Abstract
Organophosphate esters (OPEs) are widely used as flame retardants and plasticizers in consumer products, which contributes to widespread exposure in humans. OPE metabolites in urine have been used as biomarkers of human exposure to these chemicals. Little is known, however, about occurrence and temporal variability in urinary concentrations of OPE metabolites in humans. In this study, 11 OPE metabolites were measured in 213 urine samples collected from 19 volunteers from Albany, New York, United States, at 3-day intervals for five weeks to investigate temporal variability in urinary concentrations. Diphenyl phosphate (DPHP) and bis(1,3-dichloro-2-propyl) phosphate (BDCIPP) were the major OPE metabolites, detected in all urine samples at specific gravity (SG)-adjusted concentrations (geometric mean, GM) of 1,060 and 414 pg/mL and creatinine (Cr)-adjusted concentration (GM) of 404 and 156 ng/g, respectively. Inter-day variability in urinary OPE concentrations in 19 individuals was evaluated by intraclass correlation coefficients (ICCs). The inter-day variability in Cr-adjusted OPE concentrations (ICC: 0.31–0.67) was lower than those of SG-adjusted (ICC: 0.19–0.71) and unadjusted urinary concentrations (ICC: 0.24–0.74). BDCIPP (ICC: 0.68) and bis(2-chloroethyl) phosphate (BCEP) (ICC: 0.67) concentrations showed a moderate-to-strong reliability over the sampling period, whereas the other nine OPE metabolites exhibited a moderate reliability (ICC: 0.31–0.55). Urine samples were further stratified by gender, age, ethnicity, and body mass index (BMI). The concentrations of BDCIPP and DPHP were significantly lower in males with normal BMI (BMI: 18.5–25 kg/m2) than in females and for other BMI categories (p < 0.01). Relatively high ICCs, indicating low inter-day variability, were observed for males (ICC: 0.35–0.71) of 30–40 years of age (ICC: 0.34–0.87) with normal BMI (ICC: 0.28–0.64). The daily exposure doses to OPEs were estimated from urinary concentrations of corresponding OPE metabolites. The estimated doses of triphenyl phosphate (TPHP) and triethyl phosphate (TEP), based on median urinary concentrations of their metabolites, were 19.4 and 24.0 ng/kg bw/day, and the exposure dose to total ΣOPEs was estimated at 65.3 ng/kg bw/day. Overall, our results indicate a high ICC for Cr-adjusted urinary concentration of 11 OPE metabolites in urine.
Keywords: organophosphate esters, urinary metabolites, temporal variability, exposure assessment
1. Introduction
Organophosphate esters (OPEs) have received considerable attention in recent years due to their widespread use as flame retardants and plasticizers in consumer products, including furniture, textile, and electronics (van der Veen et al., 2012). OPEs are substitutes for brominated flame retardants (BFRs) (Wei et al., 2015) and have been reported to occur in indoor air (Fromme et al., 2014; Hartmann et al., 2004), atmosphere (Rauert et al., 2018), dust (Wang et al., 2018; Xu et al., 2016; Zheng et al., 2015), soil (Cui et al., 2017; Wang et al., 2018), sediment (Wang et al., 2017), water (Kim et al., 2017; Kim et al., 2018; Lee et al., 2016), and biota (Hallanger et al., 2015). Toxicological studies have linked exposure to OPEs with reproductive and developmental effects (Behl et al., 2015), genotoxicity (Du et al., 2016), and endocrine effects (Chen et al., 2015). Due to OPEs’ carcinogenic and neurotoxic properties (van der Veen et al., 2012), human exposure to these chemicals is a matter of concern.
Following exposure, OPEs, such as triphenyl phosphate (TPHP) and tris(1,3-dichloro-2-propyl) phosphate (TDCIPP), are hydrolyzed in the human body to yield diester metabolites, which are excreted in urine (Cequier et al., 2015). Therefore, urinary OPE metabolites have been used as biomarkers of OPE exposure (Dodson et al., 2014; Hoffman et al., 2014). Recent studies have reported the occurrence of OPE metabolites in urine from children (Butt et al., 2016; Chen et al., 2018; Zhang et al., 2018), adults (Meeker et al., 2013; Lu et al., 2017), and pregnant women (Romano et al., 2017) from around the world. Diphenyl phosphate (DPHP), diethyl phosphate (DEP) and bis(1,3-dichloro-2-propyl) phosphate (BDCIPP) were the most dominant OPE metabolites reported in most human biomonitoring studies (He et al., 2018; Ospina et al., 2018; Sun et al., 2018). On the basis of the urinary concentrations of OPE metabolites, daily urine excretion volume, and molar fraction of the metabolites excreted, human exposure dose to OPEs has been estimated (Fromme et al., 2014). For instance, exposure to tri-n-butyl phosphate (TNBP) in children was estimated from urinary dibutyl phosphate (DNBP) measurements with an exposure dose to TNBP at 1.03 μg/kg bw/day (Fromme et al., 2014). In general, studies that report daily variations in OPE metabolite concentrations in urine are scarce. Such studies are needed to assess the value and interpretability of data from single-spot urine collected and analyzed for epidemiologic studies. Interclass correlations can be used in the assessment of consistency or reproducibility of quantitative measurements made longitudinally over time from the same individual.
The half-life of OPEs in humans is not well known, and the urinary concentration of OPE metabolites can vary in the same individual, depending on the sampling time. The temporal variability in such biomarker concentrations can introduce significant uncertainty in exposure assessment (Perrier et al., 2016). Several studies have examined reproducibility and inter-day variability in urinary concentrations of bisphenols and phthalates metabolites (Dewalque et al., 2015; Vernet et al., 2018). Temporal variability in two urinary OPE metabolite concentrations, BDCIPP and DPHP, has been reported (Hoffman et al., 2014; Hoffman et al., 2015; Meeker et al., 2013). Studies have reported variability in urinary concentrations of bis(2-chloroethyl) phosphate (BCEP) (Romano et al., 2017) and bis(methylphenyl) phosphate (BMPP) (Tao et al., 2018). Temporal variability in the concentrations of DEP, DNBP, bis(butoxyethyl) phosphate (BBOEP), and bis(1-chloro-2-propyl) phosphate (BCIPP), however, has not been examined, especially in healthy adult individuals. In this study, 11 OPE metabolites were analyzed in 213 daily urine samples, collected every three days from 19 volunteers over five weeks in Albany, New York, United States (U.S.), to describe inter-day variability in urinary concentrations and to estimate exposure doses to OPEs.
2. Materials and methods
2.1. Standards and reagents
DEP was purchased from AccuStandard (New Haven, CT, U.S.). Dipropyl phosphate (DPRP), DNBP, diisobutyl phosphate (DIBP), BBOEP, bis(2-ethylhexyl) phosphate (BEHP), BCEP, BCIPP, BDCIPP, DPHP, and BMPP were purchased from Toronto Research Chemicals (North York, ON, Canada). Nine deuterated OPE compounds were used as internal standards, which comprise dibutyl phosphate-d18 (DNBP-d18), diisobutyl phosphate-d14 (DIBP-d14), bis(butoxyethyl) phosphate-d8 (BBOEP-d8), bis(2-ethylhexyl) phosphate-d34 (BEHP-d34), bis(2-chloroethyl) phosphate-d8 (BCEP-d8), bis(1-chloro-2-propyl) phosphate-d12 (BCIPP-d12), bis(1,3-dichloro-2-propyl) phosphate-d10 (BDCIPP-d10), diphenyl phosphate-d10 (DPHP-d10), and bis(methylphenyl) phosphate-d14 (BMPP-d14) were purchased from Toronto Research Chemicals. All standard solutions were prepared in HPLC-grade acetonitrile. HPLC-grade water (J. T. Baker, Center Valley, PA, U.S.), acetonitrile (J. T. Baker), and methanol (Fisher Scientific, Fair Lawn, NJ, U.S.) were used in the analysis. The physico-chemical properties of target chemicals are provided in Table S1 of the Supplementary Information (SI).
2.2. Sample collection
De-identified individual samples collected from February to April 2018 from 19 healthy volunteers who were living in Albany, New York, U.S., were used. Each volunteer provided information regarding gender (males, n= 11; females, n=8), age (33.8 ± 12 years), ethnicity (Asians, n= 13; Caucasians, n=6), body weight (67.6 ± 17 kg), height (1.68 ± 0.1 m), and smoking habits (none was an active smoker) (Table S2). Volunteers were well educated with >89% with a Bachelor or higher degree and none indicated any occupational exposure to OPEs. The archived samples, originally collected for another study and stored at −20° C, were used. Early morning-void urine samples were collected from each volunteer every three days over five weeks in polypropylene (PP) containers which were precleaned with methanol. For personal reasons, the collection intervals of a few samples ranged from 2 to 10 days, and the number of samples from each volunteer ranged from 6 to 14. In total, 213 urine samples were analyzed in this study. The Wadsworth Center, New York State Department of Health, institutional review board approvals were obtained for the analysis of samples.
2.3. Extraction
Urine samples were extracted by following a previously published method with minor modifications (Sun et al., 2018). Briefly, urine samples (1 mL) were spiked with an internal-standards mixture (2 ng), and pH was adjusted by the addition of 1 mL of ammonium acetate buffer (10 mM, pH = 5.0). After vortexing for 1 min, samples were loaded on a STRATA-X-AW cartridge (60 mg 3 cm3; Phenomenex Inc., Torrance, CA, U.S.) that was conditioned with 2.0 mL of methanol and 2.0 mL of water. One milliliter of water was used to wash the cartridge after loading the sample, and the target chemicals were eluted by 2 mL of 5% ammonium hydroxide in MeOH. The eluents were concentrated to near dryness under a gentle nitrogen stream and reconstituted with 100 μL of acetonitrile. After micro-centrifugation (0.22 μm nylon filter, Spin-X; Costar, Corning Inc., Corning, NY, U.S.), samples were analyzed by HPLC-MS/MS.
2.4. Instrumental analysis
High-performance liquid chromatography (HPLC, Agilent 1260 series; Agilent Technologies Santa Clara, CA, U.S.), coupled with electrospray triple quadrupole mass spectrometry (API QTRAP 4500, ESI-MS/MS; Applied Biosystems, Foster City, CA. U.S.), was used in the identification and quantification of target compounds. Eleven OPE metabolites and 9 internal standards were separated by a HILIC column (100 mm × 2.1 mm, 5 μm; Thermo, Waltham, MA, U.S.) connected to a Betasil C18 guard column (20 mm × 2.1 mm, 5 μm; Thermo). The mobile phase consisted of HPLC-grade water (10 mM ammonium acetate): methanol (2:3 v/v) (A) and acetonitrile (B), used at a flow rate of 200 μL/min. The initial mobile phase flow was set at 96.5% B. After a 2-min hold, Phase B was decreased linearly to 88.5% in 1.5 min and then decreased linearly to 86.0% in 3 min, held for 8.5 min before reverting to the initial condition in 0.5 min, and allowed to equilibrate for 9.5 min, with a total run time of 25 min. Electrospray negative ionization (ESI-) and multiple reaction monitoring (MRM) modes were used for the identification and quantification of OPE diesters. Detailed MS/MS parameters used in the analysis of OPE diesters are shown in Table S3.
2.5. Quality assurance (QA) and quality control (QC)
Calibration standards at concentrations that range from 0.01 to 100 ng/mL (with 20 ng/mL internal standards mixture) were used for the calculation of target chemicals’ concentration in samples. The regression coefficients of calibration curves were >0.99. Procedural blank samples were analyzed to check for background levels of contamination. DEP, DNBP, DIBP, BBOEP, BEHP, and DPHP were found at trace levels in blanks (2.1–9.8 ng/L). OPE metabolite concentrations in samples were subtracted from blank values. For those analytes that were not found in procedural blanks, the method detection limits (MDLs) were calculated at a signal-to-noise ratio of 10 (of the lowest point of the calibration standard) by taking into account the sample volume and concentration factor. For those analytes that were present as procedural blanks, MDLs were calculated as three times the standard deviation (SD) of blank values, and sample volume and concentration factors were taken into consideration. The recoveries of target chemicals spiked into urine at high (25 μg/L) and low (5 μg/L) levels were 74.8–104% and 84.5–113%, respectively. The recoveries of internal standards spiked into each of the samples were between 80% and 108%. Further details of the QA/QC parameters are presented in Table S4.
2.6. Statistical analysis
Urinary OPE metabolite concentrations were corrected by specific gravity (SG) and creatinine (Cr). Only analytes with detection frequency (DF) >50% were considered in the statistical analysis. A value of 1/2 the MDL was used for those compounds that were below the detection limit (He et al., 2018; Sun et al., 2018) and non-detects (n.d.s) were assigned a value of zero. Nonparametric statistical tests (Mann-Whitney U test and Kruskal-Wallis H test) were used to compare differences in individual OPE metabolite concentrations with demographic variables such as gender, age, body mass index (BMI; normal: 18.5–25, overweight: 25.1–30, obese: >30), and ethnicity. Spearman’s correlation analysis was used to examine the relationships among several OPE metabolite concentrations in urine. Statistical significance was set at p < 0.05. All statistical analyses were performed with SPSS software (Version 22.0, SPSS Inc., Armonk, NY, U.S.).
Temporal variability in urinary concentrations of 11 OPE metabolites was evaluated by intraclass correlation coefficients (ICCs) using one-way random-effect ANOVA models (Vernet et al., 2018). All urine samples from each volunteer (6–14 spot urine samples per person) were used for ICC calculation. ICC values near zero indicate high inter-day variability and less reliability for predicting concentrations over a period, whereas values close to one indicate less variability and high reliability for predicting concentrations over time (ICCs >0.80 were considered as high, 0.40–0.79 as moderate, and <0.40 as low reliability) (Sakhi et al., 2018). Negative ICCs values were not computable in the current model (Vernet et al., 2018). To characterize the inter-individual variability, we defined ICC as the ratio of intra-individual variance to the total variance (sum of inter- and intra-variance) and evaluated by the same models as temporal variability evaluation.
The exposure doses of parent OPEs (Dp) were calculated from the urinary concentrations of OPE metabolites. Studies of toxico-kinetics and the metabolism of OPEs are limited. Urinary excretion rates of DNBP and BDCIPP metabolites in orally exposed rats have been reported (Lynn et al., 1981; Suzuki et al., 1984). The molar fraction (FUE) values of urine-excreted DNBP of 0.18 (Suzuki et al., 1984) and BDCIPP of 0.63 (Lynn et al., 1981) were used in the calculation of Dp of other OPE metabolites by following the equation (eq. 1) (Chen et al., 2018; Fromme et al., 2014):
| (1) |
where Cm is the unadjusted concentration of the metabolite in urine (ng/mL), and UVexcr is the daily urine excretion volume of 20 mL/kg bw/day for adults and 22.2 mL/kg bw/day for children (Chen et al., 2018; Wittassek et al., 2011). FUE is the molar fraction of the urine-metabolite excreted (0.18 for DEP, DPRP, DNBP, DIBP, BBOEP, and BEHP; 0.63 for BCEP, BCIPP, BDCIPP, DPHP, and BMPP) (Lynn et al., 1981; Suzuki et al., 1984), and MWp and MWm are the molecular weights of parent and corresponding metabolites, respectively.
3. Results and discussion
3.1. Urinary concentrations of OPE metabolites
Of the 11 OPE metabolites measured, DEP, DIBP, BDCIPP, and DPHP were found in all urine samples (DF = 100%), followed by DNBP, BEHP, BBOEP, BCEP, DPRP, and BCIPP, found at a DF of 64% to 98% (Table 1). BMPP was found in only <19% of the samples, and, therefore, this compound was removed from further statistical analysis.
Table 1.
Distribution of specific gravity (SG) -adjusted and creatinine (Cr) -adjusted organophosphate ester (OPE) metabolite concentrations in urine (n=213) collected every 3 days for 5 weeks from 19 individuals from Albany, New York, United States
| OPE metabolites | Percentile | Range | GM | AM | DF (%) | MDL | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | ||||||
| SG-adjusted (pg/mL) | ||||||||||
| DEP | 63.6 | 178 | 403 | 689 | 1500 | 26.2–3160 | 348 | 531 | 100 | 1.7 |
| DPRP | ND | ND | 8.7 | 21.3 | 77.1 | ND-726 | 15.4 | 29.9 | 73 | 7.2 |
| DNBP | <MDL | 8.6 | 18.4 | 33.6 | 104 | ND-836 | 16.8 | 34.8 | 98 | 2.9 |
| DIBP | <MDL | 27.5 | 45.6 | 77.1 | 191 | <MDL-721 | 49.1 | 70.7 | 100 | 25 |
| BBOEP | ND | 13.8 | 28.5 | 55.3 | 132 | ND-284 | 32.6 | 47.2 | 92 | 11 |
| BEHP | <MDL | 6.1 | 14.7 | 25.8 | 67.9 | ND-148 | 13.4 | 22.9 | 96 | 2.3 |
| BCEP | ND | 146 | 302 | 534 | 1580 | ND-4250 | 354 | 519 | 87 | 2.7 |
| BCIPP | ND | ND | 33.2 | 106 | 606 | ND-1400 | 83.8 | 180 | 64 | 16 |
| BDCIPP | 54.6 | 199 | 359 | 962 | 2540 | 21.3–5650 | 414 | 737 | 100 | 10 |
| DPHP | 303 | 517 | 919 | 2060 | 4890 | 151–13500 | 1060 | 1630 | 100 | 3.5 |
| BMPP | ND | ND | ND | ND | 70.7 | ND-480 | 42 | 78.1 | 19 | 0.6 |
| Cr-adjusted (ng/g) | ||||||||||
| DEP | 22.1 | 58.2 | 147 | 281 | 620 | 5.58–1390 | 132 | 216 | ||
| DPRP | ND | ND | 4.11 | 9.57 | 37.9 | ND-533 | 4.85 | 11.6 | ||
| DNBP | <MDL | 3.40 | 6.63 | 12.6 | 31.1 | ND-615 | 6.56 | 15.5 | ||
| DIBP | <MDL | 10.9 | 16.2 | 31.8 | 85.5 | <MDL-530 | 18.7 | 32.2 | ||
| BBOEP | ND | 6.09 | 10.6 | 22.0 | 58.1 | ND-157 | 11.9 | 18.1 | ||
| BEHP | <MDL | 1.97 | 5.84 | 11.5 | 38.3 | ND-109 | 4.83 | 10.1 | ||
| BCEP | ND | 51.7 | 108 | 193 | 719 | ND-1900 | 73.6 | 182 | ||
| BCIPP | ND | ND | 18.4 | 42.6 | 164 | ND-342 | 19.4 | 39.2 | ||
| BDCIPP | 24.7 | 89.6 | 150 | 318 | 887 | 7.38–1880 | 156 | 255 | ||
| DPHP | 106 | 192 | 329 | 850 | 1980 | 74.7–3990 | 404 | 627 | ||
| BMPP | ND | ND | ND | ND | 31.4 | ND-184 | 0.53 | 6.86 | ||
ND= Not detected
GM= Geometric mean
AM= Arithmetic mean
DF= Detect frequency
MDL= Method determination limit
Urinary concentrations of OPE metabolites reported in previous studies were either SG-adjusted or unadjusted values. We compared our SG-adjusted OPE concentrations with those reported in the literature. The concentrations (geometric mean; GM) of DPHP (1,060 pg/mL), BDCIPP (414 pg/mL), BCEP (354 pg/mL), and DEP (348 pg/mL) were significantly higher than those of other OPE metabolites measured in this study (Table 1). BCIPP was found at a concentration of 83.8 pg/mL, and the remaining OPE metabolites were found below 50 pg/mL. Several previous studies reported the predominance of DPHP and BDCIPP in urine samples (Ospina et al., 2018; Thomas et al., 2017; Tao et al., 2018). In our study, the urinary concentration of DPHP (1,060 pg/mL) was higher than that reported for adults and children from China (32.4–280 pg/mL) (Sun et al., 2018; Tao et al., 2018) (Table S5), comparable to that reported for adults from the U.S. (1,200 pg/mL) (Butt et al., 2016), and children from the U.S. (845 pg/mL) (Ospina et al., 2018) and Norway (1,000 pg/mL) (Cequier et al., 2015). Few studies reported significantly higher urinary DPHP concentrations in adults (1,800–2,990 pg/mL) (Preston et al., 2017) and children (3,000–8,200 pg/mL) (Butt et al., 2014; Thomas et al., 2017) from the U.S. BDCIPP concentration measured in this study (414 pg/mL) was comparable to those reported previously for adults from the U.S. (130–408 pg/mL) (Carignan et al., 2013; Meeker et al., 2013) and children from Central China (230 pg/mL) (Tao et al., 2018) but significantly lower than those reported for women (2,400–3,300 pg/mL) (Butt et al., 2014; Butt et al., 2016) and children (2,700–10,900 pg/mL; 856 pg/mL) (Butt et al., 2016; Thomas et al., 2017; Ospina et al., 2018) from the U.S. Urinary concentrations of OPE metabolites other than DPHP and BDCIPP have rarely been reported in the literature. A few studies reported DEP, DNBP, BBOEP, and BCEP concentrations in urine samples (Dodson et al., 2014; Petropoulou et al., 2016; Sun et al., 2018; Ospina et al., 2018). DEP concentrations (348 pg/mL) determined in our study were similar to those reported from China (376 pg/mL) (Sun et al., 2018), whereas DNBP (16.8 pg/mL), and BCEP (354 pg/mL) concentrations were lower than those reported from China (Chen et al., 2018) and the U.S. (Ospina et al., 2018).
The occurrence of high DPHP concentrations in urine samples suggests exposure to its parent compound TPHP and other compounds that could metabolize into DPHP. TPHP and 2-ethylhexyl diphenyl phosphate (EHDPP) were found in indoor dust samples from the U.S. (Clark et al., 2017; Hoffman et al., 2017a). Further, metabolic transformation of several aryl-OPEs, such as t-butylphenyl diphenyl phosphate (BPDP), cresyl diphenyl phosphate (CDDP), isodecyl diphenyl phosphate (IDDP), and resorcinol bis(diphenyl phosphate) (RDP), which are widely used in polyurethane foams, hydraulic fluids, and polyvinyl chloride (PVC) plastics (van der Veen et al., 2012), can yield DPHP. In general, tris(2-chloroisopropyl) phosphate (TCIPP) and tris(2-butoxyethyl) phosphate (TBOEP) were found at the highest concentrations in abiotic matrices (Wei et al., 2015), whereas their metabolites, BCIPP and BBOEP, were found at concentrations lower than those of DPHP in urine. In contrast, triethyl phosphate (TEP) and TDCIPP were found at moderate concentrations in abiotic matrices, but their metabolites DEP and BDCIPP were abundant in urine samples. Aryl-OPEs were hydrolyzed more rapidly than were alkyl- and chlorinated ones (Su et al., 2016); further, the fraction of BDCIPP excreted in urine was (0.63) higher than that of DNBP (0.18) (Lynn et al., 1981; Suzuki et al., 1984). Therefore, the differences in the profiles of parent OPEs in abiotic samples and the metabolites in urine suggest variabilities in metabolic transformation and urinary excretion rates.
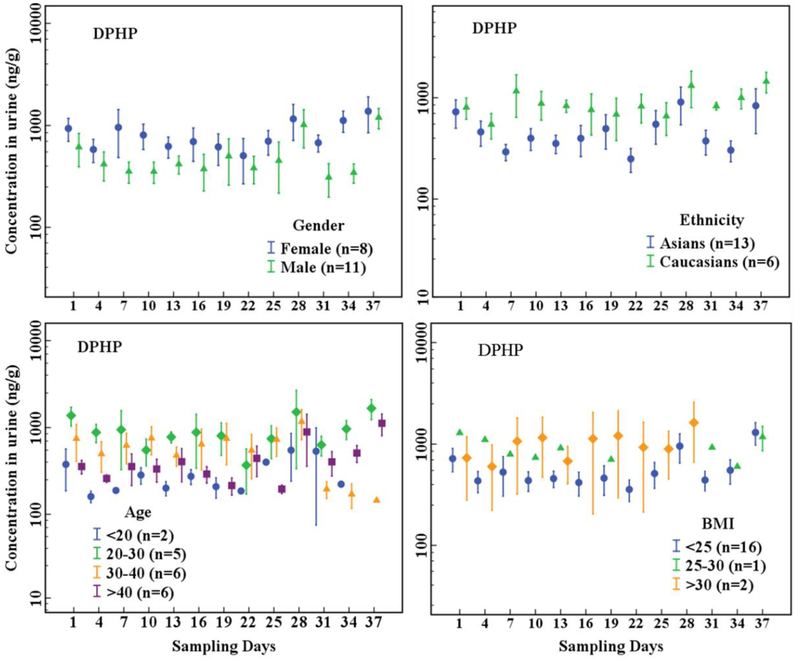
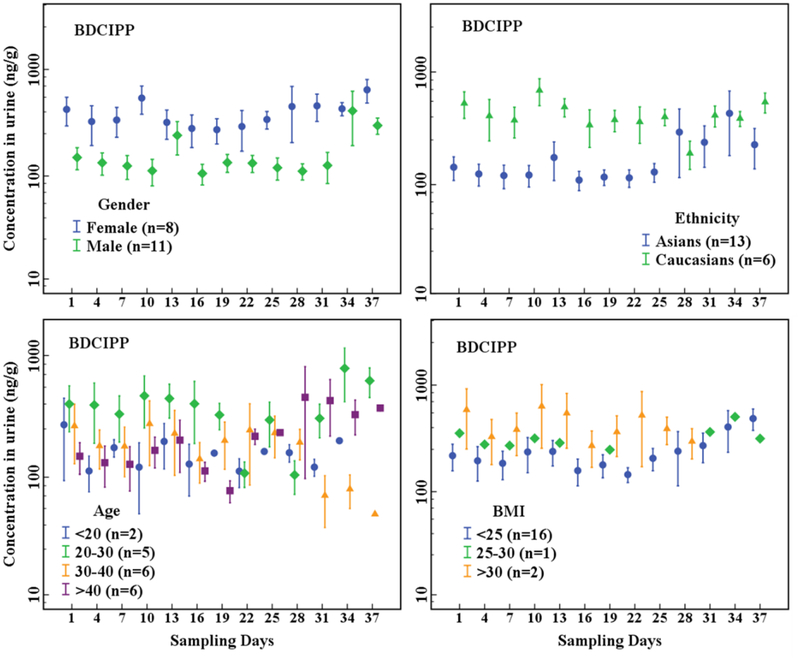
The Cr-adjusted urinary concentrations of OPE metabolites (Figure 1, Figure 2, Table S6) were compared between gender, ethnicity, age, and BMI categories. Female urinary concentrations of DPHP (p < 0.001), BDCIPP (p < 0.001), and DEP (p < 0.001) were significantly higher than those of males, whereas the concentrations of BEHP (p = 0.925) and BCIPP (p = 0.259) were similar between females and males. Urinary concentrations of DPHP (p < 0.001) and BDCIPP (p < 0.001) of Caucasians were significantly higher than those of Asians. Among the age groups, individuals between 20 and 30 years old had significantly higher urinary concentrations of DPHP and BDCIPP (p < 0.05) than did the other age groups. Similarly, all OPE metabolite concentrations were significantly different among the three BMI categories (p ≤ 0.001–0.023). Urinary concentrations of DPHP (p < 0.001), BDCIPP (p < 0.001), and BCIPP (p < 0.001) in obese individuals (BMI>30 kg/m2) were higher than those in individuals with normal BMI (18.5–25 kg/m2), whereas urinary concentrations of DEP (p = 0.012), BBOEP (p = 0.023), and BEHP (p = 0.004) in obese individuals were lower than those with normal BMI.
Figure 1.
Temporal variation in Cr-adjusted concentrations (ng/g) of DPHP in urine collected every 3 days from 19 individuals grouped by gender, age, ethnicity and BMI (n = participant number). The dots represent arithmetic means and whiskers represent standard error (SE).
Figure 2.
Temporal variation in Cr-adjusted concentrations (ng/g) of BDCIPP in urine collected every 3 days from 19 individuals grouped by gender, age, ethnicity and BMI (n = participant numbers). The dots represent arithmetic means and whiskers represent standard error (SE).
3.2. Temporal variability of urinary concentrations of OPE metabolites
The ICCs were calculated for OPE concentrations measured in urine samples collected longitudinally (every three days) from 19 individuals over five weeks (Table 2). The inter-day variability for unadjusted OPE concentrations was moderate for a majority of the compounds analyzed (ICCs = 0.40–0.74), except for DPRP (0.24; 95% CI: 0.09–0.52), DIBP (0.25; 95% CI: 0.10–0.53), and BEHP (0.20; 95% CI: 0.06–0.48), which showed high variability in concentrations over the sampling period (Table 2). Much higher ICC values were observed for BDCIPP (0.69; 95% CI: 0.51–0.87) and BCIPP (0.74; 95% CI: 0.0.57–0.89), which suggests that concentrations of these compounds varied little across time.
Table 2.
Inter-day variability in urinary organophosphate ester metabolite concentrations in samples collected every 3 days for 5 weeks from 19 individuals.
| OPE metabolites | Unadjusted | SG-adjusted | Cr-adjusted | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variance (pg/mL) | CV (%) | ICC | 95% CI | Variance (pg/mL) | CV (%) | ICC | 95% CI | Variance (ng/g) | CV (%) | ICC | 95% CI | |
| DEP | 162 | 70.0 | 0.40 | 0.20–0.67 | 519 | 74.9 | 0.19 | 0.05–0.46 | 207 | 76.3 | 0.32 | 0.17–0.54 |
| DPRP | 9.78 | 80.4 | 0.24 | 0.09–0.52 | 23.1 | 86.4 | 0.30 | 0.13–0.58 | 10.9 | 96.0 | 0.31 | 0.16–0.53 |
| DNBP | 17.3 | 94.9 | 0.52 | 0.32–0.76 | 32.7 | 92.4 | 0.49 | 0.28–0.74 | 14.3 | 94.8 | 0.39 | 0.23–0.61 |
| DIBP | 20.2 | 57.4 | 0.25 | 0.10–0.53 | 69.9 | 62.6 | 0.16 | 0.03–0.42 | 31.0 | 74.7 | 0.39 | 0.23–0.61 |
| BBOEP | 17.6 | 77.0 | 0.42 | 0.23–0.69 | 44.7 | 66.1 | 0.36 | 0.17–0.64 | 17.4 | 68.3 | 0.35 | 0.19–0.57 |
| BEHP | 6.21 | 69.9 | 0.20 | 0.06–0.48 | 21.6 | 76.8 | 0.28 | 0.12–0.56 | 9.76 | 84.6 | 0.44 | 0.27–0.65 |
| BCEP | 202 | 94.2 | 0.65 | 0.46–0.84 | 427 | 85.2 | 0.64 | 0.45–0.84 | 170 | 89.7 | 0.67 | 0.52–0.83 |
| BCIPP | 58.6 | 78.5 | 0.74 | 0.57–0.89 | 122 | 66.9 | 0.71 | 0.53–0.88 | 38.2 | 70.8 | 0.55 | 0.38–0.74 |
| BDCIPP | 260 | 77.1 | 0.69 | 0.51–0.87 | 712 | 65.4 | 0.70 | 0.52–0.87 | 245 | 60.3 | 0.68 | 0.52–0.83 |
| DPHP | 660 | 71.1 | 0.44 | 0.24–0.70 | 1622 | 54.9 | 0.58 | 0.38–0.80 | 616 | 54.0 | 0.54 | 0.37–0.74 |
All urine samples were collected every 3 days for 5 weeks from 19 individuals
ICCs (Intraclass correlation coefficients) are calculated from log10-transformed OPE metabolite concentrations
CV= coefficient of variation
SG= specific gravity
Cr= creatinine
CI = confidence intervals
Overall, these results indicate that OPE metabolites in single-spot urine over a period of 5 weeks entail moderate to high dependability (except for DPRP, DIBP, and BEHP). SG-adjusted and Cr-adjusted concentrations of OPE metabolites are presented in Table 2. The ICCs of OPE concentrations did not improve significantly after adjustment for SG, whereas the ICCs of DEP (0.19; 95% CI: 0.05–0.46) and DIBP (0.16; 95% CI: 0.03–0.42) decreased. Nevertheless, Cr-adjustment of OPE concentrations improved the ICCs of DPRP, DIBP, and BEHP. The significant Spearman rank correlations between OPE metabolite concentrations and creatinine explain the reason for improvement in ICCs following Cr-adjustment (Table S7). For further data analysis, we used Cr-normalized concentrations of OPEs, as discussed below.
Between the two genders, the ICCs of OPE concentrations in males were >0.35, whereas those for DEP, DPRP, DIBP, and BEHP in females were between 0.05 and 0.19, indicating that urinary concentrations of OPEs were more variable in females than males (Table S8). The urinary concentrations of OPEs in Asians showed higher variability (ICC: 0.20–0.26) than did those of Caucasians (ICC: 0.32–0.67). There was no significant difference (p = 0.437) in variability in OPE concentrations between the age groups of 30–40 years (ICC: 0.34–0.87) and >40 years (ICC: 0.17–0.70). DEP (0.03; 95% CI: 0–0.51), DPRP (0.03; 95% CI: 0–0.52), and BCIPP (0.05; 95% CI: 0–0.56) concentrations, however, exhibited higher variability in the age group of 20–30 years. Individuals with BMI>25 kg/m2 showed a low ICC for BBOEP (ICC: 0.04), which suggested that overweight or obese individuals show a propensity for high variability in OPE metabolite concentrations in urine.
A few studies have examined temporal variability in urinary concentrations of environmental phenols (Koch et al., 2014; Vernet et al., 2018) and phthalate metabolites (Dewalque et al., 2015; Fisher et al., 2015). The calculated variability in OPE metabolite concentrations in urine is higher than those reported for bisphenol A (ICC: 0.6, 95% CI: 0.3–0.89), methyl paraben (ICC: 0.84, 95% CI: 0.69–1.0), and triclosan (ICC: 0.89, 95% CI: 0.78–1.0) (Vernet et al., 2018). The temporal variability in mono-ethylhexyl phthalate (MEHP) (ICC: 0.16–0.22, 95% CI: 0.15–0.45) (Dewalque et al., 2015; Fisher et al., 2015) was higher than that found for OPE metabolites in urine. To the best of our knowledge, no previous study investigated inter-day variabilities in urinary concentrations of OPE metabolites, except for BDCIPP, DPHP, and BCEP. The inter-day variabilities calculated for DPHP (ICC: 0.54, 95% CI: 0.37–0.74) were similar to those reported in urine from pregnant women (ICC: 0.6, 95% CI: 0.4–0.7) (Hoffman et al., 2014), healthy adults (ICC: 0.51, 95% CI: 0.43–0.63) (Hoffman et al., 2015), and women (ICC: 0.42, 95% CI: 0.36–0.50) (Romano et al., 2017) from the U.S. Similarly, our results for the ICC for BCIPP were comparable to those reported in the literature (Hoffman et al., 2014; Meeker et al., 2013; Romano et al., 2017). Overall, urinary OPE concentrations exhibited a moderate temporal reproducibility, and Cr-adjustment improved the reproducibility.
3.3. Inter-individual variability and composition of OPE metabolites
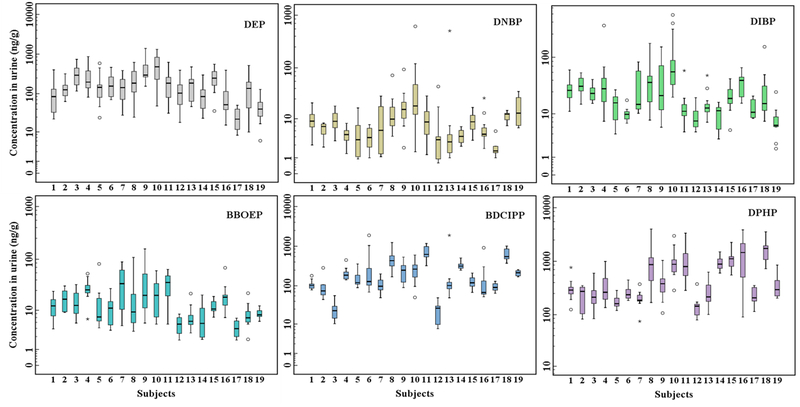
The inter-individual variance (Cr-adjusted) and coefficient of variation (CV%) in concentrations of DEP, BDCIPP, and DPHP among the 19 individuals were 207 (98%) ng/g, 270 (107%) ng/g, and 620 (100%) ng/g, respectively (Figure 3, Table S9). The highest concentrations of BCIPP (102 ng/g) and DPHP (1543 ng/g) were found in an individual with the highest BMI (Table S2). The highest concentrations of DEP (391 ng/g), DPRP (28.3 ng/g), DNBP (24.7 ng/g), DIBP (72.1 ng/g), and BEHP (17.6 ng/g) were found in a 56-year-old Caucasian female with a normal BMI. The concentrations of DPHP and BDCIPP accounted for 30–85% and 5–25%, respectively, of the total concentrations of 11 metabolites (Figure S1). The composition profile of OPE metabolites was similar to that reported earlier in urine from the U.S. with DPHP accounting for 35–41% of the total concentrations (Dodson et al., 2014; Ospina et al., 2018) and Australia (DPHP 84%) (He et al., 2018) but different from that reported for China, where BCEP (60%) was the most abundant metabolite (Chen et al., 2018). Regional differences in the OPE metabolite composition profile in urine may indicate different exposure patterns.
Figure 3.
Cr-adjusted concentrations (ng/g) of DEP, DNBP, DIBP, BBOEP, BDCIPP and DPHP in urine collected every 3 days from 19 individuals. The black horizontal line inside each box represents median, the boxes represent 25th and 75th percentiles, whiskers represent a value of 1.5*SD and the dots represent outliers.
3.4. Exposure assessment
Exposure doses to parent OPEs were calculated based on unadjusted urinary metabolite concentrations (Table 3). The median and 95th percentile ΣOPE exposure doses were 65.3 and 355 ng/kg bw/day, respectively. Females (81.9 ng/kg bw/day), Caucasians (114 ng/kg b.w./day), and obese individuals (BMI>30 kg/m2) (165 ng/kg bw/day) had higher ΣOPE exposure doses than did the other categories. The median and 95th percentile exposure doses of TEP were 24.0 and 79.8 ng/kg bw/day, respectively, whereas those of TPHP were 19.4 and 118 ng/kg bw/day, respectively, which were significantly higher than those of other OPEs analyzed. Nail polish was reported as a source of TPHP exposure (Mendelsohn et al., 2016), which could contribute to higher exposure in females (24.8 ng/kg bw/day) than males (15.7 ng/kg bw/day).
Table 3.
Estimated daily exposure dose to organophosphate esters calculated from urinary concentration of metabolites in the United States
| Exposure dose (ng/kg bw/day) | TEP | TNBP | TIBP | TBOEP | TEHP | TCEP | TCIPP | TDCIPP | TPHP | ΣOPEs | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentile | 5th | 3.47 | 0.20 | 1.81 | 0.85 | 0.17 | 0.06 | 0.34 | 0.93 | 4.14 | 12.5 |
| 25th | 13.4 | 0.54 | 1.81 | 0.85 | 0.57 | 2.34 | 0.34 | 3.36 | 9.92 | 33.6 | |
| 50th | 24.0 | 1.09 | 2.49 | 2.10 | 1.01 | 5.97 | 0.34 | 8.51 | 19.4 | 65.3 | |
| 75th | 39.0 | 2.43 | 5.10 | 4.95 | 1.61 | 11.9 | 2.39 | 21.9 | 44.4 | 135 | |
| 95th | 79.8 | 9.59 | 11.9 | 11.4 | 3.20 | 38.6 | 14.4 | 61.9 | 118 | 355 | |
| Gender | Male | 21.1 | 0.91 | 2.56 | 1.80 | 1.02 | 4.35 | 0.34 | 5.56 | 15.7 | 53.9 |
| Female | 27.4 | 1.27 | 2.14 | 2.66 | 0.98 | 7.82 | 0.81 | 13.4 | 24.8 | 81.9 | |
| Ethnicity | Asians | 23.0 | 0.73 | 1.80 | 1.87 | 1.03 | 4.59 | 0.34 | 5.30 | 14.6 | 53.7 |
| Caucasians | 29.1 | 2.41 | 4.08 | 2.74 | 0.97 | 8.72 | 1.76 | 19.7 | 44.2 | 114 | |
| Age | <20 | 25.5 | 0.66 | 2.98 | 6.11 | 1.32 | 5.89 | 0.34 | 6.24 | 10.4 | 59.9 |
| 20–30 | 18.5 | 0.82 | 1.80 | 0.85 | 0.96 | 5.24 | 0.76 | 15.5 | 28.9 | 73.8 | |
| 30–40 | 17.2 | 1.46 | 3.62 | 1.74 | 0.81 | 2.46 | 0.88 | 8.55 | 28.9 | 66.1 | |
| >40 | 32.0 | 1.27 | 2.98 | 2.31 | 1.17 | 9.61 | 0.34 | 7.50 | 14.5 | 72.1 | |
| BMI | <25 | 24.0 | 0.94 | 1.80 | 2.06 | 1.06 | 5.77 | 0.34 | 6.24 | 15.7 | 58.5 |
| 25–30 | 23.7 | 1.11 | 1.80 | 0.85 | 0.79 | 6.67 | 0.98 | 31.7 | 79.6 | 148 | |
| >30 | 20.9 | 5.84 | 4.31 | 5.38 | 0.43 | 4.11 | 14.1 | 45.3 | 64.1 | 165 |
Estimated dose for gender, ethnicity, age and BMI was calculated based on median urinary concentrations (unadjusted).
The minimal risk levels (MRLs) of 20 μg/kg bw for TDCCIP, 200 μg/kg bw for tris(2-chloroethyl) phosphate (TCEP), and 80 μg/kg bw for TNBP have been reported (ATSDR, 2012). Similarly, a reference dose (RfD) of 50 μg/kg bw has been suggested for TBOEP (NSF, 2012). The calculated exposure doses in our study for TDCIPP, TCEP, TNBP, and TBOEP were 3–4 orders of magnitude below the MRLs or RfD.
The calculated median and high ΣOPE exposure doses were lower than those reported for children from China (Chen et al., 2018). The median and high exposure doses of TCEP were similar to those reported for pregnant women from California (Castorina et al., 2017). The estimated dose of TDCIPP was lower than those reported for U.S. infants (Hoffman et al., 2017b). Similarly, the estimated exposure dose of TNBP was significantly lower than that reported for children from Germany (Fromme et al., 2014).
Although this study presents novel information with regard to OPE exposure levels and temporal variability, the results should be interpreted with caution. All urine samples collected in this study were from only 19 individuals. In addition, there were no intra-day urine samples for the calculation of intra-day variabilities in OPE metabolite concentrations. Further, due to the lack of information on urinary molar excretion fraction of OPE metabolites, the exposure dose calculation should be considered an estimate. It is also worth to note that we did not use glucuronidase enzyme during extraction of samples and therefore our results may reflect freely available forms of OPE metabolites in urine. A few earlier studies measured OPE metabolites without enzymatic deconjugation of urine (Butt et al., 2014; Sun et al., 2018; Tao et al., 2018). Although pilot studies were conducted in our laboratory to compare two methods of extraction (with and without enzyme), we did not find a significant difference in the OPE concentrations between the two methods, although DNBP, DIBP and BBOEP concentrations in urine were slightly higher after enzymatic conjugation, but that difference between two methods was below 30%. Other OPE concentrations in urine were comparable between the two methods of extraction.
4. Conclusions
Overall, the collection of urine samples from 19 individuals longitudinally over five weeks permitted the analysis of inter-day variability in 11 OPE metabolite concentrations. DEP, BDCIPP, and DPHP were the predominant OPEs found in urine. The ICC of OPE metabolites showed lower inter-day variability and higher reproducibility of Cr-adjusted concentrations. Age, gender, and BMI have an effect on inter-day variations in OPE concentrations. The estimated exposure doses of OPEs suggest minimal risks from the current exposure levels.
Supplementary Material
Highlights.
Interday variability in urinary concentrations of OPE metabolites was studied
Diphenyl phosphate and bis(1,3-dichloro-2-propyl) phosphate were the major OPE metabolites in urine
Urine collected longitudinally for five weeks from volunteers showed moderate variability
Creatinine adjustment of urinary OPE levels improved ICC and increased reproducibility
Age, gender, race and BMI had some effects on ICC.
Acknowledgments
We thank the volunteers for donating the urine samples. Research reported in this publication was supported, in part, by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award No. U2CES026542–01 and partly by Ministry of Education of China (No. T2017002). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ATSDR (Agency for Toxic Substances and Disease Registry), 2012. Toxicological profile for phosphate ester flame retardants Atlanta, Georgia, U.S. [PubMed] [Google Scholar]
- Behl M, Hsieh JH, Shafer TJ, Mundy WR, Rice JR, Boyd WA, Freedman JH, Hunter ES 3rd, Jarema KA, Padilla S, Tice RR, 2015. Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol. Teratol 52, 181–193 [DOI] [PubMed] [Google Scholar]
- Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM, 2014. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ. Sci. Technol 48, 10432–10438 [DOI] [PubMed] [Google Scholar]
- Butt CM, Hoffman K, Chen A, Lorenzo A, Congleton J, Stapleton HM, 2016. Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environ. Int 94, 627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignan CC, McClean MD, Cooper EM, Watkins DJ, Fraser AJ, Heiger-Bernays W, Stapleton HM, Webster TF, 2013. Predictors of tris(1,3-dichloro-2-propyl) phosphate metabolite in the urine of office workers. Environ. Int 55, 56–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorina R, Butt C, Stapleton HM, Avery D, Harley KG, Holland N, Eskenazi B, Bradman A, 2017. Flame retardants and their metabolites in the homes and urine of pregnant women residing in california (the CHAMACOS cohort). Chemosphere 179, 159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cequier E, Sakhi AK, Marce RM, Becher G, Thomsen C, 2015. Human exposure pathways to organophosphate triesters - a biomonitoring study of mother-child pairs. Environ. Int 75, 159–165 [DOI] [PubMed] [Google Scholar]
- Chen G, Zhang S, Jin Y, Wu Y, Liu L, Qian H, Fu Z, 2015. Tpp and tcep induce oxidative stress and alter steroidogenesis in TM3 leydig cells. Reprod. Toxicol 57, 100–110 [DOI] [PubMed] [Google Scholar]
- Chen Y, Fang J, Ren L, Fan R, Zhang J, Liu G, Zhou L, Chen D, Yu Y, Lu S, 2018. Urinary metabolites of organophosphate esters in children in South China: Concentrations, profiles and estimated daily intake. Environ. Pollut 235, 358–364 [DOI] [PubMed] [Google Scholar]
- Clark AE, Yoon S, Sheesley RJ, Usenko S, 2017. Spatial and temporal distributions of organophosphate ester concentrations from atmospheric particulate matter samples collected across houston, TX. Environ. Sci. Technol 51, 4239–4247 [DOI] [PubMed] [Google Scholar]
- Cui K, Wen J, Zeng F, Li S, Zhou X, Zeng Z, 2017. Occurrence and distribution of organophosphate esters in urban soils of the subtropical city, Guangzhou, China. Chemosphere 175, 514–520 [DOI] [PubMed] [Google Scholar]
- Dewalque L, Pirard C, Vandepaer S, Charlier C, 2015. Temporal variability of urinary concentrations of phthalate metabolites, parabens and benzophenone-3 in a Belgian adult population. Environ. Res 142, 414–423 [DOI] [PubMed] [Google Scholar]
- Dodson RE, Van den Eede N, Covaci A, Perovich LJ, Brody JG, Rudel RA, 2014. Urinary biomonitoring of phosphate flame retardants: Levels in California adults and recommendations for future studies. Environ. Sci. Technol 48, 13625–13633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Zhang Y, Wang G, Peng J, Wang Z, Gao S, 2016. Tphp exposure disturbs carbohydrate metabolism, lipid metabolism, and the DNA damage repair system in Zebrafish liver. Sci. Rep 6, 21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Arbuckle TE, Mallick R, LeBlanc A, Hauser R, Feeley M, Koniecki D, Ramsay T, Provencher G, Berube R, Walker M, 2015. Bisphenol a and phthalate metabolite urinary concentrations: Daily and across pregnancy variability. J. Expo. Sci. Environ. Epidemiol 25, 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Lahrz T, Kraft M, Fembacher L, Mach C, Dietrich S, Burkardt R, Volkel W, Goen T, 2014. Organophosphate flame retardants and plasticizers in the air and dust in german daycare centers and human biomonitoring in visiting children (LUPE 3). Environ. Int 71, 158–163 [DOI] [PubMed] [Google Scholar]
- Hallanger IG, Sagerup K, Evenset A, Kovacs KM, Leonards P, Fuglei E, Routti H, Aars J, Strom H, Lydersen C, Gabrielsen GW, 2015. Organophosphorous flame retardants in biota from Svalbard, Norway. Mar. Pollut. Bull 101, 442–447 [DOI] [PubMed] [Google Scholar]
- Hartmann PC, Burgi D, Giger W, 2004. Organophosphate flame retardants and plasticizers in indoor air. Chemosphere 57, 781–787 [DOI] [PubMed] [Google Scholar]
- He C, Toms LL, Thai P, Van den Eede N, Wang X, Li Y, Baduel C, Harden FA, Heffernan AL, Hobson P, Covaci A, Mueller JF, 2018. Urinary metabolites of organophosphate esters: Concentrations and age trends in Australian children. Environ. Int 111, 124–130 [DOI] [PubMed] [Google Scholar]
- Hoffman K, Butt CM, Webster TF, Preston EV, Hammel SC, Makey C, Lorenzo AM, Cooper EM, Carignan C, Meeker JD, Hauser R, Soubry A, Murphy SK, Price TM, Hoyo C, Mendelsohn E, Congleton J, Daniels JL, Stapleton HM, 2017a. Temporal trends in exposure to organophosphate flame retardants in the United States. Environ. Sci. Technol. Lett 4, 112–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Daniels JL, Stapleton HM, 2014. Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ. Int 63, 169–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Garantziotis S, Birnbaum LS, Stapleton HM, 2015. Monitoring indoor exposure to organophosphate flame retardants: Hand wipes and house dust. Environ. Health. Perspect 123, 160–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Gearhart-Serna L, Lorber M, Webster TF, Stapleton HM, 2017b. Estimated tris(1,3-dichloro-2-propyl) phosphate exposure levels for U.S. Infants suggest potential health risks. Environ. Sci. Technol. Lett 4, 334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim UJ, Kannan K, 2018. Occurrence and distribution of organophosphate flame retardants/plasticizers in surface waters, tap water, and rainwater: Implications for human exposure. Environ. Sci. Technol 52, 5625–5633 [DOI] [PubMed] [Google Scholar]
- Kim UJ, Oh JK, Kannan K, 2017. Occurrence, removal, and environmental emission of organophosphate flame retardants/plasticizers in a wastewater treatment plant in New York State. Environ. Sci. Technol 51, 7872–7880 [DOI] [PubMed] [Google Scholar]
- Koch HM, Aylward LL, Hays SM, Smolders R, Moos RK, Cocker J, Jones K, Warren N, Levy L, Bevan R, 2014. Inter- and intra-individual variation in urinary biomarker concentrations over a 6-day sampling period. Part 2: Personal care product ingredients. Toxicol. Lett 231, 261–269 [DOI] [PubMed] [Google Scholar]
- Lee S, Jeong W, Kannan K, Moon H-B, 2016. Occurrence and exposure assessment of organophosphate flame retardants (OPFRs) through the consumption of drinking water in Korea. Water Res 103, 182–188. [DOI] [PubMed] [Google Scholar]
- Lu SY, Li YX, Zhang T, Cai D, Ruan JJ, Huang MZ, Wang L, Zhang JQ, Qiu RL, 2017. Effect of e-waste recycling on urinary metabolites of organophosphate flame retardants and plasticizers and their association with oxidative stress. Environ. Sci. Technol 51, 2427–2437 [DOI] [PubMed] [Google Scholar]
- Lynn RK, Wong K, Garvie-Gould C, Kennish JM, 1981. Disposition of the flame retardant, tris(1, 3-dichloro-2-propyl) phosphate, in the rat. Drug Metab. Dispos 9, 434–441 [PubMed] [Google Scholar]
- Meeker JD, Cooper EM, Stapleton HM, Hauser R, 2013. Urinary metabolites of organophosphate flame retardants: Temporal variability and correlations with house dust concentrations. Environ. Health. Perspect 121, 580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn E, Hagopian A, Hoffman K, Butt CM, Lorenzo A, Congleton J, Webster TF, Stapleton HM, 2016. Nail polish as a source of exposure to triphenyl phosphate. Environ. Int 86, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NSF, 2012. Tri-(2-butoxyethyl)-phosphate. Oral risk assessment document Ann Arbor, MI, USA: NSF international [Google Scholar]
- Ospina M, Jayatilaka NK, Wong LY, Restrepo P, Calafat AM, 2018. Exposure to organophosphate flame retardant chemicals in the U.S. general population: Data from the 2013–2014 National Health and Nutrition Examination Survey. Environ. Int 110, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier F, Giorgis-Allemand L, Slama R, Philippata C, 2016. Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiology 27, 378–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulou SS, Petreas M, Park JS, 2016. Analytical methodology using ion-pair liquid chromatography-tandem mass spectrometry for the determination of four di-ester metabolites of organophosphate flame retardants in California human urine. J. Chromatogr. A 1434, 70–80 [DOI] [PubMed] [Google Scholar]
- Preston EV, McClean MD, Claus Henn B, Stapleton HM, Braverman LE, Pearce EN, Makey CM, Webster TF, 2017. Associations between urinary diphenyl phosphate and thyroid function. Environ. Int 101, 158–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauert C, Schuster JK, Eng A, Harner T, 2018. Global atmospheric concentrations of brominated and chlorinated flame retardants and organophosphate esters. Environ. Sci. Technol 52, 2777–2789 [DOI] [PubMed] [Google Scholar]
- Romano ME, Hawley NL, Eliot M, Calafat AM, Jayatilaka NK, Kelsey K, McGarvey S, Phipps MG, Savitz DA, Werner EF, Braun JM, 2017. Variability and predictors of urinary concentrations of organophosphate flame retardant metabolites among pregnant women in Rhode island. Environ. Health 16, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhi AK, Sabaredzovic A, Papadopoulou E, Cequier E, Thomsen C, 2018. Levels, variability and determinants of environmental phenols in pairs of norwegian mothers and children. Environ. Int 114, 242–251 [DOI] [PubMed] [Google Scholar]
- Su GY, Letcher RJ, Yu HX, 2016. Organophosphate flame retardants and plasticizers in aqueous solution: Ph-dependent hydrolysis, kinetics, and pathways. Environ. Sci. Technol 50, 8103–8111 [DOI] [PubMed] [Google Scholar]
- Sun Y, Gong X, Lin W, Liu Y, Wang Y, Wu M, Kannan K, Ma J, 2018. Metabolites of organophosphate ester flame retardants in urine from Shanghai, China. Environ. Res 164, 507–515 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sasaki K, Takeda M, Uchiyama M, 1984. Metabolism of tributyl phosphate in male rats. J. Agrie. Food Chem 32, 603–610 [Google Scholar]
- Tao Y, Shang Y, Li J, Feng J, He Z, Covaci A, Wang P, Luo J, Mao X, Shi B, Hu L, Luo D, Mei S, 2018. Exposure to organophosphate flame retardants of hotel room attendants in Wuhan city, China. Environ. Pollut 236, 626–633 [DOI] [PubMed] [Google Scholar]
- Thomas MB, Stapleton HM, Dills RL, Violette HD, Christakis DA, Sathyanarayana S, 2017. Demographic and dietary risk factors in relation to urinary metabolites of organophosphate flame retardants in toddlers. Chemosphere 185, 918–925 [DOI] [PubMed] [Google Scholar]
- van der Veen I, de Boer J, 2012. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88, 1119–1153 [DOI] [PubMed] [Google Scholar]
- Vernet C, Philippat C, Calafat AM, Ye X, Lyon-Caen S, Siroux V, Schisterman EF, Slama R, 2018. Within-day, between-day, and between-week variability of urinary concentrations of phenol biomarkers in pregnant women. Environ. Health. Perspect 126, 037005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sun H, Zhu H, Yao Y, Chen H, Ren C, Wu F, Kannan K, 2018. Occurrence and distribution of organophosphate flame retardants (OPFRs) in soil and outdoor settled dust from a multi-waste recycling area in China. Sci. Total. Environ 625, 1056–1064 [DOI] [PubMed] [Google Scholar]
- Wang Y, Wu X, Zhang Q, Hou M, Zhao H, Xie Q, Du J, Chen J, 2017. Organophosphate esters in sediment cores from coastal Laizhou bay of the Bohai sea, China. Sci. Total. Environ 607–608, 103–108 [DOI] [PubMed] [Google Scholar]
- Wei GL, Li DQ, Zhuo MN, Liao YS, Xie ZY, Guo TL, Li JJ, Zhang SY, Liang ZQ, 2015. Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure. Environ. Pollut 196, 29–46 [DOI] [PubMed] [Google Scholar]
- Wittassek M, Koch HM, Angerer J, Bruning T, 2011. Assessing exposure to phthalates - the human biomonitoring approach. Mol. Nutr. Food Res 55, 7–31 [DOI] [PubMed] [Google Scholar]
- Xu F, Giovanoulis G, van Waes S, Padilla-Sanchez JA, Papadopoulou E, Magner J, Haug LS, Neels H, Covaci A, 2016. Comprehensive study of human external exposure to organophosphate flame retardants via air, dust, and hand wipes: The importance of sampling and assessment strategy. Environ. Sci. Technol 50, 7752–7760 [DOI] [PubMed] [Google Scholar]
- Zhang B Lu S, Huang M, Zhou M, Zhou Z, Zheng H, Jiang Y, Bai X, Zhang T, 2018. Urinary metabolites of organophosphate flame retardants in 0–5-year-old children: Potential exposure risk for inpatients and home-stay infants. Environ. Pollut 243, 318–325 [DOI] [PubMed] [Google Scholar]
- Zheng X, Xu F, Chen K, Zeng Y, Luo X, Chen S, Mai B, Covaci A, 2015. Flame retardants and organochlorines in indoor dust from several E-waste recycling sites in South China: Composition variations and implications for human exposure. Environ. Int 78, 1–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.