in Table 2.
Table 2.
Weight-loss treatment modules.
| Session | Topic | Objective |
|---|---|---|
| 1 | Getting Started for Success: Setting Goals | Review energy balance and weight management. Set personal calorie intake goals. |
| 2 | Taking Control of Eating Patterns | Learn difference between hunger vs. cravings. Review strategies to implement healthy eating patterns. |
| 3 | Stepping Up: Becoming Active | Review benefits of physical activity. Set personal step goals. |
| 4 | Becoming a Fat Gram Detective/Southern Cooking Made Healthier | Understand roles of fat, traditional Southern foods, and calories in long-term weight management. Review lower-fat food options. |
| 5 | Building a Healthy Diet | Review MyPlate recommended servings for each food group. Set total daily food serving goals. |
| 6 | Keep on Moving! | Discuss benefits of exercise/aerobic fitness and safety. |
| 7 | Scheduling Sleep | Identify current sleep habits and learn how sleep affects health and weight. Discuss sleep hygiene strategies. |
| 8 | Taking Charge: Eating and Activity Cues | Discuss triggers/cues for eating and physical activity. Discuss method to modify problematic eating and activity cues. |
| 9 | An Introduction to Problem Solving | Discuss steps in Problem Solving model and how to apply them to current and future problems. |
| 10 | Talking Back to Negative Thoughts | Discuss common weight-related negative thoughts and patterns. Demonstrate how to “talk back” to negative thoughts. |
| 11 | Slipping but not Falling: Preventing Relapse | Identify risk factors for experiencing a slip. Create a plan to prevent slips from becoming a relapse. |
| 12 | Coping with High-Risk Situations and Eating Out the Healthy Way | Identify high-risk situations, challenges faced, and coping strategies. Demonstrate how to budget calories for eating out. |
| 13 | Social Support and Making Social Cues Work for You | Discuss strategies for building social support and ways to effectively manage social cues. |
| 14 | Managing Stress | Discuss causes and effects related to stress. Introduce healthy strategies for stress reduction. |
| 15 | Having a Positive Body Image and Dealing with Plateaus | Discuss facts about body image and plateaus. Review tools for overcoming plateaus. |
| 16 | Looking Forward: Planning Ahead | Review current progress and discuss future goals. |
| 17 | Phase II Program Goals: Food Tracking Review | Review positive changes made, goal setting, and importance of accurate food tracking. |
| 18 | Tools for More Effective Problem Solving | Review problem solving model and apply to a current problem. |
| 19 | Meal Mastery: Build a Better Meal | Review benefits of planning ahead for meals. Identify small changes that can reduce calories in a meal. |
| 20 | Time for Health | Discuss importance of budgeting time for exercise. Set goals and problem solve how to make time for activity. |
| 21 | Plateau Busting | Review information about plateaus. Discuss ways to address a plateau. |
| 22 | Travel Triumphantly! | Identify barriers to healthy eating and activity while traveling. |
| 23 | Nutrition and Exercise During Illness | Identify strategies for overcoming illness by modifying physical activity and incorporating nutritious foods. |
| 24 | Super Sneakers, Stretching, and Safety | Identify appropriate footwear, clothing, and stretching techniques for physical activity. |
| 25 | Nutrition Myth Busters | Identify myths related to nutrition and healthy eating. |
| 26 | Planning Challenge: Perfectly Prepared | Review 7-day meal planning worksheet and strategies to prepare meals at home during busy times. |
| 27 | Planning Challenge: Food Swaps | Identify lower calorie substitutes for high calorie food, beverages, and ingredients. |
| 28 | Fitness Challenge: Hike the Himalayas | Identify barriers preventing planned exercise and problem solving strategies to overcome them. |
| 29 | Preventing Chronic Disease | Identify lifestyle changes that could prevent chronic diseases and problem solve strategies to implement them. |
| 30 | Mind Challenge: Empowering Emotions | Discuss how to positively manage emotions and review ways to relieve stress. |
| 31 | Life Lessons: Renewing Your Vows | Identify ways to overcome stress and maintain positive changes. Identify ways to modify goals and stick to them during stressful times. |
| 32 | Life Lessons: Reaching New Heights | Discuss strategies to keep healthy eating and physical activity exciting. |
| 33 | Mastering Maintenance and Continuing Goals | Review strategies from National Weight Control Registry. Discuss SMART goals. |
| 34 | Reflection and Planning for the Next Step | Reflect on healthy lifestyle changes made throughout the program. Review the importance of self-monitoring and problem solving. |
The goals of the initial lifestyle intervention were to decrease caloric intake in a nutritionally sound manner [51–53] so as to produce a weight loss of approximately 0.4–0.9 kg per week and to increase home-based walking to 210 minutes per week [54]. Initial caloric intake goals were determined by the participant’s baseline weight (e.g., 1200 kcal/day for participants weighing ≤ 113.6 kg; 1500 kcal/day for those weighing > 113.6 kg). Participants were instructed to keep daily logs of their food and drink intake, including the types and amounts of foods consumed along with corresponding caloric values, using either written logs or online programs/applications (e.g., MyFitnessPal® or Lose It!®). Participants were also instructed to keep daily records of their steps using a pedometer or activity monitor. To support these self-monitoring efforts, participants were provided, at no cost, with a Calorie King reference book [55,56], blank food logs, measuring cups, a food scale, and a clip-on pedometer. As a substitute for the use of a pedometer, participants were permitted to self-monitor physical activity via self-purchased electronic tracking devices (e.g., Fitbit®, Garmin®, or Apple Watch®).
Participants were encouraged to work toward meeting their daily caloric intake goals by selecting lower-calorie food options, decreasing consumption of “fast food,” decreasing or eliminating sugar-sweetened beverages, increasing consumption of fruits, vegetables, and whole grains, and using lower-fat methods of preparing foods. Weight-loss treatment modules included instruction on the USDA’s MyPlate to emphasize choosing whole grains, fruits, vegetables, lean meat, and low-fat dairy products [51].
Walking was the primary form of prescribed physical activity. During week 2, each participant’s steps were monitored over 7 days, and then participants were encouraged to gradually increase their planned daily walking time by 5–10 minutes (i.e., 500–1000 steps) each week as tolerated until they reached an average of 30 minutes/day (3000 steps) above their baseline level. Participants who achieved this objective were encouraged to further increase their steps toward an ultimate goal of 60 min/day of walking for exercise (i.e., 6000 steps above baseline).
2.6. Phase II Randomization and Extended-Care Lifestyle Intervention
To maximize participation in the randomized and follow-up phases (II and III) of the trial, only those individuals who attended fewer than 50% of the Phase I sessions were excluded from advancing to Phase II randomized assignments. All other participants, regardless of initial weight loss achieved, were randomly assigned to one of the three Phase II conditions (GRP, IND, or CTRL). Randomization was completed by the study statistician (MJD) using the random number generator in R statistical computing software [57]. County size, county, Phase I group, and session time were balanced during randomization. Interventionists notified participants of their Phase II group assignment during the last Phase I session. The primary objectives of Phase II (Months 5–16) are to sustain the lifestyle changes accomplished in Phase I so as to maintain lost weight and to make further adjustments in energy balance to produce additional weight loss during “campaigns” conducted in Months 8–9 and Months 13–14.
The mode of extended-care contacts differs by condition during Phase II, but the schedule of contacts is consistent across the conditions. Intervention contact across all three conditions occurs bi-weekly during Month 5 through 10 and monthly during Months 11 through 16. At each of the scheduled contact points, participants in all three conditions receive a weight-loss treatment module delivered via e-mail or U.S. mail with information and recommended behavioral activities targeted at maintaining lost weight. All participants are also provided with a supply of blank self-monitoring logs and pre-paid mailers for returning completed dietary logs to the interventionists.
2.6.1. GRP condition
Participants randomized to the GRP condition call into a teleconference line for 60-minute group sessions with the interventionist and other members from their Phase I group. The calls include three primary components: (1) a group “check-in” during which participants report progress toward goals and problems experienced since the previous session coupled with group problem solving of one or two complex issues to address barriers to progress [49]; (2) group discussion of the treatment module with the interventionist explaining new objectives and suggesting behavioral strategies to achieve those objectives; and (3) structured individual goal setting with respect to each participant’s plans for diet and/or physical activity changes to be accomplished prior to the next session. To maximize the time efficiency of the group session, all participants are asked to identify their calorie and step goals prior to the call.
The group problem-solving activities facilitated by the interventionist during the extended-care sessions incorporates the use of an evidence-based, five-step model [49,50] including: (1) orientation (i.e., developing an appropriate coping perspective -- “Problems are a normal part of managing your weight, but they can be dealt with effectively.”); (2) definition (i.e., specifying the problem and goal behaviors - “What is the particular problem facing you right now? What is your goal in this situation?”); (3) generation of alternatives (i.e., brainstorming potential solutions - “The greater the range of possible solutions you consider, the greater your chances of developing an effective solution.”); (4) decision making (i.e., anticipating the probable outcomes of different options - “What are the likely short- and long-term consequences of each of your options?”; and (5) implementation and evaluation (i.e., trying out a plan and evaluating its effectiveness - “What solution plan are you going to try and how will you know if it works?”).
2.6.2. IND condition
Participants randomized to the IND condition call into a teleconference line at a predetermined appointment time for a one-on-one, 10- to 20-minute session with the interventionist. Participants are contacted to schedule make-up calls in the event of time conflicts or missed calls. Aside from the difference in call length and the absence of other group members, IND calls include the same structure, components, and problem-solving focus of the GRP calls.
2.6.3. CTRL condition
Participants in the CTRL group receive a series of 18 written educational weight-loss treatment modules delivered via e-mail or U.S. mail delivered on the same schedule as the GRP and IND contacts during Phase II. The modules include the identical content used in the GRP and IND conditions. The CTRL condition does not include any scheduled phone or in-person interactions with the interventionists or other participants.
2.7. Measures
2.7.1. Assessment visits
Study personnel masked to participant randomization conduct in-person assessment visits at baseline and at Months 4, 10, 16, and 22. Assessment visits are conducted at the same local CES site for all time points. Self-reported medical history and a list of medications are updated at each assessment visit.
2.7.2. Anthropometrics
At baseline, height is measured using a stadiometer (ShorrBoard®), with participant shoes removed. At baseline and subsequent assessment visits, weight is measured with a calibrated digital scale (Tanita BWB-800S), with participants in light indoor clothing, pockets emptied, and shoes removed.
2.7.3. Fasting blood samples
Fasting blood samples are obtained by the study nurse at baseline and at Months 4 and 22 and analyzed for metabolic and lipid profiles by Quest Diagnostics®.
2.7.4. Resting blood pressure and heart rate
Blood pressure and heart rate are measured at baseline and Months 4 and 22. Measurements are taken while the participant is seated with feet resting on the floor for five minutes in a quiet room free of distractions. Resting systolic and diastolic blood pressure are taken three times, two minutes apart, using a Dinamap® Automated Vital Signs Monitor with appropriate cuff size; the second and third readings are entered and averaged in the study database.
2.7.5. 400 Meter Walk Test
The 400 Meter Walk Test [58], a standardized measure of physical fitness commonly used to assess individuals with chronic health conditions, is completed at baseline, and at Months 4 and 22 to assess fitness.
2.7.6. Participant questionnaires
The following questionnaires are completed by the participants at baseline and at Months 4, 10, 16, and 22.
2.7.6.1. Paffenbarger Physical Activity Questionnaire
Self-reported physical activity is collected via the Paffenbarger Physical Activity Questionnaire [59], which includes questions on distance walked, flights of stairs climbed, and time spent in other sports, recreational, or fitness activities during a typical day or week.
2.7.6.2. Medical Outcomes Study 36-Item Short Form Health Survey (SF-36)
The SF-36 [60] is a quality-of-life measure that assesses the following domains: (a) physical functioning; (b) role limitations due to physical health; (c) bodily pain; (d) general health; (e) vitality; (f) social functioning; (g) role limitations due to emotional problems; and (h) mental health [61].
2.7.6.3. Social Problem-Solving Inventory-Revised (SPSI-R)
The SPSI-R [62–64] is a 52-item questionnaire that evaluates positive versus negative problem orientation and problem-solving style.
2.7.6.4. Social Provisions Scale
The 24-item Social Provisions Scale [65,66] measures perceived support from others in one’s social network.
2.7.6.5. Cost Analysis Questionnaire
The cost analysis questionnaire [35] assesses direct and indirect costs of lifestyle interventions.
2.7.6.6. Health Thermometer
The Health Thermometer is a visual analog scale [67] that measures health-related quality of life on a scale of 0 to 100 with 0 indicating the “worst imaginable health state” to 100 indicating the “best imaginable health state.”
2.7.6.7. Program Satisfaction and Group Leader Evaluation Forms
Participant ratings of program satisfaction, the usefulness of specific treatment strategies (e.g., self-monitoring, cognitive restructuring, etc.), and the effectiveness of interventionists are completed at Months 4, 10, 16, and 22.
2.8. Participant Retention Strategies
Participants receive payments on a study-provided credit card of $50 upon completion of the Month 4 visit, $20 upon the completion of the Month 10 and Month 16 visits, and $75 upon completion of the Month 22 visit. Participants also receive $10 as travel reimbursement for each of the in-person sessions attended during Phase I.
2.9. Data Management and Statistical Analyses
2.9.1. Data management
Data are stored in a database via the Research Electronic Data Capture (REDCap) system [68], a secure web-based data management system supported by the University of Florida Clinical and Translational Science Institute. Self-report data are entered directly by participants into REDCap’s online survey module. All other data are entered by study personnel according to the protocol and in compliance with the U.S. Health Insurance Portability and Accountability (HIPAA) Privacy Rule. Data will be exported to a SAS dataset for statistical analyses [69].
2.9.2. Missing data
Data analyses for this effectiveness trial will be performed using an intent-to-treat approach that includes all randomized participants. It will be assumed that individuals who discontinue participation in the study prior to Month 22 regained weight, on average, at a rate of 0.3 kg per month after leaving the study, up to their baseline weight. This conservative approach to imputing missing weight values has been employed by numerous prior studies [34,35,70] and is consistent with published reviews of weight regain following lifestyle treatment for obesity [18,71,72]. To account for the uncertainty regarding the exact amount of weight regained, the corresponding (co-)variance of these regain values will be identified under assumptions consistent with missing at random for the covariance structure [73,74]. Sensitivity to the assumptions about the missing data [75] will be examined in our final analysis.
2.9.3. Sample size justification
The primary outcome will be change in body weight from Month 4 to Month 22, comparing the GRP and IND phone-based conditions to the education CTRL condition. It is assumed that the county size, county, and session time effects will be negligible due to the balance in the randomization assignments. The analysis will, however, take into account a random effect that allows for correlation among individuals in the same GRP sessions. Based upon prior studies [35,76], a 20% attrition rate is expected by Month 22. The recruitment target included a total of 540 individuals entering Phase I (180 per condition). Power calculations were computed using the expected attrition rate and a Bonferroni adjustment to account for comparisons of the three treatment conditions. The study is expected to have > 80% power (two-sided tests, type I error rate of 0.05) to detect a 2.5 kg difference in regain from Month 4 to Month 22 between GRP vs. CTRL and IND vs. CTRL.
2.9.4. Primary analyses
Participants’ weight change will be calculated as the difference between weight in kilograms at Month 22 and weight at Month 4. The primary hypothesis is that both the GRP and IND telephone conditions will produce greater weight reductions at Month 22 than the education CTRL condition. To test this hypothesis, a mixed model for longitudinal data will be utilized. Fixed effects will include treatment condition, time since study initiation, and session time. Random effects will include county. The GRP arm will also include a random effect for group. Treatment condition was randomly assigned, with session times serving as blocking factors. Two primary contrasts of interest will be tested for significance: GRP vs. CTRL and IND vs. CTRL. Bonferroni corrections will be used to control for type I error. Covariates (e.g., age, race, initial weight loss) can be included in the model and analyzed using the same procedure in SAS. It is not anticipated that the effects of county or session time will be of consequence.
2.9.5. Secondary analyses
For the secondary aim, the outcome of interest is the proportion of participants within a treatment condition who lost ≥ 5% of body weight by Month 4 and then maintained a ≥ 5% loss in body weight at Month 22. It is hypothesized that both the GRP and IND conditions will result in greater proportions of participants maintaining ≥ 5% losses at Month 22 than the CTRL condition. Two contrasts will be tested for significance: GRP vs. CTRL and IND vs. CTRL. Each contrast will use an exact test for binomial proportions to test the null hypothesis that the relevant proportions are equal versus the alternative that they are not equal. Bonferroni corrections will be used to control for type I error.
2.9.6. Additional analyses
2.9.6.1. Exploratory analyses.
Changes from Month 4 to Month 22 will be examined for systolic and diastolic blood pressure, resting heart rate, blood lipids profile (LDL and HDL cholesterol and triglycerides), glycemic control (HbA1c), dietary intake, self-reported physical activity, physical performance (400 Meter Walk Test [58]), and health-related quality of life. Process measures will include intervention-related activities (e.g., attendance, self-monitoring) and participants’ evaluation of program components and interventionist effectiveness. These endpoints are continuous variables, and analyses will be the same as that described for the primary endpoint. No adjustments will be made for multiple comparisons due to the exploratory nature of these analyses.
2.9.6.2. Moderator analyses.
Age, race/ethnicity, socioeconomic status, and social support differences across participants and counties [77] will be evaluated as potential moderators of the effect of the intervention conditions on weight change at Month 22.
2.9.6.3. Mediator analyses.
Completion of written self-monitoring records (percent of days with records) and change in problem-solving skills (change in SPSI-R total score) will be evaluated as key mediators of the relationship between the intervention conditions and weight change at Month 22. In a previous study conducted by our research team [35], completion of written self-monitoring records during initial treatment (number of days recorded from baseline to Month 6) was the single best behavioral predictor of weight change at Month 18 (r = .51), and change in problem-solving skills also showed a significant positive association with long-term weight change (r = .19). These findings provide support for an examination of weight-related vigilance (i.e., self-monitoring) and problem-solving abilities as potential mediators of long-term changes in body weight. Mediation analyses will be conducted using both traditional [78] and more recent nonparametric, formal causal approaches [79,80].
2.9.6.4. Cost-effectiveness analyses.
The analytic framework will follow the guidelines of the Second Panel on Cost-Effectiveness in Health and Medicine [81]. Costs of each condition will be tracked from the service provider perspective [35]. Cost effectiveness will be measured primarily as “dollars per kg loss per treatment condition” [82]. Cost effectiveness will also be measured according to the recommended clinical cut-points of maintaining ≥ 5% loss of initial weight [15]. Similar effectiveness for the GRP and IND conditions are expected, with lower effectiveness in the CTRL condition. Cost effectiveness will also be assessed based on Quality-Adjusted Life Year (QALY) gains related to weight change and will be calculated using data from the SF-36 questionnaires completed at baseline, Month 4, Month 16, and Month 22 to derive a preference score using the Short Form-6 Dimension (SF6D) method; 10 items from the SF-36 are used to calculate a QALY score [83,84].
Incremental cost effectiveness ratios (ICERs) will identify the differential costs and outcomes of switching from individual to group-based telephone counseling. A priori, it is expected that the GRP condition will be less expensive than the IND condition but with similar effectiveness. All costs will be adjusted to reflect constant 2017 dollars. Sensitivity analyses will be conducted to determine whether costs and cost-effectiveness estimates are sensitive to study-specific wage rates and other program costs. Extrapolation using sample median wage rates or national wage data will be calculated and the analysis will be rerun to determine any change in results. Additional sensitivity analyses will rely on either probabilistic models or tornado charts to gauge whether ICER results depend on various model assumptions.
2.10. Attrition
Phase I of the Rural LEAP Trial has been completed. The observed attrition rate at Month 4 was 15.7%. Based on previous studies [35,76], the expected attrition at Month 22 is 20%.
2.11. Weight Loss During Phase I
Weight loss over the first four weeks of Phase I treatment was compared for participants randomized to Phase II and those who did not qualify for randomization based on attendance at < 50% of Phase I sessions. During the first four weeks of Phase I treatment, participants randomized to Phase II had a mean weight loss of 2.1 kg (SD = 1.2), whereas those not randomized to Phase II had a mean weight loss of 0.8 kg (SD = 1.5).
Table 3 provides the mean weight and BMI changes at the completion of Phase I (Month 4) for participants who were randomized to Phase II. Table 3 also includes the percent of randomized participants who achieved different categories of weight loss.
Table 3.
Weight-loss progress at the conclusion of Phase 1 (Month 4) for participants randomized into Phase II (n = 445).
| Weight (kg) | ||
|---|---|---|
| M | SD | |
| Baseline | 99.9 | 14.6 |
| Month 4 | 91.6 | 14.0 |
| Change at Month 4 | −8.3 | 4.9 |
| Body mass index (kg/m2 ) | ||
| M | SD | |
| Baseline | 36.4 | 3.7 |
| Month 4 | 33.4 | 3.9 |
| Change at Month 4 | −3.0 | 1.7 |
| Category of weight change at Month 4 | ||
| n | % | |
| Weight loss < 5% | 100 | 22.5 |
| Weight loss ≥ 5 to < 10% | 191 | 42.9 |
| Weight loss ≥ 10% | 154 | 34.6 |
3. Discussion
Rural LEAP is a randomized trial, conducted via CES offices in rural communities and designed to evaluate the effectiveness and cost-effectiveness of extended-care programs for weight management delivered via group or individual telephone counseling compared to an education control condition. Accomplishments to date include: (a) the recruitment of a diverse sample of 528 adults with obesity from 14 rural communities in Florida into the initial weight-loss phase of the trial; (b) the achievement of mean initial weight reduction of 8.3 kg; and (c) the advancement of 445 participants (84.3%) to the randomized extended-care phase of the trial. Those not randomized were more likely to have Class III obesity and a slow rate of initial weight loss. Overall, the results to date are on par with expectations, and the magnitude of initial weight loss is comparable to the reductions observed in large multi-site efficacy trials such as the DPP [23] and Look AHEAD [30].
Comprehensive lifestyle interventions can produce weight reductions of sufficient magnitude to improve health, but the existing research is limited with respect to two critical factors: (a) the long-term maintenance of treatment effects and (b) translation and dissemination to underserved populations. Most weight-loss trials have been efficacy studies, conducted with middle-class, urban and suburban participants and delivered by teams of experts working in academic medical centers. Very few trials have been conducted in medically underserved community settings with treatment delivered by local staff, and relatively little attention has been given to the maintenance of treatment effects. Two prior trials [35,76] have demonstrated the feasibility and effectiveness of delivering lifestyle programs through the existing infrastructure of the CES, which has offices covering more than 87% of the 1,889 rural counties in the U.S. [25,85].
Beyond demonstrating the feasibility of delivering weight-loss interventions via the CES, the TOURS Trial [35] showed that the long-term effects of obesity treatment can be enhanced via extended-care programs consisting of either face-to-face treatment sessions or individual telephone counseling. However, both of these options entail relatively high costs, which inhibit their adoption in rural communities. Group-based telephone counseling may represent a lower cost option for the delivery of extended-care programs for weight management in rural communities. Two trials conducted with residents of rural communities [36,37] demonstrated the effectiveness of weight-management interventions delivered via group-based, telephone conference calls. In a pilot study, Befort and colleagues [36] found that group-based phone counseling produced weight losses equivalent to individual phone counseling, but at lower costs. In a subsequent trial, Befort et al. [37] showed that extended care delivered via group phone counseling improved the maintenance of weight loss compared with a newsletter control group. Thus, the use of conference call technology to deliver extended care combines the convenience of phone counseling with the benefits of group support, which may be especially important for rural residents who tend to be isolated, have heightened concerns about privacy, and report lower levels of quality of life [86].
Limitations of the current study should be noted, particularly with respect to the selection of an appropriate control group or comparison treatment and to the vexing challenge of widespread dissemination and implementation. Alternative control group and comparison treatment arms were considered, including the use of a no-treatment “waiting list” control group and an Internet-based comparison treatment. Rather than adopting either of these options, an education control group was included that provides participants with written modules focused on information and guidance regarding weight-loss maintenance. This option was deemed preferable to the use of a “waiting list” control group that would leave participants without ongoing assistance following initial treatment and potentially result in differential attrition and diminish the methodological soundness of the trial. Alternatively, the inclusion of a comparison treatment arm that would receive an Internet-based intervention was considered but not adopted for two reasons. First, engagement in web-based interventions has been shown to decline dramatically during extended care resulting in poor long-term outcomes [31,34], and second, 45% of rural area residents do not have Internet access with sufficient bandwidth to run interactive weight-management software [87].
Finally, it should be noted that testing innovative extended-care interventions for translation into community settings represents a necessary but not sufficient step toward providing residents of rural counties with access to effective treatment for obesity. Ultimately, the availability of lifestyle interventions for obesity via the CES infrastructure will represent a policy decision made by key leaders at county, state, and national levels. An important objective of the Rural LEAP Trial is to provide policy makers, as well as researchers and interventionists, with evidence regarding potentially effective and cost-efficient interventions that could be readily disseminated and implemented via the CES in rural communities across the country.
Acknowledgements
This work was supported by NHLBI grant R18 HL112720. NHLBI had no involvement in the study design, data collection, or analyses. The authors thank the staff members and students of the University of Florida Weight Management Program. We also gratefully acknowledge our CES partners and interventionists in Baker, Bradford, Columbia, Dixie, Flagler, Gilchrist, Hamilton, Lafayette, Levy, Madison, Putnam, Suwannee, Taylor, and Union counties. Finally, the authors thank the members of the Rural LEAP Data and Safety Monitoring Board, Drs. Vera Bittner, Jack Rejeski, and Robert Newton.
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Befort CA, Nazir N, Perri MG. Prevalence of obesity among adults from rural and urban areas of the United States: Findings from NHANES (2005–2008). J Rural Health 2012;28(4):392–7. 10.1111/j.1748-0361.2012.00411.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lundeen EA, Park S, Pan L, O’Toole T, Matthews K, Blanck HM. Obesity prevalence among adults living in metropolitan and nonmetropolitan counties-United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(23):653–58. https://doi.org/10.15585/m mwr.mm6723a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hales CM, Fryar CD, Carroll MD, Freedman DS, Aoki Y, Ogden CL. Differences in obesity prevalence by demographic characteristics and urbanization level among adults in the United States, 2013–2016. JAMA. 2018;319(23):2419–29. 10.1001/jama.2018.7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen M, Fan JX, Kowaleski-Jones L, Wan N. Rural-urban disparities in obesity prevalence among working age adults in the United States: Exploring the mechanisms. Am J Health Promot. 2018;32(2):400–08. 10.1177/0890117116689488 [DOI] [PubMed] [Google Scholar]
- 5.Knudson A, Meit, M., Tanenbaum, E., Brady, J., Gilbert, T., Klug, et al. 2016, March; Available from: https://ruralhealth.und.edu/projects/health-reform-policy-research-center/pdf/technical-notes-exploring-rural-urban-mortality-differences.pdf.
- 6.Moy E Leading causes of death in nonmetropolitan and metropolitan areas-United States, 1999–2014. MMWR Surveillance Summary; 2017; 66: Available from: http://www.cdc.gov/mmwr/volumes/66/ss/ss6601a1.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones CP TS; Ahearn M; et al. Health status and healthcare access of farm and rural populations. United States Department of Agriculture Economic Research Service, 2009. [Google Scholar]
- 8.Martin SL, Kirkner GJ, Mayo K, Matthews CE, Durstine JL, Hebert JR. Urban, rural, and regional variations in physical activity. J Rural Health. 2005;21(3):239–44. [DOI] [PubMed] [Google Scholar]
- 9.Patterson PD, Moore CG, Probst JC, Shinogle JA. Obesity and physical inactivity in rural America. J Rural Health. 2004;20(2):151–9. [DOI] [PubMed] [Google Scholar]
- 10.Rural Healthy People 2020: A Companion Document to Healthy People 2020, volume 1 Texas: A&M University Health Science Center, School of Public Health, Southwest Rural Health Research Center, 2015. [Google Scholar]
- 11.Flora CB, Flora JL, & Gasteyer SP. Rural communities: Legacy + change. Boulder, Colorado: Westview Press; 2015. [Google Scholar]
- 12.Lutfiyya MN, Chang LF, Lipsky MS. A cross-sectional study of US rural adults’ consumption of fruits and vegetables: Do they consume at least five servings daily? BMC Public Health. 2012;12:280 10.1186/1471-2458-12-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearson TA, Lewis C. Rural epidemiology: Insights from a rural population laboratory.Am J Epidemiol. 1998;148(10):949–57. [DOI] [PubMed] [Google Scholar]
- 14.McTigue KM, Harris R, Hemphill B, Lux L, Sutton S, Bunton AJ, et al. Screening and interventions for obesity in adults: Summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;139(11):933–49. [DOI] [PubMed] [Google Scholar]
- 15.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–38. 10.1161/01.cir.0000437739.71477.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatr Clin North Am. 2011. ;34(4):841 −59. 10.1016/j.psc.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wing RR. Behavioral weight control Handbook of obesity treatment. New York, NY: Guilford Press; 2002: 301–316. [Google Scholar]
- 18.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132(6):2226–38. https ://doi.org/10.1053/j.gastro.2007.03.051 [DOI] [PubMed] [Google Scholar]
- 19.Johnston CA, Tyler C, Foreyt JP. Behavioral management of obesity. Curr Atheroscler Rep. 2007;9(6):448–53. [DOI] [PubMed] [Google Scholar]
- 20.Vetter ML, Faulconbridge LF, Webb VL, Wadden TA. Behavioral and pharmacologic therapies for obesity. Nat Rev Endocrinol. 2010;6(10):578–88. 10.1038/nrendo.2010.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Pei JH, Kuang J, Chen HM, Chen Z, Li ZW, et al. Effect of lifestyle intervention in patients with type 2 diabetes: A meta-analysis. Metabolism. 2015;64(2):338–47. 10.1016/j.metabol.2014.10.018 [DOI] [PubMed] [Google Scholar]
- 22.Lin JS, O’Connor E, Evans CV, Senger CA, Rowland MG, Groom HC. Behavioral counseling to promote a healthy lifestyle in persons with cardiovascular risk factors: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161(8):568–78. 10.7326/M14-0130 [DOI] [PubMed] [Google Scholar]
- 23.Knowler wC , Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Dis J Where we live: Health care in rural vs urban America. JAMA. 2002;287(1):108–08. https://doi.org/DOI 10.1001/jama.287.1.108 [PubMed] [Google Scholar]
- 25.United States Department of Agriculture. Cooperative Extension System, National Institute of Food and Agriculture; 2015; Available from: https://nifa.usda.gov/cooperative-extension-system. [Google Scholar]
- 26.Braun B BK, Cronk L, Fox LK, Koukel S, Le Menestrel S et al. Cooperative Extension’s National Framework for Health and Wellness. 2014; Available from: http://www.aplu.org/members/commissions/food-environmentand-renewable-resources/CFERR_Library/national-framework-for-health-and-wellness/file. [Google Scholar]
- 27.Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011. ;365(21): 1959–68. 10.1056/NEJMoa1108660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bray GA, Wadden TA. Improving long-term weight loss maintenance: Can we do it? Obesity (Silver Spring). 2015;23(1):2–3. 10.1002/oby.20964 [DOI] [PubMed] [Google Scholar]
- 29.Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR, et al. Longterm maintenance of weight loss: Current status. Health Psychol. 2000;19(1S):5–16. [DOI] [PubMed] [Google Scholar]
- 30.Look AHEAD Research Group. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: Four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170(17): 1566–75. 10.1001/archinternmed.2010.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: The weight loss maintenance randomized controlled trial. JAMA. 2008;299(10):1139–48. 10.1001/jama.299.10.1139 [DOI] [PubMed] [Google Scholar]
- 32.Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, et al. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107(10):1755–67. 10.1016/j.jada.2007.07.017 [DOI] [PubMed] [Google Scholar]
- 33.Look AHEAD Research Group. The Look AHEAD study: A description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring). 2006;14(5):737–52. 10.1038/oby.2006.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355(15):1563–71. 10.1056/NEJMoa061883 [DOI] [PubMed] [Google Scholar]
- 35.Perri MG, Limacher MC, Durning PE, Janicke DM, Lutes LD, Bobroff LB, et al. Extended-care programs for weight management in rural communities: The Treatment of Obesity in Underserved Rural Settings (TOURS) randomized trial. Arch Intern Med. 2008; 168(21):2347–54. 10.1001/archinte.168.21.2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Befort CA, Donnelly JE, Sullivan DK, Ellerbeck EF, Perri MG. Group versus individual phone-based obesity treatment for rural women. Eat Behav. 2010;11(1):11–7. 10.1016/j.eatbeh.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Befort CA, Klemp JR, Sullivan DK, Shireman T, Diaz FJ, Schmitz K, et al. Weight loss maintenance strategies among rural breast cancer survivors: The rural women connecting for better health trial. Obesity (Silver Spring). 2016;24(10):2070–7. 10.1002/oby.21625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donnelly JE, Goetz J, Gibson C, Sullivan DK, Lee R, Smith BK, et al. Equivalent weight loss for weight management programs delivered by phone and clinic. Obesity (Silver Spring). 2013;21(10):1951–9. 10.1002/oby.20334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.United States Census Bureau. Geography: Urban and rural. 2016; Available from: https://www.census.gov/geo/reference/urban-rural.html
- 40.United States Health and Human Services. Health professional shortage areas of primary care, dental care, or mental health providers. 2018; Available from: http://hpsafind.hrsa.gov/HPSASearch.
- 41.Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1–253. [PubMed] [Google Scholar]
- 42.Diabetes Prevention Program Research Group. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22(4):623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renjilian DA, Perri MG, Nezu AM, McKelvey WF, Shermer RL, Anton Sd . Individual versus group therapy for obesity: Effects of matching participants to their treatment preferences. J Consult Clin Psychol. 2001;69(4):717–21. [PubMed] [Google Scholar]
- 45.Perri MG, Martin AD, Leermakers EA, Sears Sf , Notelovitz M. Effects of group- versus home-based exercise in the treatment of obesity. J Consult Clin Psychol. 1997;65(2):278–85. [DOI] [PubMed] [Google Scholar]
- 46.Bandura A Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 47.Bandura A Self-efficacy: The Exercise of Control. New York, NY: W.H. Freeman and Company; 1997. [Google Scholar]
- 48.Kanfer FH, Gaelick-Buys L Self-management methods. Pergamon general psychology series, Vol. 52 Helping people change: A textbook of methods. Elmsford, NY: Pergamon Press, 1991: 305–360. [Google Scholar]
- 49.Perri MG, Nezu AM, McKelvey WF, Shermer RL, Renjilian DA, Viegener BJ. Relapse prevention training and problem-solving therapy in the long-term management of obesity. J Consult Clin Psychol. 2001;69(4):722–6. [PubMed] [Google Scholar]
- 50.Perri MG, Nezu AM, Viegener BJ. Improving the long-term management of obesity: Theory, research, and clinical guidelines. New York: Wiley; 1992. [Google Scholar]
- 51.United States Department of Agriculture. Choose MyPlate. 2018; Available from: https://www.choosemyplate.gov
- 52.United States Department of Health and Human Services, Department of Agriculture, Dietary Guidelines Advisory Committee. Dietary Guidelines for Americans, 2010. Seventh edition Washington, D.C. [Google Scholar]
- 53.United States Department of Health and Human Services, Department of Agriculture, Dietary Guidelines Advisory Committee. Dietary Guidelines for Americans, 2015. –2020 Eighth edition Washington, D.C. [Google Scholar]
- 54.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK, et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41 (2):459–71. 10.1249/MSS.0b013e3181949333 [DOI] [PubMed] [Google Scholar]
- 55.Borushek A The Calorie King Calorie, Fat, & Carbohydrate Counter. 2014 ed. Costa Mesa CA: Family Health Publications; 2014. [Google Scholar]
- 56.Borushek A The Calorie King Calorie, Fat, & Carbohydrate Counter. 2017 ed. Costa Mesa CA: Family Health Publications; 2017. [Google Scholar]
- 57.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: 2017. [Google Scholar]
- 58.Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: The Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49(11):1544–8. [DOI] [PubMed] [Google Scholar]
- 59.Paffenbarger RS Jr., Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25(1):60–70. [DOI] [PubMed] [Google Scholar]
- 60.Jenkinson C, Wright L, Coulter A. Criterion validity and reliability of the SF-36 in a population sample. Qual Life Res. 1994;3(1 ):7–12. [DOI] [PubMed] [Google Scholar]
- 61.American Psychological Association. Medical Outcomes Scale. n.d.; Available from: http://www.apa.org/pi/about/publications/caregivers/practice-settings/assessment/tools/medical-outcomes.aspx.
- 62.Maydeu-Olivares A, & Dzurilla TJ. A factor-analytic study of the Social Problem Solving Inventory: An integration of theory and data. Cognitive Therapy and Research. 1996;20(2):115–33. [Google Scholar]
- 63.Shewchuk RM, Johnson MO, Elliott TR. Self-appraised social problem solving abilities, emotional reactions and actual problem solving performance. Behav Res Ther. 2000;38(7):727–40. [DOI] [PubMed] [Google Scholar]
- 64.D’Zurilla TJ NA, Maydeu-Olivares A. Social Problem-Solving Inventory-Revised: Technical manual. North Tonawanda, NY: Multi-Health Systems; 2002. [Google Scholar]
- 65.Cutrona CE. Objective determinants of perceived social support. J Pers Soc Psychol. 1986;50(2):349–55. [DOI] [PubMed] [Google Scholar]
- 66.Cutrona CE, & Russell DW. The provisions of social relationships and adaptation to stress. Greenwich, CT: JAI Press; 1987. [Google Scholar]
- 67.Llach XB, Herdman M, Schiaffino A. Determining correspondence between scores on the EQ-5D “thermometer” and a 5-point categorical rating scale. Med Care. 1999;37(7):671–77. https://doi.org/Doi 10.1097/00005650-199907000-00007 [DOI] [PubMed] [Google Scholar]
- 68.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.SAS Institute Inc. SAS/ACCESS® 9.4 Interface to ADABAS. Cary, NC: SAS Institute Inc.; 2013. [Google Scholar]
- 70.Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353(20):2111–20. 10.1056/NEJMoa050156 [DOI] [PubMed] [Google Scholar]
- 71.Olson K, Bond D, Wing RR. Behavioral approaches to the treatment of obesity. R I Med J. 2017;100(2):21–24. [PubMed] [Google Scholar]
- 72.Perri MG, Corsica JA. Improving the maintenance of weight lost in behavioral treatment of obesity. Handbook of Obesity Treatment. 2002. January 9;1:357–79. [Google Scholar]
- 73.Daniels MJ, Hogan JW. Reparameterizing the pattern mixture model for sensitivity analyses under informative dropout. Biometrics. 2000;56(4):1241–8. [DOI] [PubMed] [Google Scholar]
- 74.Little RJ. A class of pattern-mixture models for normal incomplete data. Biometrika. 1994;81(3):471–83. [Google Scholar]
- 75.Daniels MJ, Hogan JW. Missing data in longitudinal studies: Strategies for bayesian modeling and sensitivity analysis. Boca Raton: Chapman & Hall/CRC; 2008. [Google Scholar]
- 76.Perri MG, Limacher MC, von Castel-Roberts K, Daniels MJ, Durning PE, Janicke DM, et al. Comparative effectiveness of three doses of weight-loss counseling: Two-year findings from the Rural LITE trial. Obesity (Silver Spring). 2014;22(11):2293–300. 10.1002/oby.20832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Teixeira PJ, Going SB, Sardinha LB, Lohman TG. A review of psychosocial pretreatment predictors of weight control. Obes Rev. 2005;6(1):43–65. 10.1111/j.1467-789X.2005.00166.x [DOI] [PubMed] [Google Scholar]
- 78.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6): 1173–82. [DOI] [PubMed] [Google Scholar]
- 79.Daniels MJ, Roy Ja , Kim C, Hogan JW, Perri MG. Bayesian inference for the causal effect of mediation. Biometrics. 2012;68(4):1028–36. 10.1111/j.1541-0420.2012.01781.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim C, Daniels MJ, Marcus BH, Roy JA. A framework for bayesian nonparametric inference for causal effects of mediation. Biometrics. 2017;73(2):401 –09. 10.1111/biom.12575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost- effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–103. 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 82.Institute of Medicine. Weighing the Options: Criteria for Evaluating Weight-Management Programs. Thomas PR, ed. Washington, DC: National Academies Press; 1995. [PubMed] [Google Scholar]
- 83.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–92. [DOI] [PubMed] [Google Scholar]
- 84.Tsai AG, Glick HA, Shera D, Stern L, Samaha FF. Cost-effectiveness of a low- carbohydrate diet and a standard diet in severe obesity. Obes Res. 2005;13(10):1834–40. 10.1038/oby.2005.223 [DOI] [PubMed] [Google Scholar]
- 85.Ratcliffe M, Burd C, Holder K, Fields A. Defining Rural at the U.S. Census Bureau. 2016; Available from: https://www2.census.gov/geo/pdfs/reference/ua/Defining_Rural.pdf
- 86.Trivedi T, Liu J, Probst J, Merchant A, Jhones S, Martin AB. Obesity and obesity-related behaviors among rural and urban adults in the USA. Rural Remote Health. 2015;15(4):3267. [PubMed] [Google Scholar]
- 87.Horrigan JB, Duggan M. Home Broadband 2015. Pew Research Center, 2015. [Google Scholar]


