Abstract
Focal segmental glomerulosclerosis (FSGS) is a common kidney disease that results in nephrotic syndrome. FSGS arises from dysfunction and apoptosis of podocytes in the glomerulus of the kidney, leading to podocytopathy. The molecular mechanisms underlying podocyte apoptosis remain incompletely understood. Using an array of gene expression profiling, PCR, and in situ hybridization assay, we found here that the levels of the long noncoding RNA LOC105374325 were elevated in the renal podocytes of individuals with FSGS. We also observed that the microRNAs miR-34c and miR-196a/b down-regulated the expression of the apoptosis regulators BCL2-associated X, apoptosis regulator (Bax), and BCL2 antagonist/killer 1 (Bak) in podocytes. Competitive binding between LOC105374325 and miR-34c or miR-196a/b increased Bax and Bak levels and caused podocyte apoptosis. Of note, the mitogen-activated protein kinase P38 and the transcription factor CCAAT enhancer–binding protein β (C/EBPβ) up-regulated LOC105374325 expression. P38 inhibition or C/EBPβ silencing decreased LOC105374325 levels and inhibited apoptosis in adriamycin-treated podocytes. LOC105374325 overexpression decreased miR-34c and miR-196a/b levels, increased Bax and Bak levels, and induced proteinuria and focal segmental lesions in mice. In conclusion, activation of the P38/C/EBPβ pathway stimulates the expression of LOC105374325, which, in turn, increases Bax and Bak levels and causes apoptosis by competitively binding to miR-34c and miR-196a/b in the podocytes of individuals with FSGS.
Keywords: nephrology; podocyte; long noncoding RNA (long ncRNA, lncRNA); microRNA (miRNA); apoptosis; focal segmental glomerulosclerosis; kidney disease; nephrotic syndrome; posttranscriptional regulation
Introduction
Focal segmental glomerulosclerosis (FSGS)4 is a common pathological type of nephrotic syndrome in which the sclerosis lesion involves <50% of the total number of glomeruli and <50% of the glomerular capillary surface. FSGS accounts for 40% of the cases of nephrotic syndrome in adults (1). The degree of proteinuria is an independent risk factor for renal function decline in patients (2, 3). Under normal conditions, the podocyte slit diaphragm serves as an important domain contributing to the permeability barrier of the glomerulus. Podocyte loss is involved in the pathogenesis of FSGS, and apoptosis is the major cause of reduced podocyte number, leading to proteinuria and glomerulosclerosis (4).
Bax and its homolog Bak are the key regulators of the mitochondrial pathway of apoptosis. Under conditions of cell stress, Bax and Bak accumulate on the mitochondrial surface, where they oligomerize and mediate cytochrome c release, thereby leading to cell death. A previous study showed an increased level of Bax expression and apoptosis in the glomerular tissues of FSGS patients (5). Nevertheless, the mechanisms underlying the expression of Bax and podocyte apoptosis remain incompletely understood.
Long noncoding RNAs (lncRNAs) are longer than 200 nucleotides and have no protein-encoding capacity (6). Several lines of evidence suggest that lncRNAs are involved in the pathogenesis of podocyte injury. Hu et al. (7) reported that the level of the lncRNA MALAT1 was elevated in diabetic nephropathy and was involved in high glucose-induced podocyte injury via its interplay with β-catenin. Long et al. (8) reported that Tug1 regulates mitochondrial function in podocytes by the epigenetic targeting of the transcription factor peroxisome proliferator-activated receptor γ coactivator 1α. Ling et al. (9) showed that lncRNA ENSRNOG00000037522 is involved in the podocyte epithelial–mesenchymal transition in diabetic rats.
In this study, we performed a transcriptome analysis of glomerular tissues in 5 FSGS patients and 5 controls. Among the differentially expressed lncRNAs, the level of LOC105374325 showed the most significant increase in the glomerular tissues of FSGS patients. Whether the up-regulated LOC105374325 leads to the increase of Bax expression and podocyte injury remains unclear. We conducted both in vitro and in vivo experiments to investigate the role of LOC105374325 in podocyte injury in FSGS patients.
Results
LncRNA LOC105374325 is up-expressed in renal podocytes of FSGS patients
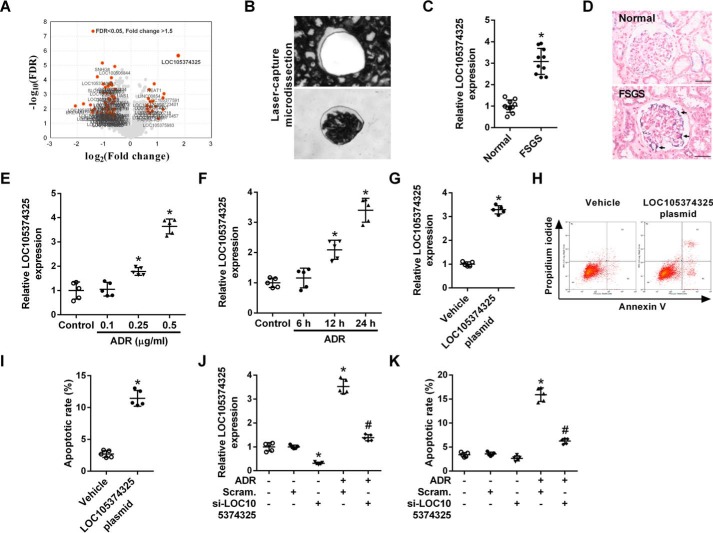
Renal glomerular tissues from 5 FSGS patients and 5 controls were microdissected, and an Affymetrix HTA 2.0 microarray was used to perform a global analysis of the gene expression pattern in the tissues. Among the differentially expressed lncRNAs, the level of LOC105374325 showed the most significant increase in the glomerular tissues of FSGS patients (Fig. 1A). RT-PCR analysis of another set of patients confirmed that the level of LOC105374325 was increased in the glomerular tissues of FSGS patients (Fig. 1, B and C). In situ hybridization (ISH) staining showed that LOC105374325 expression was up-regulated in renal podocytes of FSGS patients (Fig. 1D).
Figure 1.
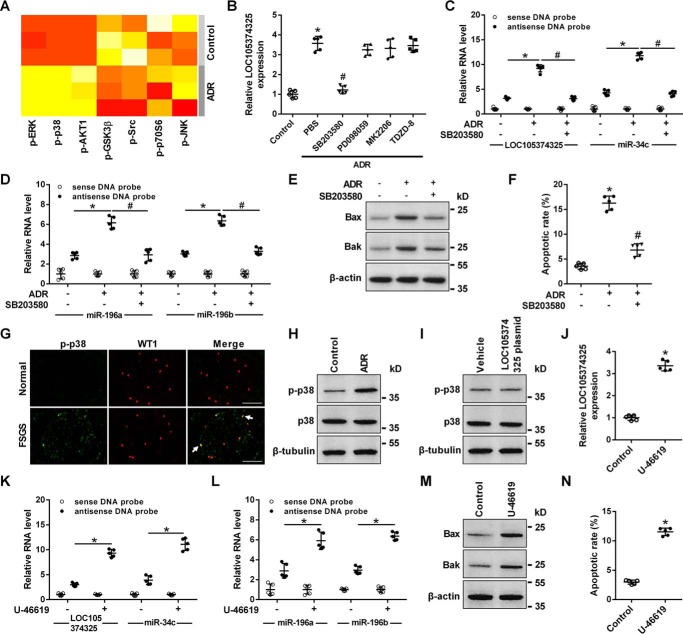
Change of LOC105374325 level in renal podocytes of FSGS patients. A, volcano plot of differentially expressed lncRNAs in glomerular tissues of FSGS patients, with cutoff values of fold-change >1.5 and false discovery rate <0.05 (n = 5); B, isolation of glomerular tissues by laser capture microdissection; C, level of LOC105374325 in glomerular tissues of FSGS patients (n = 10, validation cohort); D, ISH analysis of LOC105374325 in glomerular tissues of FSGS patients and normal controls (n = 5); E, level of LOC105374325 in podocytes treated with different doses of ADR for 24 h (n = 5); F, level of LOC105374325 in podocytes treated with ADR (0.5 μg/ml) for different times (n = 5); G, level of LOC105374325 in podocytes transfected with LOC105374325 plasmid (n = 5); H, flow cytometric analysis of apoptotic cells in podocytes transfected with LOC105374325 plasmid; I, quantitative analysis of apoptotic cells in podocytes transfected with LOC105374325 plasmid (n = 5); J, level of LOC105374325 in podocytes treated with ADR and LOC105374325 siRNA (n = 5); K, flow cytometric analysis of apoptotic cells in podocytes treated with ADR and LOC105374325 siRNA (n = 5). For statistical analysis, a two-tailed Student's t test was used for C, G, and I, and one-way ANOVA with Tukey's post hoc test was used for E, F, J, and K. *, p < 0.05 compared with control; #, p < 0.05 compared with podocytes treated with ADR. Bar, 20 μm.
Treatment with adriamycin (ADR) in cultured human podocytes increased the expression of LOC105374325 in both a dose- and time-dependent manner (Fig. 1, E and F). The level of LOC105374325 was significantly increased in podocytes treated with 0.5 μg/ml of ADR for 24 h. Overexpression of LOC105374325 alone induced the cell apoptosis in cultured podocytes (Fig. 1, G–I). Conversely, silence of LOC105374325 decreased the cell apoptosis in podocytes treated with ADR (Fig. 1, J and K).
LOC105374325 increases the expression of Bax and Bak in podocytes
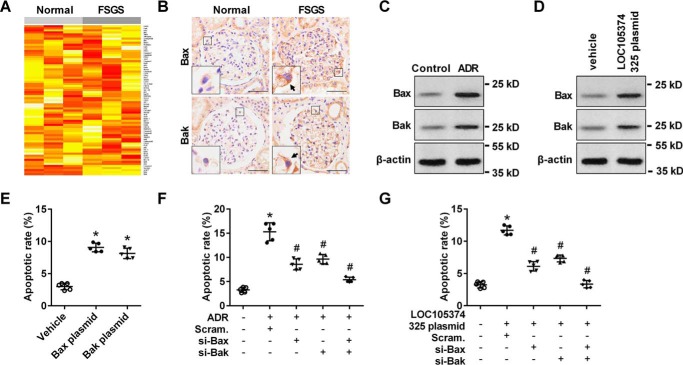
Apoptosis antibody array analysis indicated that the level of Bax and Bak were obviously increased in the glomerular tissues of FSGS patients (Fig. 2A). Bax and its homolog Bak are the key regulators of the mitochondrial pathway of apoptosis. On cell stress, Bax and Bak accumulate on the mitochondrial surface where they oligomerize and mediate cytochrome c release, leading to cell death. Immunohistochemical (IHC) analysis showed that, the level of Bax and Bak were increased in the glomerular podocytes of FSGS patients, compared with normal controls (Fig. 2B). Treatment with ADR or overexpression of LOC105374325 increased the expression of Bax and Bak in podocytes in vitro (Fig. 2, C and D).
Figure 2.
Effect of LOC105374325 on the expression of Bax and Bak in podocytes. A, apoptosis antibody array analysis of glomerular tissues of FSGS patients (n = 3); B, IHC analysis of Bax and Bak in glomerular tissues of FSGS patients (n = 5); C, Western blot analysis of Bax and Bak in podocytes treated with ADR (n = 3); D, Western blot analysis of Bax and Bak in podocytes transfected with LOC105374325 plasmid (n = 3); E, flow cytometric analysis of apoptotic cells in podocytes transfected with Bax or Bak plasmid (n = 5); F, flow cytometric analysis of apoptotic cells in podocytes treated with ADR, Bax siRNA, and Bak siRNA (n = 5); G, flow cytometric analysis of apoptotic cells in podocytes transfected with LOC105374325 plasmid, Bax siRNA, and Bak siRNA (n = 5). For statistical analysis, one-way ANOVA with Tukey's post hoc test was used for E–G. *, p < 0.05 compared with control; #, p < 0.05 compared with podocytes treated with ADR or transfected with LOC105374325 plasmid. Bar, 20 μm.
Overexpression of Bax or Bak induced the cell apoptosis in podocytes, and mimics the effect of ADR treatment or LOC105374325 overexpression (Fig. 2E). Conversely, silence of Bax or Bak decreased the cell apoptosis in both ADR-treated podocytes and LOC105374325-overexpressed podocytes (Fig. 2, F and G).
LOC105374325 up-regulates the expression of Bax by competitive binding of miR-34c in podocytes
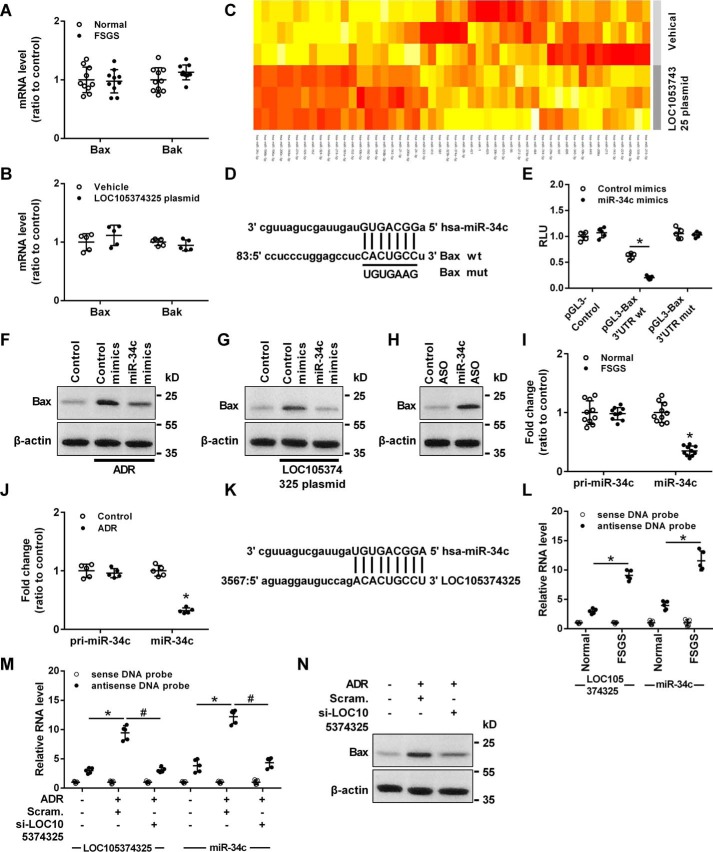
Although the protein levels of Bax and Bak were increased in glomerular tissues of FSGS patients, PCR analysis showed that the mRNA levels of Bax and Bak were not changed in the glomerular tissues of FSGS patients or in the podocytes transfected with LOC105374325 plasmid (Fig. 3, A and B), suggesting a post-transcriptional mechanism involved in the regulation of Bax and Bak in podocytes of FSGS.
Figure 3.
Effect of the binding between LOC105374325 and miR-34c on the expression of Bax in podocytes. A, level of Bax and Bak mRNA in glomerular tissues of FSGS patients (n = 10); B, level of Bax and Bak mRNA in podocytes transfected with LOC105374325 plasmid (n = 5); C, miRNA PCR array analysis of podocytes transfected with LOC105374325 plasmid (n = 3); D, the binding site in the 3′UTR of Bax mRNA targeted by miR-34c; E, normalized luciferase activity of reporter constructs containing the 3′UTR of Bax or mutant 3′UTR of Bax in cells cotransfected with miR-34c mimics (n = 5); F, level of Bax protein in podocytes treated with ADR and miR-34c mimics (n = 3); G, level of Bax protein in podocytes transfected with LOC105374325 plasmid and miR-34c mimics (n = 3); H, level of Bax protein in podocytes transfected with miR-34c ASO (n = 3); I, level of pri-miR-34c and miR-34c in glomerular tissues of FSGS patients (n = 10); J, level of pri-miR-34c and miR-34c in podocytes treated with ADR (n = 5); K, the binding site in LOC105374325 targeted by miR-34c; L, RNA pulldown and RT-PCR analysis of the LOC105374325–miR-34c complex in glomerular tissues of FSGS patients (n = 5); M, RNA pulldown and RT-PCR analysis of the LOC105374325–miR-34c complex in podocytes treated with ADR and LOC105374325 siRNA (n = 5); N, level of Bax protein in podocytes treated with ADR and LOC105374325 siRNA (n = 3). For statistical analysis, a two-tailed Student's t test was used for A, B, E, I, and J, and one-way ANOVA with Tukey's post hoc test was used for L and M. *, p < 0.05 compared with control; #, p < 0.05 compared with podocytes treated with ADR.
MiRNA PCR array analysis showed that the level of miR-34c and miR-196a/b were significantly decreased in podocytes transfected with the LOC105374325 plasmid (Fig. 3C). The absolute levels of LOC105374325, miR-34c, and miR-196a/b were quantified by the standard curve method. The levels of LOC105374325, miR-34c, and miR-196a/b were comparable in podocytes, and overexpression of LOC105374325 decreased the absolute level of miR-34c and miR-196a/b in podocytes (Fig. S1).
Computational analysis detected a miR-34c–binding sequence in Bax mRNA 3′UTR (Fig. 3D). Luciferase reporter analysis showed that miR-34c mimics significantly inhibited the reporter activity of Bax 3′UTR-Luc, and site-directed mutations rescued the miR-34c-mediated inhibition of Bax 3′UTR-Luc (Fig. 3E). Transfection with miR-34c mimics also decreased the level of Bax in podocytes treated with ADR or transfected with LOC105374325 plasmid (Fig. 3, F and G). Conversely, transfection with the miR-34c antisense oligonucleotide (ASO) increased the level of Bax in podocytes (Fig. 3H).
RT-PCR analysis showed that the level of miR-34c but not pri-miR-34c was decreased in the glomerular tissues of FSGS patients and in ADR-treated podocytes (Fig. 3, I and J). Bioinformatic analysis with RNAHybrid software showed that LOC105374325 contains the response element of miR-34c (Fig. 3K). RNA pulldown and RT-PCR analysis confirmed that miR-34c bound to LOC105374325 in glomerular tissues, and formation of the LOC105374325–miR-34c complex was increased in the tissues of FSGS patients compared with normal controls (Fig. 3L). Treatment with ADR increased the formation of the LOC105374325–miR-34c complex in podocytes, which was prevented by LOC105374325 siRNA (Fig. 3M). Silence of LOC105374325 also decreased the level of Bax in ADR-treated podocytes (Fig. 3N).
LOC105374325 up-regulates the expression of Bak by competitive binding of miR-196a/b in podocytes
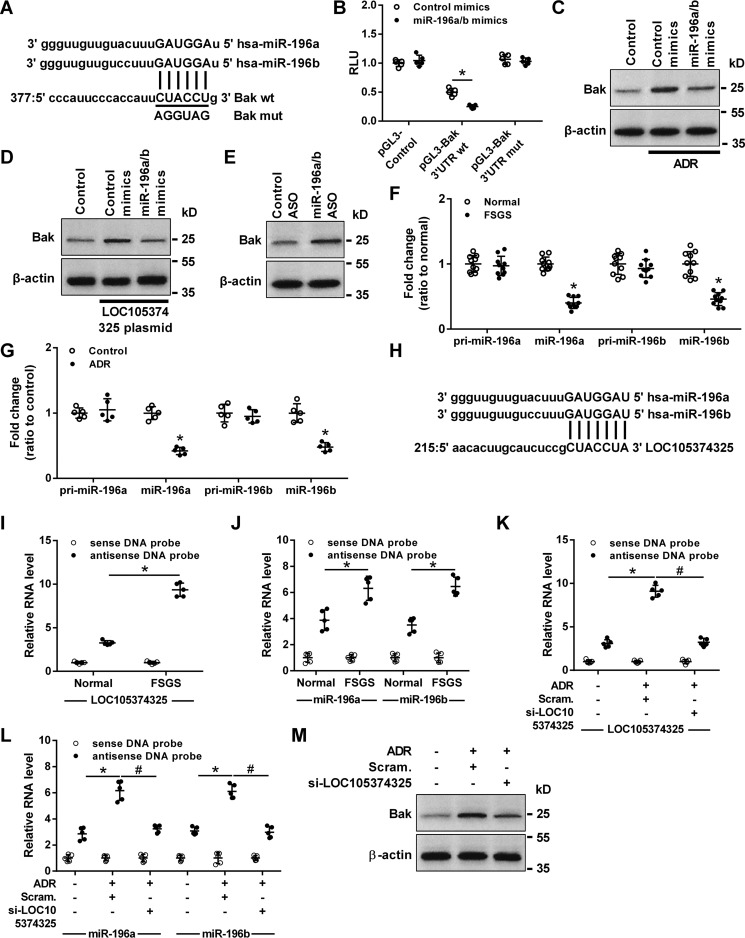
Computational analysis detected a miR-196a/b–binding sequence in Bak mRNA 3′UTR (Fig. 4A). MiR-196a/b mimics significantly inhibited the reporter activity of Bak 3′UTR-Luc, and site-directed mutations rescued the miR-196a/b-mediated inhibition of Bak 3′UTR-Luc (Fig. 4B). Transfection with miR-196a/b mimics also decreased the level of Bak in podocytes treated with ADR or transfected with LOC105374325 plasmid (Fig. 4, C and D). Conversely, transfection with miR-196a/b ASO increased the level of Bak in cultured podocytes (Fig. 4E).
Figure 4.
Effect of the binding between LOC105374325 and miR-196a/b on the expression of Bak in podocytes. A, the binding site in the 3′UTR of Bak mRNA targeted by miR-196a/b; B, normalized luciferase activity of reporter constructs containing the 3′UTR of Bak or mutant 3′UTR of Bak in cells cotransfected with miR-196a/b mimics (n = 5); C, level of Bak protein in podocytes treated with ADR and miR-196a/b mimics (n = 3); D, level of Bak protein in podocytes transfected with LOC105374325 plasmid and miR-196a/b mimics (n = 3); E, level of Bak protein in podocytes transfected with miR-196a/b ASO (n = 3); F, level of pri-miR-196a, miR-196a, pri-miR-196b, and miR-196b in glomerular tissues of FSGS patients (n = 10); G, level of pri-miR-196a, miR-196a, pri-miR-196b, and miR-196b in podocytes treated with ADR (n = 5); H, the binding site in LOC105374325 targeted by miR-196a/b; I and J, RNA pulldown and RT-PCR analysis of the LOC105374325–miR-196a/b complex in glomerular tissues of FSGS patients (n = 5); K and L, RNA pulldown and RT-PCR analysis of the LOC105374325–miR-196a/b complex in podocytes treated with ADR and LOC105374325 siRNA (n = 5); M, level of Bak protein in podocytes treated with ADR and LOC105374325 siRNA (n = 3). For statistical analysis, a two-tailed Student's t test was used for B, F, and G, and one-way ANOVA with Tukey's post hoc test was used for I–L. *, p < 0.05 compared with control; #, p < 0.05 compared with podocytes treated with ADR.
RT-PCR analysis showed that the level of miR-196a and miR-196b but not pri-miR-196a or pri-miR-196b was decreased in the glomerular tissues of FSGS patients and in ADR-treated podocytes (Fig. 4, F and G). Bioinformatic analysis with RNAHybrid software showed that, LOC105374325 also contains the response element of miR-196a/b (Fig. 4H). RNA pulldown and RT-PCR analysis confirmed that miR-196a/b bound to LOC105374325 in glomerular tissues, and the formation of LOC105374325–miR-196a/b complex was increased in the tissues of FSGS patients compared with normal controls (Fig. 4, I and J). Treatment with ADR increased the formation of LOC105374325–miR-196a/b complex in podocytes, which was prevented by LOC105374325 siRNA (Fig. 4, K and L). Silence of LOC105374325 also decreased the level of Bak in ADR-treated podocytes (Fig. 4M).
ADR treatment induces the expression of LOC105374325 by activating p38 MAPK pathway in podocytes
Phosphokinase array analysis showed that p38, ERK, AKT, and GSK3β pathways were activated in ADR-treated podocytes (Fig. 5A). The p38 inhibitor SB203580 but not the AKT inhibitor MK2206, the ERK inhibitor PD098059, or the GSK3β inhibitor TDZD-8 prevented the increase of LOC105374325 in podocytes treated with ADR (Fig. 5B). The p38 inhibitor SB203580 also suppressed formation of LOC105374325–miR-34c and LOC105374325–miR-196a/b complexes, decreased the level of Bax and Bak, and inhibited cell apoptosis in podocytes treated with ADR (Fig. 5, C–F).
Figure 5.
Role of p38 MAPK in the expression of LOC105374325 in podocytes. A, human phosphokinase array analysis in podocytes treated with ADR (n = 3); B, level of LOC105374325 in podocytes treated with ADR, SB203580, PD098059, MK2206, and TDZD-8 (n = 5); C and D, RNA pulldown and RT-PCR analysis of the LOC105374325–miR-34c and LOC105374325–miR-196a/b complexes in podocytes treated with ADR and SB203580 (n = 5); E, level of Bax and Bak protein in podocytes treated with ADR and SB203580 (n = 3); F, flow cytometric analysis of apoptotic cells in podocytes treated with ADR and SB203580 (n = 5); G, immunofluorescence staining of p-p38 in glomerular tissues of FSGS patients and normal controls (n = 5); H, level of p-p38 in podocytes treated with ADR (n = 3); I, level of p-p38 in podocytes transfected with LOC105374325 plasmid (n = 3); J, level of LOC105374325 in podocytes treated with U-46619 (n = 5); K and L, RNA pulldown and RT-PCR analysis of the LOC105374325–miR-34c and LOC105374325–miR-196a/b complexes in podocytes treated with U-46619 (n = 5); M, level of Bax and Bak protein in podocytes treated with U-46619 (n = 3); N, flow cytometric analysis of apoptotic cells in podocytes treated with U-46619 (n = 5). For statistical analysis, a two-tailed Student's t test was used for J and N, and one-way ANOVA with Tukey's post hoc test was used for B–D, F, K, and L. *, p < 0.05 compared with control; #, p < 0.05 compared with podocytes treated with ADR. Bar, 20 μm.
Level of p-p38 was increased in renal podocytes of FSGS patients and in podocytes treated with ADR (Fig. 5, G and H). Overexpression of LOC105374325 alone did not change the level of p-p38 in podocytes (Fig. 5I). Conversely, treatment with the p38 activator U-46619 increased the level of LOC105374325, promoted formation of LOC105374325–miR-34c and LOC105374325–miR-196a/b complexes, increased the level of Bax and Bak, and caused cell apoptosis in podocytes (Fig. 5, J–N).
Transcription factor C/EBPβ regulates the expression of LOC105374325 in podocytes
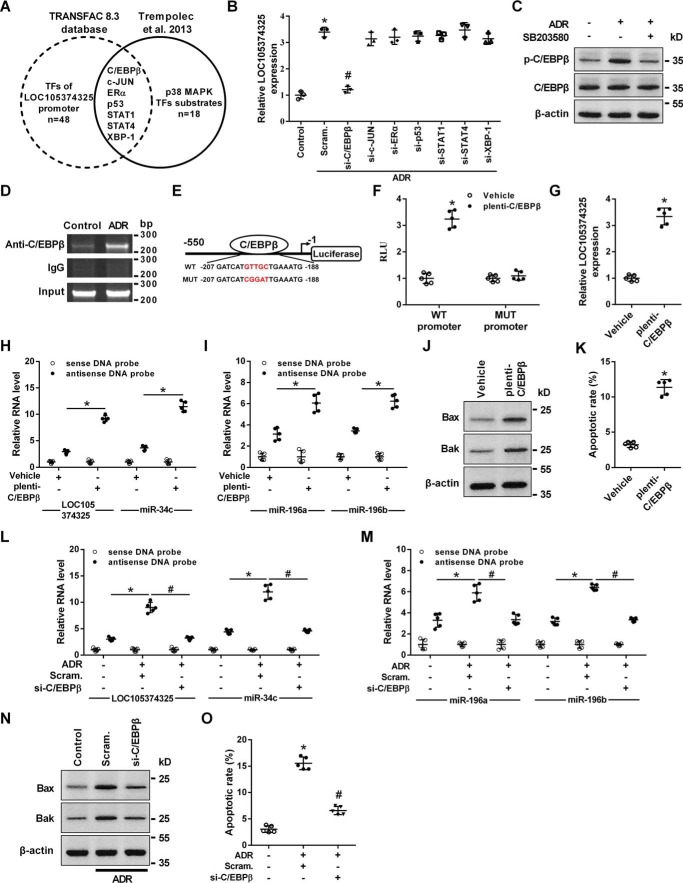
The PROMO (TRANSFAC 8.3) software was applied to analyze the sequence of the LOC105374325 promoter region. Among the predicted transcription factors, 7 factors have been reported to be the downstream substrates of p38 MAPK (Fig. 6A) (10). Knockdown of C/EBPβ but not other factors significantly inhibited the expression of LOC105374325 in podocytes treated with ADR (Fig. 6B). The phosphorylation of C/EBPβ was increased in podocytes treated with ADR, and inhibition of p38 prevented the ADR-induced phosphorylation of C/EBPβ (Fig. 6C).
Figure 6.
Role of C/EBPβ in the expression of LOC105374325 in podocytes. A, prediction of transcription factors binding to LOC105374325 promoter region; B, level of LOC105374325 in podocytes treated with ADR, C/EBPβ siRNA, c-JUN siRNA, ERα siRNA, p53 siRNA, STAT1 siRNA, STAT4 siRNA, and XBP-1 siRNA (n = 3); C, level of C/EBPβ and p-C/EBPβ protein in podocytes treated with ADR and SB203580 (n = 3); D, ChIP analysis of the binding between C/EBPβ and LOC105374325 promoter in podocytes treated with ADR (n = 3); E, schematic of the constructed LOC105374325 promoter-luciferase reporter plasmids; F, normalized luciferase activity of reporter constructs in podocytes cotransfected with C/EBPβ plasmid (n = 5); G, level of LOC105374325 in podocytes transfected with C/EBPβ plasmid (n = 5); H and I, RNA pulldown and RT-PCR analysis of the LOC105374325–miR-34c and LOC105374325–miR-196a/b complexes in podocytes transfected with C/EBPβ plasmid (n = 5); J, level of Bax and Bak protein in podocytes transfected with C/EBPβ plasmid (n = 3); K, flow cytometric analysis of apoptotic cells in podocytes transfected with C/EBPβ plasmid (n = 5); L and M, RNA pulldown and RT-PCR analysis of the LOC105374325–miR-34c and LOC105374325–miR-196a/b complexes in podocytes treated with ADR and C/EBPβ siRNA (n = 5); N, level of Bax and Bak protein in podocytes treated with ADR and C/EBPβ siRNA (n = 3); O, flow cytometric analysis of apoptotic cells in podocytes treated with ADR and C/EBPβ siRNA (n = 5). For statistical analysis, a two-tailed Student's t test was used for F, G, and K, and one-way ANOVA with Tukey's post hoc test was used for B, H, I, L, M, and O. *, p < 0.05 compared with control; #, p < 0.05 compared with podocytes treated with ADR.
Chromatin immunoprecipitation (ChIP) analysis confirmed that C/EBPβ bound to the promoter region of LOC105374325 in podocytes, which was enhanced by ADR treatment (Fig. 6D). Overexpression of C/EBPβ increased the expression of the luciferase reporter construct containing the binding sequence, and site-directed mutations rescued the C/EBPβ-mediated up-regulation of the LOC105374325 promoter-luciferase reporter plasmid (Fig. 6, E and F). Overexpression of C/EBPβ also increased the level of LOC105374325, promoted the formation of LOC105374325–miR-34c and LOC105374325–miR-196a/b complexes, increased the level of Bax and Bak, and caused cell apoptosis in podocytes (Fig. 6, G–K). Conversely, knockdown of C/EBPβ suppressed the formation of LOC105374325–miR-34c and LOC105374325–miR-196a/b complexes, decreased the level of Bax and Bak, and inhibited cell apoptosis in ADR-treated podocytes (Fig. 6, L–O).
Overexpression of LOC105374325 causes podocyte injury in mice
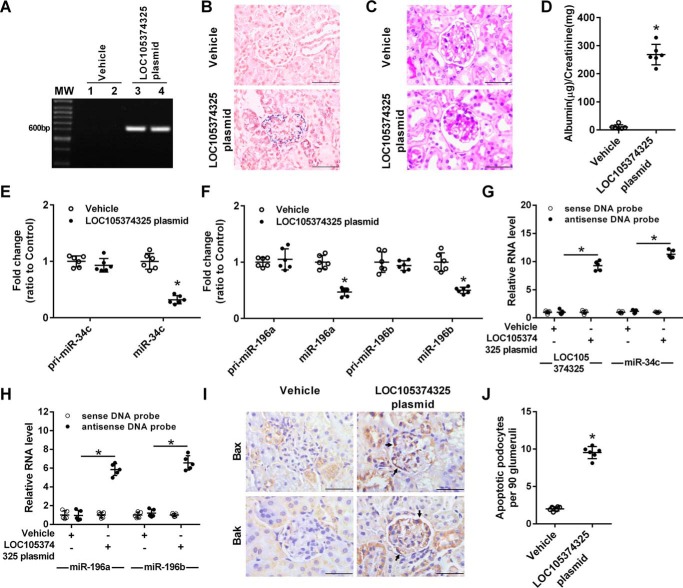
The miR-34c–binding site in Bax 3′UTR and the miR-196a/b–binding site in Bak 3′UTR were conserved in mouse, but LOC105374325 was not conserved in mouse (Fig. S2). To analyze the pathogenic role of LOC105374325 in vivo, we elevated renal LOC105374325 expression by a hydrodynamic-based delivery of LOC105374325-expressing plasmid in mice. After 4 weeks of treatment, LOC105374325 was obviously expressed in the podocyte of mice (Fig. 7, A and B). Mice overexpressing LOC105374325 developed proteinuria and focal segmental lesions (Fig. 7, C and D).
Figure 7.
Effect of exogenous LOC105374325 expression on podocyte injury in mice. A, PCR analysis of LOC105374325 in glomerular tissues of mice treated with control plasmid or LOC105374325-expressing plasmid; B, ISH analysis of LOC105374325 in glomerular tissues of mice (n = 6); C, periodic acid-Schiff staining of renal sections in mice (n = 6); D, urinary albumin excretion in mice (n = 6); E, level of pri-miR-34c and miR-34c in glomerular tissues of mice (n = 6); F, level of pri-miR-196a, miR-196a, pri-miR-196b, and miR-196b in glomerular tissues of mice (n = 6); G and H, RNA pulldown and RT-PCR analysis of the LOC105374325–miR-34c and LOC105374325–miR-196a/b complexes in glomerular tissues of mice (n = 6); I, IHC analysis of Bax and Bak in glomerular tissues of mice (n = 6); J, apoptotic podocytes in glomerular tissues of mice (n = 6). For statistical analysis, a two-tailed Student's t test was used for D, E, F, and J, and one-way ANOVA with Tukey's post hoc test was used for G and H. *, p < 0.05 compared with vehicle control mice. Bar, 20 μm.
Overexpression of LOC105374325 did not change the level of pri-miR-34c, pri-miR-196a, or pri-miR-196b, instead it decreased the level of miR-34c and miR-196a/b in the glomerular tissues of mice (Fig. 7, E and F). RNA pulldown and RT-PCR analysis showed that the formation of LOC105374325–miR-34c and LOC105374325–miR-196a/b complexes was increased in the glomerular tissues of mice overexpressing LOC105374325 (Fig. 7, G and H). Meanwhile, overexpression of LOC105374325 increased the expression of Bax and Bak in the podocytes of mice (Fig. 7I). As a result, the apoptotic podocytes were obviously increased in the mice overexpressing LOC105374325 (Fig. 7J).
Discussion
FSGS accounts for ∼40% of nephrotic syndrome in adults (1). Most FSGS patients have massive nonselective proteinuria and severe podocyte injury at disease onset (11). The prognosis of FSGS is poor, with a renal survival rate of only 43.8% at 10 years after renal biopsy (3). Podocyte loss is the central pathological mechanism underlying glomerular sclerosis in FSGS patients (12). Although several signaling pathways have been implicated in podocyte loss, the mechanisms underlying podocyte apoptosis in FSGS patients remain incompletely understood (13, 14).
LncRNAs have been shown to play important roles in renal disease (15). Puthanveetil et al. (16) reported that the lncRNA MALAT1 regulated the glucose-induced up-regulation of inflammatory mediators IL-6 and tumor necrosis factor α through the activation of SAA3 in endothelial cells. Sun et al. (17) reported that the lncRNA Erbb4-IR promoted renal fibrosis by suppressing miR-29b in db/db mice. To date, the role of lncRNAs in podocyte injury in FSGS patients has not been revealed. We performed a glomerular transcriptome analysis and found that the level of LOC105374325 showed the most significant increase in FSGS patients, and the overexpression of LOC105374325-induced apoptosis in podocytes.
Bax and its homolog Bak are key regulators of the mitochondrial pathway of apoptosis. Aviles et al. (18) showed that the level of pro-apoptotic Bax and cFLIP were elevated following IL-2 exposure in podocytes. Lei et al. (19) reported that high glucose treatment caused the up-regulation of Bax and Bak and the down-regulation of Bcl-2 in podocytes. Burlaka et al. (20) reported that albumin uptake in renal epithelial cells was accompanied by the mitochondrial accumulation of Bax, the down-regulation of Bcl-xL and mitochondrial membrane depolarization. Aberrant increased expression of Bax and decreased expression of Bcl-2 have been observed in glomerular tissues of ADR-treated mice (21). We found that the level of Bax and Bak protein, but not of mRNA, were increased in the renal podocytes of FSGS patients, suggesting that a posttranscriptional mechanism is involved in the regulation of Bax and Bak in the podocytes of FSGS.
Competing endogenous RNA is an important mechanism by which lncRNA participates in the post-transcriptional regulation of gene expression (15, 22). We found that the level of miR-34c and miR-196a/b were most significantly decreased in LOC105374325-overexpressed podocytes, and miR-34c and miR-196a/b were predicted and confirmed to regulate the expression of Bax and Bak in podocytes. The levels of miR-34c and miR-196a/b have been shown to be enriched in renal tissues by Solexa sequencing (23). Li et al. (24) reported that the level of miR-34c in differentiated podocytes was 187 times higher than those in undifferentiated podocytes. Liu et al. (25) reported that miR-34c was down-regulated in high glucose-treated podocytes, and the overexpression of miR-34c inhibited high glucose-induced podocyte apoptosis. Our previous studies showed that the urinary miR-196a level was correlated with disease activity in FSGS patients, and miR-196a/b mitigated renal fibrosis by targeting transforming growth factor-β receptor 2 in the tubular cells of FSGS (23, 26, 27). These results indicated the important role of miR-34c and miR-196a/b in maintaining renal homeostasis. The relationship between miR-34c or miR-196a/b and apoptosis has also been indicated in other types of cells. Catuogno et al. (28) showed that miR-34c protected lung cancer cells from paclitaxel-induced apoptosis. Wang et al. (29) reported that miR-196b inhibited late apoptosis in pancreatic cancer cells. Cao et al. (30) reported that overexpression of miR-196b enhanced viability and inhibited apoptosis of human HeLa and 293T cells. We found that the response elements of miR-34c and miR-196a/b existed in the sequence of LOC105374325. The overexpression of LOC105374325 decreased the level of miR-34c and miR-196a/b, increased the level of Bax and Bak, and caused podocyte apoptosis in mice.
The activation of p38 MAPK has been reported to be involved in podocyte apoptosis by independent studies. Koshikawa et al. (31) showed that the enhanced phosphorylation of p38 was detected in the nuclei of podocytes in biopsy specimens from FSGS patients. Bao et al. (32) showed that treatment with puromycin aminonucleoside induced the activation of p38 in podocytes. Liu et al. (33) reported that the inhibition of p38 reduced Bax expression and attenuated apoptosis in podocytes treated with high glucose. Van Laethem et al. (34) reported that the activation of p38 was required for Bax translocation to mitochondria, cytochrome c release, and apoptosis in human keratinocytes exposed to UVB irradiation. We confirmed that the activation of p38 phosphorylated C/EBPβ, which bound to the promoter and increased the expression of LOC105374325 in podocytes. Chen et al. (35) reported that the level of C/EBPβ was increased in renal tissues after ischemia/reperfusion-induced injury. Liu et al. (36) reported that C/EBPβ was up-regulated in heme-induced endothelial cell apoptosis. Chen et al. (37) showed that the overexpression of C/EBPβ abrogated the effect of atorvastatin on increasing Bcl-2/Bax, whereas the early and late apoptosis rate was increased in cardiomyocytes. Silencing C/EBPβ decreased the level of LOC105374325, suppressed the formation of LOC105374325–miR-34c and LOC105374325–miR-196a/b complexes, decreased the level of Bax and Bak, and inhibited apoptosis in podocytes treated with ADR. In conclusion, activation of the p38/C/EBPβ pathway induces the expression of LOC105374325 in podocytes, and LOC105374325 increases the level of Bax and Bak and causes cell apoptosis by the competitive binding of miR-34c and miR-196a/b in the podocytes of FSGS patients.
Materials and methods
Patients and control subjects
Five FSGS patients were recruited for glomerular transcriptome analysis (Table 1). Another 10 FSGS patients were enrolled for the confirmation study. Control tissues were obtained from the unaffected portion of surgical nephrectomies, and were confirmed to be normal through light microscope analysis. The study was carried out in accordance with the principles of the Declaration of Helsinki and was approved by the ethics committees of Jinling Hospital. Renal specimens were kept in Renal Biobank of National Clinical Research Center of Kidney Diseases at Jinling Hospital. Informed consent has been obtained from each participant.
Table 1.
Demographic characteristics in the discovery and validation cohorts
| Group | Number | Age | Gender | Disease course |
|---|---|---|---|---|
| years | % female | months | ||
| Discovery cohort | ||||
| Normal | 5 | 34.6 ± 7.3 | 20 | |
| FSGSa | 5 | 32.2 ± 15.7 | 20 | 6.0 (0.7–18.0) |
| Validation cohort | ||||
| Normal | 10 | 38.5 ± 6.1 | 40 | |
| FSGS | 10 | 35.1 ± 16.6 | 40 | 5.6 (0.9–11.6) |
a FSGS, focal segmental glomerulosclerosis.
Glomerulus isolation
For array analysis, pink-colored, spherical-shaped glomeruli were manually isolated under a stereomicroscope using 2 dissection needle holders in RNAlater at 4 °C. RNA quality and quantity were determined using the Laboratory-on-Chip Total RNA Pico Kit (Agilent BioAnalyzer, Waldbronn, Germany). For PCR analysis, glomeruli were isolated by a laser capture microdissection system (Leica Microsystems AG, Wetzlar, Germany). Approximately 50 glomerular cross-sections were captured from each case (38). The expression ratio of WT1 in laser-captured glomerular versus tubulointerstitial tissues was 53.5.
Gene expression profile analysis
The Affymetrix HTA2.0 microarrays were used for the global profiling of human lncRNAs in microdissected glomerular tissues. The accession number for the microarray data reported in this paper is GEO accession number GSE121233.
RT-PCR analysis
Total RNA was extracted using miRNeasy Mini Kit (217004, Qiagen, CA). Reverse transcription was carried out with miScript II RT Kit (218161, Qiagen). QuantiTect SYBR Green PCR Master Mix (204143, Qiagen) was used for gene expression level measurement. The primers for PCR analysis were listed in Table S1. The levels of miR-34c, miR-196a, and miR-196b were quantified by the TaqMan miRNA assay (Applied Biosystems, Foster City, CA) with small nuclear RNA U6 as an endogenous control (38).
In situ hybridization analysis of LOC105374325
Paraffin tissue sections were deparaffinized with xylene, rehydrated with ethanol dilution series and treated with 15 μg/ml of proteinase K (03508811103, Roche Applied Science, Switzerland) at 37 °C for 15 min. Then slides were fixed in 4% paraformaldehyde and hybridized with 5′ digoxin-labeled LOC105374325 probe (Exiqon, Vedbaek, Denmark) at 55 °C overnight. After washing, slides were treated with blocking buffer for 30 min. Slides were then incubated with anti-digoxin-AP in blocking buffer for 1 h. LOC105374325 was visualized in a staining reaction with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate solution (39–41).
Podocyte culture and treatment
Immortalized temperature-sensitive human podocytes (gift from Dr. M Saleem) were propagated at permissive temperature (33 °C) in RPMI 1640 medium supplemented with 10% FBS and insulin/transferrin/selenium (42). Cells were then allowed to differentiate for 2 weeks under nonpermissive conditions at 37 °C in the same medium. Podocytes were treated with ADR (0.5 μg/ml) for 24 h. For intervention studies, 10 μm p38 MAPK inhibitor SB203580 (sc-3533, Santa Cruz), 10 μm Akt inhibitor MK2206 (sc-364537, Santa Cruz), 50 μm ERK inhibitor PD98059 (sc-3532, Santa Cruz), 5 μm GSK3β inhibitor TDZD-8 (sc-221692, Santa Cruz), or 1 μm U-46619 (sc-201242, Santa Cruz) was given 30 min before treatments. To infect podocytes with plenti-CMV-LOC105374325, plenti-CMV-Bax, plenti-CMV-Bak, or plenti-CMV-C/EBPβ plasmid, the lentiviral stock was mixed with Polybrene (1 μg/ml) and added to podocytes. BAX siRNA (sc-29212), BAK siRNA (sc-29786), C/EBPβ siRNA (sc-44251), c-JUN siRNA (sc-29223), ERα siRNA (sc-29305), p53 siRNA (sc-29435), STAT1 siRNA (sc-44123), STAT4 siRNA (sc-36568), and XBP-1 siRNA (sc-38627) were bought from Santa Cruz Biotechnology (Dallas, TX). LOC105374325 siRNA were bought from Thermo Fisher (4390771, Carlsbad, CA). Transfection of siRNA, miRNA mimics, or miRNA ASO was conducted with Lipofectamine 2000.
Apoptosis analysis with flow cytometry
Detection of apoptotic podocytes was performed using FITC Annexin V Apoptosis Detection Kit I (556547, Biosciences, San Jose, CA).
Apoptosis antibody array analysis
Apoptosis antibody array featured highly specific and well-characterized antibodies related to apoptosis (APP069, Full Moon BioSystems). The level of apoptosis-related proteins in glomerular tissues were analyzed with the apoptosis antibody array according to the manufacturer's protocol.
Immunohistochemical staining
Paraffin-embedded sections were deparaffinized, rehydrated, and stained using Super SensitiveTM IHC Detection System kit (BD5001, Bioworld, MN). Endogenous peroxidase was blocked with 0.3% hydrogen peroxide for 30 min. The sections were incubated for 1 h with primary antibody. Negative controls were obtained by omission of the primary antibody from the staining procedure.
Western blot analysis
Tissues or cells were lysed in RIPA buffer supplemented with protease inhibitors. Protein concentrations were determined using a bicinchoninic acid protein assay kit (BCA1, Sigma) (43). Samples with equal amounts of total protein (30 μg) were fractionated on 10% SDS-polyacrylamide gels and analyzed by Western immunoblot (44). The antibodies were listed in Table S2.
MiRNA PCR array assay
MiRNAs of LOC105374325-overexpressed podocytes were converted to cDNA using the miRNA first-strand kit. The level of miRNAs were detected using the Human miFinder miScript miRNA PCR Array (MIHS-3001, Exiqon, Vedbaek, Denmark) (38).
Luciferase assays
Podocytes were transiently cotransfected with 0.1 μg of the reporter constructs, 0.02 μg of the Renilla construct, and 50 nm synthetic miRNA mimics. The firefly and Renilla luciferase activity were determined using the Dual-Luciferase Reporter Assay System (E1910, Promega, Madison, WI). Values were normalized with Renilla luciferase (38, 45).
Biotin pulldown assay
Tissue or cells were lysed in 10% glycerol, 20 mm Tris (pH 8), 0.2 mm EDTA, 0.5% Nonidet P-40, 0.5 m KCl, 1 mm DTT (46). The biotinylated DNA probe complementary to LOC105374325 (100 pmol) was incubated with Dynabeads M-280 Streptavidin (11205D, Invitrogen) at room temperature for 10 min to generate probe-coated beads according to the manufacturer's protocol. Then, tissue or cell lysates were incubated with the probe-coated beads, and the RNA complexes bound to these beads were extracted for RT-PCR analysis (47, 48).
Human phosphokinase array analysis
The relative phosphorylation level of AKT1(Ser-473), ERK(Thr-202/Tyr-204), GSK3β(Ser-9), JNK(Thr-183/Tyr-185), p38(Thr-180/Tyr-182), p70-S6(Thr-421/Ser-424), and Src(Tyr-416) in ADR-treated podocytes were measured by human phosphokinase array (Phospho RTK4, Labospace, Milano, Italy).
ChIP analysis of C/EBPβ DNA binding
ChIP assay was performed with the ChIP-IT Express Magnetic Chromatin Immunoprecipitation kit (53008, Active Motif, Carlsbad, CA) and anti-C/EBPβ or IgG antibodies (38).
Overexpression of LOC105374325 in mice
Animal studies were approved by the Institutional Animal Care and Use Committee of Jinling Hospital. We obtained the male C57BL/6 mice (8 weeks) from the Model Animal Research Center of Nanjing University (Nanjing, China). LOC105374325 was not conserved in mouse. For introducing exogenous LOC105374325 in the kidney of mice, mice were administrated LOC105374325 expression plasmid by a hydrodynamic-based gene transfer technique (23). Briefly, we mixed LOC105374325-expressing plasmid (20 μg) into ∼2.6 ml of TransIT-EE Hydrodynamic Delivery Solution (Mirus, Madison, WI). Then we injected the mixture into mice via the tail vein in 5 s. Each mouse received injections once a week for a total of 4 weeks. Urine samples were collected in metabolic cages, and the albumin and creatinine levels were measured using Albuwell M and Creatinine Companion Kits (1101 Exocell, Philadelphia, PA).
Isolation of mouse glomeruli
Mice glomeruli were isolated by the magnetic bead-based isolation technique (49–51). Mice were anesthetized and the kidney was perfused with 5 ml of saline containing 8 × 107 Dynabeads M-450 (Dynal Biotech ASA, Oslo, Norway). The kidneys were then cut into 1-mm3 pieces and then digested in a digestion buffer containing 1 mg/ml of collagenase A for 30 min at 37 °C. The tissue was then pressed gently through a 100-μm cell strainer (Falcon, Bedford, MA) and washed with ice-cold sterilized PBS. Glomeruli-containing Dynabeads were gathered using a magnetic particle concentrator.
Analysis of podocyte apoptosis in renal tissue
Apoptotic cell death in kidney sections was detected by a FITC-labeled in situ TUNEL assay (11684795910, Roche Molecular Biochemicals, Mannheim, Germany) following the manufacturer's protocol. The cells labeled by 4′,6-diamidino-2-phenylindole, WT1, and TUNEL were counted as apoptotic podocytes.
Statistics
All of the data are expressed as the mean ± S.D. The data from multiple groups were analyzed with a one-way ANOVA followed by a Tukey's post hoc test. Data from two groups were compared by t tests. p values <0.05 were considered significant.
Author contributions
S. H., R. H., J. S., X. Z., W. Q., and C. Z. investigation; S. H., R. H., J. S., X. Z., W. Q., and C. Z. methodology; S. H. and H. B. writing-original draft; S. H., H. B., and Z. L. writing-review and editing; H. B. conceptualization; H. B. and Z. L. funding acquisition; H. B. and Z. L. project administration.
Supplementary Material
Acknowledgments
We thank the physicians, patients, and volunteers who contributed to this study.
This work was supported by National Natural Science Foundation of China Grants 81873610 and 81200516, Natural Science Foundation of Jiangsu Province Grants BK20171330 and BK2012372, Deng Feng Scholars of Nanjing University Grant 2015–105, Fundamental Research Funds for the Central Universities Grant 021414380347, National Key Research and Development Program of China Grant 2016YFC0904100, Major International (Regional) Joint Research Project Grant 81320108007, and Key Research and Development Program of Jiangsu Province Grant BE2016747. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1 and S2 and Tables S1 and S2.
The accession number for the microarray data reported in this paper is GEO accession number GSE121233.
- FSGS
- focal segmental glomerulosclerosis
- lncRNAs
- long noncoding RNAs
- ISH
- in situ hybridization
- ADR
- adriamycin
- IHC
- immunohistochemical
- miR
- microRNA
- ASO
- antisense oligonucleotide
- GSK3β
- glycogen synthase kinase 3β
- ERK
- extracellular signal-regulated kinase
- IL
- interleukin
- C/EBP
- CCAAT enhancer–binding protein
- MAPK
- mitogen-activated protein kinase
- CMV
- cytomegalovirus
- STAT
- signal transducers and activators of transcription
- ANOVA
- analysis of variance.
References
- 1. D'Agati V. D., Kaskel F. J., and Falk R. J. (2011) Focal segmental glomerulosclerosis. N. Engl. J. Med. 365, 2398–2411 10.1056/NEJMra1106556 [DOI] [PubMed] [Google Scholar]
- 2. Alexopoulos E., Stangou M., Papagianni A., Pantzaki A., and Papadimitriou M. (2000) Factors influencing the course and the response to treatment in primary focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 15, 1348–1356 10.1093/ndt/15.9.1348 [DOI] [PubMed] [Google Scholar]
- 3. Tang X., Xu F., Chen D. M., Zeng C. H., and Liu Z. H. (2013) The clinical course and long-term outcome of primary focal segmental glomerulosclerosis in Chinese adults. Clin. Nephrol. 80, 130–139 10.5414/CN107607 [DOI] [PubMed] [Google Scholar]
- 4. Xiao B., Wang L. N., Li W., Gong L., Yu T., Zuo Q. F., Zhao H. W., and Zou Q. M. (2018) Plasma microRNA panel is a novel biomarker for focal segmental glomerulosclerosis and associated with podocyte apoptosis. Cell Death Dis 9, 533 10.1038/s41419-018-0569-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maidannyk V. G., and Burlaka I. A. (2014) Violations in apoptosis control system in children with nephrotic syndrome. Pharma Innovation J. 3, 43–46 10.7897/2277-4572.031104 [DOI] [Google Scholar]
- 6. Jiang X., and Zhang F. (2017) Long noncoding RNA: a new contributor and potential therapeutic target in fibrosis. Epigenomics 9, 1233–1241 10.2217/epi-2017-0020 [DOI] [PubMed] [Google Scholar]
- 7. Hu M., Wang R., Li X., Fan M., Lin J., Zhen J., Chen L., and Lv Z. (2017) LncRNA MALAT1 is dysregulated in diabetic nephropathy and involved in high glucose-induced podocyte injury via its interplay with β-catenin. J. Cell. Mol. Med. 21, 2732–2747 10.1111/jcmm.13189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Long J., Badal S. S., Ye Z., Wang Y., Ayanga B. A., Galvan D. L., Green N. H., Chang B. H., Overbeek P. A., and Danesh F. R. (2016) Long noncoding RNA Tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. J. Clin. Invest. 126, 4205–4218 10.1172/JCI87927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ling L., Tan Z., Zhang C., Gui S., Hu Y., and Chen L. (2018) Long noncoding RNA ENSRNOG00000037522 is involved in the podocyte epithelial-mesenchymal transition in diabetic rats. Int. J. Mol. Med. 41, 2704–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trempolec N., Dave-Coll N., and Nebreda A. R. (2013) SnapShot: p38 MAPK substrates. Cell 152, 924–924.e1 10.1016/j.cell.2013.01.047 [DOI] [PubMed] [Google Scholar]
- 11. Floege J., and Amann K. (2016) Primary glomerulonephritides. Lancet 387, 2036–2048 10.1016/S0140-6736(16)00272-5 [DOI] [PubMed] [Google Scholar]
- 12. Kriz W., Gretz N., and Lemley K. V. (1998) Progression of glomerular diseases: is the podocyte the culprit. Kidney Int. 54, 687–697 10.1046/j.1523-1755.1998.00044.x [DOI] [PubMed] [Google Scholar]
- 13. Shankland S. J. (2006) The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 69, 2131–2147 10.1038/sj.ki.5000410 [DOI] [PubMed] [Google Scholar]
- 14. Wu J., Zheng C., Fan Y., Zeng C., Chen Z., Qin W., Zhang C., Zhang W., Wang X., Zhu X., Zhang M., Zen K., and Liu Z. (2014) Downregulation of microRNA-30 facilitates podocyte injury and is prevented by glucocorticoids. J. Am. Soc. Nephrol. 25, 92–104 10.1681/ASN.2012111101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adams B. D., Parsons C., Walker L., Zhang W. C., and Slack F. J. (2017) Targeting noncoding RNAs in disease. J. Clin. Invest. 127, 761–771 10.1172/JCI84424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puthanveetil P., Chen S., Feng B., Gautam A., and Chakrabarti S. (2015) Long non-coding RNA MALAT1 regulates hyperglycaemia induced inflammatory process in the endothelial cells. J. Cell. Mol. Med. 19, 1418–1425 10.1111/jcmm.12576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun S. F., Tang P. M., Feng M., Xiao J., Huang X. R., Li P., Ma R. C., and Lan H. Y. (2018) Novel lncRNA Erbb4-IR promotes diabetic kidney injury in db/db mice by targeting miR-29b. Diabetes 67, 731–744 10.2337/db17-0816 [DOI] [PubMed] [Google Scholar]
- 18. Zea A. H., Stewart T., Ascani J., Tate D. J., Finkel-Jimenez B., Wilk A., Reiss K., Smoyer W. E., and Aviles D. H. (2016) Activation of the IL-2 receptor in podocytes: a potential mechanism for podocyte injury in idiopathic nephrotic syndrome. PLoS ONE 11, e0157907 10.1371/journal.pone.0157907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lei J., Zhao L., Zhang Y., Wu Y., and Liu Y. (2018) High glucose-induced podocyte injury involves activation of mammalian target of rapamycin (mTOR)-induced endoplasmic reticulum (ER) stress. Cell. Physiol. Biochem. 45, 2431–2443 10.1159/000488231 [DOI] [PubMed] [Google Scholar]
- 20. Burlaka I., Nilsson L. M., Scott L., Holtbäck U., Eklöf A. C., Fogo A. B., Brismar H., and Aperia A. (2016) Prevention of apoptosis averts glomerular tubular disconnection and podocyte loss in proteinuric kidney disease. Kidney Int. 90, 135–148 10.1016/j.kint.2016.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu J. B., Ye S. F., Liang C. L., Li Y. C., Yu Y. J., Lai J. M., Lin H., Zheng J., and Zhou J. Y. (2014) Qi-Dan Fang ameliorates adriamycin-induced nephrotic syndrome rat model by enhancing renal function and inhibiting podocyte injury. J. Ethnopharmacol. 151, 1124–1132 10.1016/j.jep.2013.12.028 [DOI] [PubMed] [Google Scholar]
- 22. Salmena L., Poliseno L., Tay Y., Kats L., and Pandolfi P. P. (2011) A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language. Cell 146, 353–358 10.1016/j.cell.2011.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meng J., Li L., Zhao Y., Zhou Z., Zhang M., Li D., Zhang C. Y., Zen K., and Liu Z. (2016) MicroRNA-196a/b mitigate renal fibrosis by targeting TGF-β receptor 2. J. Am. Soc. Nephrol. 27, 3006–3021 10.1681/ASN.2015040422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Z., Wang L., Xu J., and Yang Z. (2015) MiRNA expression profile and miRNA-mRNA integrated analysis (MMIA) during podocyte differentiation. Mol. Genet. Genomics 290, 863–875 10.1007/s00438-014-0960-z [DOI] [PubMed] [Google Scholar]
- 25. Liu X. D., Zhang L. Y., Zhu T. C., Zhang R. F., Wang S. L., and Bao Y. (2015) Overexpression of miR-34c inhibits high glucose-induced apoptosis in podocytes by targeting Notch signaling pathways. Int. J. Clin. Exp. Pathol. 8, 4525–4534 [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang C., Liang S., Cheng S., Li W., Wang X., Zheng C., Zeng C., Shi S., Xie L., Zen K., and Liu Z. (2018) Urinary miR-196a predicts disease progression in patients with chronic kidney disease. J. Transl. Med. 16, 91 10.1186/s12967-018-1470-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang W., Zhang C., Chen H., Li L., Tu Y., Liu C., Shi S., Zen K., and Liu Z. (2014) Evaluation of microRNAs miR-196a, miR-30a-5P, and miR-490 as biomarkers of disease activity among patients with FSGS. Clin. J. Am. Soc. Nephrol. 9, 1545–1552 10.2215/CJN.11561113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Catuogno S., Cerchia L., Romano G., Pognonec P., Condorelli G., and de Franciscis V. (2013) miR-34c may protect lung cancer cells from paclitaxel-induced apoptosis. Oncogene 32, 341–351 10.1038/onc.2012.51 [DOI] [PubMed] [Google Scholar]
- 29. Wang H. L., Zhou R., Liu J., Chang Y., Liu S., Wang X. B., Huang M. F., and Zhao Q. (2017) MicroRNA-196b inhibits late apoptosis of pancreatic cancer cells by targeting CADM1. Sci. Rep. 7, 11467 10.1038/s41598-017-11248-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cao D., Hu L., Lei D., Fang X., Zhang Z., Wang T., Lin M., Huang J., Yang H., Zhou X., and Zhong L. (2015) MicroRNA-196b promotes cell proliferation and suppress cell differentiation in vitro. Biochem. Biophys. Res. Commun. 457, 1–6 10.1016/j.bbrc.2014.11.085 [DOI] [PubMed] [Google Scholar]
- 31. Koshikawa M., Mukoyama M., Mori K., Suganami T., Sawai K., Yoshioka T., Nagae T., Yokoi H., Kawachi H., Shimizu F., Sugawara A., and Nakao K. (2005) Role of p38 mitogen-activated protein kinase activation in podocyte injury and proteinuria in experimental nephrotic syndrome. J. Am. Soc. Nephrol. 16, 2690–2701 10.1681/ASN.2004121084 [DOI] [PubMed] [Google Scholar]
- 32. Bao W., Xia H., Liang Y., Ye Y., Lu Y., Xu X., Duan A., He J., Chen Z., Wu Y., Wang X., Zheng C., Liu Z., and Shi S. (2016) Toll-like receptor 9 can be activated by endogenous mitochondrial DNA to induce podocyte apoptosis. Sci. Rep. 6, 22579 10.1038/srep22579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu W. T., Peng F. F., Li H. Y., Chen X. W., Gong W. Q., Chen W. J., Chen Y. H., Li P. L., Li S. T., Xu Z. Z., and Long H. B. (2016) Metadherin facilitates podocyte apoptosis in diabetic nephropathy. Cell Death Dis. 7, e2477 10.1038/cddis.2016.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van Laethem A., Van Kelst S., Lippens S., Declercq W., Vandenabeele P., Janssens S., Vandenheede J. R., Garmyn M., and Agostinis P. (2004) Activation of p38 MAPK is required for Bax translocation to mitochondria, cytochrome c release and apoptosis induced by UVB irradiation in human keratinocytes. FASEB J. 18, 1946–1948 10.1096/fj.04-2285fje [DOI] [PubMed] [Google Scholar]
- 35. Chen H. H., Lan Y. F., Li H. F., Cheng C. F., Lai P. F., Li W. H., and Lin H. (2016) Urinary miR-16 transactivated by C/EBPβ reduces kidney function after ischemia/reperfusion-induced injury. Sci. Rep. 6, 27945 10.1038/srep27945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu M., Wilson N. O., Hibbert J. M., and Stiles J. K. (2013) STAT3 regulates MMP3 in heme-induced endothelial cell apoptosis. PLoS ONE 8, e71366 10.1371/journal.pone.0071366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen Y., Yu S., Zhang N., Li Y., Chen S., Chang Y., Sun G., and Sun Y. (2017) Atorvastatin prevents angiotensin II induced myocardial hypertrophy in vitro via CCAAT/enhancer-binding protein β. Biochem. Biophys. Res. Commun. 486, 423–430 10.1016/j.bbrc.2017.03.057 [DOI] [PubMed] [Google Scholar]
- 38. Bao H., Chen H., Zhu X., Zhang M., Yao G., Yu Y., Qin W., Zeng C., Zen K., and Liu Z. (2014) MiR-223 downregulation promotes glomerular endothelial cell activation by upregulating importin α4 and α5 in IgA nephropathy. Kidney Int. 85, 624–635 10.1038/ki.2013.469 [DOI] [PubMed] [Google Scholar]
- 39. Gupta R. A., Shah N., Wang K. C., Kim J., Horlings H. M., Wong D. J., Tsai M. C., Hung T., Argani P., Rinn J. L., Wang Y., Brzoska P., Kong B., Li R., West R. B., et al. (2010) Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464, 1071–1076 10.1038/nature08975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lv X. B., Lian G. Y., Wang H. R., Song E., Yao H., and Wang M. H. (2013) Long noncoding RNA HOTAIR is a prognostic marker for esophageal squamous cell carcinoma progression and survival. PLoS ONE 8, e63516 10.1371/journal.pone.0063516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bao H., Hu S., Zhang C., Shi S., Qin W., Zeng C., Zen K., and Liu Z. (2014) Inhibition of miRNA-21 prevents fibrogenic activation in podocytes and tubular cells in IgA nephropathy. Biochem. Biophys. Res. Commun. 444, 455–460 10.1016/j.bbrc.2014.01.065 [DOI] [PubMed] [Google Scholar]
- 42. Saleem M. A., O'Hare M. J., Reiser J., Coward R. J., Inward C. D., Farren T., Xing C. Y., Ni L., Mathieson P. W., and Mundel P. (2002) A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 13, 630–638 [DOI] [PubMed] [Google Scholar]
- 43. Gong R., Latif S., Morris D. J., and Brem A. S. (2008) Co-localization of glucocorticoid metabolizing and prostaglandin synthesizing enzymes in rat kidney and liver. Life Sci. 83, 725–731 10.1016/j.lfs.2008.09.015 [DOI] [PubMed] [Google Scholar]
- 44. Bao H., Ge Y., Zhuang S., Dworkin L. D., Liu Z., and Gong R. (2012) Inhibition of glycogen synthase kinase-3β prevents NSAID-induced acute kidney injury. Kidney Int. 81, 662–673 10.1038/ki.2011.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hu S., Bao H., Xu X., Zhou X., Qin W., Zeng C., and Liu Z. (2015) Increased miR-374b promotes cell proliferation and the production of aberrant glycosylated IgA1 in B cells of IgA nephropathy. FEBS Lett. 589, 4019–4025 10.1016/j.febslet.2015.10.033 [DOI] [PubMed] [Google Scholar]
- 46. Nonne N., Ameyar-Zazoua M., Souidi M., and Harel-Bellan A. (2010) Tandem affinity purification of miRNA target mRNAs (TAP-Tar). Nucleic Acids Res. 38, e20 10.1093/nar/gkp1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang P., Liu Y. H., Yao Y. L., Li Z., Li Z. Q., Ma J., and Xue Y. X. (2015) Long non-coding RNA CASC2 suppresses malignancy in human gliomas by miR-21. Cell. Signal. 27, 275–282 10.1016/j.cellsig.2014.11.011 [DOI] [PubMed] [Google Scholar]
- 48. Ma M. Z., Chu B. F., Zhang Y., Weng M. Z., Qin Y. Y., Gong W., and Quan Z. W. (2015) Long non-coding RNA CCAT1 promotes gallbladder cancer development via negative modulation of miRNA-218–5p. Cell Death Dis. 6, e1583 10.1038/cddis.2014.541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ilatovskaya D. V., Palygin O., Chubinskiy-Nadezhdin V., Negulyaev Y. A., Ma R., Birnbaumer L., and Staruschenko A. (2014) Angiotensin II has acute effects on TRPC6 channels in podocytes of freshly isolated glomeruli. Kidney Int. 86, 506–514 10.1038/ki.2014.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li C., Ge Y., Dworkin L., Peng A., and Gong R. (2016) The β isoform of GSK3 mediates podocyte autonomous injury in proteinuric glomerulopathy. J. Pathol. 239, 23–35 10.1002/path.4692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ilatovskaya D. V., Palygin O., Levchenko V., Endres B. T., and Staruschenko A. (2017) The role of angiotensin II in glomerular volume dynamics and podocyte calcium handling. Sci. Rep. 7, 299 10.1038/s41598-017-00406-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.