Abstract
The Diabetes Sleep Treatment Trial (DSTT) is a multi-site, double-blinded, randomized, sham-controlled trial. The study objective is to test whether treatment of obstructive sleep apnea (OSA) with continuous positive airway pressure (CPAP) treatment results in improved glycemic control and diabetes self-management behavior compared to participants on a sham-CPAP (sub-therapeutic) device in participants with type 2 diabetes mellitus (T2DM) and co-morbid OSA. The purpose of this paper is to describe the premise for the DSTT, the study design, and the methodology used in this on-going trial. The target enrollment is 210 randomly assigned participants recruited from two sites. The primary outcome for glucose control is HbA1C; additional outcomes for diabetes self-management include objectively measured steps walked and subjectively measured diabetes-related distress, diabetes empowerment, and diabetes knowledge. All participants receive individual diabetes education and counseling for 6 weeks over two individual sessions and three telephone calls. Participants are randomized to receive either sham or active CPAP for 12 weeks, after which, they “guess” their group assignment; this will assist in determining the success of blinding participants to treatment group assignment. Participants revealed to be on active CPAP will be encouraged to continue CPAP for an additional 12 weeks; participants who had been on sham devices will be encouraged to have a repeat CPAP titration study and to crossover to active CPAP treatment for 24 weeks. An intention-to-treat approach will be used for efficacy analyses. The trial is registered with Clinicaltrials.gov (NCT01901055).
Keywords: Clinical trial, type 2 diabetes, sleep apnea, glucose control
1.1. Introduction
People who are overweight or obese, and who now represent nearly two-thirds of the adult U.S. population1, are at high risk for developing obstructive sleep apnea (OSA), type 2 diabetes mellitus (T2DM) and other chronic disorders. Successful treatment of these chronic conditions includes the need for patient engagement and self-management as a way of reducing risk for early morbidity and mortality due to primarily adverse cardiovascular outcomes.
Behavioral interventions to reduce time spent in sedentary behavior and increase engagement in lifestyle interventions can reduce the impact of an abnormal body mass index (BMI) and T2DM on adverse cardiovascular events. Diabetes self-management education and counseling (DEC) has been observed to improve glycemic control and improve long terms outcomes in overweight and obese subjects with T2DM, 2,3 but the Center for Disease Prevention and Health Promotion estimated that in 2010 only 58% of patients with T2DM report had received formal diabetes education.4 Among those who have received diabetes self-management education, many patients have difficulty complying with the recommendations offered.
Excessive daytime sleepiness and cognitive impairment represent potential barriers to compliance with lifestyle interventions for people with T2DM.5–7 The prevalence of OSA in adults withT2DM is notably greater than that for the general population, with estimates of 40 – 97% depending on severity of OSA and age of the population studied.8–11 Prior studies suggest that OSA and T2DM not only co-exist, but that sleep-related breathing disorders directly and adversely affect glucose homeostasis.12–19 Currently, the most effective medical treatment for OSA is the use of continuous positive airway pressure (CPAP), which eliminates apneas and hypopneas during sleep and results in improved self-reported daytime functioning.15–18
Although several studies report that OSA and OSA severity are associated with impaired glucose metabolism, there is no definitive evidence that CPAP results in improved glucose control. Conflicting results examining the effect of OSA therapy on glycemic outcomes may be due to small sample sizes20–25 and lack of attention to behaviors that impact glycemic control. In a preliminary study that included persons with T2DM, excessive daytime sleepiness was identified as a barrier to effective diabetes self-management, and poor sleep was associated with suboptimal diabetes self-management.26,27 However, the effect of OSA on behavioral aspects of diabetes self-management and the effect of CPAP treatment on patient behaviors following DEC have not been studied.
The underlying premise of this study is that repeated apneas and hypopneas associated with OSA are not only associated with altered glucose metabolism, but also hinder diabetes self-management in adults with T2DM because of low mood and motivation. The purpose of this paper is to describe the premise, design and methodology of a randomized controlled trial that was designed to examine if participants with co-morbid T2DM and OSA, who are randomized to treatment with CPAP, have improved glycemic control and diabetes self-management outcomes when compared to those randomized to a sham-CPAP (placebo) group.
2. Design and methods
2.1. Aims and hypothesis.
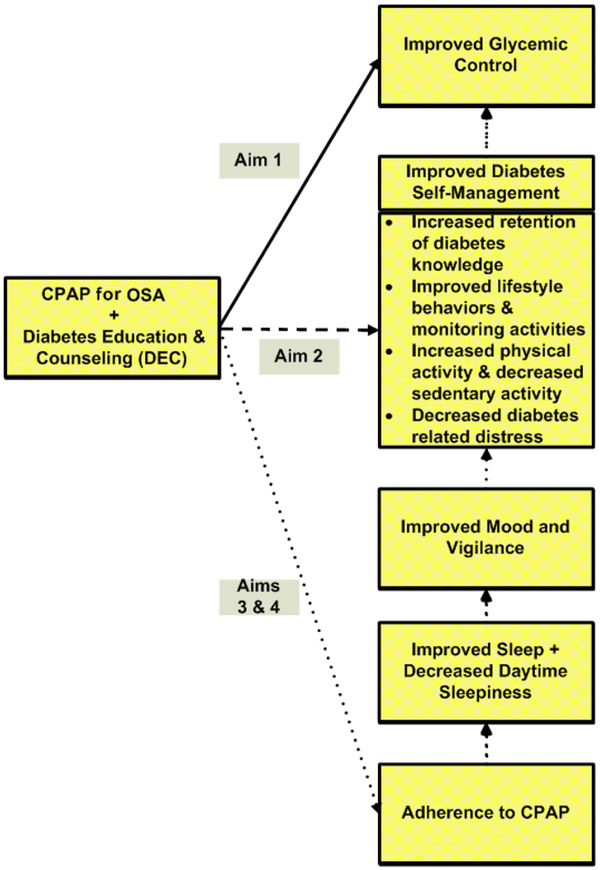
Figure 1 depicts the conceptual framework of the primary variables and how they relate to each specific aim. This study was designed to evaluate the following aims:
Figure 1:
Conceptual Framework and Specific Aims for Study
CPAP= continuous positive airway pressure
OSA= Obstructive sleep apnea
To determine if CPAP combined with DEC is superior to sham-CPAP with DEC for improving glycemic control. Hypothesis 1: Participants who receive CPAP with DEC will have improved glycemic control compared to participants on sham-CPAP who receive DEC at 6 and 12 weeks as measured using HbA1C (primary) and fructosamine (secondary).
To determine if CPAP with DEC is superior to sham-CPAP with DEC for improving diabetes self-management outcomes. Hypothesis 2: Participants who receive CPAP with DEC will have better diabetes self-management outcomes (retention of information taught during the diabetes education and counseling classes (i.e., diabetes knowledge), improved lifestyle behaviors, self-reported monitoring activities, objectively measured increased physical activity, and decreased diabetes-related distress) compared to participants on sham-CPAP with DEC at 6 weeks and 12 weeks.
(Secondary) To explore adherence to CPAP (dose) and specific neuro-behavioral and physiological mechanisms (sleep quality, daytime sleepiness, mood, and vigilance that may mediate the effects of CPAP treatment on glycemic control and diabetes self-management outcomes.
(Secondary) To explore the association of adherence to CPAP (dose) to glycemic control (HbA1C) and diabetes self-management outcomes 24 weeks after starting active CPAP.
2.2. Study design
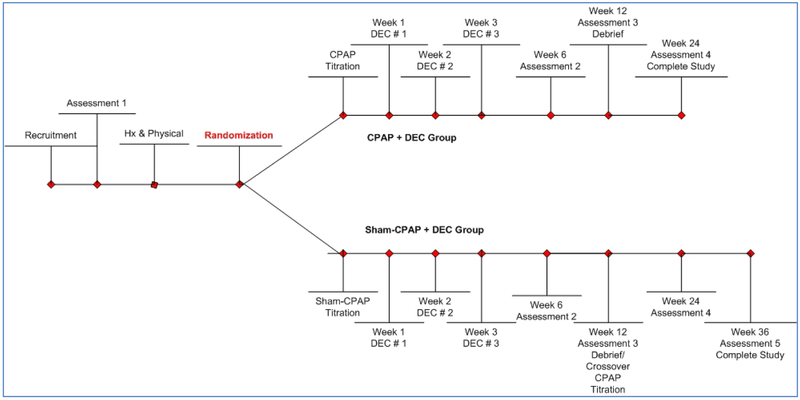
The DSTT is an on-going multi-center clinical trial that commenced in February 2014 with a parallel, 2-group, randomized, sham-controlled design with treatment cross-over in persons originally on Sham-CPAP (see Figure 2). Approval of the study protocol was obtained from the Institutional Review Board at each of the sites. Written informed consent for each study participant is obtained prior to any data collection. The study is registered on ClinicalTrials.gov (NCT01901055) and conforms to the Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Figure 2:
Study Design
Hx= History, CPAP= continuous positive airway pressure, DEC= diabetes education and counseling
2.3. Study Organization and Coordinating Center
Adults with T2DM at high risk for OSA are recruited and evaluated at two sites: University of Pittsburgh (PITT) and the Veterans Administration Pittsburgh Healthcare System (VAPHS). The University of Pittsburgh site, the coordinating center for the study, is responsible for training of research staff at each site for all of the research procedures; randomization and tracking participant enrollment; maintaining records of any serious adverse events or unanticipated problems, and for federal reporting of the study.
2.4. Sample eligibility criteria
Inclusion criteria include a physician diagnosis of T2DM with suboptimal glucose control (HbA1C ≥ 6.5%). Before randomization, participant’s primary care provider is contacted to inform him/her of the potential participant’s interest and to request their approval, if appropriate. See Table 1 for a full list of the study’s inclusion and exclusion criteria. The proposed sample is 210 randomized participants who met all eligibility criteria. Using information from our pilot/feasibility study,28 we estimate that 4 to 5 participants will need to be evaluated in order to recruit 1 subject who meets all eligibility criteria.
Table 1:
Eligibility Criteria
| Inclusion Criteria | |
| 1. | Apnea + Hypopnea Index (AHI) greater than ≥ 10 |
| 2. | Type 2 diabetes mellitus |
| 3. | Age 18 years or older |
| 4. | Willing to be randomized to continuous positive airway pressure (CPAP) or sham-CPAP |
| Exclusion Criteria | |
| 1. | Type 1 DM or pregnancy-induced diabetes |
| 2. | HbA1C <6.5% or > 11% |
| 3. | Sleep duration less than four hours per day |
| 4. | Change in diabetes medications during the previous 3 months |
| 5. | Acute medical or surgical conditions or hospitalization ≤ 3 months |
| 6. | Previous diagnosis of another sleep disorder |
| 7. | Oxygen or bi-level positive airway pressure (PAP) required |
| 8. | Not CPAP naïve |
| 9. | O2 sat. < 75% for > 25% of the ApneaLinkPlus® sleep study |
| 10. | Cardiovascular events noted during overnight titration sleep study |
| 11. | Persons in household with CPAP |
| 12. | History of near-miss or auto accident during last 12 months |
| 13. | Employed in safety sensitive job (i.e. truck driver or airline pilot) |
| 14. | Non-ambulatory |
| 15. | Regular use of hypnotic or alerting (e.g. modafinil) medications |
| 16. | Excessive consumption of alcohol |
| 17. | Claustrophobia to wearing mask |
| 18. | Unable to speak, read & write English |
| 19. | Pregnant or planning to become pregnant |
| 20. | Primary care provider |
2.5. Sample size justification
The projected study sample size of 210 for this trial was determined focusing on the testing of hypotheses associated with the primary aims (Hypotheses 1 and 2), and considering the results of the pilot/feasibility study, the adjustment of the testwise significance level to .01 to limit inflation of type 1 error due the multiple testing of hypotheses at 6- and 12-weeks follow-up for several outcomes, and the anticipated participant attrition during the 12-weeks of follow-up. Regarding Aim 1 and its corresponding hypothesis (Hypothesis 1), our pilot/feasibility study suggests that CPAP improves glycemic control as measured by serum fructosamine.28 Therefore, we anticipate large effects (in terms of standardized mean differences, d) on the order of d=−0.71 may be detected with 0.80 power with a group sample size of 43 (86 total) when comparing mean changes from baseline to 6 weeks and baseline to 12 weeks in serum fructosamine between the CPAP+DEC and sham-CPAP+DEC groups using group comparisons of means (assuming a normal distribution) at testwise two-tailed level of significance of .01. Regarding Aim 2 and its corresponding hypothesis (Hypothesis 2), we have observed effect sizes with magnitudes ranging from 0.22 to 1.27 (median effect size of 0.75) for 4-week changes from baseline in physical activity, sleep, vigor, fatigue and vigilance measures.28 With a minimum group size of 84 participants (168 total) we would be able to detect effect sizes (in terms of the standardized mean difference, d) as small as d=±0.53 in mean changes from baseline to 6 weeks and baseline to 12 weeks between the treatment groups using group comparisons of means (assuming a normal distribution) with 0.80 power at testwise two-tailed level of significance of .01. Although efforts will be made to retain study participants, some attrition is possible. Our recent pilot/feasibility study of active-CPAP with a 4-week follow-up in adults with T2DM demonstrated a low attrition of 5%. In the CATNAP trial evaluating the effect of CPAP versus sham-CPAP in persons with milder OSA (AHI=5–30), the investigators observed approximately 10% attrition at 8 weeks.29 To ensure an adequate number of participants complete the study by conservatively adjusting sample size for approximately 20% attrition, 210 subjects (105 per group) will be enrolled to have at least 168 participants (84 per group) complete the study.
2.6. Recruitment and initial screening
A wide array of participant recruitment strategies are used including recruitment from diabetes and sleep clinics, use of electronic medical record review, resources from the Clinical & Translational Science Institute (CTSI), focused mailings, flyers within the community, and social media advertisements.
A brief phone or in-person screening is done to inform potential participants about the study and determine initial eligibility (self-reported T2DM, no prior CPAP use, willing to be randomized). Research staff meet with respondents to the recruitment solicitations and explain the study requirements. If the individual is willing, the staff provides a brief in-person or telephone interview to determine if they meet the initial eligibility criteria listed above. The screening interview includes administering the Multivariable Apnea Prediction (MAP) Index to identify persons at moderate-to-high risk for OSA. The MAP uses binary logistic regression with multiple explanatory covariates with frequency of snoring, breath holding, and snorting, age, sex, and self- reported height and weight to estimate the probability of OSA.30 According to Maislin et al., the MAP’s positive predictive ability of AHI≥10 was 0.79 (p<0.0001) in patients presenting at a sleep disorder center.30 However, a score on the MAP was not used as an eligibility criterion because of the high prevalence of OSA (i.e. over 86%) in a study by Foster and colleagues in persons (60% female) with obesity and T2DM.31
2.7. Assessments and Procedures
Protocol fidelity is maintained between the two sites with standardized procedure manuals, staff training sessions prior to initiating the study, and staff training on an ongoing basis with weekly phone calls between the coordinating center project manager and site managers.
Assessment 1.
Assessment 1 includes screening for eligibility criteria and obtaining baseline data on participants who are later randomized. After informed consent has been obtained, the participant’s ability to read at the 5th grade level and comprehend information are evaluated with questions that ask “if you feel sleepy, should you drive a car or use dangerous machines?” The baseline Assessment 1 includes additional questionnaires for demographic information, health history, evaluation of health literacy, and a urine pregnancy test, if appropriate, in women with childbearing potential. Anthropometric measurements (height, weight, waist circumference, body composition) and a venipuncture are performed for HbA1C, fructosamine, and lipid profile. Participants are instructed on how to wear the ApneaLinkPlus® device that night when they go to sleep, wearing the BodyMedia SenseWear Armband® and filling out a modified Consensus Sleep Diary32 when they wake up and before bedtime for the next 7 days. Materials are returned to the research center in padded pre-paid mail packets. Data from the ApneaLinkPlus® are reviewed by a registered polysomnography technician and reviewed by a physician board certified by the American Association of Sleep Medicine. Participants eligible after Assessment 1 undergo a history and physical to determine if there are other factors that may exclude them from the study. The principal investigator visits with each participant who is being randomized, to either CPAP or sham-CPAP, to answer any questions and to reinforce that the study is a clinical trial and that they may be randomized to sham-CPAP.
Follow-up assessments.
Assessments 2, 3, 4, and 5 are very similar to the baseline assessment without repeating the screening measures. Assessment 2 is conducted at approximately 6-weeks of CPAP/sham-CPAP use. Participants are mailed the questionnaires, BodyMedia SenseWear Armband® and the sleep diary to use for 7-days. They return the materials to the study site at which time they complete Assessment 2, which includes anthropometric measurements and a venipuncture to obtain a sample for HbA1C, fructosamine and lipid profile measures. Assessment 3 occurs at approximately 12 weeks of CPAP/sham-CPAP use. Procedures during Assessment 3 are essentially the same as before except for the addition of a “guess questionnaire.” Prior to being informed of his/her treatment condition and debriefed, participants are asked which arm of the study (CPAP or sham-CPAP) they think they were assigned and how confident are they of their “guess”.
Participants originally on active CPAP are encouraged to continue in the study for 12 more weeks. Participants who were on sham-CPAP are invited to continue in the study for 24 weeks after having a crossover titration to active CPAP. During Assessment 4, approximately 12 weeks later, all participants are reassessed as described above, which is the final assessment for those who were initially on active CPAP. Those participants who crossed-over to active CPAP will have their final assessment performed after 24 weeks of active CPAP (assessment 5) as described above. All participants receive a final debriefing; a copy of their sleep and laboratory data is given to them and sent to their primary care provider.
3.0. Intervention
3.1. CPAP and sham-CPAP devices
Participants in both arms of the study are treated with the Respironics System One Auto® CPAP System. While participants in the active-CPAP arm receive an actual device, the device in the sham-CPAP group contains a sham circuit that creates a CPAP placebo along with a hidden leak in the connector between the mask and CPAP tubing that allows air to escape and to prevent the rebreathing of carbon dioxide. The device pressure is set to 0.5–1 cm H2O at the mask to generate sufficient airflow to convince the CPAP naïve participant that they are receiving treatment, however; the pressure is insufficient to prevent apneas. The device airflow generates a similar degree of blower noise from both circuits, providing CPAP naïve participants with audible and tactile cues that do not differ between sham and active-CPAP and thus maintains blinding. Previous studies found that the sham-CPAP device does not deliver therapeutic pressure nor produce clinically meaningful alterations in pre-treatment AHI, the nadir of oxygen desaturation, arousal index, and sleep efficiency.33,34 The duration of the blinded intervention with sham comparison is 12 weeks. After the completion of the 12-week intervention, CPAP treatment is provided to those participants who were initially randomized to sham-CPAP. All participants are followed for a total of 24 weeks of therapeutic CPAP treatment to evaluation durability of adherence to CPAP, glycemic control, and behavioral outcomes.
4.0. Study Measures.
The study’s constructs evaluated, measures, and time points for administration are listed in Table 2. Participants undergo a venipuncture for HbA1C, the primary outcome of glycemic control, and for a fructosamine level as a secondary measure. In addition, participants have blood for lipid levels drawn and are weighed on a Tanita scale. Objective measurement of OSA presence and severity are done with an ApneaLinkPlus® home sleep study; aspects of impaired sleep are measured by the Pittsburgh Sleep Quality Index,35 Epworth Sleepiness Scale,36 Insomnia Severity Index,37,38 Functional Outcomes of Sleepiness Questionnaire, 39 state sleepiness by the Stanford Sleepiness Scale,40 and the Brief Psychomotor Vigilance Test.41 Physical activity is measured by the SenseWear Pro Armband® that includes information on the length of time the device is worn, steps accrued, intensity of the activity, and sleep duration. Measures of diabetes self-management and diet habits include the Summary of Diabetes Self-Care Activities,42 Diabetes Knowledge Test,43 Diabetes Empowerment Scale - Short Form,44 the Rapid Eating & Activity Assessment for Patients,45 and the Connor Diet Habit Survey.46 Questionnaires to assess for dysphoric mood include the Profile of Mood States47 and the Problem Areas in Diabetes.48 The Newest Vital Sign (NVS)49 serves as a screening test for limited health literacy. The NVS is administered using an enlarged, laminated food label that requires interpretation of the information and basic calculations of percent calories and fat (paper/pencil offered for calculation); less than 4 correct answers indicate the possibility of limited health literacy.
Table 2.
Construct Evaluated, Measures, and Visit
| Construct | Measure | Visit |
|---|---|---|
| OSA presence and severity | ApneaLinkPlus®* | Assessment 1 |
| Glucose control-last 3 months | HbA1C | Assessment 1,2,3,4,5 |
| Glucose control- last 2 weeks | Fructosamine | Assessment 1,2,3,4,5 |
| Lipid profile | Total cholesterol, HDL, LDL & triglycerides | Assessment 1,2,3,4,5 |
| Demographic information | Sociodemographic and Lifestyle Questionnaire.50 | Assessment 1 |
| Health history | Health History Questionnaire* | Assessment 1 |
| Health literacy | Newest Vital Sign49 | Assessment 1 |
| Participant blinding to CPAP/sham-CPAP | “Guess” questionnaire | Assessment 3 |
| Physical activity | BodyMedia SenseWear Pro Armband®* | Assessment 1,2,3,4,5 |
| Diabetes self-management behaviors | Summary of Diabetes Self-Care Activities (SDSCA)42* | Assessment 1,2,3,4,5 |
| Diabetes distress | Problem Areas in Diabetes (PAID)48* | Assessment 1,2,3,4,5 |
| Diabetes knowledge | Diabetes Knowledge Test (DKT)43 | Assessment 1 & 3 |
| Diabetes self-efficacy | Diabetes Empowerment Scale-Short Form44* | Assessment 1,2,3,4,5 |
| Diet behavior | Rapid Eating & Activity Assessment for Pts. (REAP)45 | Assessment 1,2,3,4,5 |
| Eating habits and diet composition | Connor Diet Habit Survey (Connor DHS)46* | Assessment 1,2,3,4,5 |
| Overweight/obesity | BMI/ Body Composition | Assessment 1,2,3,4,5 |
| Mood disturbance | Profile of Mood States (POMS)47 | Assessment 1,2,3,4,5 |
| Physical and mental health-related quality of life | SF-12v2 Health Survey51* | Assessment 1,2,3,4,5 |
| Sleep quality | The Pittsburgh Sleep Quality Index (PSQI)35* | Assessment 1,2,3,4,5 |
| Daytime sleepiness | Epworth Sleepiness Scale (ESS)36,52* | Assessment 1,2,3,4,5 |
| Insomnia symptoms | Insomnia Severity Index (ISI)37,38* | Assessment 1,2,3,4,5 |
| Functional outcomes that are sensitive to sleepiness | Functional Outcomes of Sleep Quality (FOSQ)39* | Assessment 1,2,3,4,5 |
| Daily sleep habits/behavior | Consensus Sleep Diary32 (modified)* | Assessment 1,2,3,4,5 |
| State sleepiness | Stanford Sleepiness Scale.40 | Pre/post diabetes education |
| Ability to maintain vigilance | Brief Psychomotor Vigilance Test (PVT-B).41 | Pre/post diabetes education |
OSA= obstructive sleep apnea, BMI= body mass index kg/m2
Completed at home
4.1. Reduction of participant burden with assessments
The risk of participant burden was considered in planning assessment. Strategies to reduce burden include use of a home sleep studies to evaluate the presence and severity of OSA (ApneaLink ApneaLinkPlus®*), a low burden activity monitor (BodyMedia SenseWear Pro Armband®), and sending home with the participant at the baseline assessment, ten of the questionnaires (indicated with an asterisk in Table 2) so they can be completed when convenient and mailed back after one week with the activity monitor. For the other assessments, questionnaires and devices are mailed to the participant approximately 2 weeks before their appointment and brought back with them to the evaluation.
5.0. Procedures
5.1. Randomization and blinding.
Participants who meet all inclusion/exclusion criteria are randomly assigned by computer program using a minimization algorithm with equal allocation to one of two treatment groups: 1) active CPAP set at a level of pressure that will abolish nocturnal respiratory disturbances, or 2) sham-CPAP (placebo) where the level of pressure will not abolish nocturnal respiratory disturbances. Use of a minimization algorithm insures treatment balance on the marginal distributions of four factors: 1) the participant’s baseline physical activity levels in terms of mean number of steps accrued per day (< 5,000, ≥ 5000),53 2) whether the participant had previous diabetes education, 3) whether the participant is prescribed insulin, and 4) by site. Minimization is a form of adaptive treatment allocation in which the probability of assignment to the treatment groups does not remain constant, but is determined by the current balance and/or composition of the groups on the pre-randomization factors. Participants and all research team personnel involved in further data collection are blinded as to which group the participants are assigned. If there is a serious adverse event, anticipated to be an uncommon occurrence, then the treatment group assignment would be unmasked for the safety of the particular participant.
5.2. CPAP Titration Study/Sham-CPAP Titration.
The nighttime CPAP titration PSG is performed to determine the optimal level of CPAP needed to eliminate the participant’s apnea. The CPAP titration PSG is performed using standard techniques,54 with effective CPAP level defined as an AHI <5 an hour with the minimum effective pressure for each participant. Participants randomized to the sham-CPAP arm have titration studies that are performed identically to the CPAP titration except the standard CPAP hose is replaced with the sham-CPAP hose, the modified expiratory valve was attached and pressure at the nose mask is ≤ 1 cm H2O. The sham-CPAP blower is set at a pre-set level where pressure is not transmitted to the participant but produces the blower sound to help blind the participant to treatment group status. Participants are loaned, without charge, either the CPAP or the sham-CPAP machines by the manufacturer (Respironics, Inc.) to take home for their duration in the study. Participants receive education prior to starting CPAP therapy on what is OSA and why treatment is important. All participants are shown various masks and permitted to select the most comfortable mask interface.
5.3. Adherence monitoring
Participants are encouraged to use CPAP/sham-CPAP every night; adherence is monitored closely during the first week with phone calls after their first night at home with the device to assist with any problems participants may be experiencing and there is continued monitoring of CPAP/sham-CPAP adherence with phone calls to the participants at least weekly. Adherence is monitored with the EncoreAnywhere™ system that record the amount of time participants use their devices. EncoreAnywhere™ contains a wireless modem that records adherence to CPAP nightly treatment and transmits the data to a password protected, web-based secure server. The standardized procedure to ensure adherence is utilized in both treatment arms and across sites. As a backup to prevent the loss of data, adherence is also assessed with a Smartcard® that is inserted in every machine in case the wireless modem does not function. The Smartcard® can be downloaded at the assessment visit to determine the amount of time the participant used their device.
5.4. Diabetes Education/Counseling.
The diabetes educator is blinded to group assignment; their goal is to work with each participant to help them achieve improved diabetes management. All participants in each group spend the same amount of time with the diabetes educator with two (2) one-on-one sessions (a 90-minute session and a 60-minute session), and a minimum of 3 follow-up phone calls of approximately 15–30 minutes. The diabetes education and counseling sessions begin approximately one week after participants have started CPAP or sham-CPAP to allow them time to become comfortable using their devices. Participant education is provided using published educational materials from the American Diabetes Association and the American Association of Diabetes Educators. Although the content is interactive and tailored to match each individual’s needs and cultural influences, all participants received standardized diabetes education and counseling based on the AADE patient competencies (AADE7).55 Participants are provided a pedometer to wear each day and a daily step goal, which is increased over the 12 weeks with a general goal to reach at least 7,500 steps per day.56 Participants are given 7-day paper diaries and asked to record their daily food and beverage intake. The diabetes educator reviews the diaries at each of the two intervention sessions and provides feedback to the participant on progress made toward improving their eating and physical activity habits. In order to estimate if sleepiness is a barrier to diabetes education, participants are questioned on sleepiness before and after the session with the Stanford Sleepiness Scale.40 Vigilance is tested before and after each diabetes education and counseling session with the PVT-B.41
5.5. Compensation for participation.
To minimize attrition, in addition to the education/counseling session and telephone calls, we offer study participants a modest monetary compensation for their time. Participants who complete the entire study (24 weeks) with active-CPAP will receive a maximum compensation of $270; those on sham-CPAP who then continue to active treatment for an additional 24 weeks will receive a total compensation of $300. Participants are compensated after designated assessment time-points. Excluded participants are compensated for activities they complete. As part of the total compensation, participants who complete the 12 weeks of CPAP or sham-CPAP when they were blinded to treatment group received a $25 bonus if they are adherent to CPAP for at least an average of 4 hours a night over the entire period. Participants who are adherent to CPAP for an average of 6 hours a night over the 12 weeks receive an additional $25 bonus.
6.0. Data Management
The Center for Research and Evaluation at the University of Pittsburgh serves as the data processing center for all data collected for this study. Data are obtained via paper-and-pencil instruments, electronic monitors, and biological assays. For paper-and-pencil instruments including biomedical data obtained during the clinical assessments, TELEform Elite (version 10, Verity, Inc., Sunnyvale, CA), a Windows-based software package for automated data entry/verification, is being used for data entry and verification then exported to the project’s Oracle database which runs on a secure server at the University of Pittsburgh Network Operating Center, a 24/7 facility with uninterruptable power and continuous backups. ApneaLinkPlus® sleep study data is being visually reviewed and scored by certified sleep technologists and then evaluated by board certified sleep physicians at each site. Data collected using electronic data capture methods (SenseWear Pro Armband®) are uploaded from the monitoring devices to the project computer, interpreted using the software, exported as an ASCII file, and then merged with identifying information in the Oracle database.
7.0. Statistical Analysis
Data will be analyzed using SAS (version 9.4., SAS Institute, Inc., Cary, NC) to conduct descriptive, exploratory and repeated measures analyses.
7.1. Preliminary analyses
Detailed exploratory data analyses will be performed initially to describe univariate/bivariate distributions; reveal associations among study variables, including key dependent variables, with possible covariates/confounders and imbalances between treatment groups; assess the amount of and patterns in missing data; and check for violations in statistical assumptions underlying the planned analyses. Covariates/confounders will be included in planned analyses secondarily and their impact on the results will be evaluated. The randomness of missing data will be investigated using available information on participant characteristics to help discern patterns in the missing data, identify possible missing data mechanisms, and inform the strategies employed to handle missing data. It is anticipated that the primary reason for data missingness will be participant attrition. We will closely monitor attrition and use binary logistic regression with multiple explanatory covariates to identify participant characteristics that are associated with participant attrition. If the data are ignorably missing (i.e., either missing completely at random or missing at random), the likelihood based estimation procedures to be used will produce unbiased estimates, while allowing us to retain cases with missing values on the outcome variables. Multiple imputation will be used to impute missing values on the covariates. If the data are not ignorably missing (i.e., nonrandom missingness), we will use pattern mixture or selection modeling to investigate the sensitivity of the results given the missing data pattern assumed.
7.2. Analysis Strategy for Aim 1
An “intent-to-treat” (ITT) approach will be used when investigating efficacy of CPAP+DEC compared to sham-CPAP+DEC. All participants will be included in efficacy analyses in the groups to which they were randomly assigned, regardless of adherence to protocol, treatment received, withdrawal or deviation from protocol. Adherence with the assigned protocol will be closely monitored throughout the duration of the study. With this information, the sensitivity of the results under the ITT model will be explored to identify the effects of the amount of intervention received and the deviations in protocol on the efficacy of the intervention. Since the primary endpoint of interest, glycemic control, is assessed repeatedly with HbA1C as the primary outcome and fructosamine as a secondary outcome, linear mixed modeling (LMM) will be used to examine the change in each outcome separately over time between treatment groups. A flexible modeling approach, LMM allows for unequally spaced repeated assessments, fixed and time-varying covariates, dependent variables with data that are missing at random, and modeling the covariance/correlation among repeated measures responses. We will consider the baseline value both as an assessment point as well as a possible covariate in analyses. The primary independent variable is the randomized assignment to treatment (CPAP+DEC vs. sham-CPAP+DEC). Fixed and/or time-dependent covariates (including baseline outcome values) may be included in the repeated measures model as indicated secondarily based on the results on the preliminary data analysis to adjust for group imbalances and/or variables related to the dependent variable. Prior to fitting the repeated measures model via LMM, the structure of the estimated variance-covariance matrix for the repeated measures will be carefully examined to determine best fitting structure using standard fit criteria (e.g., AIB, BIC). When fitting models, time will be included as a continuous, fixed, repeated within-subjects factor, while treatment assignment will be treated as a fixed, between-subjects factor, with an interaction between time and treatment group assignment. F-tests will be used to test the main and interaction effects included in the model. The individual regression parameters will also be estimated and reported with their confidence intervals. Sensitivity analyses will be performed to discern the impact of possible influential cases on results. To test the associated hypothesis (Hypothesis 1), the estimates of the interaction effects from the fitted linear mixed model will be used to compare, relative to baseline values, the CPAP+DEC and sham-CPAP+DEC groups on: 1) the 6-week values of fructosamine and HbA1C and the 2) the 12-week values of fructosamine and HbA1C. Confidence intervals will be obtained for these time point specific estimates.
7.3. Analysis Strategy for Aim 2
An approach similar to that described for Aim 1 using linear mixed modeling to compare 6-week and 12-week values for diabetes self-management outcomes (retention of information taught during the DEC classes [DKT], improved self-management behaviors [SDSCA, DES-SF], diabetes monitoring, increased physical activity [SenseWare Armband activity counts], and decreased diabetes-related distress [PAID]) and their changes relative to baseline values between subjects on CPAP+DEC and those receiving sham-CPAP+DEC. Generalized linear mixed modeling or nonlinear mixed modeling will be used to analyze longitudinal categorical outcomes should the continuous type outcome variables need to be categorized for analysis. Along with test statistics and p-values, point and interval estimates will be obtained for the group comparisons at the 6 and 12 weeks.
7.4. Analysis Strategy for Aim 3
We will explore adherence to CPAP (dose) and specific neuro-behavioral and physiological mechanisms (nighttime sleep, daytime sleepiness, mood, and vigilance during DEC sessions) as possible intervening, or mediator, variables that may mediate the effects of CPAP treatment on glycemic control and diabetes self-management outcomes. For mediational analyses, Mplus (version 8.2, Los Muthén & Muthén, Los Angeles, CA) will be used. Initially, we will fit simple mediational models (predictor, single mediator, single outcome) and estimate whether the effects of CPAP (predictor) on changes in glycemic control and diabetes self-management outcomes are mediated through changes in specific neuro-behavioral and physiological variables (i.e., nighttime sleep, daytime sleepiness, mood, and vigilance during DEC sessions). Standardized parameter estimates of path linkages (with confidence intervals) will be obtained to summarize associations between the exogenous treatment variables, suspected mediators, and distal outcomes, as well as the indirect effect of the treatment group on the targeted outcomes through the suspected mediators and the direct effect of the treatment group on the outcomes. The goodness-of-fit of a mediational model will be assessed using standard fit indices (e.g., RMSEA, CFI) as well as through residual analyses. Depending on the results for testing of individual mediators, we may consider the mediators jointly as a multiple mediator model to develop a more complete picture of the hypothesized mechanisms of action for CPAP.
7.5. Analysis Strategy for Aim 4
For this aim we will combine the 6 months of data for the two groups receiving CPAP (including the participants in the delayed treatment group who crossover from sham-CPAP to active CPAP) to evaluate the maintenance of treatment effects and to explore the association between the durability of CPAP treatment of OSA (i.e., adherence to CPAP) and 1) sleep quality [PSQI], 2) daytime sleepiness [ESS]), 3) mood [POMS], 4) diabetes self-management [SDSA, DES-SF, PAID] and 5) glycemic control [fructosamine, HbA1C] after 24 weeks of active CPAP.
8.0. Discussion
The goal of this study is to determine whether treatment of OSA improves glycemic control in adults with T2DM who are overweight or obese. The novelty of this study is in directly examining the effect of reducing apneas and hypopneas as well as the indirect effect of decreasing daytime sleepiness on diabetes self-management as a contributor to changes in glycemic control. We hypothesize that participants who receive CPAP therapy for OSA and DEC will have lower HbA1C levels, improved retention of diabetes education, and improved self-management behaviors compared to those receiving sham CPAP.
Several recent important studies have been published that attempted to answer this question – what is the effect of treatment of OSA on glycemic control? In a small (N=19) one-week “proof of concept” study 57 that randomized participants with co-existing T2DM and OSA to either real or sham-CPAP, there was a significant decrease in 24-hour and nighttime glucose levels in the group receiving real CPAP. The participants had identical meals and spent their nights in the laboratory where CPAP/sham-CPAP use over a period of 8 hours was supervised. Another large RCT (N=298) 58 compared persons with co-morbid OSA and T2DM (HbA1C 6.5%−8.5%) who were randomized to CPAP or usual care. Patients receiving insulin were excluded from the study. There were no significant differences for HbA1C or fasting glucose after 6 months of treatment either in an intent-to-treat analysis or when limited to those adherent to CPAP (CPAP≥ 4 hours/night for 70% of nights). In subgroup analyses, participants who were adherent to CPAP had a significant decrease in their diastolic blood pressure, daytime sleepiness, and SF-36 Mental Component Summary Score. 59 In a third study, there were no differences at 3 months in HbA1C, fasting glucose, or fasting insulin among participants with co-existing T2DM and OSA (N=50) randomly assigned to CPAP when compared to sham-CPAP. However, a measure of insulin sensitivity, QUICKI, was significantly improved in the CPAP group at 3 months. At 6 months participants in the CPAP group had significantly lower HbA1C, fasting glucose, fasting insulin, HOMA-IR, and QUICKI values compared to those in the sham-CPAP group. These three studies suggest that CPAP is effective in improving glucose control in a controlled environment but that adherence to CPAP among free-living persons may affect the efficacy of treatment and account for the inconsistent results obtained. Furthermore, these studies did not examine the effect of nighttime CPAP on daytime health behaviors that are associated with improvements in glycemic control.
The knowledge obtained from our study may help in the understanding of both the effect of CPAP treatment of OSA in this population on glycemic control and on behaviors critical for optimal self-management of this chronic disease. A recent state of the science review of OSA and diabetes 60 included the three studies discussed above with four additional small studies with 23 to 64 participants. This review concluded that the available conflicting evidence regarding the effect of CPAP on glucose metabolism suggests that further study is needed to answer this question. None of the studies in this review examined the contribution of DEC to any observed improvement with CPAP - an area that our study hopes to elucidate.
An anticipated challenge for the DSTT is recruitment to accrue the desired sample size in this relatively intensive protocol that utilizes sham-CPAP. We intend to monitor subject recruitment on a regular basis and to utilize consultants for future suggestions. In addition, we will investigate other recruitment sites, as needed. Another potential challenge is to recruit an adequate number of women and minority participants. Our goal is to enroll approximately 50% women in the study. We were successful in the recruitment of women in the completed pilot/feasibility study with the sample screened including 55% women and the randomized sample having 41% women.28 Our goal is to have a heterogeneous, racially diverse sample by enrolling at least 40% minorities in the study. We exceeded that number in the completed pilot/feasibility study that included 53% minority subjects screened (41% African American, 3% Asian, 9% Bi-racial [African American and American Indian]) and 45% minority subjects randomized (41% African American and 4% Bi-racial).28 The estimated prevalence of T2DM in African Americans is two to four times that of Whites. 61 We are over-recruiting women and minorities and will do focused recruitment, if required, to achieve this goal.
In conclusion, the overall impact of this study will be to increase knowledge about the effect of diabetes self-management on outcomes following treatment with CPAP in subject with comorbid T2DM and OSA. Expanding our knowledge of the effect of sleep disturbances on diabetes self-management may affect millions of persons with diabetes and lead to improved guidelines for screening and treatment of OSA in this population. Results from this study may also suggest that treatment of impaired sleep improves symptoms and self-management behavior in populations with other chronic diseases.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK096028) and through the Clinical +Translational Research Institute grants UL1TR001857 and UL1TR000005. ApneaLink Plus devices were obtained by a loan agreement from ResMed, Inc. CPAP and sham-CPAP devices obtained by a loan agreement from Philips-Respironics, Inc.
References
- 1.Overweight & Obesity Statistics. 2018. at https://www.nxiddk.nih.gov/health-information/health-statistics/overweight-obesity.)
- 2.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care 2002;25:1159–71. [DOI] [PubMed] [Google Scholar]
- 3.Thorpe LE, Upadhyay UD, Chamany S, et al. Prevalence and control of diabetes and impaired fasting glucose in New York City. Diabetes Care 2009;32:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diabetes Topics and Objectives. U.S. Department of Health and Human Services, 2018. (Accessed 4/20/2018, at https://www.healthypeople.gov/2020/data-search/Search-the-Data-objid=4111;.)
- 5.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep 2003;26:298–307. [DOI] [PubMed] [Google Scholar]
- 6.Chasens ER, Korytkowski M, Sereika SM, Burke LE. Effect of poor sleep quality and excessive daytime sleepiness on factors associated with diabetes self-management. Diabetes Educ 2013;39:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferini-Strambi L, Baietto C, Di Gioia MR, et al. Cognitive dysfunction in patients with obstructive sleep apnea: Partial reversibility after continuous positive airway pressure. Brain Research Bulletin 2003;61:87–92. [DOI] [PubMed] [Google Scholar]
- 8.Brooks B, Cistulli PA, Borkman M, et al. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. Journal of Clinical Endocrinology & Metabolism 1994;79:1681–5. [DOI] [PubMed] [Google Scholar]
- 9.Chasens ER, Umlauf MG, Pillion DJ, Wells JA. Nocturnal polyuria in Type 2 Diabetes Mellitus: A symptom of obstructive sleep apnea. Diabetes Educator 2002;28:424–34. [DOI] [PubMed] [Google Scholar]
- 10.Foster GD, Sanders MH, Millman R, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care 2009;32:1017–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. American Journal of Respiratory and Critical Care Medicine 2002;165:677–82. [DOI] [PubMed] [Google Scholar]
- 12.Al-Delaimy WK, Manson JE, Willett WC, Stampfer MJ, Hu FB. Snoring as a risk factor for type II diabetes mellitus: a prospective study. American Journal of Epidemiology 2002;155:387–93. [DOI] [PubMed] [Google Scholar]
- 13.Elmasry A, Lindberg E, Berne C, et al. Sleep-disordered breathing and glucose metabolism in hypertensive men: A population-based study. Journal of Internal Medicine 2001;249:153–61. [DOI] [PubMed] [Google Scholar]
- 14.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive Sleep Apnea Is Independently Associated with Insulin Resistance. Am J Respir Crit Care Med 2002;165:670–6. [DOI] [PubMed] [Google Scholar]
- 15.Punjabi NM, Ahmed MM, Polotsky VY, Beamer BA, O’Donnell CP. Sleep-disordered breathing, glucose intolerance, and insulin resistance. Respir Physiol Neurobiol 2003;136:167–78. [DOI] [PubMed] [Google Scholar]
- 16.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. American Journal of Respiratory & Critical Care Medicine 2005;172:1590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiihonen M, Partinen M, Narvanen S. The severity of obstructive sleep apnoea is associated with insulin resistance. Journal of Sleep Research 1993;2:56–61. [DOI] [PubMed] [Google Scholar]
- 18.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. Journal of Clinical Endocrinology & Metabolism 2000;85:1151–8. [DOI] [PubMed] [Google Scholar]
- 19.West SD, Nicoll DJ, Stradling JR. Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax 2006;61:945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Archives of Internal Medicine 2005;165:447–52. [DOI] [PubMed] [Google Scholar]
- 21.Harsch IA, Schahin SP, Radespiel-Troger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2004;169:156–62. [DOI] [PubMed] [Google Scholar]
- 22.Lam JC, Lam B, Yao TJ, et al. A randomised controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur Respir J 2010;35:138–45. [DOI] [PubMed] [Google Scholar]
- 23.Trenell MI, Ward JA, Yee BJ, et al. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab 2007;9:679–87. [DOI] [PubMed] [Google Scholar]
- 24.Vgontzas AN, Zoumakis E, Bixler EO, et al. Selective effects of CPAP on sleep apnoea-associated manifestations. Eur J Clin Invest 2008;38:585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax 2007;62:969–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chasens ER, Olshansky E. The experience of being sleepy while managing type 2 diabetes. Journal of the American Psychiatric Nurses Association 2006;12:272–8. [Google Scholar]
- 27.Chasens ER, Olshansky E. Daytime sleepiness, diabetes, and psychological well-being. Issues Ment Health Nurs 2008;29:1134–50. [DOI] [PubMed] [Google Scholar]
- 28.Chasens ER, Korytkowski M, Sereika SM, Burke LE, Drumheller OJ, Strollo PJ Jr. Improving activity in adults with diabetes and coexisting obstructive sleep apnea. West J Nurs Res 2014;36:294–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weaver TE, Mancini C, Maislin G, et al. Continuous Positive Airway Pressure Treatment of Sleepy Patients with Milder Obstructive Sleep Apnea: Results of the CPAP Apnea Trial North American Program (CATNAP) Randomized Clinical Trial. Am J Respir Crit Care Med 2012;186:677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maislin G, Pack AI, Kribbs NB, et al. A survey screen for prediction of apnea. Sleep 1995;18:158–66. [DOI] [PubMed] [Google Scholar]
- 31.Foster G, Kuna S, Sanders M, et al. Sleep Apnea in obese adults with type 2 diabetes: Baseline results from the Sleep Ahead Study. Sleep 2005;28:A204. [Google Scholar]
- 32.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep 2012;35:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kushida CA, Nichols DA, Quan SF, et al. The Apnea Positive Pressure Long-term Efficacy Study (APPLES): rationale, design, methods, and procedures. J Clin Sleep Med 2006;2:288–300. [PubMed] [Google Scholar]
- 34.Rodway GW, Weaver TE, Mancini C, et al. Evaluation of sham-CPAP as a placebo in CPAP intervention studies. Sleep 2010;33:260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 36.Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep 1991;14:540–5. [DOI] [PubMed] [Google Scholar]
- 37.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001;2:297–307. [DOI] [PubMed] [Google Scholar]
- 38.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep 1997;20:835–43. [PubMed] [Google Scholar]
- 40.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology 1973;10:431–6. [DOI] [PubMed] [Google Scholar]
- 41.Basner M, Mollicone DJ, Dinges DF. Validity and sensitivity of a brief psychomotor vigilance test (PVT-B) to total and partial sleep deprivation. Acta Astronautica 2011;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care 2000;23:943–50. [DOI] [PubMed] [Google Scholar]
- 43.Fitzgerald JT, Funnell MM, Hess GE, et al. The reliability and validity of a brief diabetes knowledge test. Diabetes Care 1998;21:706–10. [DOI] [PubMed] [Google Scholar]
- 44.Anderson RM, Funnell MM, Fitzgerald JT, Marrero DG. The Diabetes Empowerment Scale: a measure of psychosocial self-efficacy. Diabetes Care 2000;23:739–43. [DOI] [PubMed] [Google Scholar]
- 45.Gans KM, Risica PM, Wylie-Rosett J, et al. Development and evaluation of the nutrition component of the Rapid Eating and Activity Assessment for Patients (REAP): a new tool for primary care providers. J Nutr Educ Behav 2006;38:286–92. [DOI] [PubMed] [Google Scholar]
- 46.Connor SL, Gustafson JR, Sexton G, Becker N, Artaud-Wild S, Connor WE. The Diet Habit Survey: a new method of dietary assessment that relates to plasma cholesterol changes. J Am Diet Assoc 1992;92:41–7. [PubMed] [Google Scholar]
- 47.McNair D, Lorr M, Druppleman L. EITS Manual for the Profile of Mood States. San Diego: Educational and Industrial Test Services; 1971. [Google Scholar]
- 48.Welch GW, Jacobson AM, Polonsky WH. The Problem Areas in Diabetes Scale. An evaluation of its clinical utility. Diabetes Care 1997;20:760–6. [DOI] [PubMed] [Google Scholar]
- 49.Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: The newest vital sign. Ann Fam Med 2005;3:514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sereika SM, Engberg SJ. Development of standardized sociodemographic and co-morbidity questionnaires. Sigma Theta Tau International Honor Society of Nursing 17th International Nursing Research Congress; Montreal, Quebec, Canada: 2006. [Google Scholar]
- 51.Ware J Jr., Kosinski M, Keller SD, Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care 1996;34:220–33. [DOI] [PubMed] [Google Scholar]
- 52.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep 1992;15:376–81. [DOI] [PubMed] [Google Scholar]
- 53.Friedman LM, Furberg CD, DeMets DL. Fundamentals of clinical trials. Littleton,MA: PSG Publishing Co., Inc.; 1996. [Google Scholar]
- 54.American Thoracic Society. Indications and standards for use of nasal continuous positive airway presssure (SPAP) in sleep apnea sydromes. Am J Respir Crit Care Med 1993;16:1738–45. [DOI] [PubMed] [Google Scholar]
- 55.Funnell MM, Brown TL, Childs BP, et al. National Standards for diabetes self-management education. Diabetes Care 2011;34 Suppl 1:S89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tudor-Locke C, Bassett DR Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med 2004;34:1–8. [DOI] [PubMed] [Google Scholar]
- 57.Mokhlesi B, Grimaldi D, Beccuti G, et al. Effect of One Week of 8-Hour Nightly Continuous Positive Airway Pressure Treatment of Obstructive Sleep Apnea on Glycemic Control in Type 2 Diabetes: A Proof-of-Concept Study. Am J Respir Crit Care Med 2016;194:516–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaw JE, Punjabi NM, Naughton MT, et al. The Effect of Treatment of Obstructive Sleep Apnea on Glycemic Control in Type 2 Diabetes. Am J Respir Crit Care Med 2016;194:486–92. [DOI] [PubMed] [Google Scholar]
- 59.Martinez-Ceron E, Barquiel B, Bezos AM, et al. Effect of Continuous Positive Airway Pressure on Glycemic Control in Patients with Obstructive Sleep Apnea and Type 2 Diabetes. A Randomized Clinical Trial. Am J Respir Crit Care Med 2016;194:476–85. [DOI] [PubMed] [Google Scholar]
- 60.Reutrakul S, Mokhlesi B. Obstructive Sleep Apnea and Diabetes: A State of the Art Review. Chest 2017;152:1070–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Statistics About Diabetes. American Diabetes Association, 2014. at http://www.diabetes.org/diabetes-basics/statistics/.)